Abstract
Background
In the recent decades controlling postoperative pain has become a popular topic as it leads to the patients’ wellbeing and improved life quality, while it reduces the costs for both patients and medical facilities.
Objectives
This study aimed at comparing intravenous magnesium sulfate versus intravenous sufentanil on the duration of analgesia and postoperative pain in patients undergoing tibia fracture surgery.
Methods
This double blind clinical trial study was performed on 70 candidates of tibia fractures between the ages of 18 and 55 years with American society of anesthesiologists (ASA) class I and II. The patients were randomly divided to 2 groups, 1 receiving magnesium sulfate (M) and another receiving sufentanil (S). Both of the groups underwent spinal anesthesia with 10 mg bupivacaine 0.5%. One hour after ensuring the sensorimotor blockade, in the S group 0.1 µg/kg/hour and in the M group 8 mg/kg/hour was diluted in 1 liter of Ringer’s solution and infused. In this study, full weakness of the lower limb was considered as the sign of sensorimotor blockade initiation. The postoperative pain intensity was measured using the Visual Analog Scale (VAS), 0, 1, 4, 8, 16, and 24 hours after the end of anesthesia duration. In case of VAS ≥ 3, the patients received 0.3 mg/kg pethidine, intravenously. At last, the time of requesting the first narcotic drug and the total usage of pethidine were recorded.
Results and Conclusions
Sufentanil was found to be more effective than magnesium sulfate in reducing postoperative pain and the time of first narcotics request was later in patients receiving sufentanil (P < 0.05).
Keywords: Tibia Fracture, Spinal Anesthesia, Postoperative Pain, Magnesium Sulfate, Sufentanil
1. Background
Postoperative pain is one of the most common complications of patients undergoing operations (1). Despite various researches and availability of different painkillers, many patients still experience low to high intensities of pain after the operation (2). In the recent decades, managing postoperative pain, its side effects, and control have attracted a lot of attention. It is believed that controlling pain and the resulting physiological processes can lead to patients’ satisfaction and result in an increase in their quality of life after surgery, and reduction of expenses for both the patients and the medical facilities (3).
Different ways to control pain usually include usage of narcotics, non-steroidal anti-inflammatory drugs (NSAIDs), and other pain controlling methods. Since pain is a multifactorial phenomenon, it usually cannot be controlled using one treatment with narcotics or other common drugs (4). One of the most commonly used compounds in various fields of anesthesiology is magnesium sulfate. Magnesium sulfate reinforces local anesthetic action on peripheral nerves. It is a muscle relaxant and can be used as protection from myocardial infarction, during treatment of eclampsia and pre-eclampsia, as a tocolytic in preterm delivery, hypokalemia, and as a medicine for respiratory disorders in infants and pulmonary hypertension (5). Magnesium is a non-alkyl cation with different compounds available in pharmaceutics. Magnesium naturally acts as a calcium antagonist that prevents transmission of pain impulses by allowing the entrance of calcium to the cells. In this way, magnesium can be used to control pain and prevent low blood circulation with regards to existence of NMDA receptors environmentally and local pain control methods for magnesium sulfate, such as direct influence on nerve properties (6).
One of the other groups of drugs used as painkillers is narcotics, such as sufentanil (7). Narcotics have various side effects and the fear of these side effects, especially respiratory depression, has led to administration of insufficient dosage that is usually not sufficient for proper pain control (8).
With regards to what was mentioned regarding the importance of controlling postoperative pain and necessity to achieve low cost methods with minimum side effects, the effects of intravenous magnesium sulfate in comparison with sufentanil on the postoperative pain in patients with tibia fracture were investigated in this work.
2. Objectives
This study aimed at comparing intravenous magnesium sulfate versus intravenous sufentanil on the duration of analgesia and postoperative pain in patients undergoing tibia fracture surgery.
3. Methods
This double blind clinical trial study was performed in 2015 to 2016 with permission number IR.AJUMS.REC.1394.628 of the medical moral committee of Ahvaz Jundishapur University of Iran. In this study, 70 candidates of tibia fractures were randomly divided to 2 groups through a computer-generated list of random numbers. One group received intravenous magnesium sulfate solution 10% (Pasteur Institute of Iran) and another received intravenous sufentanil solution (Janssen, UK).
The inclusion criteria were age of 18 to 55 years with body mass index (BMI) of 19 to 30, American society of anesthesiologists (ASA) class I and II, and provision of a written consent. The exclusion criteria were absolute and relative spinal contraindication such as the patient’s refusal, high intracranial pressure (ICP), coagulopathy, infection of the skin or tissue where the needle is penetrated, peripheral neuropathy of lower limb, history of kidney complications as well as cardiac arrhythmias, usage of sedatives, antipsychotic, and calcium-channel blockers. The level of serum creatinine was measured before surgery for all patients.
After inserting peripheral venous cannula, 10 cc/kg of liquid colloidal crystal was administered. The patients underwent spinal anesthesia in sitting position using 10 mg of bupivacaine 5% (Aguettant, France) with needle number 25 (Dr. J-Japan) on L4 - L5 segment. The patients underwent surgery after ensuring neuraxial blockade and a lack of feeling the sharp tip of the needle in dermatome T10. In the sufentanil group with dosage of 0.1 µg/kg/hour (Janssen, UK) was diluted in 1 liter of Ringer and infused for 1 hour after ensuring the sensorimotor blockade. In the magnesium sulfate group, 8 mg/kg/h intravenous magnesium sulfate 10% (Pasteur Institute - Iran) was diluted in 1 liter of Ringer and infused 1 hour after ensuring the sensorimotor blockade.
During the operation at 5-minute intervals, systolic and diastolic blood pressure, arterial oxygen percentage content and heartbeat were monitored. In case of systolic pressure of lower than 100 mmHg or 20% drop of blood pressure, 5 mg ephedrine was administered and in case of heartbeat less than 60 bpm, 0.5 mg atropine was administered with repeated doses if needed. In this study, the start time of sensory block was estimated using the pinprick technique and by asking the patients if there was a tingling sensation in their legs. The start time of motor block was estimated upon noticing full muscle weakness of the lower limb as a consequence of spinal anesthesia and the duration of the block was assessed using the Modified Bromage scale. During recovery after the surgery, heartbeat, percentage of arterial oxygen, blood pressure, and breathing of the patients were monitored until the end of the sensorimotor blockade period. The average postoperative pain intensity was measured using the visual analog scale (VAS).
Considering the termination of anesthesia in recovery as the start time of postoperative pain intensity, the patients were examined at 0, 1, 4, 8, 16, and 24 hours after the end of anesthesia in recovery and in case of VAS equal or larger than 3, 0.3 mg/kg pethidine was administered. At the end, the request time of the first analgesic and also the used pethidine were recorded.
3.1. Statistical Methods
The findings were reported based on mean ± standard deviation. In order to compare the groups after analyzing the natural distribution of the findings and homogeneity of variances, the data was investigated using the T independent, Chi-squared, and ANOVA tests. The data was considered meaningful at P < 0.05. The statistical analysis was conducted using the SPSS software version 20.
4. Results
Throughout this study 1 patient left the magnesium sulfate group due to lack of consent and was replaced by another candidate.
Table 1 shows the demographic characteristics of the participants and the duration of the operation. There was no statistically significant difference between the demographic characteristics of the 2 groups, such as age, gender, and body mass index (BMI) as well as the duration of the surgery (121.88 ± 33.42 and 122.65 ± 11.36) (P > 0.05). In order to account for the probable effect of gender on the results, t independent test showed that there were no statistically significant differences between the number of male and female participants in the two groups (P > 0.05) (Figure 1).
Table 1. Demographic Characteristics of the Participants and the Duration of Operationa.
| Group | Age, y | BMI | Gender (F:M) | Duration of Operation |
|---|---|---|---|---|
| Sufentanil | 32.29 ± 10.22 | 25.95 ± 2.99 | 23:12 | 122.65 ± 11.36 |
| Magnesium | 31.21 ± 11.27 | 25.14 ± 3.61 | 21:14 | 121.88 ± 33.42 |
| P Value | 0.655 | 0.924 | 0.605 | 0.710 |
aData are presented as mean ± SD (P < 0.05).
Figure 1. First Analgesic Request Time.
Table 2 shows the first analgesic request time after the surgery in the 2 groups. It was found that there was a statistically significant difference in the request time of the first pethidine between group M (220.29.47 ± 133.49 minutes) and group S (401.47 ± 86.76 minutes), (P < 0.05).
Table 2. First Analgesic Request Time (minutes)a.
| Group | Value |
|---|---|
| Sufentanil | 401.47 ± 86.76 |
| Magnesium sulfate | 290.29 ± 133.49 |
| P value | 0.0001 |
aData are presented as mean ± SD (P > 0.05).
The total usage of pethidine (mg) using the average ± standard deviation is shown in Table 3. There was a statistically significant difference between group M (91.64 ± 33.27) and group S (37.29 ± 28.41), (P < 0.05).
Table 3. Total Used Dosage of Analgesic (mg)a.
| Group | Value |
|---|---|
| Sufentanil | 37.29±28.41 |
| Magnesium sulfate | 91.64±33.27 |
| P value | 0.0001 |
aData are presented as mean ± SD (P > 0.05).
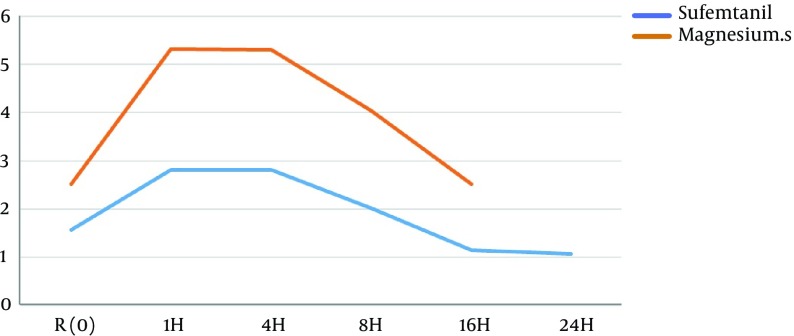
The average of postoperative pain intensity based on VAS index at different time intervals is presented in Table 4. The pain intensity at 1, 4, 8, 16, and 24 hours after surgery was significantly less in group S than in group M, (P < 0.05). Post postoperative pain intensity is shown in Figure 2.
Table 4. Postoperative Pain Intensitya.
| Group | R | 1H | 4H | 8H | 16H | 24H |
|---|---|---|---|---|---|---|
| Sufentanil | 1.57 ± 0.675 | 2.82 ± 1.23 | 2.82 ± 1.00 | 2.02 ± 0.69 | 1.15 ± 0.36 | 1.08 ± 1.68 |
| Magnesium | 2.52 ± 1.41 | 5.32 ± 1.99 | 5.30 ± 1.77 | 4.05 ± 1.75 | 2.52 ± 1.50 | 1.52 ± 2.67 |
| P value | 0.001 | 0.005 | 0.003 | 0.0001 | 0,0001 | 0.0001 |
aData are presented as mean ± SD (P < 0.05)
Figure 2. Postoperative Pain Intensity.
5. Discussion
The major finding of this clinical trial was that intraoperative administration of intravenous magnesium sulfate versus intravenous sufentanil had no significant effect on early postoperative opioid requirement or pain after tibia fracture surgery, however, no complications due to magnesium administration were evident with the doses used.
Orthopedic surgeries are often painful operations, and well-planned pain management is crucial for decreasing morbidity after these surgeries. Pain as stress, induces psychological and physiological responses and the patient’s response to pain is different; the consequences of pain have a direct effect on mortality and postoperative complications, recovery time, and patient satisfaction with the health system (9). However, systemic opioids are easy to use, cheap, and preferred by many clinicians, yet, alternative methods are necessary to obtain analgesia in patients. Adjuvant analgesics to opioids are being studied to decrease the required dose and the consequent unwarranted effects of opioids. For this purpose, employing adjuvant medication for prolonging motor and sensory block, and proper analgesia is very important (10). Regional magnesium sulfate and narcotics are known adjuvants used in surgeries. Sufentanil is a narcotic painkiller that is used as a complementary drug as well as for induction of anesthesia. The intrathecal administration of this medicine is an effective anesthetic during and after the operation. Sufentanil passes the blood-brain barrier easily and its accumulation in fat tissues can result in prolonged effects (11).
Magnesium sulfate is a physiological inhibitor of calcium channels. It has antagonist effects on N-Methyl-D-Aspartate (NMDA) receptor. By inhibiting the receptor, it causes electricity to flow through the membrane. Magnesium sulfate results in the release of neurotransmitters in all the synaptic junctions and can strengthen the local anesthetics’ activities (12).
There have been many studies on the effects of these medications on postoperative pain intensity; however, in the majority of these studies, the medications have been intrathecally administered and the effect of intravenous administration on variables of this study has rarely been discussed. In addition, to the best of our knowledge there are currently no studies, which have particularly investigated the effect of intravenous magnesium sulfate versus intravenous sufentanil. Contradictory results were obtained by different studies as some found magnesium sulfate to be effective in reducing postoperative pain while others found these effects to be limited or negligible. In this study, the effect of intravenous magnesium sulfate versus intravenous sufentanil on postoperative pain in patients with tibia fracture was investigated.
The results indicate that sufentanil infusion in comparison with magnesium sulfate is more effective in reducing pain intensity and the amount of patient’s requested narcotics.
In a study conducted by Kahraman and Eroglu in 2013 in Turkey, the effect of intravenous infusion of magnesium sulfate on spinal block duration as well as the postoperative pain of patients with abdominal hysterectomy was examined. These results indicated that motor and sensory block duration in the magnesium recipient group was significantly longer and the patients in this group experienced less postoperative pain (13). In this study the effect of magnesium sulfate on postoperative pain was investigated and the results were in agreement with our findings.
In another study conducted by Haghighi et al. during 2015 in Iran, the effect of adding magnesium sulfate to lidocaine on prolonging the duration of motor and sensory axillary plexus blockade in upper body orthopedic surgery was examined. The results showed that the average motor and sensory blockade was significantly higher in the intervening group than the control group (14).
In a study performed by Faiz et al. in Iran during year 2013, the effects of intrathecal injection of magnesium sulfate in 72 females undergoing elective cesarean section were investigated. The results showed noticeable improvement in perioperative shivering (15). In a 2012 study by the same group, magnesium sulfate proved to be a safe and effective adjuvant for increasing the onset time of motor block in 90 patients undergoing lower extremities surgeries (16).
In a study conducted in India during year 2015 by Maulik et al., the role of magnesium sulfate was investigated in prolonging the analgesic effect of spinal bupivacaine for cesarean section in patients with severe preeclampsia. It was concluded that prescribing intravenous magnesium sulfate results in a decrease in postoperative pain intensity as well as decrease in the amount of narcotics needed in comparison with the control group (17). The effectiveness of using magnesium sulfate on reducing postoperative pain in this study was in agreement with our findings.
In a study conducted in Iran during year 2010 by Alavi et al., the effect of intravenous sufentanil and morphine were investigated on post-cardiac surgery pain control using Patient Controlled Analgesia (PCA) device. Administration of sufetanil PCA was found effective in reducing post-operative intensity (18).
In a 2013 study performed by Sedighinejad et al. in Iran, the affectivity of magnesium sulfate and sufentanil combined together was compared with sufentanil alone in orthopedic surgery. It was found that the combination of magnesium sulfate and sufentanil was only effective in controlling pain (19).
Despite the findings of the present study, Mehraein et al. during year 2007 in Iran concluded that magnesium sulfate with a dosage of 25 and 50 mg/kg did not affect postoperative pain of inguinal hernia repair patients (20). The result was not in agreement with our findings; albeit, in their study, magnesium sulfate was used as push and divided doses, which could justify the difference between their findings and that of the present study.
In a study conducted in Italy during year 2015 by Frassanito et al., the effects of intravenous infusion of magnesium sulfate on postoperative analgesia in total knee arthroplasty was investigated. It was concluded that the injection of magnesium sulfate before the operation does not have any effect on controlling pain and the amount of analgesia (21). The result was not in agreement with our findings.
5.1. Conclusion
Sufentanil is found to be more effective than magnesium sulfate in controlling postoperative pain. Subsequently, in the sufentanil-administered group, a significant decrease in usage of narcotics and easing the postoperative pain in patients with tibia fracture was observed. It can be concluded that narcotics are still the most effective pain control drug category and the main basis in controlling postoperative pain protocol. Other medicinal and non-medicinal drug interventions can be employed for complementarity purposes. More studies need to be carried out in this field in order to draw more solid conclusions.
Acknowledgments
We sincerely thank the vice-president of research and technology and the Research Center of Jundishapur University of Ahvaz for their support and providing the resources and equipment needed for this study.
Footnotes
Authors’ Contribution:Study concept and design, Ali Reza Olapour and Ahmad Reza Mohtadi; data collection, Maryam Jafari; data analysis and manuscript preparation, Mansour Soltanzadeh, Ali Ghomeishi, Reza Akhondzadeh, and Maryam Jafari; critical revision of the manuscript for important intellectual content, Ali Reza Olapour.
Funding/Support:Financial support was provided by Ahvaz Jundishapur University of Medical Sciences, vice chancellor for research and technology.
References
- 1.Chung JW, Lui JC. Postoperative pain management: study of patients' level of pain and satisfaction with health care providers' responsiveness to their reports of pain. Nurs Health Sci. 2003;5(1):13–21. doi: 10.1046/j.1442-2018.2003.00130.x. [DOI] [PubMed] [Google Scholar]
- 2.Imani F, Faiz HR, Sedaghat M, Hajiashrafi M. Effects of adding ketamine to fentanyl plus acetaminophen on postoperative pain by patient controlled analgesia in abdominal surgery. Anesth Pain Med. 2014;4(1):ee12162. doi: 10.5812/aapm.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joshi GP, Bonnet F, Kehlet H, Prospect collaboration Evidence-based postoperative pain management after laparoscopic colorectal surgery. Colorectal Dis. 2013;15(2):146–55. doi: 10.1111/j.1463-1318.2012.03062.x. [DOI] [PubMed] [Google Scholar]
- 4.Nesioonpour S. Pain. first ed. Tehran: Taimourzadeh publisher; 2004. [Google Scholar]
- 5.Ryu JH, Kang MH, Park KS, Do SH. Effects of magnesium sulphate on intraoperative anaesthetic requirements and postoperative analgesia in gynaecology patients receiving total intravenous anaesthesia. Br J Anaesth. 2008;100(3):397–403. doi: 10.1093/bja/aem407. [DOI] [PubMed] [Google Scholar]
- 6.Imani F. Postoperative pain management. Anesth Pain Med. 2011;1(1):6–7. doi: 10.5812/kowsar.22287523.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regeh N, Ahmadi F, Mohammadi E, Anoosheh M. Pain management: Patients’ perspective. Iran J Nurs. 2007;20(52):7–20. [Google Scholar]
- 8.Minozzi S, Amato L, Davoli M. Development of dependence following treatment with opioid analgesics for pain relief: a systematic review. Addiction. 2013;108(4):688–98. doi: 10.1111/j.1360-0443.2012.04005.x. [DOI] [PubMed] [Google Scholar]
- 9.Akhondzade R, Pipelzade MR, Gousheh MR, Sarrafan N, Mahmoodi K. Comparison of the analgesic effect of intra-articular and extra-articular injection of morphine and ketamine compound in arthrotomy lower limb surgery under spinal anesthesia. Pak J Med Sci. 2014;30(5):942–5. doi: 10.12669/pjms.305.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahimzadeh P, Imani F, Faiz SH, Nikoubakht N, Sayarifard A. Effect of intravenous methylprednisolone on pain after intertrochanteric femoral fracture surgery. J Clin Diagn Res. 2014;8(4):GC01–4. doi: 10.7860/JCDR/2014/8232.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon SB, Koltzenburg M, Tracey I, Turk D. Wall & Melzack's textbook of pain. Elsevier Health Sciences; 2013. [Google Scholar]
- 12.Weetman SC. Martindade: the complete drug reference. 38th ed. London: Pharmaceutical Press; 2014. [Google Scholar]
- 13.Kahraman F, Eroglu A. The effect of intravenous magnesium sulfate infusion on sensory spinal block and postoperative pain score in abdominal hysterectomy. Biomed Res Int. 2014;2014:236024. doi: 10.1155/2014/236024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haghighi M, Soleymanha M, Sedighinejad A, Mirbolook A, Naderi Nabi B, Rahmati M, et al. The effect of magnesium sulfate on motor and sensory axillary plexus blockade. Anesth Pain Med. 2015;5(1):ee21943. doi: 10.5812/aapm.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faiz SH, Rahimzadeh P, Imani F, Bakhtiari A. Intrathecal injection of magnesium sulfate: shivering prevention during cesarean section: a randomized, double-blinded, controlled study. Korean J Anesthesiol. 2013;65(4):293–8. doi: 10.4097/kjae.2013.65.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faiz SH, Rahimzadeh P, Sakhaei M, Imani F, Derakhshan P. Anesthetic effects of adding intrathecal neostigmine or magnesium sulphate to bupivacaine in patients under lower extremities surgeries. J Res Med Sci. 2012;17(10):918–22. [PMC free article] [PubMed] [Google Scholar]
- 17.Maulik. Magsulph and severe preeclampsia. J Bas Clin Reprod Sci . 2015;4(1):25. [Google Scholar]
- 18.Alavi SM, Kish RF, Farsad F, Imani F, Sheikhvatan M. Intravenous sufentanil and morphine for post-cardiac surgery pain relief using patient-controlled analgesia (PCA) device: A randomized double-blind clinical trial. Pak J Med Sci. 2010;26(1):137–41. [Google Scholar]
- 19.Sedighinejad A, Haghighi M, Naderi Nabi B, Rahimzadeh P, Mirbolook A, Mardani-Kivi M, et al. Magnesium sulfate and sufentanil for patient-controlled analgesia in orthopedic surgery. Anesth Pain Med. 2014;3(3) doi: 10.5812/aapm.11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehraein A, Azad MA, Sadeghi M. The analgesic effect of magnesium sulfate in postoperative pain of inguinal hernia repair [In Persian]. Tehran Univ Med J. 2007;65(4):55–8. [Google Scholar]
- 21.Frassanito L, Messina A, Vergari A, Colombo D, Chierichini A, Della Corte F, et al. Intravenous infusion of magnesium sulfate and postoperative analgesia in total knee arthroplasty. Minerva Anestesiol. 2015;81(11):1184–91. [PubMed] [Google Scholar]