Abstract
Background
Type 2 diabetes mellitus (T2DM) might aggravate the carotid plaque vulnerability, and increase the risk for ischemic stroke. Few studies reported the acute stroke subtype with carotid plaque characteristics in T2DM patients. This study aimed to investigate the association between carotid plaque characteristics and acute cerebral infarct (ACI) lesion features determined by MRI in T2DM patients.
Methods
Patients with acute cerebrovascular syndrome in internal carotid artery territory were recruited. All patients were stratified into T2DM and non-T2DM groups and underwent both carotid and brain MRI scans. Ipsilateral carotid plaque morphological and compositional characteristics, intracranial and extracranial carotid artery stenosis were also determined. Stroke subtype based on the Trial of ORG 10172 in Acute Stroke Treatment classification and ACI lesion patterns were evaluated.
Results
Of the recruited 140 patients, 68 (48.6%) patients had T2DM (mean age 64.16 ± 11.38 years, 40 males). T2DM patients exhibited higher prevalence of carotid type IV–VI lesions, larger plaque burden as well as larger lipid-rich necrotic core (LRNC) compared with non-T2DM patients. Among the patients with carotid LRNC on symptomatic side, more concomitant large perforating artery infarct patterns and larger ACI size in the internal carotid artery territory were found in T2DM group than those in non-T2DM group. Carotid plaque with LRNC% > 22.0% was identified as an independent risk factor for the presence of ACI lesions confined to the carotid territory in T2DM patients, regardless of other risk factors.
Conclusions
This study shows that more concomitant large perforating artery infarct patterns and larger ACI size in the internal carotid artery territory were found in the T2DM patients with ipsilateral carotid LRNC plaque than those in non-T2DM patients. Quantification of the carotid plaque characteristics, particularly the LRNC% by MRI has the potential usefulness for stroke risk stratification.
Keywords: Diabetes mellitus, Acute stroke, Carotid plaque, Lipid-rich necrotic core, MR imaging
Background
Diabetes mellitus is a well-established risk factor for atherosclerosis [1]. Type 2 diabetes mellitus (T2DM) might increase the carotid plaque vulnerability by aggravating inflammation, increasing vasa-vasorum neovascularization, and promoting lipid-core expansion [2]. Although several studies using carotid ultrasound and multidetector CT (MDCT) have reported several ultrasound and CT features of carotid plaques in T2DM patients [3, 4], carotid ultrasound and MDCT could not accurately provide critical tissue characteristics of carotid plaque vulnerability [i.e., lipid-rich necrotic core (LRNC) and intraplaque hemorrhage (IPH)] [5].
MRI can assess both qualitative and quantitative vulnerable characteristics of carotid plaque [6, 7] using multi-contrast vessel wall sequences and acute cerebral infarct (ACI) lesion features on diffusion-weighted imaging (DWI). Few studies have reported the acute stroke subtype with carotid plaque characteristics in T2DM subjects.
Thus, in this study we (1) qualitatively and quantitatively compared the vulnerable characteristics of carotid plaques by MRI in subjects with and without T2DM; and (2) determined the association between the carotid plaque characteristics and ACI lesion features on MRI in T2DM patients with acute stroke.
Methods
Study population
Patients with acute cerebrovascular syndrome hospitalized in Shanghai Jiao Tong University Renji Hospital were consecutively recruited from the neurology department between September 2011 and July 2014. The patients with ischemic stroke in the internal carotid artery territory underwent carotid vessel wall and brain MR examinations including diffusion-weighted imaging (DWI) and MR angiography (MRA) within 1 week of symptom onset. The exclusion criteria were as follows: (1) amaurosis fugax; (2) high-risk cardioembolic sources identified by electrocardiogram or transthoracic echocardiography (e.g., atrial fibrillation); (3) previous carotid endarterectomy on the index side or previous neck irradiation; (4) other etiologies, such as vasculitis, moyamoya disease or cancer-related stroke; and (5) contraindications to MRI. The symptomatic carotid artery was defined as being ipsilateral to the acute lesion in the internal carotid artery territory or responsible for the neurological symptoms [8]. The study protocol was approved by the Institutional Review Board of Renji Hospital. Informed consent was obtained from all the subjects.
Cardiovascular risk factors
Data from medical records including the neurological examination [National Institutes of Health Stroke Scale (NIHSS) score], laboratory analysis [i.e., lipid level, high-sensitivity C-reactive protein (hs-CRP), serum creatinine (Scr), glomerular filtration rate (GFR), hemoglobin A1c (HbA1)], and patient’s baseline information, including age, sex, body mass index (BMI), and cardiovascular risk factors [e.g., T2DM, dyslipidemia, hypertension, current smoking, and a prior transient ischemic attack (TIA)/stroke] were collected upon admission. The patients with T2DM were diagnosed based on their blood glucose levels, i.e., either a fasting plasma glucose (FPG) level of ≥7.0 mmol/l or an OGTT result of ≥11.1 mmol/l. Hypertension was defined as a systolic blood pressure (l) either a fasastolic blood pressure ≥90 mmHg, or current treatment with antihypertensive agents. Dyslipidemia was defined as TC/HDL-C ratio ≥5; measured LDL-C ≥ 3.5 mmol/l; or taking lipid-modifying medications [9]. Smoking status was assessed at the time of the ischemic event, and the patients were dichotomized into two groups: current smoker (recent 3 years) or not a current smoker.
Classification of stroke subtypes
Stroke subtypes were classified into 5 categories based on etiology, using the TOAST classification: (1) large-artery atherosclerosis, (2) small-artery occlusion, (3) cardioembolism (CE), (4) stroke of other determined etiology, and (5) stroke of undetermined etiology [10]. We modified the definition of large-artery atherosclerosis as with ≥50% stenosis in carotid artery or middle cerebral artery (MCA) on TOF MRA; small vessel occlusion as an ACI lesion diameter of <15 mm and located in a subcortical area on DWI [11].
ACI lesions in the internal carotid artery territory were also allocated to one of the following 6 patterns [12]: single lesion—(1) small perforating artery infarct (PAI) with diameter ≤1.5 cm; (2) large PAI with diameter >1.5 cm; (3) pial infarct; (4) large territorial infarct; (5) borderzone infarct; and (6) multiple lesions.
MR imaging
All the subjects underwent brain and carotid MRI on a 3.0-T whole-body scanner (Philips Achieva, Best, the Netherlands). A routine MR protocol including diffusion weighted imaging (DWI) and time of flight (TOF) MRA, was used for brain imaging. The imaging parameters of DWI sequence are as follows: repetition time (TR)/echo time (TE) 1598/46 ms, b values = 0.1000 s/mm2, matrix of 128 × 128, slice thickness of 6 mm. Three-dimensional (3D) TOF MRA was also acquired for intracranial arteries with the following parameters: TR/TE 23/3.5 ms, flip angle 18°, field of view 199 × 199 mm2, matrix 500 × 332, and slice thickness 1.2 mm.
For carotid vessel wall imaging, a previously published multi-contrast protocol was used for plaque detection [3D TOF, T1-weighted, T2-weighted, 3D magnetization-prepared rapid acquisition gradient-echo (MPRAGE) and post-contrast T1-weighted sequences] [13]. Post-contrast T1-weighted images were acquired at about 5 min after contrast agent injection (Magnevist, Bayer Healthcare, Berlin, Germany). The longitudinal coverage of the vessel wall sequences were 32 mm (16 sections). Carotid MRA images were reconstructed from the 3D TOF images. The total acquisition time was approximately 35 min.
Image analysis
Two trained reviewers (X.Z. and H.Z.; more than 3 years of experience in carotid plaque imaging) interpreted the carotid MR images in the symptomatic side via consensus using a custom-designed software (CASCADE, Seattle, WA, USA) [14]. The reviewers were blinded to the brain MRI scans and clinical information. Image quality rating was assigned using a 4-point scale (1 = poor, 4 = excellent). Slices with image quality <2 were excluded from review. The morphological measurements, including the maximum wall thickness (max WT) and percent wall volume were measured and calculated for each artery. The luminal stenosis of the symptomatic carotid arteries was measured using the NASCET criteria [15]. The presence or absence of carotid plaque component [e.g., LRNC, IPH, calcification, or fibrous cap rupture (FCR)] was identified based on previously published criteria which were validated by histology [7]. Quantitative assessment of the volume of calcification, LRNC and IPH was determined. The percent volume of each component was described as the percent cross-sectional volume of the total plaque. Carotid atherosclerotic plaque was defined as lesions with presence of any plaque component (e.g., CA, LRNC, FCR or IPH) on MR images. According to the modified American Heart Association (AHA) criteria [6], type IV–V was assigned to plaques characterized by a lipid or necrotic core surrounded by fibrous tissue with possible calcification. Type VI was assigned to complex plaques with a possible FCR, IPH or thrombus.
Brain MR images were evaluated by two experienced radiologists (Q.L. and X.G., >5 years of experience in neuroradiology) with consensus blinded to clinical information and carotid MR images. ACI lesions in the internal carotid artery territory were localized by using DWI, which were defined when lesions had hyperintense on DWI images and hypointense on the apparent diffusion coefficient map. The sum size for ACI lesions in the internal carotid artery territory was recorded on the symptomatic side of each subject. The stenosis of middle cerebral artery (MCA) M1 segment on TOF MRA was also evaluated using the NASCET criteria and graded as <50% diameter stenosis and ≥50%.
Statistical analysis
Univariate and multivariate logistic regression analyses were performed to assess the risk factors for the presence of LRNC plaques and ACI lesions. The quantitative and categorical data are presented as mean ± SD and percentages, respectively. The continuous variables were compared via the independent-sample t test when the data were normally distributed or the Mann–Whitney U test when the data were non-normally distributed. The categorical variables were compared using the Chi square test. A receiver operator curve (ROC) analysis was performed to evaluate the discriminative strength of plaque features for ACI lesion in T2DM patients and non-T2DM patients. The ROC analysis was performed using Medcalc version 11.4.2.0 and the other analyses in this study were performed using R 2.11.0 (R De-velopment Core Team 2010). All of the tests were 2-tailed, and P < 0.05 were considered significant.
Results
Baseline characteristics of symptomatic patients with and without DM
During the study period, 512 patients with ischemic stroke in the internal carotid artery territory were consecutively hospitalized at our hospital. A total of 149 patients meeting the inclusion criteria were recruited and successfully performed the carotid and brain MRI for this study. Nine patients were finally excluded because of inadequate MR scan quality. Of the remaining 140 patients, 84 (60%) were male, 81 (57.9%) had hypertension, and 68 (48.6%) had T2DM. The baseline clinical characteristics of T2DM patients and non-T2DM patients are presented in Table 1. T2DM patients had higher Hs-CRP with significant difference (P = 0.006) and higher dyslipidemia, Scr and history of stroke at the very edge of significance compared to non-T2DM patients. The presence of hypertension, current smoking and NIHSS score was not significantly different between these two groups (all P > 0.05).
Table 1.
Baseline characteristics in T2DM and non-T2DM patients
| Mean ± SD or n (%) | P value | ||
|---|---|---|---|
| T2DM (n = 68) | Non-T2DM (n = 72) | ||
| Risk factors | |||
| Male | 40 (58.8%) | 44 (61.1%) | 0.842 |
| Age, years | 64.16 ± 11.38 | 62.45 ± 9.73 | 0.429 |
| BMI, kg/m2 | 24.89 ± 2.68 | 24.07 ± 2.81 | 0.151 |
| Hypertension | 44 (64.7%) | 37 (51.3%) | 0.533 |
| Current smoking | 39 (57.4%) | 44 (61.1%) | 0.651 |
| Dyslipidemia | 51 (75.0%) | 43 (59.7%) | 0.054 |
| Scr, µmol/l | 79.04 ± 24.65 | 69.51 ± 16.46 | 0.056 |
| GFR, ml/min | 86.67 ± 30.70 | 94.59 ± 25.01 | 0.106 |
| Hs-CRP, mg/l | 7.14 ± 6.88 | 3.85 ± 4.61 | 0.006 |
| History of stroke/TIA | 23 (33.8%) | 14 (19.4%) | 0.054 |
| NIHSS score | 4.48 ± 3.59 | 4.26 ± 3.43 | 0.710 |
| Carotid plaque features | |||
| Presence of plaque | 56 (82.4%) | 42 (58.3%) | 0.002 |
| AHA type IV–VI | 53 (77.9%) | 38 (52.8%) | 0.002 |
| Luminal stenosis (%) | 31.2 ± 33.17 | 19.42 ± 25.91 | 0.013 |
| Max WT (mm) | 3.22 ± 1.73 | 2.26 ± 1.17 | 0.001 |
| Percent wall volume | 42.76 ± 11.85 | 37.78 ± 11.05 | 0.011 |
| LRNC prevalence | 53 (77.9%) | 38 (52.8%) | 0.002 |
| % volume of LRNCa,b | 35.35 ± 26.99 | 15.96 ± 17.28 | 0.001 |
| IPH or FCR prevalence | 9 (13.2%) | 6 (8.3%) | 0.349 |
| % volume of IPHa,b | 3.00 ± 9.30 | 2.21 ± 8.43 | 0.667 |
| CA prevalence | 35 (51.5%) | 29 (40.3%) | 0.184 |
| % volume of CAa,b | 5.84 ± 14.63 | 5.49 ± 8.83 | 0.891 |
T2DM type 2 diabetes mellitus, BMI body mass index, Scr serum creatinine, GFR glomerular filtration rate, Hs-CRP high-sensitivity C-reactive protein, TIA transient ischemic attack, Max WT maximum wall thickness, LRNC lipid-rich necrotic core, IPH intraplaque hemorrhage, FCR fibrous cap rupture, CA calcification
aComponent% = Corresponding component volume/Plaque volume
bOnly including those with plaque present
Comparison of carotid plaque characteristics on symptomatic side between T2DM and non-T2DM patients
The MRI characteristics of the carotid plaques on symptomatic side in patients with and without T2DM are presented in Table 1. The prevalence of carotid plaque and AHA type IV–VI lesions was higher in T2DM patients compared to non-T2DM patients (82.4% vs 58.3%, P = 0.002; 77.9% vs 52.8%, P = 0.002). Carotid plaque burden were significantly larger in T2DM patients than in non-T2DM patients, as evidenced by higher luminal stenosis (31.20% ± 33.17 vs 19.42% ± 25.91, P = 0.013), max WT (3.22 mm ± 1.73 vs 2.26 mm ± 1.17, P = 0.001) and percent wall volume (42.76% ± 11.85 vs 37.78% ± 11.05, P = 0.011). A higher prevalence of LRNC and larger LRNC% volume was found in T2DM patients than in non-T2DM patients (77.9% vs 52.8%, P = 0.002; 35.35% ± 26.99 vs 15.96% ± 17.28, P = 0.001). Furthermore, T2DM was identified as an independent risk factor for the presence of LRNC plaques (OR = 3.35; 95% CIs = 1.33–8.43), regardless of prior stroke/TIA, age (per 10 years) and current smoking (Fig. 1).
Fig. 1.
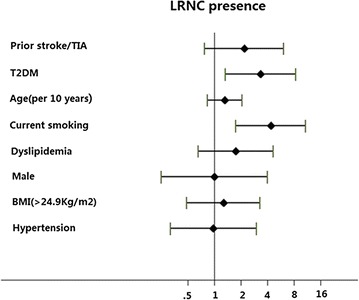
Independent predictors for the presence of carotid LRNC plaques on symptomatic side
Patients with carotid LRNC plaque on symptomatic side under stroke subtype in T2DM and non-T2DM patients
The characteristics of enrolled patients with carotid LRNC plaque on symptomatic side in T2DM patients and non-T2DM patients are shown in Table 2. Based on the results, patients were grouped according to the TOAST classification. The prevalence of these subtype have no significant difference between T2DM and non-T2DM group. However, T2DM patients with carotid LRNC plaque had more concomitant large PAI and less concomitant small PAI as compared with non-T2DM patients (34.0% vs 13.2%, P = 0.024; 13.2% vs 31.6%, P = 0.033; respectively).
Table 2.
Patients with carotid LRNC plaque on symptomatic side under stroke subtype
| T2DM (n = 53) | Non-T2DM (n = 38) | P value | |
|---|---|---|---|
| Etiological subtypes | |||
| Large-artery atherosclerosis | 21 (39.6%) | 14 (36.8%) | 0.788 |
| Small-artery occlusion | 20 (37.7%) | 21 (55.2%) | 0.097 |
| Stroke of other etiology | 12 (22.6%) | 5 (13.2%) | 0.252 |
| ACI lesion patterns | |||
| Small PAI (diameter ≤1.5 cm) | 7 (13.2%) | 12 (31.6%) | 0.033 |
| Large PAI (diameter >1.5 cm) | 18 (34.0%) | 5 (13.2%) | 0.024 |
| Pial infarct | 0 (0%) | 3 (7.9%) | 0.069 |
| Large territorial infarct | 9 (17.0%) | 4 (10.5%) | 0.386 |
| Borderzone infarct | 1 (1.9%) | 0 (0%) | 0.582 |
| Multiple lesions | 13 (24.5%) | 7 (18.4%) | 0.488 |
| ACI lesion size | |||
| ACI size (ml)a | 15.45 ± 8.97 | 9.09 ± 8.64 | 0.011 |
| ACI size in patients concurrent carotid IPH or FCR (ml)a | 18.28 ± 6.88 | 11.51 ± 12.51 | 0.225 |
| ACI size in patients concurrent carotid ≥50% stenosis (ml)a | 22.25 ± 5.32 | 14.18 ± 11.94 | 0.212 |
| ACI size in patients concurrent MCA ≥50% stenosis (ml)a | 19.43 ± 7.29 | 9.45 ± 13.12 | 0.100 |
LRNC lipid-rich necrotic core, ACI acute cerebral infarct, PAI large artery atherosclerosis, MCA middle cerebral artery, IPH intraplaque hemorrhage, FCR fibrous cap rupture
aPatients with ACI lesions
No significant difference in the prevalence of ACI lesions in the internal carotid artery territory was found in these two groups (90.6% vs. 86.8%, P = 0.211). However, T2DM patients had a larger ACI size than non-T2DM patients (15.45 ml ± 8.97 vs. 9.09 ml ± 8.64, P = 0.011). In contrast, no significant difference in ACI size was found in patients concurrent carotid or MCA ≥ 50% stenosis, and concurrent carotid IPH or FCR between T2DM and non-T2DM group.
In T2DM patients, ROC analysis showed that 22.0% was the optimal threshold of LRNC% to predict the presence of ACI in the internal carotid artery territory (Fig. 2a). The use of this value yielded a sensitivity of 83.87% and specificity of 97.8% (AUC = 0.927). Meanwhile, the ROC curve showed that 11.6% was the optimal cutoff value of stenosis level to predict the presence of ACI (AUC = 0.916, sensitivity, 87.1%; specificity, 86.7%) (Fig. 2a).
Fig. 2.
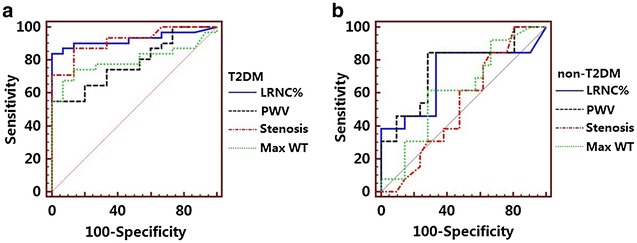
Optimal threshold of carotid plaque features to predict the presence of ACI in ICA region in T2DM subjects (a) and non-T2DM subjects (b). T2DM type 2 diabetes mellitus, LRNC% % volume of LRNC, PWV Percent wall volume, Max WT maximum wall thickness
In contrast, 4.32% was shown to be the optimal threshold of LRNC% to predict the presence of ACI in the internal carotid artery territory in non-T2DM patients. The use of this value yielded a sensitivity of 84.62% and specificity of 66.67% (AUC = 0.714) (Fig. 2b).
Association between carotid LRNC plaques and ACI features in T2DM patients
For symptomatic carotid arteries with plaque, significant association of carotid LRNC% > 22.0% and the ACI presence in internal carotid artery territory was found during univariate analysis (OR = 12.41; 95% CIs = 1.42–108.31, P = 0.023, Table 3). After adjusting for significant demographic factors, the association remained statistically significant (multivariate adjusted OR = 12.5; 95% CIs = 2.81–55.43 P = 0.001). Figure 3 are examples showing subjects with carotid LRNC and ipsilateral ACI lesions.
Table 3.
Univariate and multivariate logistic regression of vascular bed variables for the presence of ACI lesions in the internal carotid artery territory in T2DM patients with carotid plaque (n = 56)
| Univariate | Multivariatea | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Carotid stenosis ≥50% | 2.93 | 0.25–35.01 | 0.396 | 3.24 | 0.33–32.39 | 0.316 |
| MCA stenosis ≥50% | 1.62 | 0.11–24.82 | 0.728 | 1.56 | 0.13–19.37 | 0.728 |
| IPH or rupture | 0.84 | 0.06–11.85 | 0.899 | 0.84 | 0.06–11.85 | 0.899 |
| LRNC% > 22% | 12.41 | 1.42–108.31 | 0.023 | 12.50 | 2.81–55.43 | 0.001 |
T2DM type 2 diabetes mellitus, MCA middle cerebral artery, IPH intraplaque hemorrhage, FCR fibrous cap rupture, LRNC% % volume of lipid-rich necrotic core
aAdjusted for significant demographic factors, gender, age, BMI, hypertension, current smoking, dyslipidemia, history of stroke/TIA
Fig. 3.
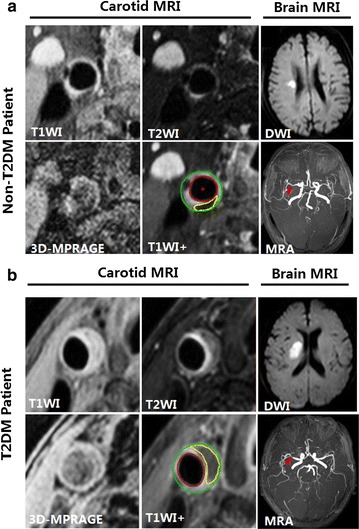
Representative MR images of a T2DM and a non-T2DM subject. a An atherosclerotic plaque with small LRNC (yellow outline) is detected in the right carotid artery of a non-T2DM patient: iso-intensity on T1WI and T2WI; no enhancement in T1WI+; hypo-intensity on MP-RAGE image. Cerebral DWI demonstrates a small perforating artery infarct (hyper-intensity) at right hemisphere. Brain MRA demonstrates right MCA M1 segment with mild stenosis (red arrow). b An atherosclerotic plaque with large LRNC (yellow outline) with mild to moderate stenosis is detected in the right carotid artery of a T2DM patient. LRNC appears as iso-intensity on T1WI; hypo-intensity on corresponding T2WI; no enhancement in T1WI+; hypo-intensity on MP-RAGE image. Cerebral DWI demonstrates large volume of ACI (hyperintensity) at right hemisphere. Brain MRA demonstrates right MCA M1 segment with moderate stenosis (red arrow). ACI acute cerebral infarct, LRNC lipid-rich necrotic core, WI weighted image, T1WI+ contrast enhanced T1-weighted image, MCA middle cerebral artery
Discussion
The present study using high-resolution MRI to access the carotid atherosclerotic plaque characteristics, intracranial arterial stenosis and ACI lesion features in symptomatic subjects with non-cardioembolism stroke. We found that: (1) T2DM patients exhibited higher prevalence of carotid type IV–VI lesions, larger plaque burden as well as larger LRNC plaques compared with non-T2DM patients; (2) among the patients with carotid LRNC plaque on symptomatic side, more concomitant large PAI patterns and larger ACI size in the internal carotid artery territory were found in T2DM patients than those in non-T2DM patients; (3) carotid plaque with LRNC% > 22.0% was identified as an independent risk factor of the presence of ACI lesions confined to the carotid territory in T2DM patients, regardless for other risk factors. Our results suggest that MRI-identified vulnerable features, especially large LRNC%, are closely related to ACI patterns and size in T2DM patients suffering an acute stroke.
The present study showed that T2DM patients with acute stroke exhibit higher prevalence of carotid type IV–VI lesions and larger plaque burden than non-T2DM patients. Furthermore, we found that T2DM was independently associated with MRI-identified LRNC plaques in the symptomatic carotid arteries, regardless of other risk factors. The above results of our study are consistent with observations from the study of Esposito et al. which showed that T2DM was associated with AHA IV–VI lesion type plaques in moderate to severe carotid artery stenosis without stroke [16]. Alonso’s study [17] showed that diabetic retinopathy in T2DM patients without cardiovascular disease and with normal renal function is associated with a higher atherosclerotic burden in the carotid arteries. Postmortem studies have demonstrated that coronary artery lesions from T2DM subjects had larger mean necrotic cores than non-T2DM subjects [18, 19]. Pre-clinical studies showed that hyperglycemia and some of its end products can cause severe inflammatory infiltration and larger LRNCs in diabetic atheroma [19]. Huang et al. [20] and our previous study [21] found a quantitative relationship between the HbA1c levels and plaque features in ultrasonic or MR images of atherosclerotic patients. These results support the findings that T2DM may have an adverse effect on carotid plaque vulnerability. In contrast, some other studies, particularly the Rotterdam Study [22] and AIM-HIGH Study [23], have shown that diabetes mellitus is not associated with a higher risk of the presence of LRNC. These disparate conclusions might be due to differences in study designs, cohorts or analytic approaches. The patients recruited in our study were with acute cerebrovascular syndrome, whereas the Rotterdam Study participants were included mainly by carotid wall thickening ≥2.5 mm, and the subjects in AIM-HIGH Study were enrolled with more females, more metabolic syndrome and fewer DM. Additionally, there were significant differences in racial distribution among these MRI sub-studies. Previous studies revealed that the composition and morphology of carotid atherosclerotic lesions were found to be different between ethno-racial groups [11, 24] and the status of metabolic level [25].
In this study, we accessed the association of carotid plaque characteristics with ACI lesions confined to the carotid territory in patients with and without T2DM. We used TOAST classification and the pattern of ACI lesions on DWI help to reveal the mechanism of the cerebral ischemic events [12]. The prevalence of TOAST subtypes had no significant difference between T2DM and non-T2DM patients. However, we found that T2DM patients with carotid LRNC plaques had concomitant more large PAI and less small PAI patterns as compared with non-T2DM patients on the symptomatic side. Previous studies [17, 26–28] have shown that T2DM have unequivocal association with the increased risk for ischemic stroke or worsened outcome following stroke. Chung et al. [11] studied 2702 acute ischemic stroke Asian patients, and found that large-artery atherosclerosis was the leading TOAST subtype in border zone infarction (89.9%) as well as internal carotid territory infarction (51.5%). Large-artery atherosclerosis and small-artery occlusion were the two most common subtypes (38.5 and 22.8%) in single internal carotid territory infarction. Lee et al. [29] revealed that perforating artery infarcts, whether single or occurring in addition to pial or border-zone infarcts, were lesion patterns specific for MCA disease. Multiple ACI lesions are thought to be markers of embolism. Diabetes promotes inflammation infiltration and lipid-core expansion, induced large proportion of LRNC in diabetic atheromas, which are associated with surface disruption and small thrombus formation [30]. It should be noted that the low prevalence of IPH or FCR (only 13.2% in T2DM patients) and mild to moderate luminal stenosis (31.2 ± 33.17% in T2DM patients) were found on the symptomatic carotid arteries in our study, suggesting that the carotid plaques identified in our study were mostly asymptomatic lesions [31]. Xu et al. [32] found that co-existing intracranial and extracranial carotid artery plaques were prevalent in symptomatic patients and the number of co-existing plaques was independently associated with the risk of recurrent stroke.
In our study, ROC analysis showed that 22.0% was the optimal threshold of LRNC% to predict the presence of ACI, yielded a sensitivity of 83.87% and specificity of 97.8% in T2DM patients. Multivariate analysis demonstrated that carotid plaque LRNC% > 22.0% was an independent risk factor for ACI presence confined to the corresponding territory after adjustment of clinical demographic factors. Our findings were in accordance with several carotid ultrasound studies observing an association between the presence of echolucent plaques, an ultrasound signature for unstable plaque, and increased risk of ischemic cerebrovascular events in T2DM patients [33–35]. Meanwhile, this study extended our previous findings which demonstrated a close relationship between higher HbA1c level and ACI severity in stroke patients [21]. An explanation for the association in our findings may be that atherosclerosis is a systemic process. There is evidence that the composition and clinical consequences of plaques at different locations within an individual are similar, and DM promotes inflammation infiltration and lipid-core expansion which might exacerbate the severity of ACI. The results of our study demonstrate that quantification of the carotid plaque characteristics, particularly the LRNC% by MRI may be useful to stratify risk for patients with low-grade carotid stenosis.
Limitations of this study should be noted. First, this was a retrospective single-center study of stroke patients. The selection bias may exist since this study was intended for patients with cerebral infarction, and the generality of the study results is limited. A prospective population-based study should be performed to verify the predictive value. Second, we performed the analyses based on a “yes or no” diagnosis of T2DM upon admission and did not determine the history or glycemic control of diabetes from these patients. Third, because of the generally long acquisition time (>30 min) and limited coverage (<40 mm centered on the carotid bifurcation) of the multi-contrast MR sequences, it was difficult to obtain sufficient cooperation from the patients with severe symptoms. This limited the detection of atherosclerotic lesions occurring in more distal or more proximal segments. Recently, fast 3D multi-contrast vessel wall techniques were proposed for joint detection of intra- and extracranial artery plaques. These techniques may be useful to capture the criminal plaque [36, 37]. In addition, diagnostic modality for noninvasively evaluating cerebral hemodynamics is useful to explore the mechanisms behind acute cerebrovascular syndrome and to determine the ideal therapies [38].
Conclusions
This study shows that T2DM patients with ipsilateral carotid LRNC plaque exhibited more concomitant large PAI patterns and larger ACI size confined to carotid artery territory than non-T2DM patients. Carotid plaque with LRNC% > 22.0% was identified as an independent risk factor of the presence of ACI lesions in T2DM patients regardless of other risk factors. Our results indicate that quantification of the carotid plaque characteristics, particularly the LRNC% by MRI has the potential usefulness for stroke risk stratification.
Authors’ contributions
BS and XL wrote and drafted the manuscript. XG and QL performed the MR examination. HZ, XZ, QL and XG contributed to the image analysis. HZ and BS performed the statistical analysis. HZ, XL and JP participated in the study design and JX reviewed the manuscript. All of the authors contributed to the discussion. All authors read and approved the final manuscript.
Acknowledgements
No.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethic Committee of Shanghai Renji Hospital and the study protocols were performed in accordance with the approved guidelines. The subjects were recruited with individual informed consent.
Funding
This study was supported by National Natural Science Foundation of China (Grants 81401374 and 81571630), National Science Fund for Distinguished Young Scholars (81625002), Young Researcher Grant from Shanghai Municipal Commission of Health and Family Planning (20144Y0076), Shanghai Jiao Tong University (YG2016MS56) and Shanghai Jiao Tong University School of Medicine (15ZH1003 and 14XJ10019), Shanghai ShenKang Hospital Development Center (16CR3034A), Shanghai Science and Technology Committee (15411963600), Shanghai Municipal Education Commission Gaofeng Clinical Medicine Grant Support (20152209).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ACI
acute cerebral infarct
- BMI
body mass index
- CA
calcification
- DWI
diffusion weighted imaging
- FCR
fibrous cap rupture
- FPG
fasting plasma glucose
- GFR
glomerular filtration rate
- HbA1c
hemoglobin A1c
- HDL-C
high-density lipoprotein cholesterol
- Hs-CRP
high-sensitivity C-reactive protein
- IPH
intraplaque hemorrhage
- ICA
internal carotid artery
- LDL-C
low-density lipoprotein cholesterol
- LRNC
lipid-rich necrotic core
- Max WT
maximum wall thickness
- MCA
middle cerebral artery
- PAI
perforating artery infarct
- Scr
serum creatinine
- TC
total cholesterol
- T2DM
type 2 diabetes mellitus
- TG
triglyceride
- TIA
transient ischemic attack
- 3D TOF
three-dimensional time-of-flight
- 3D MPRAGE
three-dimensional magnetization-prepared rapid acquisition gradient-echo
Footnotes
Beibei Sun and Xiao Li contributed equally to this work and are co-first authors
Huilin Zhao, Xiaosheng Liu and Jun Pu are co-corresponding authors
Contributor Information
Beibei Sun, Email: alisabeibei@163.com.
Xiao Li, Email: lixiao3606@163.com.
Xiaosheng Liu, Email: liu.mia@qq.com.
Xiaoqian Ge, Email: 474067607@qq.com.
Qing Lu, Email: luqing_1973@163.com.
Xihai Zhao, Email: xihaizhao@tsinghua.edu.cn.
Jun Pu, Email: pujun310@hotmail.com.
Jianrong Xu, Email: renjixjr@163.com.
Huilin Zhao, Email: huilinzhao2013@163.com.
References
- 1.Idris I, Thomson GA, Sharma JC. Diabetes mellitus and stroke. Int J Clin Pract. 2006;60(1):48–56. doi: 10.1111/j.1368-5031.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 2.Moreno PR, Fuster V. New aspects in the pathogenesis of diabetic atherothrombosis. J Am Coll Cardiol. 2004;44(12):2293–2300. doi: 10.1016/j.jacc.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 3.Ibebuogu UN, Nasir K, Gopal A, Ahmadi N, Mao SS, Young E, Honoris L, Nuguri VK, Lee RS, Usman N, et al. Comparison of atherosclerotic plaque burden and composition between diabetic and non diabetic patients by non invasive CT angiography. Int J Cardiovasc Imaging. 2009;25(7):717–723. doi: 10.1007/s10554-009-9483-9. [DOI] [PubMed] [Google Scholar]
- 4.He C, Gu M, Jiang R, Li JH. Noninvasive assessment of the carotid and cerebrovascular atherosclerotic plaques by multidetector CT in type-2 diabetes mellitus patients with transient ischemic attack or stroke. Diabetol Metab Syndr. 2013;5(1):9. doi: 10.1186/1758-5996-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosseini AA, Simpson RJ, Altaf N, Bath PM, MacSweeney ST, Auer DP. Magnetic resonance imaging plaque hemorrhage for risk stratification in carotid artery disease with moderate risk under current medical therapy. Stroke. 2017;48(3):678–685. doi: 10.1161/STROKEAHA.116.015504. [DOI] [PubMed] [Google Scholar]
- 6.Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106(11):1368–1373. doi: 10.1161/01.CIR.0000028591.44554.F9. [DOI] [PubMed] [Google Scholar]
- 7.Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, Hatsukami TS, Yuan C. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005;25(1):234–239. doi: 10.1161/01.ATV.0000149867.61851.31. [DOI] [PubMed] [Google Scholar]
- 8.Grimm JM, Schindler A, Freilinger T, Cyran CC, Bamberg F, Yuan C, Reiser MF, Dichgans M, Freilinger C, Nikolaou K, et al. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaques using parallel imaging and 3 T black-blood in vivo CMR. J Cardiovasc Magn Reson. 2013;15:44. doi: 10.1186/1532-429X-15-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardiovascular disease risk factors: new areas for research. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1994;841:1–53. [PubMed]
- 10.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 11.Chung JW, Park SH, Kim N, Kim WJ, Park JH, Ko Y, Yang MH, Jang MS, Han MK, Jung C, et al. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification and vascular territory of ischemic stroke lesions diagnosed by diffusion-weighted imaging. J Am Heart Assoc. 2014;3(4):e001119. doi: 10.1161/JAHA.114.001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Man BL, Fu YP, Chan YY, Lam W, Hui AC, Leung WH, Mok V, Wong KS. Lesion patterns and stroke mechanisms in concurrent atherosclerosis of intracranial and extracranial vessels. Stroke. 2009;40(10):3211–3215. doi: 10.1161/STROKEAHA.109.557041. [DOI] [PubMed] [Google Scholar]
- 13.Liu XS, Zhao HL, Cao Y, Lu Q, Xu JR. Comparison of carotid atherosclerotic plaque characteristics by high-resolution black-blood MR imaging between patients with first-time and recurrent acute ischemic stroke. AJNR Am J Neuroradiol. 2012;33(7):1257–1261. doi: 10.3174/ajnr.A2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerwin W, Xu D, Liu F, Saam T, Underhill H, Takaya N, Chu B, Hatsukami T, Yuan C. Magnetic resonance imaging of carotid atherosclerosis: plaque analysis. Top Magn Reson Imaging. 2007;18(5):371–378. doi: 10.1097/rmr.0b013e3181598d9d. [DOI] [PubMed] [Google Scholar]
- 15.Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, Fox AJ, Rankin RN, Hachinski VC, Wiebers DO, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325(7):445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 16.Esposito L, Saam T, Heider P, Bockelbrink A, Pelisek J, Sepp D, Feurer R, Winkler C, Liebig T, Holzer K, et al. MRI plaque imaging reveals high-risk carotid plaques especially in diabetic patients irrespective of the degree of stenosis. BMC Med Imaging. 2010;10:27. doi: 10.1186/1471-2342-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso N, Traveset A, Rubinat E, Ortega E, Alcubierre N, Sanahuja J, Hernandez M, Betriu A, Jurjo C, Fernandez E, et al. Type 2 diabetes-associated carotid plaque burden is increased in patients with retinopathy compared to those without retinopathy. Cardiovasc Diabetol. 2015;14:33. doi: 10.1186/s12933-015-0196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno PR, Murcia AM, Palacios IF, Leon MN, Bernardi VH, Fuster V, Fallon JT. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation. 2000;102(18):2180–2184. doi: 10.1161/01.CIR.102.18.2180. [DOI] [PubMed] [Google Scholar]
- 19.Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24(7):1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 20.Huang XW, Zhang YL, Meng L, Qian M, Zhou W, Zheng RQ, Zheng HR, Niu LL. The relationship between HbA(1)c and ultrasound plaque textures in atherosclerotic patients. Cardiovasc Diabetol. 2016;15:98. doi: 10.1186/s12933-016-0422-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun B, Zhao H, Liu X, Lu Q, Zhao X, Pu J, Xu J. Elevated hemoglobin A1c is associated with carotid plaque vulnerability: novel findings from magnetic resonance imaging study in hypertensive stroke patients. Sci Rep. 2016;6:33246. doi: 10.1038/srep33246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Bouwhuijsen QJ, Vernooij MW, Hofman A, Krestin GP, van der Lugt A, Witteman JC. Determinants of magnetic resonance imaging detected carotid plaque components: the Rotterdam Study. Eur Heart J. 2012;33(2):221–229. doi: 10.1093/eurheartj/ehr227. [DOI] [PubMed] [Google Scholar]
- 23.Zhao XQ, Hatsukami TS, Hippe DS, Sun J, Balu N, Isquith DA, Crouse JR, 3rd, Anderson T, Huston J, 3rd, Polissar N, et al. Clinical factors associated with high-risk carotid plaque features as assessed by magnetic resonance imaging in patients with established vascular disease (from the AIM-HIGH Study) Am J Cardiol. 2014;114(9):1412–1419. doi: 10.1016/j.amjcard.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saam T, Cai JM, Cai YQ, An NY, Kampschulte A, Xu D, Kerwin WS, Takaya N, Polissar NL, Hatsukami TS, et al. Carotid plaque composition differs between ethno-racial groups: an MRI pilot study comparing mainland Chinese and American Caucasian patients. Arterioscler Thromb Vasc Biol. 2005;25(3):611–616. doi: 10.1161/01.ATV.0000155965.54679.79. [DOI] [PubMed] [Google Scholar]
- 25.Wasserman BA, Sharrett AR, Lai S, Gomes AS, Cushman M, Folsom AR, Bild DE, Kronmal RA, Sinha S, Bluemke DA. Risk factor associations with the presence of a lipid core in carotid plaque of asymptomatic individuals using high-resolution MRI: the multi-ethnic study of atherosclerosis (MESA) Stroke. 2008;39(2):329–335. doi: 10.1161/STROKEAHA.107.498634. [DOI] [PubMed] [Google Scholar]
- 26.Kurosaki Y, Yoshida K, Fukuda H, Handa A, Chin M, Yamagata S. Asymptomatic carotid T1-high-intense plaque as a risk factor for a subsequent cerebrovascular ischemic event. Cerebrovasc Dis. 2017;43(5–6):250–256. doi: 10.1159/000455973. [DOI] [PubMed] [Google Scholar]
- 27.Bamberg F, Hetterich H, Rospleszcz S, Lorbeer R, Auweter SD, Schlett CL, Schafnitzel A, Bayerl C, Schindler A, Saam T, et al. Subclinical disease burden as assessed by whole-body MRI in subjects with prediabetes, subjects with diabetes, and normal control subjects from the general population: the KORA-MRI Study. Diabetes. 2017;66(1):158–169. doi: 10.2337/db16-0630. [DOI] [PubMed] [Google Scholar]
- 28.Tziomalos K, Spanou M, Bouziana SD, Papadopoulou M, Giampatzis V, Kostaki S, Dourliou V, Tsopozidi M, Savopoulos C, Hatzitolios AI. Type 2 diabetes is associated with a worse functional outcome of ischemic stroke. World J Diabetes. 2014;5(6):939–944. doi: 10.4239/wjd.v5.i6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee DK, Kim JS, Kwon SU, Yoo SH, Kang DW. Lesion patterns and stroke mechanism in atherosclerotic middle cerebral artery disease: early diffusion-weighted imaging study. Stroke. 2005;36(12):2583–2588. doi: 10.1161/01.STR.0000189999.19948.14. [DOI] [PubMed] [Google Scholar]
- 30.Falk E. Why do plaques rupture? Circulation. 1992;86(6 Suppl):III30–42. [PubMed] [Google Scholar]
- 31.Milosevic D, Pasternak J, Popovic V, Nikolic D, Milosevic P, Manojlovic V. The analysis of the connection between plaque morphology of asymptomatic carotid stenosis and ischemic brain lesions. Vojnosanit Pregl. 2013;70(11):993–998. doi: 10.2298/VSP110519020M. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Yuan C, Zhou Z, He L, Mi D, Li R, Cui Y, Wang Y, Wang Y, Liu G, et al. Co-existing intracranial and extracranial carotid artery atherosclerotic plaques and recurrent stroke risk: a three-dimensional multicontrast cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2016;18(1):90. doi: 10.1186/s12968-016-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katakami N, Takahara M, Kaneto H, Sakamoto K, Yoshiuchi K, Irie Y, Kubo F, Katsura T, Yamasaki Y, Kosugi K, et al. Ultrasonic tissue characterization of carotid plaque improves the prediction of cardiovascular events in diabetic patients: a pilot study. Diabetes Care. 2012;35(12):2640–2646. doi: 10.2337/dc12-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irie Y, Katakami N, Kaneto H, Takahara M, Nishio M, Kasami R, Sakamoto K, Umayahara Y, Sumitsuji S, Ueda Y, et al. The utility of ultrasonic tissue characterization of carotid plaque in the prediction of cardiovascular events in diabetic patients. Atherosclerosis. 2013;230(2):399–405. doi: 10.1016/j.atherosclerosis.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Hirano M, Nakamura T, Kitta Y, Sano K, Kodama Y, Kobayashi T, Fujioka D, Saito Y, Yano T, Watanabe K, et al. Assessment of carotid plaque echolucency in addition to plaque size increases the predictive value of carotid ultrasound for coronary events in patients with coronary artery disease and mild carotid atherosclerosis. Atherosclerosis. 2010;211(2):451–455. doi: 10.1016/j.atherosclerosis.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Zhao H, Wang J, Liu X, Zhao X, Hippe DS, Cao Y, Wan J, Yuan C, Xu J. Assessment of carotid artery atherosclerotic disease by using three-dimensional fast black-blood MR imaging: comparison with DSA. Radiology. 2015;274(2):508–516. doi: 10.1148/radiol.14132687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Z, Li R, Zhao X, He L, Wang X, Wang J, Balu N, Yuan C. Evaluation of 3D multi-contrast joint intra- and extracranial vessel wall cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2015;17:41. doi: 10.1186/s12968-015-0143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kashiwazaki D, Akioka N, Kuwayama N, Noguchi K, Tanaka K, Kuroda S. Pathophysiology of acute cerebrovascular syndrome in patients with carotid artery stenosis: a magnetic resonance imaging/single-photon emission computed tomography study. Neurosurgery. 2015;76(4):427–433. doi: 10.1227/NEU.0000000000000655. [DOI] [PubMed] [Google Scholar]


