Abstract
AMP-activated protein kinase (AMPK), an enzyme that plays a role in cellular energy homeostasis, modulates myocardial signaling in the heart. Myocardial dysfunction is a common complication of sepsis. Autophagy is involved in the aging related cardiac dysfunction. However, the role of AMPK in sepsis-induced cardiotoxicity has yet to be clarified, especially in aging. In this study, we explored the role of AMPK in lipopolysaccharide (LPS)-induced myocardial dysfunction and elucidated the potential mechanisms of AMPK/mTOR pathway regulating autophagy in young and aged mice. We harvested cardiac tissues by intraperitoneal injection of LPS treatment. The results by echocardiography, pathology, contractile and intracellular Ca2+ property as well as western blot analysis revealed that LPS induced remarkable cardiac dysfunction and cardiotoxicity in mice hearts and cardiomyocytes, which were more seriously in the aged mice. Western blot analysis indicated that the underlying mechanisms included inhibition autophagy mediated by AMPK/mTOR activation. LPS overtly promoted the expression of AMPK upstream regulator PP2A and PP2Cα. Pharmacological activation of AMPK improved cardiac function and upregulated cardiac autophagy induced by LPS in the aged mice. Collectively, our findings suggest that upregulation of autophagy by administration of AMPK could attenuate LPS-induced cardiotoxicity, which enhances our knowledge to explore new drugs and strategies for combating cardiac dysfunction induced by sepsis.
Keywords: AMPK, mTOR, Aging, Endotoxemia
1. Introduction
Sepsis is a major healthcare issue in hospitalized patients, especially in intensive care units. It can result in the high mortality and costs. Cardiac functional alterations induced by sepsis are considered as major components in outcome. Lipopolysaccharides (LPS), component of outer membrane of gram-negative bacteria, can induce experimental endotoxemia that mimicks sepsis-induced cardiac dysfunction in clinic. Intraperitoneal injection of LPS to mice is a widely used animal model to assess sepsis-induced cardiac dysfunction. Nonetheless, the precise mechanistic progression involved in the heart failure under septic shock is still need to be elucidated.
AMP-activated protein kinase (AMPK) has been found to be a key character against cardiovascular diseases and cellular stress. When activated by certain stress, AMPK regulates sugars and fatty acids that are good or detrimental to the heart. For example, targeting AMPK phosphorylation is known to protect against ischemia reperfusion-induced injury [1,2]. The mammalian target of rapamycin complex 1 (mTORC1) has a central role among the intracellular signal transduction pathways adapting growth, metabolism and aging [3]. Genetic alterations of mTORC1 elements affect aging in mice [4]. It is helpful to clarify the molecular targets downstream of mTORC1 which specifically affect age-related disorders. S6 kinases 1 and 2 (S6Ks) are mTORC1 substrates that possess serine/threonine kinase enzymatic activity. S6K1 and S6K2 are homologous proteins sharing similar modes of regulation and substrate specificities. S6K activity increases during aging and has been associated to lifespan [5].
Autophagy mainly maintains a balance between manufacture of cellular components and break down of damaged or unnecessary organelles and other cellular constituents. If some exogenous stimuli such as microbial invasion of the body insult the occurrence of endotoxemia, autophagy could trigger cell death pathways to protect or adapt the response. Researchers have focused concentration on identifying small molecules acting as chaperones to stimulate or stabilize proteins that regulate autophagy [6]. There is evidence indicated that LPS can induce autophagy in macrophages and mice [7,8]. It was reported that cardiac-specific Atg5-defcient mice showed age-related cardiomyopathy. Continuous constitutive autophagy plays an important role in maintaining cardiac structure and function [9].
The elderly population grows more rapidly in Asia now, especially in China. The elderly population will overtake the young in three decades later [10]. Aging is an important risk factor for heart diseases which the end stage is heart failure. The mechanisms of sepsis-induced heart failure have been rised concerns both in basic and clinic research. An ample of molecular mechanisms such as apoptosis, immune regulation, mitochondria, and energy metabolism have been revealed [11–13]. Autophagy is associated with accelerated cardiac aging. Reduced autophagic potential leads to aging and increased autophagy delays aging [14,15]. However, the role of autophagy and cardiac dysfunction in sepsis of aging are not clearly till now.
Due to the precise mechanisms and their role in the pathogenesis of septic cardiac dysfunction in aging remain incompletely understood. Thus the purpose of this study is to investigate the functional role and relevant mechanism of AMPK and autophagy in septic cardiac dysfunction in young and aged mice exposed to LPS. Furthermore, we assessed the relationship between AMPK and mTOR/S6 signaling molecules using A769662, a selective AMPK activator.
2. Material and methods
Experimental animals and LPS treatment
All animal procedures were approved by the Animal Care and Use Committee at University of Mississippi Medical Center. Sex-matched C57BL/6 young (3–4 months) and aged mice (18–20 months) were used. All animals were kept in our institutional animal facility with free access to laboratory chow and tap water. On the day of experimentation, both young and aged mice were injected intraperitoneally with 4 mg/kg Escherichia Coli LPS (Sigma-Aldich, St. Louis, MO) dissolved in sterile saline or an equivalent volume of pathogen-free saline (for control groups). The dosage of LPS injection was chosen based on previous observation of overt myocardial dysfunction without significant mortality [16]. We observed the condition of treated mice 4 h until they were used for experimentation. Four hours following LPS challenge, mice were sacrificed by breaking the neck for experimentation. Activation of AMPK in vivo was assessed in LPS treated young and aged mice following intraperitoneal injection of AMPK activator 30 mg/kg A769662 (Selleckchem, Houston, TX) 30mins before LPS treated [17].
Echocardiography examination
The representative randomly selected animals from each group were anaesthetized (isoflurane) and transthoracic M-mode echocardiography (Vevo 770, Visual-Sonics, Toronto, Canada) was performed. Left ventricular end-diastolic dimension, left ventricular end-systolic dimension and left ventricular diastolic interventricular septum thickness were measured [18]. Left ventricular ejection fraction was also calculated from M-mode echocardiograms. Data from three to five consecutive selected cardiac cycles were analyzed and averaged [19].
Immunohistochemistry
Hearts from young and aged mice were collected at the indicated times, fixed overnight in 10% formalin, and embedded in paraffin. Serial 5-μm heart sections from each group were analyzed. Samples were stained with H&E for routine histologic examination. Immunohistochemical staining was performed as described previously [20]. The histological sections were stained with primary antibodies against Mac-2 (1:200, Abcam) at 4 °C overnight. The bound antibodies were labeled using a second antibody (Vectastain ABC Kit, VECTOR Laboratories, Inc., Burlingame, CA). Images were captured using a Zeiss Axioimager Motorized fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
Isolation of murine cardiomyocytes
Mice were given 100 units of heparin i.p. (Sagent Pharmaceuticals, Schaumburg, IL) for anticoagulation before anaesthetized with 100 mg/kg sodium pentobarbital i.p. (Sigma, St. Louis, MO). The heart was excised and fastened onto the cardiomyocyte perfusion apparatus (Radnoti, Monrovia, CA) and perfusion was initiated in the Langendorff mode. Hearts were perfused at 37 °C with a Ca2+-free Krebs-Henseleit based buffer (pH 7.3) containing: 0.6 mM KH2PO4, 0.6 mM Na2HPO4, 10 mM HEPES, 14.7 mM KCl, 1.7 mM MgSO4, 120.3 mM NaCl, 4.6 mM NaHCO3, 30 mM taurine, 10 mM glucose, and 10 mM 2,3-butanedione monoxime that was bubbled with 95% O2/5% CO2. After a few minutes of stabilization, the heart was then digested with the same perfusion buffer containing 0.067 mg/mL Liberase Blendzyme 4 (Roche, Indianapolis, IN). After digestion, the heart was removed and minced. Extracellular Ca2+ was added back to the cells to reach a final concentration of 1 mM.
Cell shortening/relengthening
The mechanical properties of cardiomyocytes were assessed by using a SoftEdge MyoCam system (IonOptix Corporation, Milton, MA) [21]. Cardiomyocytes were placed in a chamber and stimulated with a suprathreshold voltage at a frequency of 0.5 Hz. IonOptix SoftEdge software was used to capture changes in sarcomere length during shortening and re-lengthening. Cell shortening and re-lengthening were assessed using the following indices: peak shortening (PS), the amplitude myocytes shortened on electrical stimulation, which is indicative of peak ventricular contractility; time-to-90% relengthening (TR90), the duration of myocytes to reach 90% relengthening, an indicative of diastolic duration; and maximal velocities of shortening and re-lengthening (±dL/dt); time to peak shortening (TPS).
Intracellular Ca2+ fluorescence measurement
Intracellular Ca2+ was measured by using a dual-excitation, single emission photomultiplier system (IonOptix). Cardiomyocytes were loaded with fura 2-AM(2 μM) and were exposed to light emitted by a 75 W halogen lamp through either a 340- or 380-nm filter while being stimulated to contract at a frequency of 0.5 Hz. Fluorescence emissions were then detected [21].
Western blot analysis
The protein concentrations of all samples were measured by using Bradford dye-binding method (Dye Reagent Concentrate, Bio-Rad Protein Assay). Proteins from the heart were separated by SDS–PAGE, transferred to nitrocellulose membranes (Millipore, Bedford, MA), and probed with primary antibodies against p-AMPK, p-ACC, PP2A, PP2Cα, p62, atg5, LC3 I/II, p-mTOR, p-S6 and followed by incubation with horseradish peroxidase (HRP)-coupled anti-rabbit secondary antibody (Cell Signaling Technology Inc, Beverly, MA). Blue x-ray film (Phenix, Candler, NC) was used for photon detection and image development. Films were scanned with the Bio-Rad GS-700 scanner in the core facility of the SMBS and the relative density of the bands on the film was determined by Image J software.
Statistical analysis
Data were Mean ± SEM and analyzed by using GraphPad Prism 7.0 statistical analysis software. Difference was assessed using analysis of variance (ANOVA) followed by a Tukey’s post hoc test or Student’s t-test. A p value < 0.05 was considered statistically significant.
3. Results
3.1. Morphological properties of young and aged mice treated with LPS
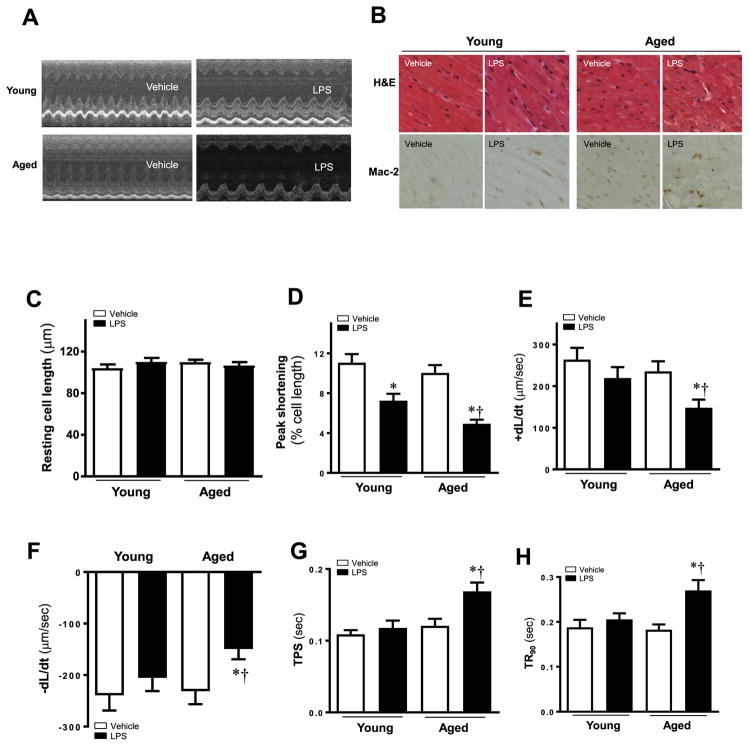
Echocardiographic analyses were conducted to evaluate the sepsis induced cardiac dysfunction in aged mice. LPS was intraperitoneally administrated to mice to induce septic myocardial dysfunction. Echocardiographic assessment revealed no changes in heart weight, heart rate and interventricular septum thickness between young and aged mice with or without LPS challenge. However, LPS treatment overtly increased myocardial volume in Left ventricular end-diastolic dimension and left ventricular end-systolic dimension, accompanying the decreased global left ejection fraction (Table 1 and Fig. 1A) in aged mice. These findings suggest aged mice were more sensitive to LPS challenge compared to young mice. Represent pictures in Fig. 1B showed the HE and Mac-2 staining of different groups. The arrangement of cardiomyocyte was disorder in aged mice challenged with LPS. More stained macrophages can be detected in aged mice challenged with LPS.
Table 1.
Heart weight and echocardiographic parameters of LPS-treated C57BL/6J young and aged mice.
| Parameter | Young | Young + LPS | Aged | Aged + LPS |
|---|---|---|---|---|
| Heart weight (mg) | 116 ± 9 | 121 ± 18 | 119 ± 12 | 119 ± 10 |
| Heart rate (bpm) | 422 ± 39 | 441 ± 21 | 423 ± 30 | 394 ± 26 |
| Interventricular septum thickness (mm) | 0.93 ± 0.04 | 0.89 ± 0.06 | 0.83 ± 0.06 | 0.77 ± 0.07 |
| Left ventricular end-diastolic dimension (mm) | 2.17 ± 0.14 | 2.30 ± 0.21 | 2.21 ± 0.28 | 2.99 ± 0.19*# |
| Left ventricular end-systolic dimension (mm) | 1.21 ± 0.17 | 1.49 ± 0.14 | 1.19 ± 0.15 | 1.99 ± 0.15*# |
| Ejection fraction (%) | 80.3 ± 5.1 | 68.8 ± 5.9* | 83.7 ± 3.0 | 50.6 ± 2.4*# |
Mean ± SEM, n = 3 mice per group,
p < 0.05 vs. young group,
p < 0.05 vs. young-LPS group.
Fig. 1.
(A) Representative echocardiographic recordings from four mice groups. (B) Representative pathological images of HE staining and Mac-2 stained for inflammation measurement. Cardiomyocyte contractile properties in young and aged mice treated with or without LPS. (C) Resting cell length; (D) Peak shortening (PS, normalized to cell length); (E) Maximal velocity of shortening (+dL/dt); (F) Maximal velocity of relengthening (- dL/dt); (G) Time-to-PS (TPS); and (H) Time-to-90% relengthening (TR90). Mean ± SEM, 3–4 mice per group, *p < 0.05 vs. their littermates; †p < 0.05 vs. young -LPS group.
3.2. Effect of LPS on contractile responses and intracellular Ca2+ properties of murine cardiomyocytes
Contractile properties were evaluated to confirm the difference of LPS-induced cardiomyocyte mechanical anomalies in young and aged mice. Average values were showed representative cell shortening and relengthening obtained after exposure to LPS. Neither LPS nor age affected resting cell length. However, LPS challenge markedly reduced PS and ±dL/dt in aged mice compared with young mice. Compared with young mice group TPS and TR90 are prolonged in aged mice group (Fig. 1C–H). These results favored a detrimental role of aging in LPS-induced cardiomyocyte contractile defect.
To further evaluate the possible mechanisms of action behind aging-elicited mechanical derangement, intracellular Ca2+ property was evaluated using the Fura-2 fluorescence technique.
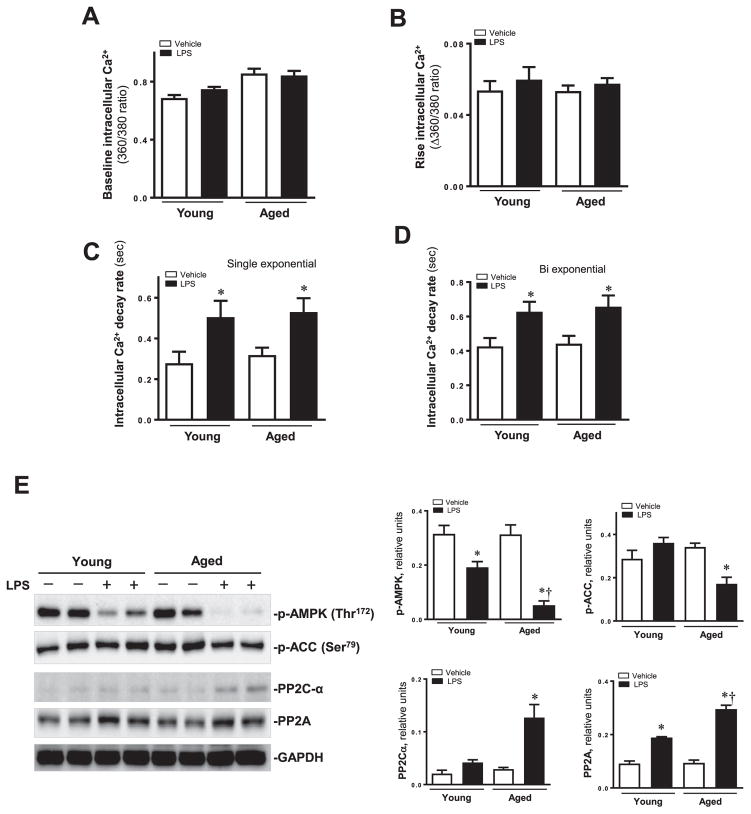
Our data revealed that LPS challenge did not elicit any notable changes in resting and rise intracellular Ca2+ levels although it significantly prolonged intracellular Ca2+ clearance. (Fig. 2A–D).
Fig. 2.
Intracellular Ca2+ properties in cardiomyocytes from young and aged mice treated with or without LPS. (A) Resting intracellular Ca2+; (B) Electrically-stimulated rise in intracellular Ca2+; (C) and (D) Single and bi-exponential intracellular Ca2+ decay rate. Mean ± SEM, 3–4 mice per group, *p < 0.05 vs. their littermates. (E) Western blot analysis in myocardium from young and aged mice treated with or without LPS. Expression levels of p-AMPK, p-ACC, PP2C-α and PP2A A proteins using specific antibodies. Mean ± SEM, n = 3–4 samples per group, *p < 0.05 vs. their littermates; †p < 0.05 vs. young -LPS group.
3.3. Effect of LPS-induced changes of AMPK-mTOR signaling
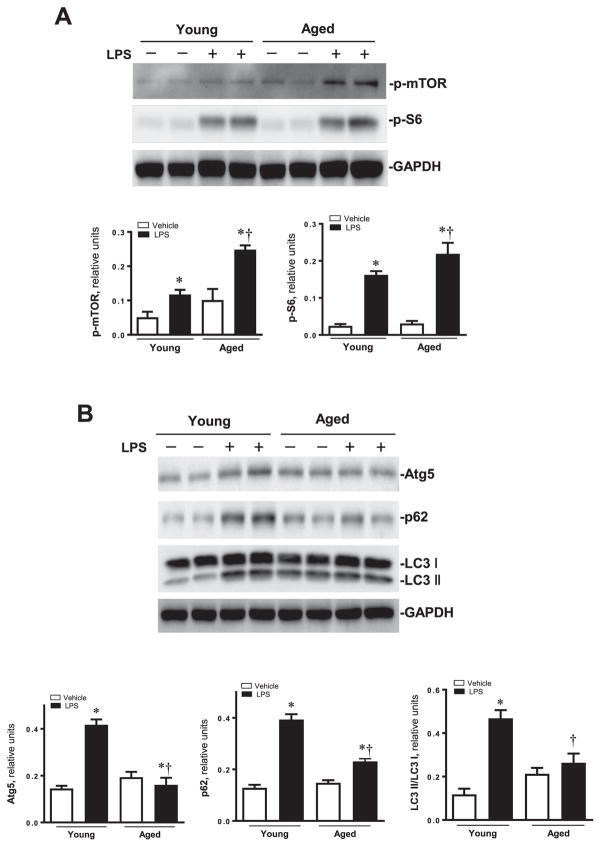
Given that AMPK is an important intracellular regulator for cardiac function under various pathological conditions, we wondered to know whether the facilitation of cardiomyocytes contractile function was due to induced AMPK phosphorylation. We examined the AMPK and its downstream signaling target acetyl-CoA carboxylase (ACC) and other related proteins. Phosphorylation of AMPK and downstream target p-ACC were significantly suppressed following LPS challenge in murine hearts, especially in aged mice. PP2C-α and PP2A A were activated in aged mice treated with LPS (Fig. 2E). LPS overtly promoted phosphorylation of mTOR and S6, respective downstream targets for AMPK and mTOR (Fig. 3A).
Fig. 3.
(A) Expression levels of p-mTOR and p-S6 proteins using specific antibodies; Pooled data of above proteins normalized to GAPDH. Mean ± SEM, n = 3–4 samples per group, *p < 0.05 vs. their littermates; †p < 0.05 vs. young -LPS group. (B) Western blot analysis about the autophagy markers in myocardium young and aged mice treated with or without LPS. Expression levels of Atg5, p62 and LC3I/II; Pooled data of above proteins normalized to GAPDH. Mean ± SEM, n = 3–4 samples per group, *p < 0.05 vs. their littermates; †p < 0.05 vs. young -LPS group.
3.4. Effect of LPS on the activation of autophagy
Given the pivotal role of autophagy in the maintenance of cardiac geometry and function, we explore if autophagy contributes to LPS induced cardiac dysfunction, levels of autophagic markers Including Atg5, p62 and LC3 I/II were evaluated. Results shown in Fig. 3B depicted that aging itself did not affect the expression of these autophagy markers Atg5, LC3-II/LC3-I ratio, and the autophagy adaptor protein p62, while LPS significantly increased the expression in Atg5, LC3II/LC3I ratio and p62 in young-LPS group compared to aged-LPS group.
3.5. Effect of AMPK activator A769662 on mTOR, S6 signaling, autophagic markers and cardiomyocyte contractile responses
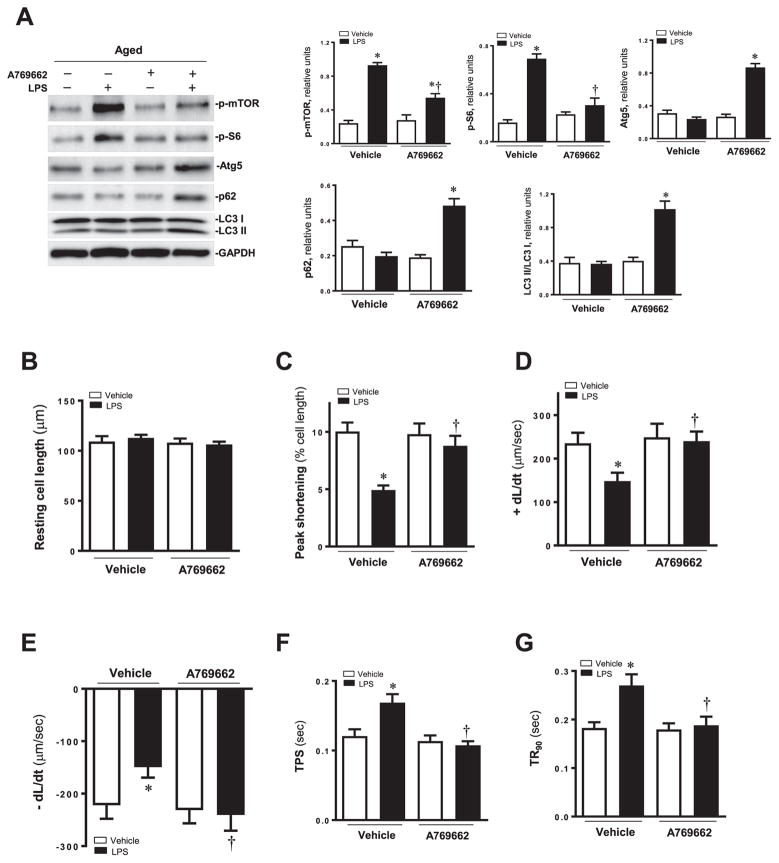
To better elucidate a cause-effect relationship for AMPK and autophagy in LPS-induced cardiac contractile response, aged mice were exposed to LPS in the presence or the absence of the AMPK activator A769662. Our data revealed that the activation of AMPK significantly decreased the expression of p-mTOR, p-S6 and increased the level of autophagic markers Atg5, p62 and LC3-II/LC3-I ratio (Fig. 4A).
Fig. 4.
(A) Western blot analysis in myocardium of aged mice treated with LPS and AMPK activator A769662. Expression levels of p-mTOR, p-S6, Atg5, p62 and LC3I/II; Pooled data of above proteins normalized to GAPDH. Mean ± SEM, n = 3–4 samples per group, *p < 0.05 vs. aged group; †p < 0.05 vs. aged -LPS group. Effect of AMPK activator A769662 on LPS induced cardiomyocyte contractile anomalies in aged mice. (B) Resting cell length; (C) Peak shortening (PS, normalized to cell length); (D) Maximal velocity of shortening (+dL/dt); (E) Maximal velocity of relengthening (− dL/dt); (F) Time-to-PS (TPS); and (G) Time-to-90% relengthening (TR90). Mean ± SEM, 3–4 mice per group, *p < 0.05 vs. aged group; †p < 0.05 vs. aged-LPS group.
We also observed the contractile properties with or without AMPK activator A769662 under the treatment of LPS in aged mice. The results showed the depressed maximal velocity of shortening/relengthening and prolonged duration of relengthening were restored significantly in LPS treated aged mice. There was no influence in resting cell length in different groups (Fig. 4B–G).
4. Discussion
Cardiac dysfunction, common complication of severe sepsis, is one cause of death in intensive care units. Accumulated evidence revealed the regulatory effect of autophagy on sepsis-induced cardiac dysfunction [15,22], although the mechanisms in young and aging are not elucidated. Our study demonstrated a notable increase in autophagy level in young mice hearts challenged with LPS. Also, we detected a significant attenuation of p-AMPK expression level in the aged mice with septic cardiac dysfunction, suggesting a closed relevance of AMPK in sepsis-induced cardiac dysfunction. Given the demonstration that both phosphorylated AMPK and phosphorylated mTOR contributed to the stimulation of cardiomyocytes contractility, we wondered to know whether the crosstalk between AMPK and mTOR exists. We found that pre-treatment of A769662, an activator of AMPK, restored the cardiomyocytes contractility partly due to the blunted phosphorylation of S6 which is the downstream protein of mTOR. Our study further demonstrated preserved autophagy against LPS in aged mice following A769662 treatment. It is highly needed to clarify the functional roles of autophagy in sepsis-cardiac dysfunction in young and aged mice. Interestingly, our data show that pharmacological induction of AMPK that enhanced the autophagy improves contractile responses in aged murine cardiomyocytes challenged with LPS. A new published study showed that myocardial aging is a T-cell-mediated phenomenon that heart-directed immune responses may spontaneously arise in the elderly [23]. Improvement of LPS-induced mitochondrial dysfunction by fasudil was attributed to inhibition of ROCK-dependent Drp1 phosphorylation and activation of autophagic processes [24]. Overall our data indicate the relationship between AMPK and autophagy response to LPS treatment in heart dysfunction.
Autophagy which has been demonstrated to be essential for cellular homeostasis, is the catabolic process for delivering cytosolic cargo to the lysosome for degradation. The role of autophagy in cardiovascular injuries of different experimental models is still a controversial topic. Excessive autophagy can induce cell death, the process of which is called autophagic cell death. A study had indicated that inhibition of autophagy protects the heart from pathological cardiac dysfunction in animal models of ischemia-reperfusion and hypertrophy [25]. Alleviation of autophagy might have therapeutic benefit in treating several cardiac diseases [26]. A study newly demonstrates that TFEB mediated autophagy is crucial for protection against LPS induced myocardial injury particularly in aging senescent heart [15]. In our results, the Atg5, p62 and LC3 were elevated in response to LPS treatment in mice hearts in this study. This may indicate that LPS can stimulate severe autophagy, which is reported in previous studies [27]. A study showed that the a maladaptive role for autophagy in hearts subjected to LPS challenge [28]. Our data suggested that LPS challenge-induced myocardial autophagy is an adaptive response as evidenced by the favorable response from A769662 against LPS-induced cardiac injury which is expected to stimulate AMPK. More importantly, our results indicated that aging accentuated LPS-induced myocardial dysfunction and survival possibly through abating myocardial autophagy induction in response to LPS challenge demonstrating by cardiomyocytes contractility and intracellular Ca2+ homeostasis.
Although induction of AMPK has been demonstrated to prevent cardiac injury after LPS treatment in aged mice, the interaction between cardiac dysfunction and myocardial autophagy during sepsis still remains to be clarified, especially whether there is a “bridge” between them. In order to elucidate the upstream and downstream signals that regulate autophagy in our study, we examined several key molecules which may be involved in. The data here showed that PP2A, PP2Cα, mTOR and S6 were key factors in this process. It is known that The PP2 heterotrimeric protein phosphatase is ubiquitously expressed. Its serine/threonine phosphatase activity has broad substrate specificity and diverse cellular functions. Moreover, the mTOR contributes to cell survival in cardiomyocytes and regulates cell proliferation, apoptosis, cell migration and metabolism. In our study, PP2A and PP2Cα decreased the phosphorylation of AMPK accompanied by inhancing autophagy. Further experiments showed that inhibition of AMPK could increase the p-mTOR and p-S6 proteins expression. The expression of phosphorylation of mTOR and S6 decreased in the presence of the AMPK activator A769662. These results favor the formation the AMPK/mTOR/S6 pathway.
Two future works of this study should be highlighted. Firstly, Long time observation is needed to make sure whether the response of autophagy is a “double-edged sword” for cardiac function over a long period of time. Secondly, more clinical plasma samples of patients are needed to confirm this finding if autophagy biomarkers could be founded for sepsis-induced cardiac dysfunction.
In conclusion, this study shows that autophagy is blunted in sepsis of aged mouse hearts. AMPK/mTOR pathway is involved the role for controlling it. Pharmacological induction of AMPK improves cardiac function in aged mice hearts with sepsis. To modulate the AMPK and autophagy might be a potential novel therapeutic target for septic myocardial dysfunction for aging.
Acknowledgments
These studies were supported by American Diabetes Association 1-17-IBS-296, NIH R21AG044820, R01AG049835, P01HL051971, and P20GM104357, and Primary research & development plan of Shandong province, China (2015GSF118180) and Medical health science and technology development plan of Shandong province (2016WS0417).
Footnotes
Conflict of interest statement
The authors have declared that no competing interest exists.
Transparency document
Transparency document related to this article can be found online at http://dx.doi.org/10.1016/j.bbrc.2017.08.034.
References
- 1.Su HH, Chu YC, Liao JM, Wang YH, Jan MS, Lin CW, Wu CY, Tseng CY, Yen JC, Huang SS. Phellinus linteus mycelium alleviates myocardial ischemia-reperfusion injury through autophagic regulation. Front Pharmacol. 2017;8:175. doi: 10.3389/fphar.2017.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pu T, Liao XH, Sun H, Guo H, Jiang X, Peng JB, Zhang L, Liu Q. Augmenter of liver regeneration regulates autophagy in renal ischemia-reperfusion injury via the AMPK/mTOR pathway. Apoptosis. 2017;22:955–969. doi: 10.1007/s10495-017-1370-6. [DOI] [PubMed] [Google Scholar]
- 3.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamming DW, Ye L, Sabatini DM, Baur JA. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest. 2013;123:980–989. doi: 10.1172/JCI64099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barilari M, Bonfils G, Treins C, Koka V, De Villeneuve D, Fabrega S, Pende M. ZRF1 is a novel S6 kinase substrate that drives the senescence programme. EMBO J. 2017;36:736–750. doi: 10.15252/embj.201694966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lalazar G, Ilyas G, Malik SA, Liu K, Zhao E, Amir M, Lin Y, Tanaka KE, Czaja MJ. Autophagy confers resistance to lipopolysaccharide-induced mouse hepatocyte injury. Am J Physiol Gastrointest Liver Physiol. 2016;311:G377–G386. doi: 10.1152/ajpgi.00124.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong Z, Jiang B, Zhang L, Liu Y, Gao M, Jiang Y, Li Y, Lu Q, Yao Y, Xiao X. HSF-1 is involved in attenuating the release of inflammatory cytokines induced by LPS through regulating autophagy. Shock. 2014;41:449–453. doi: 10.1097/SHK.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y, Yang L, Ma J, Lu L, Wang X, Ren J, Yang J. Rutin attenuates doxorubicin-induced cardiotoxicity via regulating autophagy and apoptosis. Biochim Biophys Acta. 2017;1863:1904–1911. doi: 10.1016/j.bbadis.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, Nishida K, Shimizu T, Hori M, Komuro I, Takuji Shirasawa TS, Mizushima N, Otsu K. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6:600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 10.Palomba H, Correa TD, Silva E, Pardini A, Assuncao MS. Comparative Analysis of Survival between Elderly and Non-elderly Severe Sepsis and Septic Shock Resuscitated Patients. 2015;13:357–363. doi: 10.1590/S1679-45082015AO3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimitrijevic M, Stanojevic S, Blagojevic V, Curuvija I, Vujnovic I, Petrovic R, Arsenovic-Ranin N, Vujic V, Leposavic G. Aging affects the responsiveness of rat peritoneal macrophages to GM-CSF and IL-4. Biogerontology. 2016;17:359–371. doi: 10.1007/s10522-015-9620-x. [DOI] [PubMed] [Google Scholar]
- 12.Go M, Kou J, Lim JE, Yang J, Fukuchi KI. Microglial response to LPS increases in wild-type mice during aging but diminishes in an Alzheimer’s mouse model: implication of TLR4 signaling in disease progression. Biochem Bio-phys Res Commun. 2016;479:331–337. doi: 10.1016/j.bbrc.2016.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linehan E, Dombrowski Y, Snoddy R, Fallon PG, Kissenpfennig A, Fitzgerald DC. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging Cell. 2014;13:699–708. doi: 10.1111/acel.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knuppertz L, Osiewacz HD. Orchestrating the network of molecular pathways affecting aging: role of nonselective autophagy and mitophagy. Mech Ageing Dev. 2016;153:30–40. doi: 10.1016/j.mad.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Li F, Lang F, Zhang H, Xu L, Wang Y, Hao E. Role of TFEB mediated autophagy, oxidative stress, inflammation, and cell death in endotoxin induced myocardial toxicity of young and aged mice. Oxid Med Cell Longev. 2016;2016:5380319. doi: 10.1155/2016/5380319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao P, Turdi S, Dong F, Xiao X, Su G, Zhu X, Scott GI, Ren J. Cardiac-specific overexpression of insulin-like growth factor I (IGF-1) rescues lipopolysaccharide-induced cardiac dysfunction and activation of stress signaling in murine cardiomyocytes. Shock. 2009;32:100–107. doi: 10.1097/SHK.0b013e31818ec609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bojic LA, Telford DE, Fullerton MD, Ford RJ, Sutherland BG, Edwards JY, Sawyez CG, Gros R, Kemp BE, Steinberg GR, Huff MW. PPARdelta activation attenuates hepatic steatosis in Ldlr−/− mice by enhanced fat oxidation, reduced lipogenesis, and improved insulin sensitivity. J Lipid Res. 2014;55:1254–1266. doi: 10.1194/jlr.M046037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao P, Zhang J, Yin XG, Maharaj P, Narraindoo S, Cui LQ, Tang YS. The effect of trimetazidine on cardiac function in diabetic patients with idiopathic dilated cardiomyopathy. Life Sci. 2013;92:633–638. doi: 10.1016/j.lfs.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Zhang X, Cui Y, Ferdous M, Cui L, Zhao P. Different postconditioning cycles affect prognosis of aged patients undergoing primary percutaneous coronary intervention. Cardiol J. 2017 Jul 17; doi: 10.5603/CJ.a2017.0083. http://dx.doi.org/10.5603/CJ.a2017.0083 [Epub ahead of print] [DOI] [PubMed]
- 20.Sun W, Quan N, Wang L, Yang H, Chu D, Liu Q, Zhao X, Leng J, Li J. Cardiac-specific deletion of the Pdha1 gene sensitizes heart to toxicological actions of ischemic stress. Toxicol Sci. 2016;151:193–203. doi: 10.1093/toxsci/kfw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S, Wang Z, Xu B, Mi X, Sun W, Quan N, Wang L, Chen X, Liu Q, Zheng Y, Leng J, Li J. The modulation of cardiac contractile function by the pharmacological and toxicological effects of Urocortin2. Toxicol Sci. 2015;148:581–593. doi: 10.1093/toxsci/kfv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim MJ, Bae SH, Ryu JC, Kwon Y, Oh JH, Kwon J, Moon JS, Kim K, Miyawaki A, Lee MG, Shin J, Kim YS, Kim CH, Ryter SW, Choi AM, Rhee SG, Ryu JH, Yoon JH. SESN2/sestrin2 suppresses sepsis by inducing mitophagy and inhibiting NLRP3 activation in macrophages. Autophagy. 2016;12:1272–1291. doi: 10.1080/15548627.2016.1183081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramos GC, van den Berg A, Nunes-Silva V, Weirather J, Peters L, Burkard M, Friedrich M, Pinnecker J, Abesser M, Heinze KG, Schuh K, Beyersdorf N, Kerkau T, Demengeot J, Frantz S, Hofmann U. Myocardial aging as a T-cell-mediated phenomenon. Proc Natl Acad Sci U S A. 2017;114:E2420–E2429. doi: 10.1073/pnas.1621047114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preau S, Delguste F, Yu Y, Remy-Jouet I, Richard V, Saulnier F, Boulanger E, Neviere R. Endotoxemia engages the RhoA kinase pathway to impair cardiac function by altering cytoskeleton, mitochondrial fission, and autophagy. Antioxid Redox Signal. 2016;24:529–542. doi: 10.1089/ars.2015.6421. [DOI] [PubMed] [Google Scholar]
- 25.Ma S, Wang Y, Chen Y, Cao F. The role of the autophagy in myocardial ischemia/reperfusion injury. Biochim Biophys Acta. 2015;1852:271–276. doi: 10.1016/j.bbadis.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Wu X, He L, Cai Y, Zhang G, He Y, Zhang Z, He X, Luo J. Induction of autophagy contributes to the myocardial protection of valsartan against ischemiareperfusion injury. Mol Med Rep. 2013;8:1824–1830. doi: 10.3892/mmr.2013.1708. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Wang XL, Chen HL, Wu D, Chen JX, Wang XX, Li RL, He JH, Mo L, Cen X, Wei YQ, Jiang W. Ghrelin inhibits doxorubicin cardiotoxicity by inhibiting excessive autophagy through AMPK and p38-MAPK. Biochem Pharmacol. 2014;88:334–350. doi: 10.1016/j.bcp.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 28.Ren J, Xu X, Wang Q, Ren SY, Dong M, Zhang Y. Permissive role of AMPK and autophagy in adiponectin deficiency-accentuated myocardial injury and inflammation in endotoxemia. J Mol Cell Cardiol. 2016;93:18–31. doi: 10.1016/j.yjmcc.2016.02.002. [DOI] [PubMed] [Google Scholar]