Abstract
We report a first case of a patient experiencing reactivation of pulmonary tuberculosis (TB) during treatment of oral tyrosine kinase inhibitor (TKI) with non-small cell lung cancer (NSCLC). A 44-year-old male patient visited the hospital with cough. He had been treated with erlotinib (oral TKI) for 8 months after being diagnosed as NSCLC with sensitive epidermal growth factor receptor mutation in our clinic. At initial chest imaging, the patient had fibroatelectatic calcified granuloma in the right upper lobe (RUL) apex as well as 1.9 cm × 2.5 cm sized cancer mass encasing the RUL bronchus. He had not been treated for active pulmonary TB before. He had no known history of contact with active TB patients. During the past treatment period, he had shown overall stable response to erlotinib for 8 months. However, chest computed tomography taken for the fourth response evaluation showed increased number and size of nodules with bronchial luminal narrowing in RUL compared to the last exam, suggesting disease progression. We performed bronchoscopy to re-biopsy the cancer mass. Mucosal biopsy and bronchial washing fluid culture revealed active endobronchial pulmonary TB rather than lung cancer progression. Based on these study results, we started anti-TB medications without changing chemotherapy regimen. After 7 months of treatment for pulmonary TB with erlotinib maintenance, he has been shown successful regression of pulmonary TB with stable chemotherapeutic response. Previously, several reports have described the effect of anti-cancer therapy on the treatment of active TB. However, there has been no case report presenting TB reactivation during oral TKI treatment in NSCLC. Therefore, we suggest that the risk of TB reactivation should be considered in patients with solid organ malignancies even if targeted agents are used. Moreover, misdiagnosis of disease progression must be ruled out.
Keywords: Lung cancer, erlotinib, pulmonary tuberculosis
Introduction
World Health Organization (WHO) supported by European Respiratory Society (ERS) has recently emphasized that treatment of latent tuberculosis infection (LTBI) is a key strategy to eliminate TB in low incidence countries. In areas with high incidence of TB, the main goal is to treat active cases (1,2). In South Korea, annual incidence of TB was 63/100,000 in 2015. Such incidence was considered as intermediate (3). The Korean TB guidelines recommend treatment of LTBI in selected population depending on contact history with infectious TB patients. For subjects without contact history, LTBI treatment is conducted for moderate and high risk groups such as patients with HIV infection, those with immunosuppressive therapy or long term systemic steroids, and those with silicosis, chronic respiratory failure, diabetes mellitus, or history of gastrectomy (4).
Previous literatures have reported simultaneous appearance of lung cancer and TB (5,6). Lung cancer has been reported to be associated with the development of TB (7,8). Considering that solid-organ malignancy could be a risk factor for TB, preventive strategy needs to be established for cancer patients, especially during the treatment period. However, in Korea, guideline for treatment of LTBI in patients with solid-organ malignancies is lacking. Although the parenteral systemic chemotherapy had been considered as one of immunosuppressive therapeutic agents, case reports showing the risk of pulmonary TB infection during the oral TKI treatment had not been reported yet.
Herein, we report a case of pulmonary TB reactivation during treatment with oral tyrosine kinase inhibitor (TKI) for non-small cell lung cancer (NSCLC).
This manuscript was approved by the Institutional Review Board of Uijeongbu St. Mary’s Hospital, which waived the requirement from informed consent (No UC17ZESI0067).
Case presentation
A 44-year-old man visited our clinic due to cough. He was diagnosed as stage IV NSCLC (adenocarcinoma) about 8 months ago. The initial presentation was left side partial seizure with motor weakness. Numerous metastatic lesions at various sizes were noted in both cerebral and cerebellar hemispheres, including basal ganglia and thalamus in brain magnetic resonance imaging (MRI). Chest computed tomography (CT) revealed fibroatelectatic calcified granuloma in RUL apex and a 1.9 cm × 2.5 cm sized heterogeneously enhancing lung mass encasing the RUL bronchus with numerous military opacities in both lungs (Figure 1A). Moreover, metastatic lymph nodes were suspected at the right supraclavicular area, right upper area, both lower paratracheal area, prevascular, subcarinal, and both hilar areas. Positron emission tomography-computed tomography showed multiple bone metastatic lesions at skull base, vertebra, both scapula, both pelvis, and left femur. Based on these findings, initial clinical staging was T4N3M1b. Bronchoscopy was performed to obtain tissues. There were irregular mucosal erosions at proximal RUL bronchus (Figure 1B). Mucosal biopsy with immunostaining showed adenocarcinoma with micropapillary features originating from the lung. Bacterial culture and acid-fast bacilli (AFB) culture results from bronchoscopic washing fluid specimen all showed negative results. There was old TB scar in RUL, however, he had no history of past pulmonary TB treatment. Diagnosis of active pulmonary TB was ruled out due to negative culture result of the bronchial washing specimen. He was a 25-pack-year current smoker, however we conducted gene mutation study due to his young age and the aggressive metastatic nature. From mucosal biopsy tissues taken from bronchoscopy, there were exon 20 (S768I) and exon 21 (L858R) point mutations at epidermal growth factor (EGFR) gene. Anaplastic lymphoma kinase rearrangement was negative. He had been prescribed with EGFR tyrosine kinase inhibitor (TKI) erlotinib for first line systemic chemotherapy. Radiation therapy was also performed to the whole brain, left femur, and both pelvis. For the past 8 months, he had received both radiation and oral chemotherapy without any side effects. Several follow up chest CT scans taken at 2-month intervals demonstrated that primary lung cancer mass and numerous military metastatic nodules were decreased in size and number (Figure 2A). The overall treatment response was stable until March 2016. In April 2016, chest CT for the fourth response assessment demonstrated interval increase in wall thickening of right main bronchus and luminal narrowing of RUL bronchus. In addition, the number and size of RUL nodules were increased compared to those two months ago (Figure 2B). He had mild cough and sputum without fever or hemoptysis in our clinic. We performed bronchoscopy to re-biopsy the primary cancer mass because cancer progression was strongly suspected. On bronchoscopic evaluation, whitish mucosal plaque with mucus secretion drained from RUL bronchus was noted. However, there was no luminal narrowing of RUL bronchus. Lumens of segmental bronchi were all intact. Previously noted mucosal cancer infiltration was improved. Mucosal biopsy at the plaque and bronchial washing was done (Figure 2C). Biopsy specimen revealed chronic granulomatous inflammation with caseous necrosis. AFB stain showed positive rod compatible with endobronchial TB (Figure 3) without any cancer cell infiltration. Moreover, bronchial washing fluid specimen showed 1+ AFB stain and Mycobacterium tuberculosis polymerase chain reaction showed positive results. Aggravated chest CT finding was due to active pulmonary TB rather than cancer progression. Erlotinib was resumed and anti-TB medications were started. Since then, he has been taking anti-Tb medication for 7 months (up to Feb 2017) and 1st line TKI erlotinib for 17 months without cancer progression. As regimen of anti-TB medications, Isoniazid 300 mg/Pyrazinamide 1,500 mg/Moxifloxacin 400 mg were selected due to possible drug-reaction between rifampin and erlotinib and initial decreased visual acuity of patient due to the choroidal metastasis (ethambutol was skipped). The anti-Tb medications are planned to be continued for 18 months.
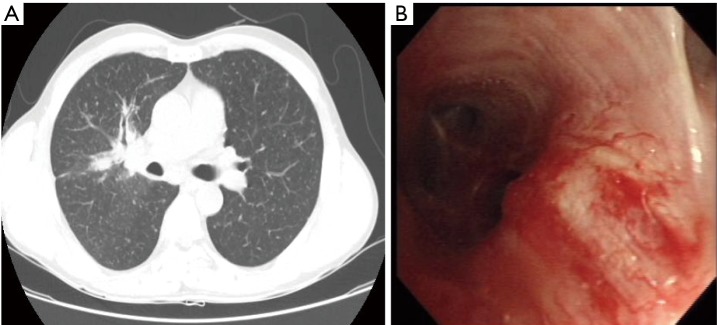
Figure 1.
Patients’ chest imaging and bronchoscopic findings at the diagnosis. (A) Initial chest tomography (CT) finding of the patient showing a 1.9 cm × 2.5 cm sized main mass at right upper lobe (RUL) bronchus and scattered nodular opacities in both lungs; (B) initial bronchoscopic findings of the patient. Mucosal elevation and irregular cancer infiltration were suspected at RUL.
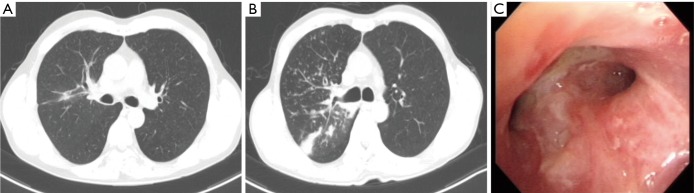
Figure 2.
Follow up chest imaging and bronchoscopic findings during the cancer treatment. (A) Chest CT taken after 2 months of erlotinib treatment and (B) after 8 months showing interval increased bronchial wall thickening and nodular opacities in RUL compared to those taken 6 months ago; (C) bronchoscopic findings of the patient after 8 months’ treatment. Whitish mucosal plaques with mucus secretion were noted at RUL bronchus. Mucosal cancer infiltration was improved compared to previous exam. RUL, right upper lobe.
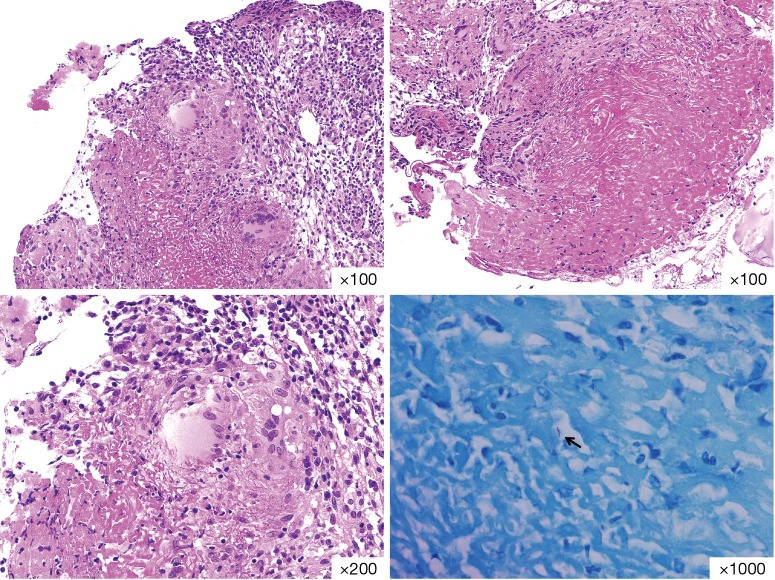
Figure 3.
Haematoxylin and eosin staining of bronchial mucosal biopsy from the RUL bronchial lesion. There were no cancer cells. Instead, chronic granulomatous inflammation with caseous necrosis and acid fast stain positive rod (indicator) were seen. RUL, right upper lobe.
Discussion
In this report, we presented a case of TB reactivation during oral TKI treatment in a patient who was diagnosed as NSCLC. His chest CT findings suggested cancer progression during TKI treatment were diagnosed as active endobronchial pulmonary TB by bronchoscopic mucosal biopsy and microbiological studies. Therefore, active re-biopsy and work up of abnormal findings on chest CT should be done during systemic chemotherapy to provide crucial evidence which can improve patient’s treatment outcome. Moreover, old pulmonary TB scar without anti-TB treatment history could be reactivated during systemic chemotherapy or radiation therapy. Although treatment of LTBI in patients with solid organ malignancies is not generally accepted, strategies for screening and treating LTBI should be reconsidered later with more case reports.
The risk of pulmonary TB in patients with solid organ malignancies has been reported in several previous reports. Kim et al. (8) have described the results of a retrospective cohort study in patients with solid-organ malignancies including lung cancer. In that study, patients with cancer had an increased risk [incidence rate ratio (IRR): 4.69, 95% confidence interval (CI): 1.52–14.46] of developing TB compared to the control group. Additional results showed that old healed TB on chest X-ray (IRR: 45.05, 95% CI: 5.74–353.88) and anticancer chemotherapy (IRR: 4.32, 95% CI: 1.10–16.89) were risk factors of chronic renal failure and the development of TB. A meta-analysis performed in 2015 presented that among 593 TB cases in 324,041 cancer patients between 1950 to 2011 in United States, lung cancer had the third high risk of active TB (83/100,000 population; IRR =9) compared to those without cancer (9). Other meta-analysis has described that active TB can occur concurrently or soon after cancer diagnosis in more than half of lung cancer patients (10). TB was discovered synchronously with malignancy in 30% of patients and was discovered in 21% of patients after 18 months of cancer diagnosis (11). These results—high risk of active TB in patient with solid organ malignancies and early time point of active TB diagnosis- support the importance of screening active TB and LTBI as well as using targeted LTBI therapy at initial cancer diagnosis and follow up periods.
The mechanisms involved in the development of lung cancer in TB patients have not been completely understood yet. It has been reported that scarring of the lung after tuberculosis might predispose patients to lung cancer, especially adenocarcinoma (9,12). Interestingly, Luo’s manuscript published in 2012 showed that among 275 Taiwan patients with pulmonary adenocarcinoma, 72 (26.1%) had old TB lesions on chest CT scans and patients with old TB lesions had higher incidence of EGFR mutation than those without (P=0.018). They additionally found that patients with old TB lesions had shorter 1-year overall survival than those without (HR: 2.12, P=0.006). The author explained that lung cancer patients with lung which were infected or damaged by TB might be more vulnerable to secondary infections. In our case, the patient had old TB scar with lung cancer and mutation-positive EGFR genes. Chronic inflammation caused by previous TB infection might have promoted lung cancer carcinogenesis. Lung cancer treatment might have provoked reactivation of old TB lesion. Fortunately, oral TKI treatment can be continued under active TB infection. Our patient has been effectively treated by both oral TKI and anti-TB medications.
Treatment of active TB in cancer patient is challenging. Moreover, combined medical comorbidities and poor general condition usually make it difficult to take anti-TB medications. Clinical outcome and treatment responses of TB in cancer patients have been reported previously. In 1996, Chen et al. (10) described that lung cancer patients who initially presented with active TB had shorter survival than in those who did not have TB. In addition, Yong et al. (13) presented results in South Korea and showed that, among 418 active TB patients, 35 (8.4%) of them had lung cancer. Patients with active TB and lung cancer showed significantly higher 12-month mortality (43.3% vs. 14.6%, P=0.003) than those with active TB without lung cancer. However, Kim et al. (14) have suggested that anti-cancer chemotherapy is not an obstacle in treatment of tuberculosis based on a retrospective case-control study done in South Korea. They revealed that bacteriologic/radiographic responses to treatment and toxicity of anti-TB medication sufficient to change or stop were not significantly different in cancer patients compared to those in the control group. Since Kim’s study only enrolled 8% of lung cancer patients among a total of 24 patients, the clinical outcome of these patients with active TB and lung cancer are debatable. Further prospective research is needed.
Based on our case and previous literatures, patients with old TB scar might have increased risks to lung cancer and those with anti-cancer therapy might at risk of developing active TB. Among anti-cancer therapies, systemic chemotherapies such as intravenous platinum doublet agents have been usually considered. However, the use of oral target agents in adenocarcinoma has been significantly increased as the main treatment strategy. To the best of our knowledge, this is the first case report presenting TB reactivation during oral TKI treatment which was initially suspected as cancer progression. The patient was successfully treated with anti-TB medications and continuing TKI.
Acknowledgements
None.
Informed Consent: This manuscript was approved by the Institutional Review Board of Uijeongbu St. Mary’s Hospital, which waived the requirement from informed consent (No UC17ZESI0067).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Dobler CC, Martin A, Marks GB. Benefit of treatment of latent tuberculosis infection in individual patients. Eur Respir J 2015;46:1397-406. 10.1183/13993003.00577-2015 [DOI] [PubMed] [Google Scholar]
- 2.Lönnroth K, Migliori GB, Abubakar I, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J 2015;45:928-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for disease control and prevention in Korea, 2016, Available online: http://cdc.go.kr/CDC
- 4.Korean guideline for tuberculosis, 2014, Available online: http://www.lungkorea.org/
- 5.Evman S, Baysungur V, Alpay L, et al. Management and Surgical Outcomes of Concurrent Tuberculosis and Lung Cancer. Thorac Cardiovasc Surg 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6.Kim YI, Goo JM, Kim HY, et al. Coexisting bronchogenic carcinoma and pulmonary tuberculosis in the same lobe: radiologic findings and clinical significance. Korean J Radiol 2001;2:138-44. 10.3348/kjr.2001.2.3.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karnak D, Kayacan O, Beder S. Reactivation of pulmonary tuberculosis in malignancy. Tumori 2002;88:251-4. [DOI] [PubMed] [Google Scholar]
- 8.Kim HR, Hwang SS, Ro YK, et al. Solid-organ malignancy as a risk factor for tuberculosis. Respirology 2008;13:413-9. 10.1111/j.1440-1843.2008.01282.x [DOI] [PubMed] [Google Scholar]
- 9.Auerbach O, Garfinkel L, Parks VR. Scar cancer of the lung: increase over a 21 year period. Cancer 1979;43:636-42. [DOI] [PubMed] [Google Scholar]
- 10.Chen YM, Chao JY, Tsai CM, et al. Shortened survival of lung cancer patients initially presenting with pulmonary tuberculosis. Jpn J Clin Oncol 1996;26:322-7. 10.1093/oxfordjournals.jjco.a023240 [DOI] [PubMed] [Google Scholar]
- 11.Libshitz HI, Pannu HK, Elting LS, et al. Tuberculosis in cancer patients: an update. J Thorac Imaging 1997;12:41-6. 10.1097/00005382-199701000-00006 [DOI] [PubMed] [Google Scholar]
- 12.Luo YH, Wu CH, Wu WS, et al. Association between tumor epidermal growth factor receptor mutation and pulmonary tuberculosis in patients with adenocarcinoma of the lungs. J Thorac Oncol 2012;7:299-305. 10.1097/JTO.0b013e31823c588d [DOI] [PubMed] [Google Scholar]
- 13.Yong SJ, Lee M, Lee WY, et al. PS01.18: The Characteristics of Active Pulmonary Tuberculosis in Lung Cancer Patients: Topic: Pulmonology. J Thorac Oncol 2016;11:S279-S280. 10.1016/j.jtho.2016.09.05327969484 [DOI] [Google Scholar]
- 14.Kim DK, Lee SW, Yoo CG, et al. Clinical characteristics and treatment responses of tuberculosis in patients with malignancy receiving anticancer chemotherapy. Chest 2005;128:2218-22. 10.1378/chest.128.4.2218 [DOI] [PubMed] [Google Scholar]