Summary
Background
Data suggest selective internal radiotherapy (SIRT) in third-line or subsequent therapy for metastatic colorectal cancer has clinical benefit in patients with colorectal liver metastases with liver-dominant disease after chemotherapy. The FOXFIRE, SIRFLOX, and FOXFIRE-Global randomised studies evaluated the efficacy of combining first-line chemotherapy with SIRT using yttrium-90 resin microspheres in patients with metastatic colorectal cancer with liver metastases. The studies were designed for combined analysis of overall survival.
Methods
FOXFIRE, SIRFLOX, and FOXFIRE-Global were randomised, phase 3 trials done in hospitals and specialist liver centres in 14 countries worldwide (Australia, Belgium, France, Germany, Israel, Italy, New Zealand, Portugal, South Korea, Singapore, Spain, Taiwan, the UK, and the USA). Chemotherapy-naive patients with metastatic colorectal cancer (WHO performance status 0 or 1) with liver metastases not suitable for curative resection or ablation were randomly assigned (1:1) to either oxaliplatin-based chemotherapy (FOLFOX: leucovorin, fluorouracil, and oxaliplatin) or FOLFOX plus single treatment SIRT concurrent with cycle 1 or 2 of chemotherapy. In FOXFIRE, FOLFOX chemotherapy was OxMdG (oxaliplatin modified de Gramont chemotherapy; 85 mg/m2 oxaliplatin infusion over 2 h, L-leucovorin 175 mg or D,L-leucovorin 350 mg infusion over 2 h, and 400 mg/m2 bolus fluorouracil followed by a 2400 mg/m2 continuous fluorouracil infusion over 46 h). In SIRFLOX and FOXFIRE-Global, FOLFOX chemotherapy was modified FOLFOX6 (85 mg/m2 oxaliplatin infusion over 2 h, 200 mg leucovorin, and 400 mg/m2 bolus fluorouracil followed by a 2400 mg/m2 continuous fluorouracil infusion over 46 h). Randomisation was done by central minimisation with four factors: presence of extrahepatic metastases, tumour involvement of the liver, planned use of a biological agent, and investigational centre. Participants and investigators were not masked to treatment. The primary endpoint was overall survival, analysed in the intention-to-treat population, using a two-stage meta-analysis of pooled individual patient data. All three trials have completed 2 years of follow-up. FOXFIRE is registered with the ISRCTN registry, number ISRCTN83867919. SIRFLOX and FOXFIRE-Global are registered with ClinicalTrials.gov, numbers NCT00724503 (SIRFLOX) and NCT01721954 (FOXFIRE-Global).
Findings
Between Oct 11, 2006, and Dec 23, 2014, 549 patients were randomly assigned to FOLFOX alone and 554 patients were assigned FOLFOX plus SIRT. Median follow-up was 43·3 months (IQR 31·6–58·4). There were 411 (75%) deaths in 549 patients in the FOLFOX alone group and 433 (78%) deaths in 554 patients in the FOLFOX plus SIRT group. There was no difference in overall survival (hazard ratio [HR] 1·04, 95% CI 0·90–1·19; p=0·61). The median survival time in the FOLFOX plus SIRT group was 22·6 months (95% CI 21·0–24·5) compared with 23·3 months (21·8–24·7) in the FOLFOX alone group. In the safety population containing patients who received at least one dose of study treatment, as treated, the most common grade 3–4 adverse event was neutropenia (137 [24%] of 571 patients receiving FOLFOX alone vs 186 (37%) of 507 patients receiving FOLFOX plus SIRT). Serious adverse events of any grade occurred in 244 (43%) of 571 patients receiving FOLFOX alone and 274 (54%) of 507 patients receiving FOLFOX plus SIRT. 10 patients in the FOLFOX plus SIRT group and 11 patients in the FOLFOX alone group died due to an adverse event; eight treatment-related deaths occurred in the FOLFOX plus SIRT group and three treatment-related deaths occurred in the FOLFOX alone group.
Interpretation
Addition of SIRT to first-line FOLFOX chemotherapy for patients with liver-only and liver-dominant metastatic colorectal cancer did not improve overall survival compared with that for FOLFOX alone. Therefore, early use of SIRT in combination with chemotherapy in unselected patients with metastatic colorectal cancer cannot be recommended. To further define the role of SIRT in metastatic colorectal cancer, careful patient selection and studies investigating the role of SIRT as consolidation therapy after chemotherapy are needed.
Funding
Bobby Moore Fund of Cancer Research UK, Sirtex Medical.
Introduction
Metastatic disease affects approximately 40–50% of the more than one million patients diagnosed with colorectal cancer worldwide each year.1, 2 5-year overall survival for patients with metastatic colorectal cancer is approximately 13%.1 The liver is the dominant site of metastases in colorectal cancer and liver metastases are the commonest cause of death for patients with colorectal cancer.3
Modest, but steady, advances in systemic therapy have improved the outlook for patients with metastatic colorectal cancer over the past two decades.4 Nevertheless, surgery remains the only curative treatment. In this disease, compelling evidence suggests that improved local control of colorectal liver metastases could translate into prolonged overall survival. After complete resection of liver metastases from metastatic colorectal cancer, approximately 35–40% of patients survive for 5 years.5 In the European Organisation for Research and Treatment of Cancer (EORTC) intergroup CLOCC 400046 randomised study of the addition of radiofrequency ablation with or without surgery to chemotherapy in 119 patients with up to nine colorectal liver metastases, a significant effect on progression-free survival did translate into a significant overall survival benefit.
Research in context.
Evidence before this study
Metastatic disease affects up to 50% of the more than one million patients diagnosed with colorectal cancer worldwide every year. The liver is the dominant site of metastases in colorectal cancer; liver metastases are the commonest cause of death for patients with colorectal cancer. Amongst the liver-directed therapies that might improve local control and increase downsizing of tumours to operability, selective internal radiotherapy (SIRT) is a new technology. The efficacy of SIRT in third-line or subsequent therapy for metastatic colorectal cancer has been evaluated and there are data to suggest that SIRT has clinical benefit in patients with colorectal liver metastases with liver-dominant disease after chemotherapy. We previously published a phase 1/2 trial that established the maximum tolerated dose of oxaliplatin-fluorouracil (FOLFOX) chemotherapy to combine as concomitant radiosensitising chemotherapy with SIRT. At the time of planning our studies in 2006, we searched PubMed and Web of Science for articles published between Jan 1, 1980, and May 31, 2006, with the search terms: “colorectal cancer”, “colon cancer”, “rectal cancer”, “oxaliplatin chemotherapy”, “5-fluourouracil chemotherapy”, “fluoropyrimidine”, “selective internal radiotherapy”, “yttrium-90 microsphere”, “radio-embolization”, “radio-embolisation”, “trans-arterial radio-embolization”, “liver metastasis”, and “hepatic metastasis” Original articles and review articles, published in English, were reviewed. No meta-analyses of SIRT treatment of liver metastasis were identified at the time of protocol writing in 2006.
Added value of this study
The FOXFIRE, SIRFLOX, and FOXFIRE-Global, randomised clinical trials were designed to study whether SIRT in combination with FOLFOX chemotherapy as first-line therapy for metastatic colorectal cancer can improve overall survival compared with FOLFOX alone. To our knowledge, the combined study represents the largest, randomised analysis performed in the field of interventional oncology to address the question of whether improved local control of colorectal liver metastases impacts on overall survival. Despite higher proportions of patients achieving a response and improved liver-specific progression-free survival, the addition of SIRT to first-line oxaliplatin-fluorouracil chemotherapy for patients with liver-only and liver-dominant metastatic colorectal cancer did not improve overall survival or progression-free survival. Additionally, to our knowledge, this study provides the most comprehensive account of the risk of adverse events which can occur secondary to SIRT when it is used in combination with FOLFOX chemotherapy.
Implications of all the available evidence
Because of the absence of overall survival benefit, early use of SIRT in combination with first-line, oxaliplatin-fluorouracil-based chemotherapy in unselected patients with metastatic colorectal cancer cannot be recommended. Careful patient selection and studies investigating the role of SIRT as a post-chemotherapy consolidation therapy are required to define the role of SIRT in treating metastatic colorectal cancer.
At present, the proportion of patients eligible for surgical resection is only 20%.7 Among the liver-directed therapies that might improve local control and increase downsizing of tumours to operability, selective internal radiation therapy (SIRT) is a leading technology.8 SIR-Spheres Y-90 resin microspheres (Sirtex Medical Limited; Sydney, NSW, Australia) containing the β-emitter yttrium-90 (Y-90) are delivered into the arterial supply of the liver under fluoroscopic guidance. The delivery of the resin microspheres into branches of the hepatic artery, which supplies the majority of blood to liver tumours, results in selective targeting by high-dose radiotherapy because the healthy liver is supplied predominantly by the portal venous system and therefore relatively spared from radiation exposure.9
Building on our phase 1/2 trial, in which we established the maximum tolerated dose of oxaliplatin-fluorouracil (FOLFOX) chemotherapy to combine as concomitant radiosensitising chemotherapy with SIRT,10 the FOXFIRE, SIRFLOX, and FOXFIRE-Global clinical trials were designed to study SIRT in combination with FOLFOX chemotherapy compared with FOLFOX alone as first-line therapy for metastatic colorectal cancer.11, 12 Eligibility criteria and trial designs were pre-planned to be similar so that the three trials could be prospectively combined for analysis of overall survival and secondary endpoints. We previously reported that the combination of SIRT with FOLFOX in SIRFLOX13 increased liver-specific progression-free survival compared with FOLFOX chemotherapy alone, but there was no effect on overall progression-free survival. In this combined study, we sought to address the question of whether improved local control of colorectal liver metastases impacts on overall survival.
Methods
Study design and participants
The FOXFIRE, SIRFLOX, and FOXFIRE-Global randomised, phase 3 trials were done in 14 countries (Australia, Belgium, France, Germany, Israel, Italy, New Zealand, Portugal, South Korea, Singapore, Spain, Taiwan, the UK, and the USA): in 28 hospitals and specialist liver centres for FOXFIRE, 87 hospitals or centres for SIRFLOX, and 69 hospitals or centres for FOXFIRE-Global (appendix pp 28–34). Two complementary trials were originally planned (FOXFIRE and SIRFLOX), but the FOXFIRE study took longer than anticipated to set up and recruit, so a third study (FOXFIRE-Global) was added as an independent trial.
Patients had to be eligible for systemic chemotherapy as first-line treatment for metastatic colorectal cancer.11, 12, 13, 14 Eligibility criteria were similar between the three trials, but not identical. Similarities and differences are highlighted in the combined study protocol paper.14 Inclusion criteria included histologically confirmed colorectal cancer with liver-only or liver-dominant metastases with or without the primary tumour in situ, WHO performance status of 0 or 1, limited extrahepatic disease, age of 18 years or older, and life expectancy 3 months or longer. The full list of inclusion criteria have previously been published.11, 12, 13, 14 Eligibility was confirmed by haematological, renal, and hepatic function tests. Exclusion criteria included ascites, cirrhosis, or portal hypertension (all established by clinical or radiological assessment); thrombosis of the main portal vein; and peripheral neuropathy grade 1 or worse. Full lists of exclusion criteria have previously been published.11, 12, 13, 14
The trials were done according to the principles of ISO14155 Good Clinical Practice and the Declaration of Helsinki. All participants provided written, informed consent. The protocols for FOXFIRE and SIRLOX trials and for this combined study have previously been published.11, 12, 14
Randomisation and masking
In all three trials, patients were randomly assigned (1:1) to either FOLFOX chemotherapy alone or FOLFOX plus SIRT with minimisation, based on the strata metastasis site (liver-only vs liver plus extrahepatic metastases), extent of tumour involvement of the liver (≤25% vs >25% measured objectively on baseline CT scan), planned use of a biological agent, and investigational centre.
In FOXFIRE, patients were allocated using minimisation with a probability of 0·8 to the treatment that most reduced the imbalance of the above factors. If there were equal numbers of patients in each treatment group, then patients were allocated to each treatment with a probability of 0·5. The first 30 treatments were allocated using (simple) block randomisation (using variable block sizes of 2, 4, and 6 in a ratio of 1:2:1). In SIRFLOX and FOXFIRE-Global, an imbalance window of 5 was used; if the treatment imbalance between the two groups was less than 5, the treatment was randomly allocated. If the treatment imbalance reached 5, the next treatment allocation was forced to reduce the imbalance. In FOXFIRE, computer-based randomisation was done centrally at the Oncology Clinical Trials Office (OCTO; Oxford, UK). While the trial was in progress, access to the full randomisation lists was restricted to the Database Development Manager at OCTO and the trial statistician at the Centre for Statistics in Medicine (CSM; Oxford, UK). In SIRFLOX and FOXFIRE-Global, randomisation was done centrally at the National Health and Medical Research Council Clinical Trials Centre (Camperdown, NSW, Australia) via an interactive voice response system. Participants were enrolled by the investigators in all three trials. As none of the trials were masked, once patients were allocated to treatment, participants, all members of the trial team, and treating medical staff knew the treatment allocation.
Procedures
In FOXFIRE, systemic FOLFOX chemotherapy consisted of OxMdG (oxaliplatin modified de Gramont chemotherapy; 85 mg/m2 oxaliplatin infusion over 2 h, L-leucovorin 175 mg or D,L-leucovorin 350 mg infusion over 2 h, and 400 mg/m2 bolus fluorouracil followed by a 2400 mg/m2 continuous fluorouracil infusion over 46 h). Systemic FOLFOX chemotherapy in SIRFLOX and FOXFIRE-Global consisted of modified FOLFOX6 (85 mg/m2 oxaliplatin infusion over 2 h, 200 mg leucovorin, and 400 mg/m2 bolus fluorouracil followed by a 2400 mg/m2 continuous fluorouracil infusion over 46 h). Oxaliplatin and fluorouracil were from hospital stock; D,L-leucovorin referred to the racemic mixture. In FOXFIRE, protocol chemotherapy was 12 cycles; in SIRFLOX and FOXFIRE-Global, protocol chemotherapy continued until disease progression or dose-limiting toxicity. The oxaliplatin dose was reduced from 85 mg/m2 to 60 mg/m2 for three cycles from the cycle coinciding with SIRT administration and for two cycles thereafter, based on phase 1/2 data.10 Dose modifications were permitted in line with standard care (appendix pp 3–8). Each chemotherapy cycle lasted 14 days. We used a hepatic arteriogram and a liver-to-lung breakthrough nuclear medicine scan to assess patient suitability to receive SIRT. We used the patient's body surface area, percentage of tumour involvement, and magnitude of liver-to-lung shunting to establish the activity (GBq) per dosing chart.12 Planned SIRT was done on cycle 1 day 3 or 4 or cycle 2 day 3 or 4.10 In FOXFIRE, patients could receive anti-VEGF (eg, bevacizumab) or anti-EGFR (eg, cetuximab) from cycle 1 in the FOLFOX alone group and from cycle 7 onwards in the FOLFOX plus SIRT group. In SIRFLOX and FOXFIRE-Global, patients could receive bevacizumab from cycle 1 in the FOLFOX alone group and from cycle 4 onwards in the FOLFOX plus SIRT group. The addition of anti-VEGF or anti-EGFR treatments to protocol therapy was at the discretion of the treating physician and doses prescribed were according to local policy at the treating centre.
We assessed patients by CT scan every 8–12 weeks until hepatic progression. Follow-up assessments included clinical assessment; CT of chest, abdomen, and pelvis; and health related quality-of-life (HRQoL) assessment.11, 12, 14 Scans were independently reviewed by Pharmtrace (Berlin, Germany) for overall and hepatic progression in FOXFIRE using Response Evaluation Criteria in Solid Tumors (RECIST; version 1.0) and in SIRFLOX with RECIST (version 1.0) with minor modifications.13 Independent reviews were not done in FOXFIRE-Global. We assessed all patients for suitability for liver resection at 6 months. After protocol therapy, patients could receive any subsequent treatment as best available care determined by the treating physician. All patients were followed up until death or for a minimum of 2 years.
Health-related quality of life was assessed with the EuroQol-5D three-level questionnaire (EQ-5D-3L), a generic HRQoL instrument15 measuring health in five dimensions (mobility, self-care, usual activities, pain or discomfort, and anxiety or depression), which is summarised as a utility score.16 The EQ-5D-3L was administered during clinic visits at baseline, between the second and the third months after randomisation, at 6 months, at 12 months, and once per year until 60 months from the start of protocol treatment. Grading of adverse events used the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3).
Outcomes
The primary outcome of this analysis was overall survival, defined as the time from randomisation to death from any cause, with patients who were still alive censored at their last known follow-up date. Secondary outcomes included progression-free survival, liver-specific progression-free survival, HRQoL, tumour response, liver resection rate, and adverse event profiles. The primary and secondary outcomes in each of the individual trials are in the appendix (p 13). We defined progression-free survival as the time from randomisation to radiological progression or death from any cause, whichever occurred first. Patients who did not progress or die during the trial were censored at their last known progression-free follow-up date. Liver-specific progression-free survival was defined as the time from randomisation to radiological hepatic progression. Progression not involving the liver and death before progression were regarded as competing events. We deemed hepatic progression to have occurred immediately before non-liver progression in patients who had identical hepatic and extrahepatic progression dates. Patients who withdrew from study treatment before documented progression were censored at commencement of non-study treatment for both the progression-free survival and liver-specific progression-free survival endpoints. Time of resection was not deemed a censoring point for progression-free survival or liver-specific progression-free survival. The proportion of patients achieving an objective response in each treatment group was defined as the number of patients achieving a complete or partial response at any point during the trial, up to their first occurrence of hepatic resection, over the number randomised in that group (early deaths by any cause and unknown responses were included in the demominator). The percentage resected in each group was defined as the number of patients who underwent a hepatic resection divided by the number randomly assigned to that group.
Statistical analysis
For the primary combined overall survival analysis, a sample size of 810 was originally calculated, but subsequently updated to 1075 patients (710 deaths) using a protocol-specified hazard ratio (HR) of 0·8, with a control group median overall survival of 19·7 months and SIRT group median overall survival of 24·6 months, two-sided 5% significance, 80% power, and allowing for 5% non-compliance.14 The updated sample size allowed for a higher median overall survival, the addition of biological agents to protocol chemotherapy (planned use added as a stratification factor to the randomisation in May, 2011, for FOXFIRE and SIRFLOX, and incorporated in FOXFIRE-Global since set-up) and crossover in both directions. The study also had 80% power to detect a 6-month overall survival benefit in the subgroup of patients with metastatic disease restricted to the liver: 708 patients (463 deaths) were needed. The cutoff for analysis was chosen such that there was a minimum of 710 deaths overall and 463 deaths in the liver-only subgroup (assuming a 6-month increase in overall survival in the SIRT-treated, liver-metastasis-only patients) and a minimum follow-up of 2 years since the last patient was randomly assigned to treatment. We did two interim toxicity and safety analyses with the combined data from the FOXFIRE and SIRFLOX trials 8 months after at least 80 patients were randomly assigned (40 patients per trial) and 8 months after 300 were randomly assigned (minimum of 120 patients per trial); FOXFIRE-Global was not included in the interim analyses on the combined data.
We calculated median follow-up time using the reverse Kaplan-Meier method. We did all efficacy analyses on an intention-to-treat basis per the published statistical analysis plan.14 We estimated overall survival and progression-free survival for each trial using Kaplan-Meier survival curves, unadjusted log-rank tests, and Cox proportional hazards survival models. We checked the proportionality assumption using Schoenfeld residuals. HRs for overall survival and progression-free survival from the individual trials were combined using a two-stage, fixed-effect, inverse-variance weighted individual participant data meta-analysis approach.17 The I2 statistic indicates the proportion of variation in the treatment effect that is due to heterogeneity rather than chance. Sensitivity analyses included using only eligible patients, and using radiological scan data as reported by sites for all three trials. We assessed liver-specific progression-free survival using cumulative incidence curves, and Fine and Gray subdistribution hazard regression models18 stratified by trial. We checked model assumptions by including a time-varying coefficient for treatment. We also estimated cause-specific hazards.19 For overall survival, progression-free survival, and liver-specific progression-free survival, we investigated the potential treatment benefit in patients presenting with disease confined to the liver using survival models stratified by trial (prespecified subgroup analysis). The odds of patients having a resection or achieving a response (overall and liver-specific) were compared between treatment groups by pooling individual trial odds ratios (OR) using two-stage individual participant data meta-analysis. The EQ-5D-3L health states reported by patients were expressed as utility scores derived by weighting the responses using US EQ-5D valuations16 with a score of 1 corresponding to full health and 0 to death. A minimum clinically significant difference in EQ-5D scores of 0·08 has been suggested across all cancers.20 We used analysis of covariance to analyse differences in EQ-5D-3L utility scores between the two treatment groups, adjusted for EQ-5D-3L baseline values. We made post-hoc comparisons between treatment groups of treatment received after protocol therapy using the Mantel-Haenszel test, stratifying by trial.
We did safety analyses on patients who received at least one dose of chemotherapy in either group and on an as-treated basis. We included adverse events reported up to 28 days after the end of trial treatment or 7 months after randomisation, whichever was earlier (deemed the safety window). We combined individual trial ORs using two-stage individual participant data meta-analysis to assess the association of grade 3 or worse adverse events with treatment. An independent data and safety monitoring committee oversaw the study. Formal interim monitoring of the accumulating data was done at regular intervals (approximately every 6 months) by the independent data and safety monitoring committee for each trial separately.
Data were analysed with Stata (version 14.1) and R (version 3.3.2). All hypothesis tests were two-sided. We used a significance level of 5%. Partial dates were imputed, but no statistical imputation techniques were used.
FOXFIRE is registered with the ISRCTN registry, number ISRCTN83867919. SIRFLOX and FOXFIRE-Global are registered with ClinicalTrials.gov, numbers NCT00724503 (SIRFLOX) and NCT01721954 (FOXFIRE-Global).
Role of the funding source
The sponsor had no role in the FOXFIRE study design, data collection, or data analysis. Employees of Sirtex were involved in all stages of the SIRFLOX and FOXFIRE-Global studies, and in the process of combining the three trial datasets. The funders had no role in data analysis or interpretation for the combined study. The sponsor reviewed the manuscript and provided comments. JMo, PD, PSV, and SL had full access to all of the data in the study and the corresponding author had final responsibility for the decision to submit for publication.
Results
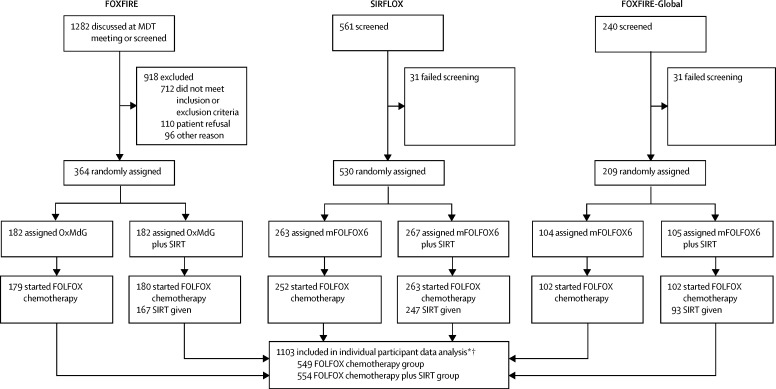
Between Oct 11, 2006, and Dec 23, 2014, 1103 patients were enrolled and randomly assigned to either FOLFOX chemotherapy (n=549) or FOLFOX plus single treatment SIRT (n=554) (figure 1). Patients were recruited for the FOXFIRE study between Nov 13, 2009, and Oct 31, 2014. Patients in the SIRFLOX study were recruited between Oct 11, 2006, and April 25, 2013. Patients were recruited for the FOXFIRE-Global study between May 20, 2013, and Dec 23, 2014. End of follow-up was Oct 31, 2016, for FOXFIRE, June 1, 2016, for SIRFLOX, and Nov 30, 2016, for FOXFIRE-Global, ensuring 2 years of follow-up after the last patient was enrolled (appendix p 13). The data lock dates for analysis were Dec 23, 2016, for SIRFLOX and FOXFIRE-Global and Feb 6, 2017, for FOXFIRE. Minimisation factors and other baseline characteristics were evenly balanced between treatment groups and between trials (table 1, appendix pp 14–15). Median follow-up was 43·3 months (IQR 31·6–58·4).
Figure 1.
Trial profile
OxMdG and mFOLFOX6 are equivalent oxaliplatin-fluorouracil-based FOLFOX chemotherapy regimens. FOLFOX=leucovorin, fluorouracil, and oxaliplatin. MDT=multidisciplinary team. mFOLFOX6=modified FOLFOX. OxMdG=oxaliplatin modified de Gramont chemotherapy. SIRT=selective internal radiotherapy. *Includes 122 ineligible patients; 49 protocol waivers and 73 retrospectively identified. †Discontinuation data were available for patients in FOXFIRE and for a subset of patients in SIRFLOX, but were not available for patients in FOXFIRE-Global.
Table 1.
Baseline characteristics of participants in the combined study
| FOLFOX alone (n=549) | FOLFOX plus SIRT (n=554) | ||
|---|---|---|---|
| Age at randomisation (years) | 62·7 (23·1–89·0) | 63·4 (28·4–89·6) | |
| Time since diagnosis of primary tumour to randomisation (months) | 1·4 (0·9–2·3) | 1·4 (0·9–2·3) | |
| Time since diagnosis of liver metastases to randomisation (months) | 1·2 (0·8–1·8) | 1·2 (0·7–1·8) | |
| Sex | |||
| Male | 361 (66%) | 363 (66%) | |
| Female | 187 (34%) | 191 (34%) | |
| Missing | 1 (<1%) | 0 | |
| WHO performance status | |||
| 0 | 347 (63%) | 354 (64%) | |
| 1 | 200 (36%) | 198 (36%) | |
| Missing | 2 (<1%) | 2 (<1%) | |
| Primary tumour site | |||
| Colon | 392 (71%) | 421 (76%) | |
| Rectum | 137 (25%) | 116 (21%) | |
| Not categorisable* | 8 (1%) | 5 (1%) | |
| Missing | 12 (2%) | 12 (2%) | |
| Primary tumour in situ | |||
| Yes | 302 (55%) | 278 (50%) | |
| No | 246 (45%) | 275 (50%) | |
| Missing | 1 (<1%) | 1 (<1%) | |
| Previous adjuvant chemotherapy | |||
| Yes | 28 (5%) | 31 (6%) | |
| No | 520 (95%) | 523 (94%) | |
| Missing | 1 (<1%) | 0 | |
| Metastases present at initial diagnosis | |||
| Yes (synchronous) | 475 (87%) | 483 (87%) | |
| No (metachronous) | 71 (13%) | 68 (12%) | |
| Missing | 3 (1%) | 3 (1%) | |
| Extrahepatic metastases status†‡ | |||
| No | 358 (65%) | 355 (64%) | |
| Yes | 191 (35%) | 199 (36%) | |
| Extent of liver involvement† | |||
| ≤25% | 380 (69%) | 374 (68%) | |
| >25% | 168 (31%) | 179 (32%) | |
| Missing | 1 (<1%) | 1 (<1%) | |
| Intention to treat with biological agents† | |||
| Yes | 299 (54%) | 298 (54%) | |
| No | 153 (28%) | 153 (28%) | |
| Not applicable§ | 97 (18%) | 103 (19%) | |
Data are median (range) for age, median (IQR) for times since diagnosis, or n (%). SIRT=selective internal radiotherapy.
Site of primary tumour recorded as both colon and rectum; the only options in FOXFIRE were colon or rectum.
Minimisation factors; treating site was also a minimisation factor.
Extrahepatic disease permitted as per trial protocols were FOXFIRE: no more than five metastases in the lung and metastases should have been, in the opinion of either the local multidisciplinary team meeting or after central review of scans arranged via the trials office, amenable to future definitive local therapy, in addition to lung metastases, a single site of other extrahepatic disease was permitted (eg, multiple lymph nodes in one lymph node region) after approval by the trials office; for SIRFLOX and FOXFIRE-Global: limited extrahepatic metastases in the lung, lymph nodes, or both were permitted, no more than five nodules in the metastases in the lung were allowed, which were no more than 1 cm in diameter or up to 1·7 cm in diameter per single lesion; involvement of lymph nodes in one single anatomic area (pelvis, abdomen, or chest) was permitted provided their longest diameter measured less than 2 cm.
Intention to treat with a biological agent was not a minimisation factor for these patients because it was introduced after these patients entered the study.
In the first 12 cycles of treatment, 3193 (59% of 5369) oxaliplatin cycles in the FOLFOX plus SIRT group and 3608 (68%) of 5344 cycles in the FOLFOX alone group were at full protocol dose. Dose reduction data were not readily available. The median number of cycles of FOLFOX treatment was 12 (IQR 7–13) in the FOLFOX plus SIRT group and 12 (7–15) in the FOLFOX alone group. Bevacizumab was given to 197 (36%) of 554 patients in the FOLFOX plus SIRT group and 256 (47%) of 549 patients in the FOLFOX alone group. In FOXFIRE, 13 (4%) of 364 patients received cetuximab (four in the FOLFOX plus SIRT group and nine in the FOLFOX group). After finishing protocol therapy, fewer patients in the FOLXFOX plus SIRT group were given irinotecan, fluoropyrimidine, anti-VEGF, or anti-EGFR systemic therapies upon progression than were patients in the FOLFOX alone group (appendix p 16). 66 (12%) of 549 patients randomly assigned to the FOLFOX alone group received SIRT in a later course of therapy, whereas 47 (8%) of 554 patients randomly assigned to SIRT did not receive SIRT.
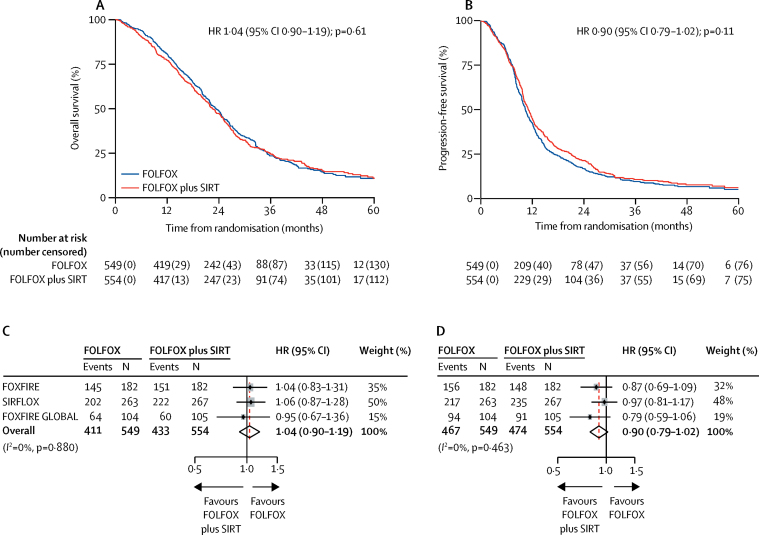
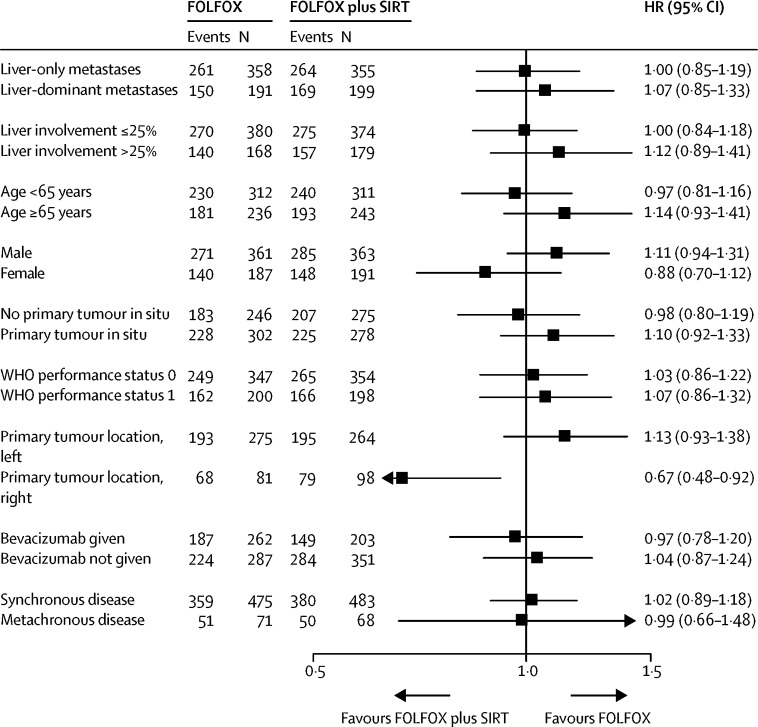
There were 844 (77%) deaths in 1103 patients in the intention-to-treat population over the follow-up period: 296 (81%) deaths in 364 patients in FOXFIRE, 424 (80%) in 530 patients in SIRFLOX, and 124 (59%) in 209 patients in FOXFIRE-Global. There were 433 (78%) deaths in 554 patients in the FOLFOX plus SIRT group and 411 (75%) in 549 patients in the FOLFOX alone group. The median survival time in the FOLFOX plus SIRT group was 22·6 months (95% CI 21·0–24·5) compared with 23·3 months (21·8–24·7) in the FOLFOX alone group; the pooled HR was 1·04 (95% CI 0·90–1·19, p=0·61; figure 2A, C). Overall survival in each trial is shown in the appendix (p 9). No difference between treatment groups was found in the prespecified, liver-metastasis-only subgroup analysis of overall survival (261 [73%] of 358 patients in the FOLFOX group, median overall survival 24·6 months, 95% CI 22·1–26·4; 264 [74%] of 355 patients in the FOLFOX plus SIRT group, 24·5 months, 22·3–26·3; pooled HR 1·00, 95% CI 0·85–1·19, p=0·96). In a sensitivity analysis of overall survival excluding ineligible patients (62 [11%] of 549 patients in the FOLFOX group and 60 [11%] of 554 patients in the FOLFOX plus SIRT group were ineligible; reasons for ineligibility not reported), there were 360 deaths (74%) in 487 patients in the FOLFOX group and 382 deaths (77%) in 494 patients in the FOLFOX plus SIRT group. Median overall survival was 23·9 months (95% CI 21·8–25·0) in the FOLFOX group and 23·4 months (21·8–25·2) in the FOLFOX plus SIRT group; pooled HR was 1·01 (95% CI 0·88–1·17, p=0·86). Subgroup comparisons for overall survival of the prespecified subgroups metastatic site, liver involvement, age, sex, WHO performance status, and tumour in situ, and the post-hoc subgroups primary tumour site, bevacizumab administration, and timing of metastasis are shown in figure 3.
Figure 2.
Overall survival (A, C) and progression-free survival (B, D) in the intention-to-treat population
HR=hazard ratio. SIRT=selective internal radiotherapy. Red line indicates the overall, pooled estimate. Size of shaded grey boxes indicates the relative weight of the study.
Figure 3.
Treatment effect on overall survival by subgroup
HR=hazard ratio. SIRT=selective internal radiotherapy.
941 (85%) of 1103 patients had an observed radiological progression or died before progression: 304 (84%) of 364 patients in FOXFIRE, 452 (85%) of 530 patients in SIRFLOX, and 185 (89%) of 209 patients in FOXFIRE-Global. 467 (85%) of 549 patients in the FOLFOX group and 474 (86%) of 554 patients in the FOLFOX plus SIRT had progression or died. Progression-free survival was not significantly different between treatment groups (pooled HR 0·90, 95% CI 0·79–1·02, p=0·11; figure 2B, D). The median progression-free survival in the FOLFOX plus SIRT group was 11·0 months (95% CI 10·2–11·8) compared with 10·3 months (9·7–10·9) in the FOLFOX alone group. Similar results were found in the prespecified subgroup analysis restricted to patients with liver-only metastatic colorectal cancer at baseline; 297 (83%) of 358 patients in the FOLFOX group had progressed or died in the liver-only subgroup analysis (median progression-free survival 11·1 months, 95% CI 10·0–12·1); 292 (82%) of 355 patients in the FOLFOX plus SIRT group had progressed or died (11·9 months, 11·0–13·8; HR 0·86, 95% CI 0·73–1·01, p=0·066). The results of a prespecified sensitivity analysis using radiological scan data as reported by sites from all three trials were consistent with the analysis of the centrally reviewed data (HR 0·91, 95% CI 0·80–1·03, p=0·15).
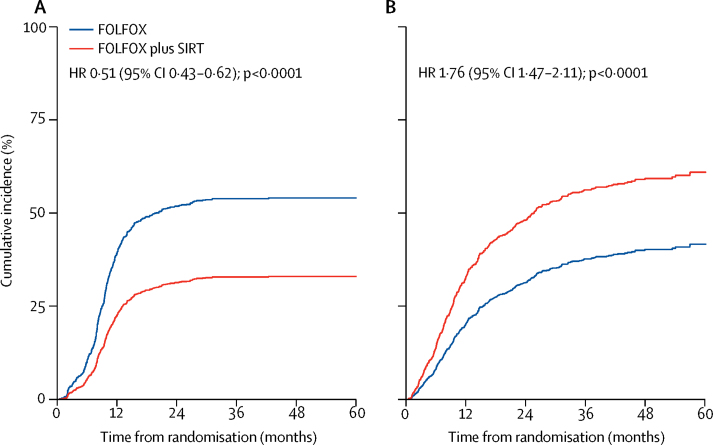
In 271 (49%) of 549 patients in the FOLFOX and 173 (31%) of 554 patients in the FOLFOX plus SIRT group, first progression events were radiologically observed in the liver (figure 4A). The cumulative incidence of first progression in the liver was lower in the FOLFOX plus SIRT group than the FOLFOX alone group (figure 4A; appendix p 17). The cumulative incidence of progression within the liver in the first 12 months of follow-up was 22% (95% CI 19–26) in the FOLFOX plus SIRT group and 39% (35–43) in the FOLFOX alone group. In 196 (36%) of 549 patients in the FOLFOX group and 301 (54%) of 554 patients in the FOLFOX plus SIRT group, first progression was extrahepatic or death occurred before recorded radiological progression (figure 4B, appendix p 17). The cumulative incidence of first progression occurring outside the liver or death before recorded radiological progression was higher in the FOLFOX plus SIRT group than in the FOLFOX alone group (figure 4B; appendix p 17). The cumulative incidence of progression outside the liver or death before recorded radiological progression in 12 months of follow-up was 33% (95% CI 29–37) in the FOLFOX plus SIRT group and 19% (16–23) in the FOLFOX alone group. Cause-specific HRs were in the same direction as, and of similar magnitudes to, the subdistribution HRs (appendix p 17).
Figure 4.
Cumulative incidence of radiological progression within the liver (A) and non-liver progression or death without radiological progression having been documented (B)
HR=hazard ratio. SIRT=selective internal radiotherapy.
An objective (complete or partial) response over the study duration was achieved in 746 (68%) of 1103 patients; 400 (72%) of 554 in the FOLFOX plus SIRT group and 346 (63%) of 549 in the FOLFOX alone group (pooled OR 1·52, 95% CI 1·18–1·96, p=0·0012; appendix pp 10, 17). An objective response was observed in more patients in the FOLFOX plus SIRT group than in the FOLFOX alone group in each of the individual trials (appendix p 17). The odds of achieving an objective response in the liver were also higher in the FOLFOX plus SIRT group than in the FOLFOX alone group (pooled OR 1·78, 95% CI 1·37–2·31, p<0·0001; appendix p 18).
Over the follow-up period, 182 (17%) of 1103 patients had at least one hepatic resection. Overall, 94 (17%) of 554 patients in the FOLFOX plus SIRT group and 88 (16%) of 549 patients in the FOLFOX alone group had a resection. The odds of undergoing a resection were not significantly different between treatment groups (pooled OR 1·07, 95% CI 0·78–1·48, p=0·67; appendix p 11).
The proportion of patients who had a grade 3 or worse adverse event at each treatment cycle by treatment group, overall and for haematological adverse events, non-haematological adverse events, and neutropenia are shown in the appendix (p 12). Of 1078 patients who received at least one dose of study treatment in the as-treated population, 755 (70%) had a grade 3 or worse adverse event (up to 28 days after the end of protocol chemotherapy or in the first 7 months after randomisation, whichever was earlier); 375 (74%) of 507 patients in the FOLFOX plus SIRT group and 380 (67%) of 571 patients in the FOLFOX alone group (table 2). The odds of a patient having a grade 3 or worse adverse event were higher in the FOLFOX plus SIRT group than in the FOLFOX alone group (pooled OR 1·42, 95% CI 1·09–1·85, p=0·0089, appendix p 13). Of 507 patients who received SIRT, 231 (46%) had a haematological grade 3 or worse adverse event, whereas 165 (29%) of the 571 patients who did not have SIRT had a haematological grade 3 or worse adverse event, the most frequent being neutropenia (186 [37%] in the FOLFOX plus SIRT group vs 138 [24%] in the FOLFOX alone group; table 2). Adverse events of grade 1 or 2 occurring in 10% of patients or more and all grade 3 or worse events are shown in the appendix (pp 18–26). In FOXFIRE, 15 (8%) of 182 patients in the FOLFOX group and 25 (14%) of 182 patients in the FOLFOX plus SIRT group discontinued treatment because of an adverse event or serious adverse event; data were not fully available for SIRFLOX and not available for FOXFIRE-Global. Serious adverse events of any grade occurred in 244 (43%) of 571 patients receiving FOLFOX alone and 274 (54%) of 507 patients receiving FOLFOX plus SIRT (appendix p 27). 10 patients in the FOLFOX plus SIRT group and 11 patients in the FOLFOX alone group died due to an adverse event during the main safety window (appendix p 26). 11 (1%) of 844 deaths were treatment-related (appendix p 27); eight in FOLFOX plus SIRT group and three in the FOLFOX alone group. Of the eight treatment-related deaths in the FOLFOX plus SIRT group, three deaths due to radiation-induced liver disease, two due to complications of surgery, one due to liver failure, one due to drug-induced pneumonitis, and one due to off-target delivery of microspheres. There were three treatment-related deaths in the FOLFOX alone group: one due to complications of surgery, one due to neutropenic sepsis, and one due to bowel perforation. With specific reference to risk of long-term toxicity to the liver from protocol treatment, during continued observation after the main safety window until the end of the follow-up period, there were two deaths due to hepatic events (hepatic failure or ascites) in the FOLFOX plus SIRT group.
Table 2.
Adverse events reported in each treatment group
|
FOLFOX alone (n=571) |
FOLFOX plus SIRT (n=507) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | ||
| Overall | 189 (33%) | 266 (47%) | 103 (18%) | 11 (2%) | 131 (26%) | 239 (47%) | 126 (25%) | 10 (2%) | |
| Haematological | 102 (18%) | 108 (19%) | 56 (10%) | 1 (<1%) | 109 (21%) | 144 (28%) | 86 (17%) | 1 (<1%) | |
| Neutropenia | 50 (9%) | 89 (16%) | 48 (8%) | 1 (<1%) | 55 (11%) | 115 (23%) | 71 (14%) | 0 | |
| Febrile neutropenia | 0 | 11 (2%) | 5 (1%) | 0 | 0 | 25 (5%) | 7 (1%) | 1 (<1%) | |
| Thrombocytopenia | 77 (13%) | 6 (1%) | 1 (<1%) | 0 | 153 (30%) | 37 (7%) | 2 (<1%) | 0 | |
| Leucopenia | 28 (5%) | 10 (2%) | 3 (1%) | 0 | 41 (8%) | 20 (4%) | 10 (2%) | 0 | |
| Non-haematological | 265 (46%) | 232 (41%) | 61 (11%) | 10 (2%) | 219 (43%) | 218 (43%) | 59 (12%) | 9 (2%) | |
| Fatigue | 275 (48%) | 28 (5%) | 0 | 0 | 261 (51%) | 43 (8%) | 0 | 0 | |
| Diarrhoea | 256 (45%) | 35 (6%) | 2 (<1%) | 0 | 189 (37%) | 33 (7%) | 1 (<1%) | 0 | |
| Pulmonary embolism | 1 (<1%) | 7 (1%) | 19 (3%) | 0 | 2 (<1%) | 4 (1%) | 24 (5%) | 0 | |
| Neuropathy peripheral | 307 (54%) | 32 (6%) | 1 (<1%) | 0 | 273 (54%) | 18 (4%) | 0 | 0 | |
| Abdominal pain | 95 (17%) | 13 (2%) | 0 | 0 | 151 (30%) | 30 (6%) | 1 (<1%) | 0 | |
| SIRT-associated | 13 (2%) | 9 (2%) | 1 (<1%) | 0 | 52 (10%) | 24 (5%) | 3 (1%) | 3 (1%) | |
| Ascites | 2 (<1%) | 4 (1%) | 0 | 0 | 23 (5%) | 6 (1%) | 0 | 0 | |
| Blood bilirubin increased | 3 (1%) | 2 (<1%) | 0 | 0 | 6 (1%) | 3 (1%) | 0 | 0 | |
| Gastric ulcer | 0 | 0 | 0 | 0 | 8 (2%) | 3 (1%) | 1 (<1%) | 0 | |
| Hyperbilirubinaemia | 1 (<1%) | 1 (<1%) | 0 | 0 | 2 (<1%) | 3 (1%) | 0 | 0 | |
| Gastrointestinal haemorrhage | 0 | 0 | 1 (<1%) | 0 | 2 (<1%) | 1 (<1%) | 2 (<1%) | 0 | |
| Radiation hepatitis | 0 | 0 | 0 | 0 | 2 (<1%) | 2 (<1%) | 0 | 2 (<1%) | |
| Duodenal ulcer | 1 (<1%) | 0 | 0 | 0 | 4 (1%) | 3 (1%) | 0 | 0 | |
| Pancreatitis | 0 | 0 | 0 | 0 | 1 (<1%) | 2 (<1%) | 0 | 0 | |
| Hepatic failure | 0 | 0 | 0 | 0 | 0 | 1 (<1%) | 0 | 1 (<1%) | |
| Jaundice | 0 | 2 (<1%) | 0 | 0 | 0 | 0 | 0 | 0 | |
| Jaundice cholestatic | 0 | 0 | 0 | 0 | 0 | 2 (<1%) | 0 | 0 | |
| Hepatic encephalopathy | 0 | 0 | 0 | 0 | 0 | 2 (<1%) | 0 | 0 | |
| Duodenitis | 0 | 0 | 0 | 0 | 4 (1%) | 1 (<1%) | 0 | 0 | |
| Portal hypertension | 1 (<1%) | 0 | 0 | 0 | 0 | 1 (<1%) | 0 | 0 | |
| Duodenal ulcer haemorrhage | 0 | 0 | 1 (<1%) | 0 | 0 | 0 | 0 | 0 | |
| Cholecystitis acute | 0 | 0 | 0 | 0 | 0 | 1 (<1%) | 0 | 0 | |
| Perihepatic abscess | 0 | 0 | 0 | 0 | 0 | 1 (<1%) | 0 | 0 | |
| Gastritis | 4 (1%) | 0 | 0 | 0 | 18 (4%) | 0 | 0 | 0 | |
| Oesophagitis | 3 (1%) | 0 | 0 | 0 | 2 (<1%) | 0 | 0 | 0 | |
| Splenomegaly | 1 (<1%) | 0 | 0 | 0 | 2 (<1%) | 0 | 0 | 0 | |
| Oesophageal ulcer | 0 | 0 | 0 | 0 | 1 (<1%) | 0 | 0 | 0 | |
Data are n (%). Table shows grade 3 or worse haematological events occurring in at least 5% of patients, grade 3 or worse non-haematological events occurring in at least 5% of patients, and all SIRT-associated adverse events. Worst grade reported per patient per category, sorted by prevalence of grade 3 or worse. SIRT=selective internal radiotherapy.
EQ-5D-3L results were reported for the first 24 months of the trial using US EQ-5D valuations; questionnaire mean utility value at each timepoint is shown in the appendix (p 27). Average unadjusted EQ-5D-3L utility scores were not significantly different between treatment groups at any time point except at 2–3 months (appendix p 27); however, this difference would not be deemed clinically meaningful.
Discussion
The FOXFIRE, SIRFLOX, and FOXFIRE-Global randomised studies designed to definitively assess the combination of SIRT with FOLFOX versus FOLFOX alone for colorectal liver metastases in the first-line setting have not shown a benefit of adding SIRT on overall survival. The combined analysis of these three randomised clinical trials shows that a global, multidisciplinary trial involving a complex liver-directed therapy can be done with adequate power to answer an important clinical question.
The efficacy of SIRT in third-line or subsequent therapy for metastatic colorectal cancer (ie, salvage therapy of chemotherapy-refractory disease) has previously been evaluated.21 A randomised trial22 of salvage in patients with metastatic colorectal cancer with liver-confined disease showed a significant benefit of chemotherapy plus SIRT versus chemotherapy alone in time to progression. These data suggest that SIRT has clinical benefit in patients with colorectal liver metastases with liver-dominant disease after chemotherapy.
Metastatic colorectal cancer in which the liver is the only site of disease occurs frequently in a subset of patients; some of these patients can be resected with long-term cures.23 In our study, the significant improvement in liver disease control assessed by competing risk analyses did not translate to a benefit in overall survival. This finding contrasts with that from the EORTC CLOCC study,6 which showed that a significant effect of radiofrequency ablation with or without surgery on progression-free survival translated into a highly significant overall survival benefit in patients who did not have primary tumour in situ or extrahepatic metastases at trial entry. The absence of benefit of the addition of SIRT to FOLFOX on progression-free survival and overall survival in our combined study could be partly explained by the high proportion of patients who developed first progression at an extrahepatic site, independently of whether the metastases were liver-only at baseline or whether there were extrahepatic metastases or primary tumour in situ at baseline. Despite the better liver control in the FOLFOX plus SIRT group compared with the FOLFOX alone group, this observation of extrahepatic progression might also explain the absence of a significant difference between the groups in the frequency of surgical resection of the liver metastases being done during or after protocol therapy. To avoid possible ascertainment bias in interpreting responses and difficulties differentiating disease progression from radiation reaction of the hepatic parenchyma in patients undergoing the interventional radiotherapy liver procedure, scans were centrally reviewed by independent readers. The absence of an overall survival benefit suggests that early use of SIRT in combination with first-line oxaliplatin-based chemotherapy cannot be recommended in unselected patients with metastatic colorectal cancer.
In our study, the SIRT-treated patients had a similar quality of life to the patients who had chemotherapy only. Toxicity was higher in the SIRT group, particularly neutropenia and SIRT-related expected toxicities. In the as-treated population in the FOLFOX plus SIRT group (n=507 patients), hepatic failure resulted in death of one patient during the main safety window and death of a further two patients during extended follow-up. The increased toxicity in the SIRT-treated patients did not translate into inferior overall survival. This study provides the most comprehensive account of the risk of adverse events that might occur secondary to SIRT when used in combination with FOLFOX chemotherapy.
One possible limitation of our study is that significant changes to the management of metastatic colorectal cancer occurred during the 8-year recruitment period with the introduction of bevacizumab and EGFR inhibitors as first-line standards of care, and increased use of liver interventions such as surgery and ablation. However, there is no reason to believe that treatment differences occurred between treatment groups. Another limitation is that some patients were found not to have met the entry criteria after randomisation. Sensitivity analyses excluding all ineligible patients indicated that the findings were robust. The non-identical design of the individual trials might also be considered a limitation, but is accounted for by the use of appropriate statistical methods.
Little progress has been reported in improving overall survival in molecularly unselected populations since the CRYSTAL, PRIME, and TRIBE studies.24, 25, 26, 27 In our study, crossover was anticipated and 12% of patients randomised to FOLFOX alone received SIRT with a later line of therapy. Furthermore, 8% of patients assigned to SIRT did not receive SIRT, a proportion consistent with our previously published data in patients with metastatic colorectal cancer.13 The difference in exposure to post-trial treatments between the two treatment groups might have affected the primary endpoint. It is possible that the improved liver disease control observed in the FOLFOX plus SIRT group might have influenced physicians to bias towards less intensive subsequent treatment, particularly as so-called treatment-holiday strategies were evolving during the recruitment and follow-up periods of these trials. Alternatively, it could be postulated that the reduced use of irinotecan after protocol therapy might have been related to increased or prolonged toxicities in the patients in the FOLFOX plus SIRT group, including the risk of myelosuppression. Our analysis of these toxicities for both groups over time showed an apparent increase of grade 3 or worse adverse events in the FOLFOX plus SIRT group, particularly haematological events, with the curves gradually converging after 6 months of protocol therapy.
In the past decade, the focus of clinical trials has been on improving outcomes for selected biological subtypes in metastatic colorectal cancer with predefined molecular criteria (eg, mutant RAS or BRAF). Several studies from the past 2 years have reported that patients with right-sided primary tumours have worse survival outcomes and it is possible that these patients benefit less from standard therapies.28, 29, 30 Molecular subtypes in our study are not currently available, but in the dataset available, 179 (25%) of 718 patients had right sided-tumours. Based on preliminary data currently available, patients with colorectal liver metastases from right-sided primary tumours could be a clinical subgroup that benefits from SIRT. Further analyses are underway to investigate this exploratory finding, with specific scrutiny of RAS mutation, BRAF mutation, and tumour location in study populations who have received SIRT in clinical trials. Further studies designed to define the potential benefit of SIRT to treat colorectal liver metastases from right-sided primaries are warranted.
In conclusion, despite better liver-specific disease control and improved radiological response, the FOXFIRE prospective trials did not show a benefit of the combination of SIRT with FOLFOX for progression-free survival or overall survival. The routine early integration of SIRT in combination with oxaliplatin-based, first-line chemotherapy cannot be recommended as therapy for metastatic colorectal cancer. Further studies are needed to study the role of SIRT in carefully selected patient populations and as a consolidation therapy after chemotherapy.
Acknowledgments
Acknowledgments
The FOXFIRE study design was reviewed and endorsed by the Clinical Trials Awards and Advisory Committee of Cancer Research UK, was approved by the National Research Ethics Service Committee South Central-Berkshire (research ethics committee reference 09/H0505/1), was developed by the National Cancer Research Institute Colorectal Clinical Study Group and the National Institute for Health Research Clinical Research Network and equivalents in devolved nations, and was sponsored by the University of Oxford. Sirtex provided an unrestricted educational grant for the FOXFIRE study. The SIRFLOX and FOXFIRE-Global studies were sponsored by Sirtex. We thank Georgie Perry for secretarial support.
Contributors
HSW, PG, SL, VG, AG, GvH, and RAS designed the study and wrote the protocol. HSW, PG, NKS, JT, VH, JR, MP, MF, AW, JMi, CW, RA, AF, JMo, PSV, PD, SL, VG, AG, GVH, and RAS led the trial, recruited patients, and collected data. JMo, PSV, PD, SL, and VG analysed the data. HSW, PG, NKS, JT, VH, JR, MP, MF, AW, JMi, CW, RA, AF, JMo, PSV, PD, SL, VG, AG, GvH, and RAS interpreted the data, wrote the manuscript, and approved the paper.
Declaration of interests
HSW reports grants, personal fees, non-financial support, and other uncompensated work from Sirtex Medical and Merck Serono; personal fees and non-financial support from Celgene; grants and non-financial support from Pfizer; personal fees and non-financial support from Roche and Lily outside the submitted work. PG reports personal fees from SIRTEX; grants and personal fees from Roche, Amgen, Pfizer, Merck, Bayer, and Servier, during the conduct of the study. NKS reports speakers' bureau and travel fees from Sirtex Medical, outside the submitted work. JT reports honoraria from Roche, Amgen, Merck, Lilly, Sanofi, Baxalta, Celgene, and Servier, outside the submitted work. VH reports grants, personal fees, and non-financial support from Sirtex Medical, Merck, and Roche, during the conduct of the study; involvement in a scientific project on radiological imaging from Sirtex Medical; grants, personal fees, and non-financial support from Merck and Roche; grants and non-financial support from Amgen; grants and personal fees from Sanofi; grants from Pfizer and Boehringer Ingelheim; and personal fees from Bristol-Myers Squibb, MSD, and Novartis, outside the submitted work. JR reports personal fees from Sirtex Medical, during the conduct of the study; and grants from Sirtex Medical, outside the submitted work. MP reports personal fees from Sirtex Medical, during the conduct of the study and outside the submitted work. MF reports grants from Sirtex Medical, during the conduct of the study. JMi reports personal fees from Sirtex Medical, during the conduct of the study; grants from Sirtex Medical; and personal fees from Sirtex Medical, Astellas, Jannessen, Lily, and Roche, outside the submitted work. CW reports receiving travel grants from Sirtext, outside the submitted work. RA reports personal fees and non-financial support from Merck Serono, Amgen, Bristol-Myers Squibb, and Sanofi, outside the submitted work. AF reports grants from Cancer Research UK; and grants and non-financial support from Sirtex Medical, during the conduct of the study. JMo reports grants from Cancer Research UK and Sirtex Medical, during the conduct of the study; and non-financial support from Sirtex Medical, outside the submitted work. PD reports grants from Cancer Research UK and Sirtex Medical, during the conduct of the study; and non-financial support from Sirtex Medical, outside the submitted work. PSV reports grants from Sirtex Medical and Cancer Research UK, during the conduct of the study. SL reports grants from Sirtex Medical and Cancer Research UK, during the conduct of the study. VG reports personal fees from Sirtex Medical, during the conduct of the study; and grants and personal fees from Sirtex Medical, outside the submitted work. AG reports grants from Cancer Research UK during the conduct of the study. GvH reports personal fees and non-financial support from Sirtex Medical, during the conduct of the study; and personal fees and non-financial support from Sirtex Medical, outside the submitted work. RAS is a consultant for and reports grants and personal fees from Sirtex Medical and BTG, during the conduct of the study; and reports personal fees from Affidea, AstraZeneca, Boston Scientific, Cancer Research Technology, Eisai, Terumo, and Varian, outside the submitted work. AW declares no competing interests.
Contributor Information
Ricky A Sharma, Email: ricky.sharma@oncology.ox.ac.uk.
FOXFIRE trial investigators:
Richard Adams, Andrew Bateman, Claire Blesing, Ewan Brown, Ian Chau, Sebastian Cummins, David Cunningham, Stephen Falk, Maher Hadaki, Marcia Hall, Tamas Hickish, Joanne Hornbuckle, Fiona Lofts, Sarah Lowndes, Astrid Mayer, Matthew Metcalfe, Gary Middleton, Jamie Mills, Amir Montazeri, Rebecca Muirhead, Andreas Polychronis, Colin Purcell, Paul Ross, Ricky A Sharma, Liz Sherwin, David Smith, Rubin Soomal, Daniel Swinson, Axel Walther, Harpreet Wasan, Andrew Weaver, Charles Wilson, and Greg Wilson
SIRFLOX trial investigators:
Pradip Amin, Bruna Angelelli, Jacques Balosso, Alex Beny, Daniel Bloomgarden, Evelyn Boucher, Michael Brown, Harald-Robert Bruch, James Bui, Matthew Burge, Giuseppe Cardaci, James Carlisle, Seungjean Chai, Yi-Jen Chen, Patrick Chevallier, Michael Chuong, Stephen Clarke, Andrew Coveler, Michael Craninx, Thierry Delanoit, Amélie Deleporte, Paul Eliadis, Francis Facchini, Thomas Ferguson, Michel Ferrante, Michael Findlay, Gary Frenette, Jacob Frick, Vinod Ganju, Michael Garofalo, Karen Geboes, Gerald Gehbauer, Benjamin George, Ravit Geva, Peter Gibbs, Michael Gordon, Kate Gregory, Seza Gulec, James Hannigan, Guy van Hazel, Norman Heching, Volker Heinemann, Thomas Helmberger, Alain Hendlisz, Koen Hendrickx, Matthew Holtzman, Richard Isaacs, Christopher Jackson, Philip James, Adeel Kaiser, Chris Karapetis, Andreas Kaubisch, Yon-Dschun Ko, Hendrik Kröning, Frank Lammert, Winston Liauw, Steven Limentani, Samy Louafi, Marc de Man, Jeffrey Margolis, Robert Martin, Andrea Martoni, Gavin Marx, Marco Matos, Els Monsaert, Veerle Moons, Louise Nott, Arnd Nusch, Anne O'Donnell, Howard Ozer, Siddarth Padia, Nick Pavlakis, Marc Peeters, David Perez, Stefan Pluntke, Marc Polus, Alex Powell, Marc Pracht, Timothy Price, David Ransom, Christine Rebischung, Jens Ricke, Karsten Ridwelski, Jorge Riera-Knorrenschild, Hanno Riess, William Rilling, Bridget Robinson, Javier Rodríguez, Federico Sanchez, Tilmann Sauerbruch, Michael Savin, Klemens Scheidhauer, Elyse Schneiderman, Grant Seeger, Eva Segelov, Einat Shaham Schmueli, Adi Shani, Jenny Shannon, Navesh Sharma, Stephen Shibata, Nimit Singhal, Denis Smith, Randall Smith, Salomon Stemmer, Oliver Stötzer, Andrew Strickland, Julien Taieb, Klaus Tatsch, Eric Terrebonne, Thomas Tichler, Ursula Vehling-Kaiser, Ruth Vera-Garcia, Thomas Vogl, Euan Walpole, Eric Wang, Samuel Whiting, and Ido Wolf
FOXFIRE-Global trial investigators:
Steven Ades, Morteza Aghmesheh, Bruna Angelelli, Miklos Auber, Hubert Ayala, Alex Beny, Daniel Bloomgarden, Patrick Boland, Eveline Bouche, Charles Bowers, Christoph Bremer, James Bui, Mathew Burge, James Carlisle, Ana Ruiz Casado, Seungjean Chai, Michael Chuong, Prasad Cooray, Martin Crain, Maike De Wit, Amelie Deleporte, Kyran Dowling, Aurelie Durand, Francis Facchini, Sandrine Faivre, Kynan Feeney, Tom Ferguson, Aurelie Ferru, Michael Findlay, Maria Fragoso, Gary Frenette, Jacob Frick, Vinod Ganju, Ravit Geva, Peter Gibbs, Cristina Granetto, Pascal Hammel, Guy van Hazel, Norman Heching, Alain Hendlisz, Koen Hendrickx, Matthew Holtzman, Richard Issacs, Renuka Iyer, Christopher Jackson, Adeel Kaiser, Andreas Kaubisch, Yeul Hong Kim, Hendrik Kröning, Jin Tung Liang, Lionel Lim, Steven Limentani, Jin Hwang Liu, Samy Louafi, Marc de Man, Gianluca Masi, Marco Matos, Els Monsaert, Stefania Mosconi, Louise Nott, Gianmauro Numico, Anne O'Donnell, Marc Peeters, Marc Polus, Marc Pracht, Lynn Ratner, Christine Rebischung, Han Sae-Won, Federico Sanchez, Adi Shani, Navesh Sharma, Madhu Singh, Nimit Singhal, Denis Smith, Patricia Stoltzfus, Andrew Strickland, Julien Taieb, Iain Tan, Eric Terrebonne, Thomas Tichler, Antonio Trogu, Craig Underhill, Ruth Vera-Garcia, Euan Walpole, Eric Wang, and Mark Westcott
Supplementary Material
References
- 1.National Cancer Institute SEER cancer stat facts: colon and rectum cancer, 2003–2009. 2013. https://seer.cancer.gov/statfacts/html/colorect.html (accessed May 30, 2017).
- 2.Cancer Research UK Bowel cancer incidence statistics. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer/incidence (accessed May 30, 2017).
- 3.Helling TS, Martin M. Cause of death from liver metastases in colorectal cancer. Ann Surg Oncol. 2014;21:501–506. doi: 10.1245/s10434-013-3297-7. [DOI] [PubMed] [Google Scholar]
- 4.Strickler JH, Hurwitz HI. Palliative treatment of metastatic colorectal cancer: what is the optimal approach? Curr Oncol Rep. 2014;16:363. doi: 10.1007/s11912-013-0363-z. [DOI] [PubMed] [Google Scholar]
- 5.Kanas GP, Taylor A, Primrose JN. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruers T, Van Coevorden F, Punt CJ. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017;109:djx015. doi: 10.1093/jnci/djx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones RP, Poston GJ. Resection of liver metastases in colorectal cancer in the era of expanding systemic therapy. Annu Rev Med. 2017;68:183–196. doi: 10.1146/annurev-med-062415-093510. [DOI] [PubMed] [Google Scholar]
- 8.Nicolay NH, Berry DP, Sharma RA. Liver metastases from colorectal cancer: radioembolization with systemic therapy. Nat Rev Clin Oncol. 2009;6:687–697. doi: 10.1038/nrclinonc.2009.165. [DOI] [PubMed] [Google Scholar]
- 9.Wang LM, Jani AR, Hill EJ, Sharma RA. Anatomical basis and histopathological changes resulting from selective internal radiotherapy for liver metastases. J Clin Pathol. 2013;66:205–211. doi: 10.1136/jclinpath-2012-201231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma RA, Van Hazel GA, Morgan B. Radioembolisation of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil and leucovorin chemotherapy. J Clin Oncol. 2007;25:1099–1106. doi: 10.1200/JCO.2006.08.7916. [DOI] [PubMed] [Google Scholar]
- 11.Dutton SJ, Kenealy N, Love SB. FOXFIRE protocol: an open-label, randomised, phase III trial of 5-fluorouracil, oxaliplatin and folinic acid (OxMdG) with or without interventional Selective Internal Radiation Therapy (SIRT) as first-line treatment for patients with unresectable liver-only or liver-dominant metastatic colorectal cancer. BMC Cancer. 2014;14:497. doi: 10.1186/1471-2407-14-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbs P, Gebski V, Van Buskirk M. Selective Internal Radiation Therapy (SIRT) with yttrium-90 resin microspheres plus standard systemic chemotherapy regimen of FOLFOX versus FOLFOX alone as first-line treatment of non-resectable liver metastases from colorectal cancer: the SIRFLOX study. BMC Cancer. 2014;14:897. doi: 10.1186/1471-2407-14-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Hazel GA, Heinemann V, Sharma NK. SIRFLOX: randomized phase III trial comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus mFOLFOX6 (plus or minus bevacizumab) plus selective internal radiation therapy in patients with metastatic colorectal cancer. J Clin Oncol. 2016;34:1723–1731. doi: 10.1200/JCO.2015.66.1181. [DOI] [PubMed] [Google Scholar]
- 14.Virdee PS, Moschandreas J, Gebski V. Protocol for combined analysis of FOXFIRE, SIRFLOX, and FOXFIRE-global randomized phase III trials of chemotherapy +/- selective internal radiation therapy as first-line treatment for patients with metastatic colorectal cancer. JMIR Res Protoc. 2017;6:e43. doi: 10.2196/resprot.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 16.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43:203–220. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey-Smith G, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. 2nd edn. BMJ Publication Group; London: 2001. [Google Scholar]
- 18.Fine J, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–508. [Google Scholar]
- 19.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 20.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seidensticker R, Denecke T, Kraus P. Matched-pair comparison of radioembolization plus best supportive care versus best supportive care alone for chemotherapy refractory liver-dominant colorectal metastases. Cardiovasc Intervent Radiol. 2012;35:1066–1073. doi: 10.1007/s00270-011-0234-7. [DOI] [PubMed] [Google Scholar]
- 22.Hendlisz A, Van den Eynde M, Peeters M. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol. 2010;28:3687–3694. doi: 10.1200/JCO.2010.28.5643. [DOI] [PubMed] [Google Scholar]
- 23.Nordlinger B, Sorbye H, Glimelius B. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Cutsem E, Kohne CH, Hitre E. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 25.Loupakis F, Cremolini C, Masi G. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609–1618. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 26.Douillard JY, Siena S, Cassidy J. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346–1355. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 27.Van Cutsem E, Lenz HJ, Kohne CH. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33:692–700. doi: 10.1200/JCO.2014.59.4812. [DOI] [PubMed] [Google Scholar]
- 28.Petrelli F, Tomasello G, Borgonovo K. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol. 2017;3:211–219. doi: 10.1001/jamaoncol.2016.4227. [DOI] [PubMed] [Google Scholar]
- 29.Tejpar S, Stintzing S, Ciardiello F. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3:194–201. doi: 10.1001/jamaoncol.2016.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venook AP, Niedzwiecki D, Innocenti F. Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance) Proc Am Soc Clin Oncol. 2016;34(suppl 15):3504. (abstr). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.