Abstract
Prior work has shown that the HIV-1 envelope of the human immunodeficiency virus (HIV) interacts directly with T-cell immunoglobulin mucin (TIM) family proteins. Herein, we demonstrate that HIV-1 envelope glycoproteins from varying HIV-1 clades bind differentially to TIM proteins and functionally similar proteins acting as phosphatidylserine (PtdSer) receptors. Using enzyme-linked immunosorbent assay (ELISA) and surface plasmon resonance (SPR) technology, we show that lysate containing HIV-1 envelope and recombinant HIV-1 envelope glycoproteins bind TIM-4 and advanced glycosylation end product-specific receptor (AGER). The complex binding of HIV-1 UG21 gp140 to TIM-4 or AGER suggests a biphasic interaction with these proteins.
Abbreviations: AGER, advanced glycosylation end product-specific receptor; CD4, cluster of differentiation 4; CMV, cytomegalovirus; ELISA, enzyme-linked immunosorbent assay; Env, envelope; HIV, human immunodeficiency virus; PtdSer, phosphatidylserine; SPR, surface plasmon resonance; TIM, T-cell immunoglobulin mucin
Keywords: Human immunodeficiency virus, Phosphatidylserine, T-cell immunoglobulin mucin, Advanced glycosylation end product-specific receptor, Surface plasmon resonance, Glycoproteins
Graphical abstract
Novel interactions between HIV-1 glycoproteins and phosphatidylserine binding proteins (AGER and TIM-4) are demonstrated in this study. Using surface plasmon resonance (SPR) analysis, TIM-4 and AGER bind to HIV-1 UG21 gp140 clade D characterized by initial complex formation followed by conformational change.
We show that there is a specific interaction between TIM-4 and various clades of HIV-1 Env glycoproteins. Using surface plasmon resonance, we showed that HIV-1 UG21 gp140 envelope protein specifically binds both TIM-4 and AGER. In addition, surface plasmon resonance analysis suggest that the binding of HIV-1 UG21 gp140 clade D to both TIM-4 and AGER demonstrates an interactions characterized by initial complex formation followed by conformational change.
Highlights
-
•
HIV-1 glycoproteins bind PS-binding proteins as confirmed by ELISA and SPR.
-
•
HIV-1 glycoproteins from multiple clades bind to bind phosphatidylserine binding proteins.
-
•
Surface plasmon resonance is used to characterize the binding kinetics of HIV-1 glycoprotein and phosphatidylserine.
-
•
HIV-1 UG21 gp140 clade D binds TIM-4 or AGER in a biphasic manner.
1. Introduction
HIV-1 is an enveloped retrovirus that acquires entry into the host cell primarily through well-studied receptor-mediated fusion interactions between the host and virus via cluster of differentiation 4 (CD4) and chemokine receptors. Although there is a vast amount of information regarding how specific proteins mediate virus-cell infection, less is known about the role of lipids in this process. The HIV-1 envelope (Env) is acquired as the virus particle buds from the cell. This Env includes proteins and lipids that are selectively obtained from the host membrane. For example, glycophospholipids in target cell membranes have been shown to increase HIV-1 infection [1], [2]. One enriched membrane phospholipid in the HIV-1 Env is phosphatidylserine (PtdSer) [3]. PtdSer has the ability to mediate cell-cell interactions, function as a ligand in the viral membrane, and may act as a factor in virus-target cell fusion [4].
The T-cell immunoglobulin mucin (TIM) family proteins, which play important roles in immunity and disease [5], [6], have three members in humans (TIM-1, 3 and 4), and eight in mice (TIM 1-8). Although the role of TIM proteins with respect to their involvement in HIV-1 binding, entry, and release remains poorly understood, TIM-1 and TIM-4 have been shown to act as PtdSer receptors in the engulfment of apoptotic cells, and also may be involved in intracellular signaling related to exosomes. TIM-4 contains one Ig-like V-type (immunoglobulin-like) domain. Despite significant sequence variations, the IgV regions of all TIM proteins contain a phospholipid binding site that is absolutely conserved [7]. Human TIM-4 has been found to be expressed mainly in macrophages and dendritic cells, and possibly act as a ligand for TIM-1, thereby facilitating T-cell activation [8], [9]. TIM-1 also has been reported to be expressed in activated CD4+ T cells [10], [11], which are the major targets of HIV-1 infections.
We recently demonstrated that TIM-4 may be involved in the cellular entry of the adenovirus (i.e., non-enveloped virus), which is facilitated by exosomes [12]. TIM proteins have also been associated with enhanced entry of a broad range of enveloped viruses [13], [14], [15]. TIM proteins on enveloped viruses (e.g., Ebola, dengue, and West Nile viruses) have been reported to enhance virus entry by binding to PtdSer molecules exposed on the virus surface [13], [16]. Such PtdSer-dependent enhancement of virus entry has been termed ‘apoptotic mimicry’, and appears to be a general mechanism of extracellular PtdSer receptors [17]. Other PtdSer receptors, such as Axl and advanced glycosylation end product-specific receptor (AGER), which do not belong to the TIM family, also block HIV-1 release, but in an isolate-dependent manner [6], [17], [18], which suggests that AGER may differentially bind to HIV-1.
Given that HIV-1 is highly enriched with PtdSer, we hypothesized that HIV-1 Env glycoproteins would interact and bind to PtdSer family receptor proteins, including TIMs and AGER. In this study, we demonstrated that the PtdSer family of receptors interact and bind differentially with HIV-1 Env glycoproteins from various HIV-1 clades. We also determined the specific association/dissociation and affinity rates/constants between HIV-1 Env glycoproteins and PtdSer receptors.
2. Materials and methods
2.1. Western blot analysis
To analyze HIV-1 protein expression, in brief, 2 × 105 HEK-293 cells were transfected for 48 h with either psh-CMV-HIV-Env (2.5 μg) or mock-transfected (control). The cell lysates were prepared and analyzed as follows: the HEK-293 cells were rinsed once in PBS and lysed in RIPA buffer on ice for 20 min. Then crude lysates were centrifuged (12,000 rpm × 10 min at 4 °C) to collect the supernatants. Equal amounts of lysate from transfected or mock-transfected cells were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were transferred to polyvinylidene fluoride membrane and western blot analysis was performed with 2F5 or 4E10 antibody (1:1000) (NIH AIDS Research and Reference Reagent Program). 2F5 antibody Cat# 1475, 4E10 antibody Cat# 10091. Goat anti-human IgG-HRP (1:2000) was applied. The membrane was developed with 3,3′-diaminobenzidine solution.
2.2. Enzyme-linked immunosorbent assay (ELISA)
The ELISA was performed essentially as described previously [12], [19]. In order to determine if expressed HIV-1 Env protein presented in lysate bound to recombinant TIM-4 protein the following experiment was performed. TIM-4-His6 protein (150 ng/well) (Sino Biological Inc., Cat# 12161-H08H) was immobilized on a 96-well plate (Nunc Maxisorp, Rochester, NY) by overnight incubation in 100 μl of 100 mM carbonate buffer (pH 9.5) per well at 4 °C. The plate was washed with 0.05% Tween 20 in Phosphate-buffered saline (PBS) and blocked in blocking solution (5% bovine serum albumin and 0.05% Tween 20 in PBS). Lysates from 1 × 106 HEK 293 cells untransfected or transfected with either psh-CMV or psh-CMV-HIV-Env (2.5 μg, transfected for 24 h) were applied to the plate at 60 μg/well. The plate was washed and blocked, followed by the application of HIV-1-specific human monoclonal antibody 2F5 or 4E10 (1:2000). Goat anti-human IgG-HRP (1:2000) was applied to the plate, which was then treated with o-phenylenediamine dihydrochloride peroxidase substrate (OPD). The ELISA plates were read at OD450 nm.
In order to determine if HIV-1 recombinant glycoproteins bound to recombinant TIM-4 or AGER proteins the following experiment was performed. The ELISA plates were incubated overnight with 200 ng/well of different recombinant gp140 proteins [NIH AIDS Research and Reference Reagent Program, UG37 cat#12063; SF162 cat#12026; CN54 cat# 12064; UG21 cat #12065; BR29 cat# 12066] or binding buffer (control) in 100 μl of 100 mM carbonate buffer (pH 9.5) per well at 4 °C. The plates were then washed and blocked as described above. Next, TIM-4-His6 protein, AGER-His6 protein or Axl-His6 protein (400 ng/well) were bound to the plate. The plates were washed and blocked followed by the addition of mouse anti-His6 monoclonal antibody (1:5000). Goat anti-mouse antibody IgG-HRP (1:5000) was applied to the plates, which were then treated with OPD peroxidase substrate and read at OD450 nm.
2.3. Surface plasmon resonance (SPR) analysis
In order to determine the binding kinetics of HIV-1 glycoproteins to recombinant TIM-4 or AGER, SPR analysis using a Biacore T200 biosensor was employed. TIM-4 or AGER proteins were amine coupled to Sensor CM5 chip surfaces according to the manufacturer's recommendations. A series of HIV-1 UG21 gp140 concentrations (0.0625-1.0 μM) were injected (30 μl/min) over a blank Sensor CM5 chip amine coupled TIM-4 or AGER surfaces. Specific binding responses (resonance units [RU]) were defined by double referencing, subtraction of non-specific binding to a blank CM5 surface, and buffer (controls). Kinetic constants were estimated using BIA evaluation software and global fits of the data, based on the two-state reaction model.
Supplemental Figures/Injection Time Test: a linked-reaction control experiment was carried out to further decipher the conformational change, which occurs in both the AGER and TIM-4 interaction with HIV-1 UG21 gp140, as evidence by the two-state kinetic fit model. This was performed by injecting a high concentration of HIV-1 UG21 gp140 (2 μM) over the immobilized AGER and TIM-4 at a flow rate of 10 ul/min with fixed times of 0.5, 3 and 10 min, respectively. The dissociation time was set at 10 min for each injection. The resultant sensorgrams were adjusted to zero response at the baseline report point and zero time at the end of the sample injection. The observed dissociation rates were then compared at the different contact times.
3. Results
3.1. Expression of HIV-1 envelope protein
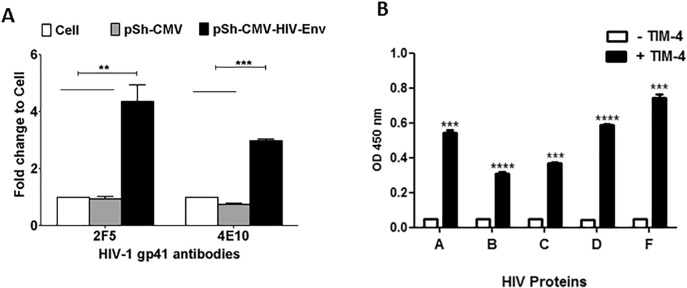
We hypothesized that HIV-1 Env interacts with TIM-4 because other RNA viruses, such as the Ebola and Marburg viruses, associate with TIM family proteins. In order to confirm this hypothesis, we first expressed HIV-1 Env proteins for subsequent experiments. HEK-293 cells were transfected with an expression plasmid encoding for HIV-1 Env, R5X4-tropic clade B strain 89.6 (GenBank: U39362.2), psh-CMV-HIV-Env [20]. HIV-1 Env expression was compared to untransfected HEK-293 cells. Cell lysates were subjected to western blot analysis with human anti-HIV antibodies 4E10 (Fig. 1A) and 2F5 (Fig. 1B). As anticipated, transfections with psh-CMV-HIV-Env [20] yielded HIV-1 specific proteins. Both 4E10 and 2F5 antibodies detected HIV-1 Env proteins at ~ 160 kDa and ~ 41 kDa, in contrast to what was observed in the control lysate sample.
Fig. 1.
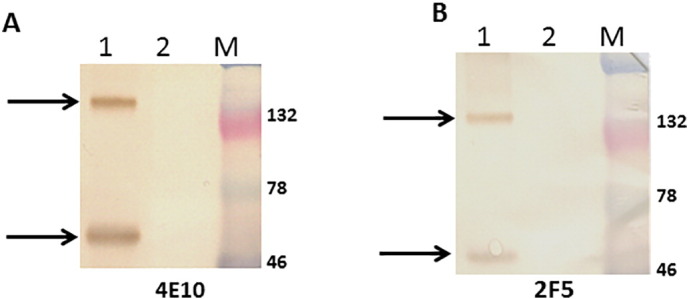
HIV-1 envelope expression in HEK-293 cells. 2 × 105 HEK-293 cells were transfected for 48 h with either psh-CMV-HIV-Env (2.5 μg) (lane 1) or mock-transfected (control) (lane 2). The cell lysates were collected and quantitated. Lysate from transfected or mock-transfected cells were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by western blot analysis with (A) 4E10 or (B) 2F5 antibody.
3.2. TIM-4 and HIV-1 Env proteins interact via enzyme-linked Immunosorbent assays
We investigated the putative interaction between TIM-4 and HIV-1 Env proteins from the clade B strain 89.6 via sandwich ELISA (Fig. 2A). ELISA plates were coated with 150 ng/well of TIM-4-His6 protein, washed, and blocked, followed by the application of three different cell lysates: cell only, psh-CMV-transfected, and psh-CMV-HIV-Env-transfected [20]. HIV-1 89.6-specific human monoclonal antibody was then applied. We found greater binding with the application of psh-CMV-HIV-Env-transfected cell lysate compared with control conditions (i.e., cell only or psh-CMV-transfected groups), suggesting a specific interaction between the TIM-4 protein and HIV-1 Env.
Fig. 2.
HIV-1 envelope binds TIM-4 protein.
(A) 1 × 106 HEK-293 cells were either untransfected or transfected with either psh-CMV or psh-CMV-HIV-Env (2.5 μg). The cell lysates were collected and protein concentration was determined. TIM-4-His6 protein was coated on ELISA plates at 150 ng/well. The plate was washed and blocked, followed by the application of three different conditioned cell lysates (60 μg/well, n = 3). HIV-1-specific human monoclonal antibody 2F5 or 4E10 was applied to the plate after washing and blocking. Goat anti-human IgG-HRP was applied to the plate, which was then treated with o-phenylenediamine dihydrochloride peroxidase substrate. Signals were read at OD450 nm and expressed as fold change. (B) ELISA plates were incubated with 200 ng/well of recombinant gp140 proteins (UG37/HIV/Clade A, SF162/HIV/Clade B, CN54/HIV/Clade C, UG21 HIV/Clade D, or BR29/HIV/Clade F). The plates were washed and blocked, followed by the application of TIM-4-His6 protein (400 ng/well, n = 3) or binding buffer (control). Next, mouse anti-His6 monoclonal antibody was applied to the plate after washing and blocking. Goat anti-mouse antibody IgG-HRP was applied to the plate, which was then treated with OPD peroxidase substrate and read at OD450 nm. Background OD values were ~ 0.004. (**) = P ≤ 0.01, (***) = P ≤ 0.001, (****) = P ≤ 0.0001.
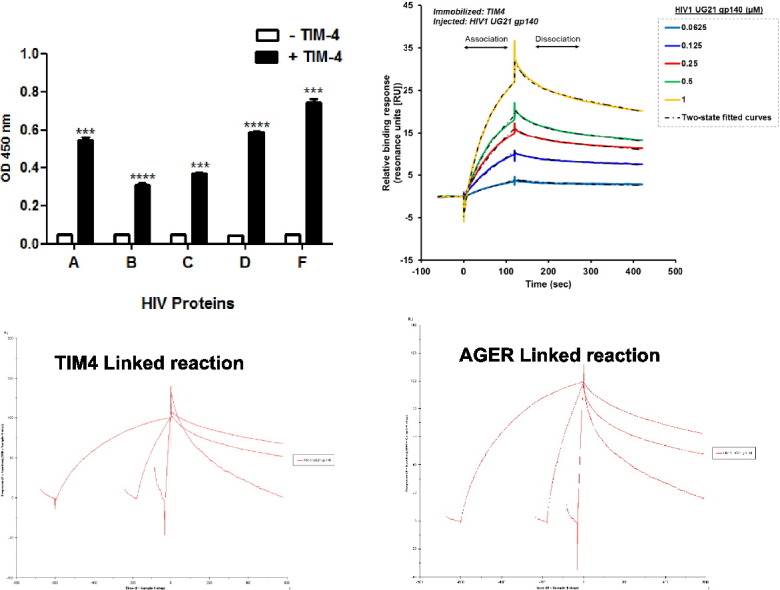
We performed additional ELISA assays to confirm the specific interaction between HIV-1 Env glycoproteins and TIM-4, as well as to uncover whether a wide spectrum interaction exists across different HIV-1 clades. For these experiments (Fig. 2B), we used the following recombinant gp140 proteins (i.e., truncated soluble forms of Env): UG37/HIV/Clade A, SF162/HIV trimer/Clade B, CN54/HIV/Clade C, UG21/HIV/Clade D, and BR29/HIV/Clade F. In brief, ELISA plates were coated with recombinant gp140 HIV-1 proteins from clades A, B, C, D, and F, followed by the addition of TIM-4 histidine (His6)-tagged protein or buffer control. We found significant binding of recombinant TIM-4 protein and all gp140 proteins (P ≤ 0.001). BR29/HIV/Clade F exhibited the highest TIM-4 binding, followed by UG21 HIV/Clade D (P ≤ 0.0001). These data confirm a specific interaction between TIM-4 and cross-clade HIV-1 Env glycoproteins. We decided to evaluate UG21 clade D for further analysis because UG21 yielded an average response in our gp140/TIM-4 ELISA; therefore, we felt that this would be a good representative gp140 protein for further analysis.
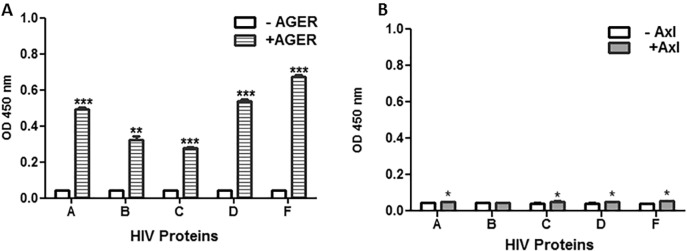
3.3. AGER and Axl interact with HIV-1 Env proteins via enzyme-linked Immunosorbent assays
Based on our findings that TIM proteins interact with gp140 proteins from different HIV-1 clades, we assessed the putative interaction between recombinant gp140 proteins and two other PtdSer receptors, AGER and Axl via ELISA method. In brief, ELISA plates were coated with recombinant gp140 HIV-1 proteins from clades A, B, C, D, and F, followed by the addition of +/− AGER His-tagged protein (Fig. 3A) or +/− Axl His-tagged protein (Fig. 3B). The plates were then incubated with mouse anti-His6 antibody, washed, blocked, and treated with goat anti-mouse IgG-HRP. We observed significant binding of recombinant AGER protein to all recombinant gp140 proteins, with BR29/HIV/Clade F exhibiting the highest AGER binding (P ≤ 0.001). (Fig. 3A). In contrast, we observed minimal binding of recombinant Axl to all recombinant gp140 proteins (Fig. 3B), although binding was significantly increased compared to controls for clades A, C, D and F. These findings suggest that only a weak interaction exists between HIV-1 gp140 and Axl, whereas the interactions between HIV-1 gp140 and TIM-4 or AGER are dominant. Based on the high binding affinity of HIV-1 gp140 proteins to TIM-4 and AGER, we evaluated the kinetics of TIM-4 and AGER to HIV-1 gp140 UG21 clade D.
Fig. 3.
HIV-1 envelope binds AGER and Axl proteins. ELISA plates were incubated with 200 ng/well of recombinant gp140 proteins (UG37/HIV/Clade A, SF162/HIV/Clade B, CN54/HIV/Clade C, UG21 HIV/Clade D, or BR29/HIV/Clade F). The plates were washed and blocked, followed by the application of (A) AGER-His6 protein (400 ng/well, n = 3) or (B) Axl-His6 protein (400 ng/well, n = 3). Binding buffer was used as controls in A and B. Next, mouse anti-His6 monoclonal antibody (1:5000) was applied to the plate after washing and blocking. Goat anti-mouse antibody IgG-HRP (1:5000) was applied to the plate, which was then treated with OPD peroxidase substrate and read at OD450 nm. (*) = P ≤ 0.05, (**) = P ≤ 0.01, (***) = P ≤ 0.001.
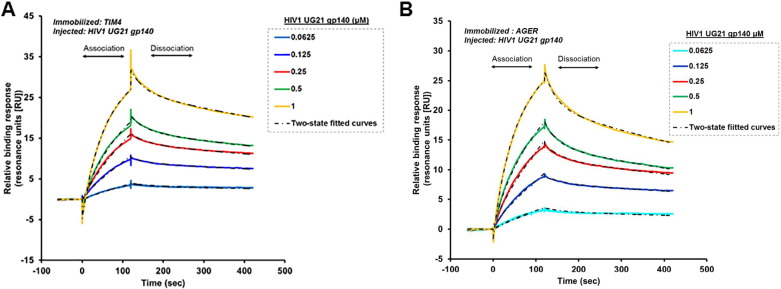
3.4. Binding of HIV-1 UG21 gp140 envelope protein to TIM-4 or AGER
The binding of the trimeric HIV-1 UG21 gp140 envelope protein to TIM-4 and AGER was determined by surface plasmon resonance (SPR) using a Biacore T200 biosensor instrument (GE Healthcare Life Sciences). We found, for the first time using SPR, that the HIV-1 UG21 gp140 envelope protein specifically binds both TIM-4 and AGER. In addition, the SPR results provide insight into the association and dissociation kinetics of the interactions between HIV-1 UG21 gp140 and TIM-4 or AGER. Complex binding of HIV-1 UG21 gp140 to both TIM-4 or AGER was observed, suggesting a biphasic interaction with both proteins. Interactions did not conform to the typical 1:1 L binding fit, but instead conformed to the two-state fit as shown in Fig. 4.
Fig. 4.
Representative SPR sensorgrams of specific binding of trimeric HIV-1 UG21 gp140. TIM-4 and AGER proteins were amine coupled to Sensor CM5 chip surfaces according to the manufacturer's recommendations. A series of HIV-1 UG21 gp140 concentrations (0.0625–1.0 μM) were injected (30 μl/min) over a blank Sensor CM5 chip amine coupled (A) TIM-4 or (B) AGER surfaces. Specific binding responses (resonance units [RU]) were defined by double referencing, subtraction of non-specific binding to a blank CM5 surface, and buffer (controls). Kinetic constants were estimated using BIA evaluation software and global fits of the data, based on the two-state reaction model, as indicated by the dotted black curves. Data are representative of at least three experiments.
The interaction of HIV-1 UG21 gp140 and TIM-4 was characterized by a relatively fast initial association constant (ka1 = 5.03 × 10E + 4 Ms− 1), indicating HIV-1 UG21 gp140-TIM-4 complex formation, followed by a much slower association constant (ka2 = 1.39 × 10E − 2 Ms− 1), indicating a conformational change in the complex (Table 1). Similar observations were made for the HIV-1 UG21 gp140-AGER interaction (association constants of ka1 = 7.83 × 10E + 4 Ms− 1 and ka2 = 1.40 × 10E − 2 Ms− 1) (Table 1). The resultant HIV-1 UG21 gp140-TIM-4 and HIV-1 UG21 gp140-AGER complexes were stable, as indicated by the slow initial dissociation constants and the final dissociation constants (HIV-1 UG21 gp140-TIM4: kd1 = 1.70 × 10E − 2 (1\s) and kd2 = 1.84 × 10E − 3 (1\s); HIV-1 UG21 gp140-AGER: kd1 = 4.17 × 10E − 2 (1\s) and kd2 = 1.91 × 10E − 3 (1\s)) (Table 1). Although the data indicate that the association (ka) of HIV-1 UG21 gp140 to AGER is faster than its association to TIM-4, its dissociation (kd) from TIM-4 is slower, which results in a > 1.5-fold increased affinity (KD) for TIM-4 compared to AGER (Table 1). Overall, the complex two-state associations of UG21 gp140 to TIM-4 and AGER may be due in part to the fact that gp140 is trimeric and is also relatively heavily glycosylated.
Table 1.
Kinetic analysis of HIV-1 UG21 gp140 binding to TIM-4 or AGER.
| Immobilized | ka1 |
ka2 |
kd1 |
kd2 |
KD |
|---|---|---|---|---|---|
| 10E + 4 (1/Ms) | 10E − 2 (1/Ms) | 10E − 2 (1/s) | 10E − 3 (1/s) | 10E − 8 | |
| TIM-4 | 5.03 | 1.39 | 1.70 | 1.84 | 3.97 |
| AGER | 7.83 | 1.40 | 4.17 | 1.91 | 6.39 |
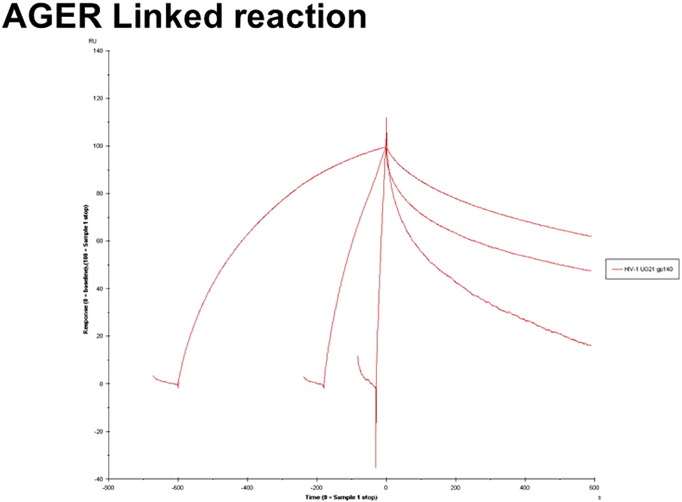
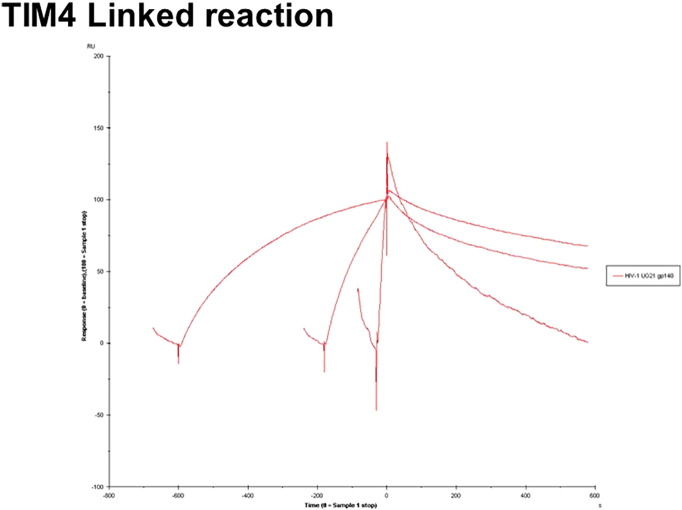
An injection time test (linked reaction control experiment) was performed to validate conformational changes at the binding time (Supplementary Fig. 1, Supplementary Fig. 2). As indicated in the Supplementary Fig. 1, Supplementary Fig. 2, the dissociation was clearly dependent on the contact time, thereby indicating linked reactions in the HIV-1 UG21 gp140-AGER and TIM-4 interactions. A linked reaction indicates that there is an initial interaction followed by a conformation change.
Supplementary Fig. 1.
The binding curves obtained with UG21 gp140 (2 μM) binding to AGER for 0.5, 3 and 10 min.
Supplementary Fig. 2.
The binding curves obtained with UG21 gp140 (2 μM) binding to TIM-4 for 0.5, 3 and 10 min.
4. Discussion
Earlier studies have shown that the HIV-1 interacts directly with TIM family proteins. In summary, we found that lysate containing HIV-1 envelope protein (clade B) binds directly to recombinant TIM-4 protein. In addition, ELISA demonstrated that varying clades of HIV-Env glycoproteins differentially bind to TIM-4 and AGER proteins. Furthermore, the HIV-1 UG21 gp140 clade D envelope protein has specificity for both TIM-4 or AGER (TIM4 > AGER). This binding was biphasic for both proteins, and our findings suggest that the binding kinetics may be dictated by conformational changes of the trimeric and glycosylation state of gp140.
In contrast, we observed minimal binding of recombinant Axl to all recombinant gp140 proteins, although binding was significantly increased compared to controls for clades A, C, D and F. These findings suggest that only a weak interaction exists between HIV-1 gp140 and Axl, whereas the interactions between HIV-1 gp140 and TIM-4 and AGER are dominant. To our knowledge, our results are the first to reveal TIM-4 and AGER as gp140 glycoprotein binding proteins, which may open a new line of investigation for HIV-1 targets. Analysis via SPR revealed that both proteins have similar binding affinities for HIV-1 UG21 gp140, whereas another PtdSer receptor, Axl, exhibited less binding affinity for UG21 gp140 (SPR data not shown). Our SPR data suggest that the binding of HIV-1 UG21 gp140 clade D to TIM-4 or AGER demonstrates an interactions characterized by initial complex formation followed by conformational change. Our work could potentially enhance the understanding of the dynamic interaction between HIV proteins/lipids and other proteins. Our work demonstrates how proteins that bind the phosphatidylserine in the viral envelope could also bind to gp41. This interaction is novel and it does not contradict any of the current knowledge of viral membrane remodeling during the fusion events [21], [22]. Theoretically, the PS-binding proteins could have a dual role in binding to both the HIV envelope lipids and HIV-specific proteins. Supplementary Fig. 1, Supplementary Fig. 2, demonstrate the dissociation was clearly dependent on the contact time, thereby indicating linked reactions in the HIV-1 UG21 gp140-AGER and TIM-4 interactions. A linked reaction indicates that there is an initial interaction followed by a conformation change. The cell specificity for TIM-4 and AGER proteins would make certain cell types more vulnerable to HIV-1 binding and subsequent entry. These novel studies, therefore, introduce novel in vitro ligand and receptor binding partners for the development of new targets and understanding HIV-1 binding.
4.1. Statistical analyses
Statistical analyses were performed with the unpaired two-tailed Student t-test, assuming unequal variance. Statistical significance was defined as p ≤ 0.05.
The following are the supplementary data related to this article.
Transparency document
Transparency document.
Acknowledgments
This work was supported by the National Institutes of Health grant #5R01AI089337-04 and Center for AIDS Research grant # P30AI027767. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. HIV-1 antibodies and Env glycoproteins were obtained from the NIH AIDS Research and Reference Reagent Program. Usage of the Biacore T200 biosensor was made possible by the UAB Multidisciplinary Molecular Interaction Core (MMIC), NIH grant # 1S10RR026935.
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- 1.Harouse J.M., Bhat S., Spitalnik S.L., Laughlin M., Stefano K. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science. 1991;253:320–323. doi: 10.1126/science.1857969. [DOI] [PubMed] [Google Scholar]
- 2.Puri A., Hug P., Jernigan K., Barchi J., Kim H.Y. The neutral glycosphingolipid globotriaosylceramide promotes fusion mediated by a CD4-dependent CXCR4-utilizing HIV type 1 envelope glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14435–14440. doi: 10.1073/pnas.95.24.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aloia R.C., Tian H., Jensen F.C. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc. Natl. Acad. Sci. U. S. A. 1993;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callahan M.K., Popernack P.M., Tsutsui S., Truong L., Schlegel R.A. Phosphatidylserine on HIV envelope is a cofactor for infection of monocytic cells. J. Immunol. 2003;170:4840–4845. doi: 10.4049/jimmunol.170.9.4840. [DOI] [PubMed] [Google Scholar]
- 5.Kuchroo V.K., Umetsu D.T., DeKruyff R.H., Freeman G.J. The TIM gene family: emerging roles in immunity and disease. Nat. Rev. Immunol. 2003;3:454–462. doi: 10.1038/nri1111. [DOI] [PubMed] [Google Scholar]
- 6.Li M., Ablan S.D., Miao C., Zheng Y.M., Fuller M.S. TIM-family proteins inhibit HIV-1 release. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E3699–E3707. doi: 10.1073/pnas.1404851111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman G.J., Casasnovas J.M., Umetsu D.T., DeKruyff R.H. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol. Rev. 2010;235:172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi N., Karisola P., Pena-Cruz V., Dorfman D.M., Jinushi M. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27:927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyahira Y., Takashima Y., Kobayashi S., Matsumoto Y., Takeuchi T. Immune responses against a single CD8 +-T-cell epitope induced by virus vector vaccination can successfully control Trypanosoma cruzi infection. Infect. Immun. 2005;73:7356–7365. doi: 10.1128/IAI.73.11.7356-7365.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umetsu S.E., Lee W.L., McIntire J.J., Downey L., Sanjanwala B. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat. Immunol. 2005;6:447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- 11.McIntire J.J., Umetsu S.E., Akbari O., Potter M., Kuchroo V.K. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat. Immunol. 2001;2:1109–1116. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 12.Sims B., Gu L., Krendelchtchikov A., Matthews Q.L. Neural stem cell-derived exosomes mediate viral entry. Int. J. Nanomedicine. 2014;9:4893–4897. doi: 10.2147/IJN.S70999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jemielity S., Wang J.J., Chan Y.K., Ahmed A.A., Li W. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moller-Tank S., Kondratowicz A.S., Davey R.A., Rennert P.D., Maury W. Role of the phosphatidylserine receptor TIM-1 in enveloped-virus entry. J. Virol. 2013;87:8327–8341. doi: 10.1128/JVI.01025-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan G., Totsuka A., Thompson P., Akatsuka T., Moritsugu Y. Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 1996;15:4282–4296. [PMC free article] [PubMed] [Google Scholar]
- 16.Meertens L., Carnec X., Lecoin M.P., Ramdasi R., Guivel-Benhassine F. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe. 2012;12:544–557. doi: 10.1016/j.chom.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morizono K., Chen I.S. Role of phosphatidylserine receptors in enveloped virus infection. J. Virol. 2014;88:4275–4290. doi: 10.1128/JVI.03287-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vlach J., Saad J.S. HIV: a vicTIM. Trends Microbiol. 2014;22:603–604. doi: 10.1016/j.tim.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Gu L., Krendelchtchikova V., Krendelchtchikov A., Farrow A.L., Derdeyn C.A. Adenoviral vectors elicit humoral immunity against variable loop 2 of clade C HIV-1 gp120 via “Antigen Capsid-Incorporation” strategy. Virology. 2016;487:75–84. doi: 10.1016/j.virol.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao C., Crews C.J., Derdeyn C.A., Blackwell J.L. Lac-regulated system for generating adenovirus 5 vaccine vectors expressing cytolytic human immunodeficiency virus 1 genes. J. Virol. Methods. 2009;160:101–110. doi: 10.1016/j.jviromet.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo N., Marin M., Kim J.H., Desai T.M., Melikyan G.B. Distinct requirements for HIV-cell fusion and HIV-mediated cell-cell fusion. J. Biol. Chem. 2015;290:6558–6573. doi: 10.1074/jbc.M114.623181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliva R.E.A., Vitiello G., De Santis A., Grimaldi M., D'Ursi A.M., Busi E., Del Vecchio P., Petraccone L., D'Errico G. On the microscopic and mesoscopic perturbations of lipid bilayers upon interaction with the MPER domain of the HIV glycoprotein gp41. Biochim. Biophys. Acta. 2016;1858:1904–1913. doi: 10.1016/j.bbamem.2016.05.007. (Epub 2016 May 12) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.