Abstract
Background
Hypotension is one of the most common complications of spinal anesthesia in parturients undergoing cesarean section. In this regard, the patient’s position may affect the incidence of hypotension.
Objectives
In this clinical trial, we evaluated the effects of 1- and 2-minute sitting positions versus immediately lying down after spinal anesthesia on hypotension and vasopressor requirements.
Methods
A total of 72 parturients, scheduled for cesarean section under spinal anesthesia, were randomly divided into 3 groups (24 subjects per group). Groups S1 and S2 remained in a sitting position for 1 and 2 minutes after the induction of spinal anesthesia, respectively, while group T was immediately placed in a lying position. Systolic, diastolic, and mean arterial blood pressure, as well as heart rate, was recorded at 1, 2, 3, and 5 minutes after anesthesia induction, every 5 minutes during the first 30 minutes of surgery, and then every 10 minutes until the end of surgery. P-value less than 0.05 was considered statistically significant.
Results
The overall frequency of hypotension was 50 (69%) cases during surgery; the reported frequency was higher in group T in comparison with group S2 (P = 0.003). The frequency of hypotension before delivery (the first 5 minutes after spinal anesthesia) was 40 (55%) cases, with a higher frequency reported in group T (20, 83%), compared to groups S1 (12, 50%) and S2 (8, 33%) (P = 0.03 and P = 0.001, respectively). The ephedrine requirement in group T (11.73 ± 7.16 mg) was higher than the other two groups (8.69 ± 7.57 and 7.82±7.95 mg in groups S1 and S2, respectively); nevertheless, the difference was not statistically significant (P = 0.19). Moreover, the difference in time to achieve T6 sensory level was only significant between group T (3.25 ± 1.1 minutes) and group S2 (4.73 ± 1.73 minutes) (P = 0.03).
Conclusions
The present study showed that 1- or 2-minute sitting position after spinal anesthesia with 2.5 cc of hyperbaric bupivacaine in elective cesarean section results in more hemodynamic stability, compared with immediately lying down.
Keywords: Patient Positioning, Anesthesia, Spinal, Cesarean Section, Hypotension
1. Background
Spinal anesthesia has been regarded as a reasonable anesthetic option for cesarean section since 1977. The advantages of this technique include avoidance of airway complications and depressant agents, as well as the mother’s ability to remain awake and enjoy the birthing experience (1, 2). This technique is simple and fast to apply and is regarded a reliable and cost-effective option, particularly compared to epidural anesthesia (3, 4). Nevertheless, patients experience more hypotension due to the greater spread of the local anesthetic in the subarachnoid space and aortocaval compression, caused by the gravid uterus; also, reduced sympathetic tone can exacerbate hypotension (1, 3).
Many different methods, including administration of fluids, vasopressors, and ondansetron, lower leg compression, and uterine displacement by wedge, have been used to decrease the risk of hypotension after the administration of spinal anesthesia. Although the incidence of hypotension is diminished by these strategies, it continues to be a challenging adverse effect of spinal anesthesia (5-7).
Some trials have suggested that the patient’s position during or after spinal anesthesia may affect the incidence of hypotension (8). Spinal anesthesia can be performed with the patient in a sitting or lying position (left, right, or Oxford). However, turning pregnant women to the lateral position after anesthesia may be difficult, especially among those with a high body mass index who are hard to lift; as a result, changing the position may present a risk to the staff and mother (9). In addition, it is technically easier to insert the needle with the patient in the sitting position (10); therefore, the sitting position may be preferred by many anesthetists (9, 10).
According to the literature, lying the patient down after anesthesia results in aortocaval compression and may lead to epidural venous engorgement and compression of the dural sac, which can force the local anesthetic towards the head and increase thoracic dermatome blockade. Therefore, allowing the patient to remain in the sitting position, instead of immediately lying down, could delay the onset of anesthesia and reduce the incidence of hypotension (11).
2. Objectives
In this randomized clinical trial, we aimed to compare the incidence of hypotension and ephedrine requirement after spinal anesthesia with 12.5 mg of hyperbaric bupivacaine 0.5%, plus 5 µg of sufentanil in 3 groups. Groups S1 and S2 remained in a sitting position for 1 and 2 minutes after the induction of spinal anesthesia, respectively, while group T was immediately placed in a lying position.
3. Methods
After approval by the ethics and research committee of Hamadan University of Medical Sciences and obtaining written informed consents from the participants, a total of 72 women (age range, 18 - 45 years), classified as ASA I-II with gestational age of > 37 weeks, were enrolled in this double-blind clinical trial. The parturients were scheduled for elective cesarean section under spinal anesthesia.
The exclusion criteria in the present study were as follows: (1) preeclampsia, (2) chronic hypertension, (3) diabetes, (4) allergy to drugs used in the study, (5) contraindication to neuraxial anesthesia, (6) blood pressure below 90 mmHg and heart rate (HR) below 50/min, (7) need for general anesthesia, (8) addiction or drug abuse, and (9) any unexpected accidents.
An 18-guage intravenous (IV) line was inserted and all the participants received 50 mg of IV ranitidine and 10 mg of IV metoclopramide at 1 hour before arrival in the operating room. Before the induction of spinal anesthesia, 300 mL of Ringer’s solution was infused, and baseline standard monitoring was performed in a supine position through automatic monitoring (Novin S1800, Saadat model).
Spinal anesthesia was induced by a 25-gauge Quincke needle (Mekon Medical Devices Co., Shanghai, China) at the L3-L4 interspace, parallel to the dural fiber, while the subject was in a sitting position. Spinal anesthesia consisted of 12.5 mg of hyperbaric bupivacaine 0.5% (2.5 mL; AstraZeneca, Austria) plus 5 µg (1 mL) of sufentanil (Sufiject Aburaihan Co., Iran), administered in 12 seconds.
The subjects were randomly allocated into 3 groups, using opaque envelopes (block randomization with a block size of 9): groups S1 and S2 in which the subjects remained in a sitting position for 1 and 2 minutes, respectively after the induction of spinal anesthesia, and group T which was immediately placed in a lying position. A 15° head-down tilt was then performed in all 3 groups. Considering the blind design of the study, an anesthesiologist administered the spinal anesthetic and randomized the subjects, while another anesthesiologist, who was unaware of classification, performed preoperative and intraoperative data collection.
All the participants received supplemented oxygen (2 - 3 L/min) via face masks. Systolic blood pressure (SBP), HR, mean arterial blood pressure (MAP), and O2 saturation (SpO2) were recorded 1 minute before (baseline) and 1, 2, 3, and 5 minutes after the induction of spinal anesthesia. Thereafter, measurements were performed in 5-minute intervals during the first 30 minutes of surgery and then every 10 minutes until the end of the surgery.
Adverse effects (ie, pruritus, nausea, vomiting, vertigo, bradycardia, or uncomfortable sensations), along with the subject’s need for ephedrine, atropine, or a rescue analgesic, were recorded. Sensory block level was assessed (based on loss of sensation to pinprick) immediately after the subject reclined and every 1 minute thereafter until she reached T6 sensory level. The measured time was recorded as the onset of complete sensory block, and at this time, surgery was allowed.
A modified Bromage scale was recorded 15 minutes after the administration of spinal anesthesia (3, no movement; 2, only able to flex the ankle and foot; 1, able to bend the knee; and 0, no paralysis and able to raise the extended leg). Hypotension (SBP ≤ 90 mmHg or > 20% decline from the baseline) was treated with 5 mg of IV ephedrine bolus; this dose was repeated as necessary to achieve an SBP of ≥ 90 mmHg. Additionally, if the mother’s HR was < 50/min, 0.5 mg of IV atropine bolus was administered.
Then, 30 IU of oxytocin was infused in 500 mL of Ringer’s solution after delivery, and a total dose of 30 mL/kg of infused Ringer’s solution continued through the end of surgery. If the parturient complained of pain at any time during surgery, 50 µg of IV fentanyl (Caspian Tamin Co., Rasht, Iran) was administered. If the analgesic was inadequate (visual analogue scale or VAS score > 4), the mentioned dose was repeated and the total rescue fentanyl dose was recorded. Upon the occurrence of nausea or vomiting, SBP was checked. In case it was ≤ 90 mmHg, treatment with 5 mg of IV ephedrine was performed; otherwise, treatment with 10 mg of metoclopramide IV bolus was selected.
The total dose of IV ephedrine and duration of surgery (from skin incision to the final skin suture) were also recorded. The newborn’s status was assessed with 1- and 5-minute Apgar scores after birth. Regression of sensory block to T10 dermatome and motor block to a modified Bromage scale of 2 were assessed every 5 minutes by an anesthesia nurse, who was blinded to the study groups in the post-anesthesia care unit (PACU). Upon pain in PACU (VAS > 4), paracetamol was infused (1 g in 100 mL of normal saline over 10 minutes) and recorded as need for the first analgesic.
The sample size was calculated with a two-tailed alpha of 0.05, power of 0.8, P1 of 47%, and P2 of 8% (frequency of ephedrine requirements in sitting and supine positions based on ref No. 5). Finally, a total of 24 women were included in each group.
Statistical analysis was performed, using SPSS version 19. Data are presented as mean ± SD, unless otherwise specified. Repeated measures analysis of variance (ANOVA) was used to analyze HR, SBP, MAP, and SpO2 measurements over time. In case of a difference, Bonferroni post-hoc test was used for comparison between the groups. Qualitative variables, as well as their associations, were analyzed, using Chi-square and Fisher’s exact tests. P value less than 0.05 was considered statistically significant.
4. Results
A total of 72 women were enrolled and evaluated in this study. They were divided into 3 groups (n = 24). No participant was excluded from the study due to inadequate blockage. The demographic and anesthesia characteristics of the study groups are shown in Table 1.
Table 1. Demographic Data and Some Anesthetic Characteristics of the Participantsa.
| Variables | Group T | Group S1 | Group S2 | P Value |
|---|---|---|---|---|
| Weight, kg | 75.75 ± 7.85 | 82.91 ± 1.4 | 77.17 ± 6.62 | 0.237 |
| Age, y | 28.6 ± 5.5 | 28.7 ± 5.1 | 29.5 ± 05 | 0.55 |
| Height, cm | 158.7 ± 5.4 | 159 ± 7.1 | 161.3 ± 2.3 | 0.51 |
| Gestational age, w | 38.17 ± 1.27 | 38.42 ± 0.79 | 38.45 ± 1.5 | 0.78 |
| Operation time, min | 50 ± 9.17 | 55.42 ± 16.9 | 54 ± 14.16 | 0.64 |
| Time to T6 level, min | 3.25 ± 1.1 | 3.77 ± 2.1 | 4.73 ± 1.73 | 0.03 |
| Ephedrine use, mg | 11.73 ± 7.16 | 8.69 ± 7.57 | 7.82 ± 7.05 | 0.19 |
| IV crystalloids, L | 1.5 ± 0.02 | 1.2 ± 0.3 | 1.3 ± 0.25 | 0.62 |
aValues are expressed as mean±SD.
Time to reach T6 dermatome sensory level (surgical anesthesia) in group S2 was longer than group T, and the difference was found to be statistically significant (P = 0.03). However, based on the findings, there was no significant difference between groups T and S1 (P = 0.67) or groups S1 and S2 (P = 0.39) (Table 1).
A modified Bromage scale was recorded 15 minutes after spinal anesthesia, and the subjects in all 3 groups reached a Bromage scale of 3 (no movement) by this time.
Although there was no significant difference in the rescue ephedrine dose between the 3 groups, more ephedrine was used in group T, compared to groups S1 and S2 (Table 1).
There was a significant difference in the frequency of hypotension during surgery between the groups, based on Chi-Square test (P = 0.007). Also, the frequency of hypotension was higher in group T in comparison with group S2, according to Fisher’s exact test results (P = 0.003). Also, there was a significant difference in the frequency of hypotension in the first 5 minutes of surgery (before delivery) between group T and groups S1 and S2 (P = 0.03 and P = 0.001, respectively) (Table 2).
Table 2. Frequency of Hypotension Before and After Deliverya,b.
| Hypotension | Group T (n = 24) | Group S1 (n = 24) | Group S2 (n = 24) | Sum | P Value |
|---|---|---|---|---|---|
| During surgery | 22 (92) | 16 (66) | 12 (50) | 50 (69) | 0.007 |
| Before delivery | 20 (83) | 12 (50) | 8 (33) | 40 (55) | 0.002 |
aValues are expressed as No. (%).
bHypotension is defined as SBP ≤ 90 mmHg.
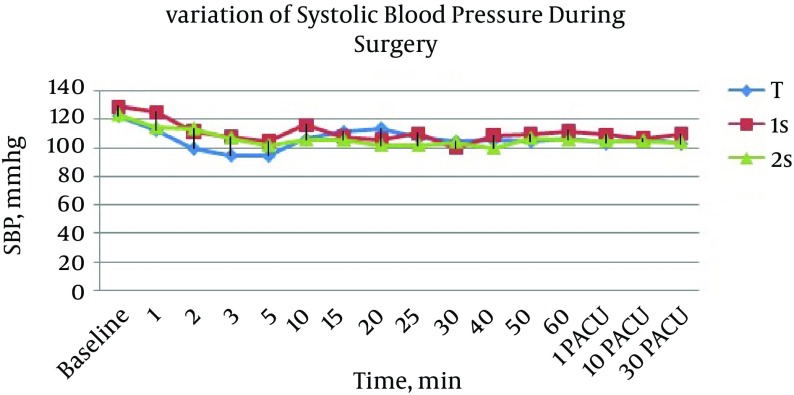
Compared to the baseline, SBP declined after the induction of spinal anesthesia in all 3 groups, and there was no significant difference in SBP over time between the groups (P > 0.05) (Figure 1).
Figure 1. Variation of SBP During Surgery in the Study Groups.
Data are presented as mean ± SD.
In group T, SBP decreased significantly from baseline at all time points during surgery: 1 minute (P = 0.02), 2 minutes (P = 0.04), 3 minutes (P = 0.03), and 5 minutes (P = 0.001) after anesthesia induction, the first 5 minutes of surgery (P = 0.00), 5 - 30 minutes of surgery (P = 0.01), and the last 30 - 60 minutes of surgery (P = 0.001). In group S1, there was no significant difference between the baseline SBP and the recorded SBP at 1 minute after spinal anesthesia. However, the decline from baseline at 2 minutes after spinal anesthesia was significant (P = 0.018), as it was at all other time intervals: 3 minutes (P = 0.006) and 5 minutes (P = 0.001) after spinal anesthesia, the first 5 minutes of surgery (P = 0.001), 5 - 30 minutes of surgery (P = 0.001), and the last 30 - 60 minutes of surgery (P = 0.001).
In group S2, there was no significant difference between the baseline SBP and SBP at 1 or 2 minutes after spinal anesthesia. However, a significant decline from the baseline was reported at 3 minutes (P = 0.001) and 5 minutes (P = 0.001) after spinal anesthesia, the first 5 minutes of surgery (P = 0.005), 5 - 30 minutes of surgery (P = 0.00), and the last 30 - 60 minutes of surgery (P = 0.001).
With respect to HR, significant differences were detected among the groups at 1 and 2 minutes after spinal anesthesia. HR was higher in group T, compared to the other two groups (group T vs. group S1, P1min = 0.02 and P2min = 0.007, respectively; group T vs. group S2, P1min = 0.008 and P2min = 0.02, respectively). Bradycardia (HR < 50 bpm) occurred in all the groups (group T, n = 6; group S1, n = 8; and group S2, n = 2), although there was no significant difference between the groups with respect to the number of patients with bradycardia (P = 0.47).
Evaluation of the secondary outcomes (ie, nausea, vomiting, shivering, pruritus, discomfort, and analgesic requirement), based on Chi-square or alternatively Fisher’s exact test, showed no significant difference between the groups (P > 0.05). The mean time of sensory block regression from T6 to T10 dermatome was not significantly different between the groups, although regression was faster in group S2 in comparison with groups S1 and T (group S2, 106 ± 20.22 minutes; group S1, 116 ± 30.55 minutes; and group T, 120 ± 22.02 minutes; P = 0.34).
Also, motor block regression time to a Bromage score of 2 was faster in group S2, compared to groups S1 and T, although the difference was not statistically significant (group S2, 118 ± 27.17 minutes; group S1, 126 ± 28.23 minutes; and group T, 130 ± 32.48 minutes; P = 0.59). In addition, there was no significant difference in the Apgar score between the groups at 1 and 5 minutes after birth (P = 0.9). Based on the findings, no participant required a rescue dose of fentanyl during surgery.
5. Discussion
Hypotension is one of the most common complications of spinal anesthesia in women undergoing cesarean section (1, 3). The effects of various methods for reducing this complication have been previously investigated (5, 6). In a number of studies, the effect of patient positioning during or after spinal anesthesia has been studied, although conflicting results have been reported (8-18).
The current study showed that the frequency of hypotension in the first 5 minutes after spinal anesthesia (before delivery) was significantly lower in women who remained seated for 1 or 2 minutes, compared to those who were immediately placed in a supine position after spinal anesthesia. The total dose of the required ephedrine was lower in the sitting groups, and the time to reach T6 sensory level was delayed in subjects who sat for 2 minutes after spinal anesthesia; however, the differences were not statistically significant.
Considering multiple variations in the design of conducted studies, it is rather difficult to analyze the influence of maternal posture during spinal anesthesia administration. In general, different local anesthetic doses, different baricities and adjuvants, and various maternal positions have been assessed. Some studies have compared the effect of maternal posture during the administration of spinal or combined spinal and epidural (CSE) anesthesia.
In this regard, Rucklidge et al. (14) compared the effects of left lateral, Oxford, and sitting positions for the induction of CSE in cesarean section, using 2.5 cc of hyperbaric bupivacaine plus 10 μg of sufentanil. Ephedrine use was less prevalent and the onset of anesthesia was slower in the sitting group, compared to the lateral and Oxford position groups. Based on the findings, there was no advantage for Oxford position over the sitting or left lateral position.
Studies comparing sitting position with left lateral or right lateral positions have reported different results. Patel et al. (16) found that injection of 10 mg of hyperbaric bupivacaine in the sitting position, compared to the left lateral position, did not provide adequate analgesia for cesarean section, while no major difference was reported in the maximum sensory block level or degree of motor block in a study by Inglis et al. (17). Patients in the lateral group required more ephedrine in the first 10 minutes after spinal anesthesia in the latter study.
Moreover, in a study by Obasuyi et al. the occurrence of hypotension was less prevalent in patients who received spinal anesthesia in the lateral position, compared to the sitting position (18). Similar studies with identical results have been performed by Yun et al. (19) and Chevuri et al. (10). Hypotension was more frequent in the sitting position in comparison with the lateral position in the study by Yun et al. and the only advantage of the sitting position, compared to the lateral posture, was easier anesthetic administration (19). Also, similar hemodynamic stability, quality of analgesia, and muscle relaxation were found in the investigation by Chevuri et al. (10).
In contrast to the abovementioned studies, Coppejans et al. found that spinal anesthesia, using 6 mg of hyperbaric bupivacaine plus 3.3 μg of sufentanil, resulted in less severe hypotension and lower ephedrine supplementation in patients in the sitting versus right lateral position (20). Also, Ortiz-Gomez et al. studied the sitting, left, and right lateral decubitus positions during spinal anesthesia induction with hyperbaric bupivacaine plus 20 μg of fentanyl. Although the incidence of hypotension and vasopressor requirements did not vary significantly, the sitting position was recommended, as it was easier to administer the anesthetic and was more comfortable for the patients (15).
In two other studies, the effect of head-down tilt with horizontal or left lateral positions was studied. Miyabe et al. found that the incidence of hypotension and ephedrine consumption in patients who were in the supine position with a 10° degree head tilt was the same as patients in a horizontal position (12). In another study, Mendonca et al. (9) demonstrated that the full left lateral position reduced the incidence of early hypotension, compared with the tilted supine position.
In another study by Wang et al. the incidence of hypotension and ephedrine requirements in patients who remained in the left lateral position was lower than patients shifted to a left-tilt supine posture after spinal anesthesia in cesarean section (21). In addition, the effects of baricity and posture on spinal anesthesia for cesarean section were studied by Hallworth et al. who demonstrated that the incidence of hypotension and ephedrine use increased with decreasing baricity, and the highest incidence of hypotension was reported in patients who received hyperbaric bupivacaine in the sitting position (22).
The effect of delayed supine positioning after the induction of spinal anesthesia in the sitting position has been also evaluated in the literature. In 2011, El-Hakeem et al. found that sitting up for 5 minutes rather than immediately lying down resulted in decreased sensory block height, reduced ephedrine and fluid requirements, and diminished some adverse effects such as nausea and vomiting, while it had no effects on SBP (8).
Kohler et al. (11) and Gori et al. (13) in similar studies found that sitting up for 3 and 2 minutes, respectively did not influence the incidence of maternal hypotension or the required ephedrine dose versus immediately lying down. The varying doses and baricities of bupivacaine, used for spinal anesthesia, might have resulted in the discrepancies between these studies and the present research. Also, use of isobaric bupivacaine in the study by Gori et al. in comparison with hyperbaric bupivaciane in the present study, as well as the higher dose of bupivacaine used by Kohler et al. could explain these conflicting results. Also, in contrast to the study by Patel et al. none of our subjects, who remained seated, had inadequate sensory block levels, which could be explained by unnecessary supplementation before careful assessment of maximum sensory block height in their study (16).
The results of the present study are consistent with the findings reported by El-Hakeem et al. There was no significant difference in SBP or MAP measurements between the groups in the current study. However, the reduced frequency of hypotension might be related to different definitions of hypotension (SBP < 90 mmHg in the current study versus SBP < 100 mmHg in the study by El-Hakeem et al.). In general, keeping the patient upright for several minutes after spinal anesthesia leads to delayed aortocaval compression and limited spread of the local anesthetic. This in fact might be the cause of the reduced incidence of hypotension and longer time to achieve T6 sensory level due to more stable hemodynamics in the first few minutes after subarachnoid block (8, 11).
To determine the maximum length of time that patients could remain seated after spinal anesthetic administration, another study by El-Hakeem et al. in 2014 showed that the maximum allowable time to sit up after spinal anesthesia was 7 minutes in patients undergoing cesarean section with the CSE technique. In fact, sitting longer than 7 minutes (9 minutes in their study) increased the need for epidural anesthesia supplementation and had no beneficial hemodynamic effects (23).
Unlike the abovementioned study, we used single-shot spinal anesthesia. Given the risk of inadequate sensory level, we chose 2 minutes as the maximum time for remaining in the sitting position and used the higher dose of hyperbaric bupivacaine. Regarding the beneficial effects of sitting for longer periods of time after spinal anesthesia, further studies with larger sample sizes, examining different time periods, are needed to determine the maximum allowable sitting time after spinal anesthesia in women undergoing cesarean section.
5.1. Conclusions
The present study revealed that the patient’s position is an important factor, which affects the frequency of hypotension and the onset of sensory block during the administration of spinal anesthesia for cesarean section. Based on the findings, keeping the parturient seated for 1 or 2 minutes after spinal anesthesia, compared to immediately lying down, could decrease the frequency of hypotension and ephedrine use in the first 5 minutes before delivery.
5.2. Limitations
In the current study, it was impossible to keep the subjects blinded to grouping; therefore, we minimized the bias by blinding the data collector and the second anesthesiologist, who assessed the subjects’ information. Also, the dose of local anesthetic and opioid, used in this study, was in the upper limit of normal range and might have affected the sensory block level in the present study.
Acknowledgments
We would like to thank Mr. Faryadras for the statistical advice and the operating room staff of Fatemieh hospital for their assistance (clinical trials.gov/.201409299014N42).
Footnotes
Authors’ Contribution:Pooran Hajian had primary responsibility for protocol development, patient screening and enrollment, outcome assessment, preliminary data analysis, and writing of the manuscript. Also, Mahshid Nikooseresht and Tayebe Lotfi contributed to patient monitoring, development of the study protocol and analytical framework, and writing of the manuscript.
References
- 1.Hingson RA, Hellman LM. Anesthesia for Obstetrics. In: Hingson RA, Hellman LM, editors. Miller's Anesthesia. Philadelphia: Saunders; 2015. [Google Scholar]
- 2.Faiz SHR, Rahimzadeh P, Sakhaei M, Imani F, Derakhshan P. Anesthetic effects of adding intrathecal neostigmine or magnesium sulphate to bupivacaine in patients under lower extremities surgeries. J Res Med Sci. 2012;17(10) [PMC free article] [PubMed] [Google Scholar]
- 3.Gogarten W. Spinal anaesthesia for obstetrics. Best Pract Res Clin Anaesthesiol. 2003;17(3):377–92. doi: 10.1016/s1521-6896(02)00116-7. [DOI] [PubMed] [Google Scholar]
- 4.Faiz SH, Rahimzadeh P, Imani F, Bakhtiari A. Intrathecal injection of magnesium sulfate: shivering prevention during cesarean section: a randomized, double-blinded, controlled study. Korean J Anesthesiol. 2013;65(4):293–8. doi: 10.4097/kjae.2013.65.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emmett RS, Cyna AM, Andrew M, Simmons SW. Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database Syst Rev. 2001;(3):CD002251. doi: 10.1002/14651858.CD002251. [DOI] [PubMed] [Google Scholar]
- 6.Sahoo T, SenDasgupta C, Goswami A, Hazra A. Reduction in spinal-induced hypotension with ondansetron in parturients undergoing caesarean section: a double-blind randomised, placebo-controlled study. Int J Obstet Anesth. 2012;21(1):24–8. doi: 10.1016/j.ijoa.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Fathi M, Imani F, Joudi M, Goodarzi V. Comparison Between the Effects of Ringer`s Lactate and Hydroxyethyl Starch on Hemodynamic Parameters After Spinal Anesthesia: A Randomized Clinical Trial. Anesth Pain Med. 2013;2(3):127–33. doi: 10.5812/aapm.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Hakeem EE, Kaki AM, Almazrooa AA, Al-Mansouri NM, Alhashemi JA. Effects of sitting up for five minutes versus immediately lying down after spinal anesthesia for Cesarean delivery on fluid and ephedrine requirement; a randomized trial. Can J Anaesth. 2011;58(12):1083–9. doi: 10.1007/s12630-011-9593-4. [DOI] [PubMed] [Google Scholar]
- 9.Mendonca C, Griffiths J, Ateleanu B, Collis RE. Hypotension following combined spinal-epidural anaesthesia for Caesarean section. Left lateral position vs. tilted supine position. Anaesthesia. 2003;58(5):428–31. doi: 10.1046/j.1365-2044.2003.03090.x. [DOI] [PubMed] [Google Scholar]
- 10.Chevuri SB, Rao JVS, Chandergutti V, Hussain MM, Khan BA. A Comparative Study of Effects of Sitting and Lateral Positions on Quality of Block during Induction of Spinal Anaesthesia in Patients Undergoing Cesarean Section. J Cont Med A Dent. 2015;3(1) [Google Scholar]
- 11.Kohler F, Sorensen JF, Helbo-Hansen HS. Effect of delayed supine positioning after induction of spinal anaesthesia for caesarean section. Acta Anaesthesiol Scand. 2002;46(4):441–6. doi: 10.1034/j.1399-6576.2002.460419.x. [DOI] [PubMed] [Google Scholar]
- 12.Miyabe M, Sato S. The effect of head-down tilt position on arterial blood pressure after spinal anesthesia for cesarean delivery. Reg Anesth. 1997;22(3):239–42. doi: 10.1016/s1098-7339(06)80008-8. [DOI] [PubMed] [Google Scholar]
- 13.Gori F, Corradetti F, Cerotto V, Peduto VA. Influence of positioning on plain levobupivacaine spinal anesthesia in cesarean section. Anesthesiol Res Pract. 2010;2010 doi: 10.1155/2010/212696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rucklidge MW, Paech MJ, Yentis SM. A comparison of the lateral, Oxford and sitting positions for performing combined spinal-epidural anaesthesia for elective Caesarean section. Anaesthesia. 2005;60(6):535–40. doi: 10.1111/j.1365-2044.2005.04178.x. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz-Gomez JR, Palacio-Abizanda FJ, Morillas-Ramirez F, Fornet-Ruiz I, Lorenzo-Jimenez AM, Bermejo-Albares ML. Effect of position on maternal haemodynamics during elective caesarean delivery under spinal anaesthesia. Anaesthesia. 2015;5:7. doi: 10.1016/j.ijoa.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Patel M, Samsoon G, Swami A, Morgan B. Posture and the spread of hyperbaric bupivacaine in parturients using the combined spinal epidural technique. Can J Anaesth. 1993;40(10):943–6. doi: 10.1007/BF03010097. [DOI] [PubMed] [Google Scholar]
- 17.Inglis A, Daniel M, McGrady E. Maternal position during induction of spinal anaesthesia for caesarean section. A comparison of right lateral and sitting positions. Anaesthesia. 1995;50(4):363–5. doi: 10.1111/j.1365-2044.1995.tb04620.x. [DOI] [PubMed] [Google Scholar]
- 18.Obasuyi BI, Fyneface-Ogan S, Mato CN. A comparison of the haemodynamic effects of lateral and sitting positions during induction of spinal anaesthesia for caesarean section. Int J Obstet Anesth. 2013;22(2):124–8. doi: 10.1016/j.ijoa.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Yun EM, Marx GF, Santos AC. The effects of maternal position during induction of combined spinal-epidural anesthesia for cesarean delivery. Anesth Analg. 1998;87(3):614–8. doi: 10.1097/00000539-199809000-00023. [DOI] [PubMed] [Google Scholar]
- 20.Coppejans HC, Hendrickx E, Goossens J, Vercauteren MP. The sitting versus right lateral position during combined spinal-epidural anesthesia for cesarean delivery: block characteristics and severity of hypotension. Anesth Analg. 2006;102(1):243–7. doi: 10.1213/01.ane.0000189049.11005.26. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Xu JM, Zhou F, He L, Cui YL, Li ZJ. Maternal position and development of hypotension in patients undergoing cesarean section under combined spinal-epidural anesthesia of intrathecal hyperbaric ropivacaine. Med Sci Monit. 2015;21:52–8. doi: 10.12659/MSM.892224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallworth SP, Fernando R, Columb MO, Stocks GM. The effect of posture and baricity on the spread of intrathecal bupivacaine for elective cesarean delivery. Anesth Analg. 2005;100(4):1159–65. doi: 10.1213/01.ANE.0000149548.88029.A2. [DOI] [PubMed] [Google Scholar]
- 23.EE AEH, Kaki AM, Alhashemi JA, Boker AM, Albasri SF. How Long Can Patients Sit Up for Before Lying Down after Combined Spinal-Epidural Anesthesia For Cesarean Delivery? A Randomized Trial. J Anesth Clin Res. 2014;5(482):2. [Google Scholar]