Abstract
Background
Seed dormancy, defined as the incapability of a viable seed to germinate under favourable conditions, is an important trait in nature and agriculture. Despite extensive research on dormancy and germination, many questions about the molecular mechanisms controlling these traits remain unanswered, likely due to its genetic complexity and the large environmental effects which are characteristic of these quantitative traits. To boost research towards revealing mechanisms in the control of seed dormancy and germination we depend on the identification of genes controlling those traits.
Methods
We used transcriptome analysis combined with a reverse genetics approach to identify genes that are prominent for dormancy maintenance and germination in imbibed seeds of Arabidopsis thaliana. Comparative transcriptomics analysis was employed on freshly harvested (dormant) and after-ripened (AR; non-dormant) 24-h imbibed seeds of four different DELAY OF GERMINATION near isogenic lines (DOGNILs) and the Landsberg erecta (Ler) wild type with varying levels of primary dormancy. T-DNA knock-out lines of the identified genes were phenotypically investigated for their effect on dormancy and AR.
Results
We identified conserved sets of 46 and 25 genes which displayed higher expression in seeds of all dormant and all after-ripened DOGNILs and Ler, respectively. Knock-out mutants in these genes showed dormancy and germination related phenotypes.
Conclusions
Most of the identified genes had not been implicated in seed dormancy or germination. This research will be useful to further decipher the molecular mechanisms by which these important ecological and commercial traits are regulated.
Electronic supplementary material
The online version of this article (10.1186/s12870-017-1098-z) contains supplementary material, which is available to authorized users.
Keywords: Arabidopsis thaliana, Delay of germination, Knockout lines, Seed performance, Transcriptromics
Background
Freshly matured seeds usually exhibit primary dormancy, a trait defined as the failure of viable seeds to germinate under favourable conditions [8]. Seed dormancy plays a crucial role in the survival of plant species, but is also important for agricultural practice to prevent pre-harvest sprouting under cool, high humidity conditions [24]. Primary dormancy can be released by either cold stratification, which is a low-temperature treatment of imbibed seeds, or by an extended period of dry seed storage (after-ripening; AR) [9].
The transition from dormancy to germination is a critical step in the life cycle of plants [25]. The plant hormone abscisic acid (ABA) has long been known to play a major role in the establishment and maintenance of seed dormancy and the inhibition of seed germination, whereas gibberellins (GAs) and several other hormones, including brassinosteroids, ethylene, and cytokinins, have been shown to promote seed germination [38]. However, it is especially the balance between ABA and GA that controls the decision to germinate or not [19]. Mutations in genes regulating ABA levels or -sensitivity result in a reduced degree of seed dormancy [34, 35]. Whereas GA biosynthesis or sensing mutants result in a block of germination [23, 31, 33]. This hormonal control is also integrated with the seed’s responses to environmental conditions, such as light [45], temperature [55, 60] and nutrients [40].
Recent advances in gene expression analysis using microarrays allow genome-wide expression studies to characterize seed dormancy and germination [10, 20, 21, 26, 27, 36, 41, 52]. Carrera et al. [12] used a targeted transcriptomics approach in imbibed non-dormant mutants (aba1 and abi1) compared to wild-type seeds that were or were not after-ripened. They concluded that, in Arabidopsis, after-ripening and dormancy are controlled by genetically separate pathways, and that ABA only affects the induction and maintenance of dormancy in imbibed seeds, but not after-ripening. The work also showed that application of exogenous ABA to after-ripened seeds does not mimic dormant seed states with respect to gene expression profiles. Recently it was shown that seed dormancy maintenance in the imbibed state was mainly controlled at the transcriptional level [3] and that transcriptional differences between dormant and non-dormant seed become visible already at early imbibition [17, 48].
Despite extensive research on dormancy and germination, many questions about the molecular mechanisms controlling these traits remain unanswered, likely due to its genetic complexity and the large environmental effects which are characteristic of these quantitative traits. Employing whole-genome scans for quantitative trait loci (QTL) is a common approach to identify genes involved in complex phenotypes. Particular attention in this method is given to the role of natural variation in the regulation of traits related to plant adaptation. Natural variation has been used to identify loci that control seed dormancy in nature. QTL analyses on six Recombinant Inbred Line (RIL) populations have identified eleven DELAY OF GERMINATION (DOG) QTL of which nine have been confirmed by near isogenic lines (NILs). The different DOG loci affect dormancy mainly by distinct genetic pathways as was concluded from the absence of strong epistatic interactions in the QTL analysis. This finding was confirmed by transcriptome analyses in freshly harvested dry seeds of the main DOGNILs, these lines showed distinct expression patterns compared to their genetic background Landsberg erecta (Ler). The genes identified in the different DOGNILs represent largely different gene ontology profiles [6].
Here we aim at identifying genes that are required for dormancy maintenance and germination of imbibed seeds. Moreover, we focus on what is in common between the different pathways. For this the transcriptome of freshly harvested (dormant) and after-ripened (AR; non-dormant) 24-h imbibed seeds of the same set of DOGNILs and Ler was investigated. We have identified sets of 46 and 25 genes that were up-regulated in seeds of all dormant (D-up) and all after-ripened (AR-up) DOGNILs and Ler, respectively. We have investigated their role in seed performance by analysing knock-out (KO) mutants in these genes. With seed performance we refer to the capacity of seeds to germinate under various environmental conditions. Traits that contribute to seed performance are seed dormancy, seed longevity (as estimated in an accelerated aging test) and germination under stress conditions, such as high salt, osmotic stress and ABA treatment [32]. In this study we have characterised several genes affecting seed performance.
Methods
Plant material
The near isogenic lines of four DELAY OF GERMINATION (DOG) loci; NILDOG1-Cvi, NILDOG2-Cvi, NILDOG3-Cvi and NILDOG6-Kas-2 and Landsberg erecta (Ler) were earlier described by Bentsink et al. [6]. Although for some of the DOG loci several NILs, containing introgression fragments from different accessions, were available we have chosen the ones with the strongest phenotypic effects. T-DNA insertional mutant lines and Columbia-0 (Col-0; N60000) were ordered from the European Arabidopsis Stock Center (NASC, www.arabidopsis.info). Details (SALK/SAIL entry, AGI code, knock out number and encoded protein) of T-DNA lines are provided in Additional file 1: Table S1.
Growth conditions
NILs: Seeds were sown in Petri dishes on water-saturated filter paper, followed by a 4-day cold treatment at 4 °C, and transferred to an acclimated room at 25 °C with 16 h light/8 h dark for 2 days before planting in 7-cm pots with standard soil. Plants were grown in an air-conditioned greenhouse at 70% relative humidity, supplemented with additional light (model SON-T plus 400 W, Philips, Eindhoven, The Netherlands) providing a day length of 16 h light (long day), with light intensity 125 mmol m−2 s−1, and maintained at a temperature of 22–25 °C (day) and 18 °C (night). NILs were grown in a randomized complete block design with eight replicates. An experimental plot consisted of a row of 12 plants. At harvest the seeds of eight plants were bulked. Three of the eight replicates were used for the microarray analyses.
T-DNA knock-out lines: Lines were screened for homozygous insertions and grown with the wild type Columbia (Col) under greenhouse conditions using Rock wool supplemented with a Hyponex solution, in a randomized complete block design with four replicates per genotype.
Sample preparation for microarray analyses
Dormant seeds were imbibed for 24 h in continuous light at 22 °C and then stored at −80 °C until RNA isolation. After-ripened seeds were imbibed for 24 h under the same conditions as the dormant seeds as soon as the seeds reached 100% germination in the germination experiment, also these seeds were stored at −80 °C until RNA isolation.
Microarray analysis
Total RNA was prepared from 24-h imbibed seeds using RNAqueous columns with Plant RNA isolation aid (Ambion, Austin, TX, USA) according to the manufacturer’s protocol. The RNA was further purified through precipitations with isopropanol and a high salt solution containing 0.24 M sodium citrate and 0.16 M sodium chloride and subsequently with 2 M lithium chloride. RNA was qualitatively assessed and quantified using an Agilent 2100 Bioanalyzer with the RNA 6000 Nano Labchip® kit (Agilent, Santa Clara CA, USA) and Nanodrop1000™ spectrometry (NanoDrop Technologies, Inc., Wilmington, DE, USA). RNA was processed and cRNA synthesized according to the 3′ GeneChips OneCycle kit and hybridized on the ATH1 GeneChip (Affymetrix Inc., Santa Clara, CA, USA). The GeneChip data were analyzed using the R statistical programming environment and the Bioconductor packages [29, 51, 54]. The data was normalized using the RMA algorithm and a linear model was fitted to the data for comparisons of dormant to after-ripened seed within each genotype, the empirical Bayes method was used to reduce the gene wise sample variance [49]. The P values were then adjusted for multiple testing with the Benjamini and Hochberg method to control for false positives [5]. The microarray data were deposited in NCBI’s Gene Expression Omnibus (GEO number GSE90162). Microarray quality and reproducibility data is presented in Additional file 2: Figure S1. Dormancy up regulated genes (D-up) represent genes up-regulated (P > 0.0001) in the following comparisons, Ler dormant vs Ler after-ripened, NILDOG1 dormant vs NILDOG1 after-ripened, NILDOG2 dormant vs NILDOG2 after-ripened, NILDOG3 dormant vs NILDOG3 after-ripened, NILDOG6 dormant vs NILDOG6 after-ripened and vice versa After-ripening up regulated genes (AR-up) represent genes up-regulated (P > 0.0001) in the following comparisons, Ler after-ripened vs Ler dormant, NILDOG1 after-ripened vs NILDOG1 dormant, NILDOG2 after-ripened vs NILDOG2 dormant, NILDOG3 after-ripened vs NILDOG3 dormant, NILDOG6 after-ripened vs NILDOG6 dormant.
T-DNA knock-out genotype analyses
A quick isolation method modified from [13] was performed to extract genomic DNA from leaves. In short, samples were ground in an extraction buffer containing 2 M NaCl, 200 mM Tris–HCl (pH 8), 70 mM EDTA and 20 mM Na2S2O5. The grinding was conducted with a stainless steel ball at 30 Hz for 1 min (96-well plate shaker, Mo Bio Laboratory). Then samples were incubated at 65 °C for 1 h. Supernatants were collected after centrifugation at maximum speed for 10 min. DNA was precipitated by adding iso-propanol and 10 M NH4Ac with ratio of 1:1/2:1 to the supernatant. This mixture was incubated at room temperature for at least 15 min, then centrifuged for 20 min at maximum speed. The DNA pellet was retrieved and rinsed with 70% ethanol followed by centrifugation for 5 min at maximum speed to recover the pellet. After drying, the DNA pellet was dissolved in distilled water. Homozygous T-DNA insertion lines were screened with gene specific primers (left and right) and insert border primers (Additional file 1: Table S1). T-DNA plants that amplified only the insertion product were consider to be homozygous mutants.
Polymerase chain reactions (PCR) were performed in a 12.5 μL-volume containing approximately 30 ng DNA, 25 μM of each dNTP, 25 ng of forward and reverse primers, 0.05 U of DNA polymerase (Firepol, Solis BioDyne), 312.5 μM of MgCl2. The reaction protocol was as follows; denaturation at 95 °C for 5 min followed by 30s at 95 °C, 30s annealing at 52 to 57 °C and a 45 s to 2 min extension at 72 °C, this cycle was repeated for 35 times, and ended with a final amplification for 10 min at 72 °C. The polymorphism was detected by agarose gel electrophoresis at concentrations from 1.5% and higher (w/v) depending on size of differences.
Germination assays
Germination tests to follow the release of seed dormancy were performed as described by Alonso-Blanco et al. [2] with small adjustments. In short, at several time intervals during seed dry storage until all seed batches reached 100% germination aliquots of 50 to 100 seeds of each genotype were evenly sown on a filter paper soaked with 0.7 ml demineralized water in a 6-cm Petri dish. Petri dishes were placed in moisture chambers consisting of plastic trays containing a filter paper saturated with tap water and closed with transparent lids. Chambers were stored for 1 week in a climate chamber illuminated with 38-W Philips TL84 fluorescent tubes at 8 W m2 in continuous light at 22 °C. After that, the total number and the number of germinating seeds was scored and the percentage of germinating seeds was calculated.
Germination under stress conditions was performed on fully after-ripened seeds. Stress conditions were: osmotic stress (−1 MPa mannitol; Sigma-Aldrich), salt stress (130 mM NaCl; Sigma-Aldrich), ABA stress (0.15 μM ABA; Duchefa Biochemie). ABA was dissolved in 10 mM MES buffer (Sigma-Aldrich) and the pH adjusted to 5.8. To measure seed longevity, an accelerated aging test was performed by incubating seeds above a saturated ZnSO4 solution (40 °C, 85% relative humidity) in a closed tank for 5 days. Then the seeds were taken out and germinated on demineralized water as described before.
Results
Identification of seed dormancy and after-ripening up-regulated genes
Seeds of Ler, NILDOG1-Cvi, NILDOG2-Cvi, NILDOG3-Cvi and NILDOG6-Kas-2 were investigated for their dormancy status. After-ripening was followed by performing germination tests during a time course of dry seed storage (Fig. 1a). After 120 days all genotypes had lost dormancy and showed 100% germination. NILDOG2 was less dormant and NILDOG3, NILDOG6 and NILDOG1 were more dormant when compared to Ler. Both dormant and after-ripened seeds of each genotype were sampled 24 h after sowing (HAS) for microarray analysis, allowing the comparison of the dormant and after-ripened seed transcriptomes of these five genotypes with varying levels of primary dormancy.
Fig. 1.
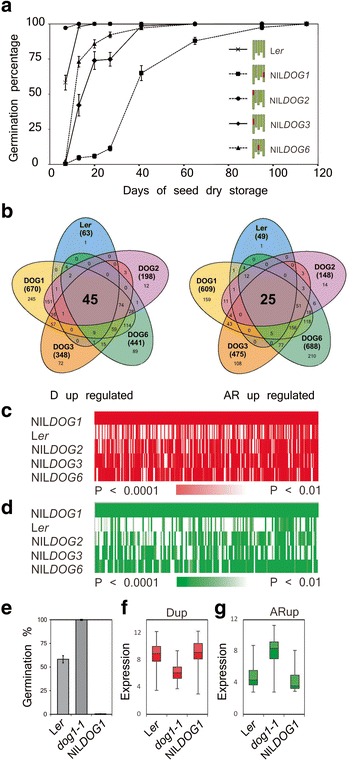
Microarray analysis of dormant and after-ripened seeds after 24 h of imbibition of five genotypes with differing dormancy levels; Ler, NILDOG1, NILDOG2, NILDOG3 and NILDOG6. a After-ripening requirement of the five genotypes. On the right graphical representations of the NILs are depicted showing the 5 chromosomes with the introgressed regions (in red) in an otherwise Ler background (in green). b Venn diagrams showing the number of genes that are differentially expressed (P < 0.0001) in dormant (D-up) and after ripened (AR-up) 24-h imbibed seeds of different genotypes. For each genotype the total number of differential expressed genes is indicated between brackets. In the intersection of all genotypes the number of genes that are investigated in this study are presented, 46 and 25 for the D-up and AR-up set, respectively. c Heat map consisting of 245 NILDOG1 D-up genes (P < 0.0001). The significance of these genes in the other genotypes is indicated, the white color indicates the genes that are not significantly different in the other genotypes (P < 0.01). d Heat map consisting of 159 NILDOG1 AR-up genes (P < 0.0001). The significance of these genes in the other genotypes is indicated, the white color represents the genes that are not significantly different in the other genotypes (P < 0.01). e Germination behaviour of freshly harvested seeds of Ler, dog1 and NILDOG1. f Box plot showing the expression of the 45 D-up genes in freshly harvested imbibed Ler, dog1 and NILDOG1 seeds (expression data taken from Dekkers et al. [16]). g Box plot showing the expression of the 25 D-up genes in freshly harvested imbibed Ler, dog1 and NILDOG1 seeds
The transcriptome data was investigated to identify genes that are up-regulated in 24 h imbibed dormant (D) Ler, NILDOG1, NILDOG2, NILDOG3 and NILDOG6 seeds and genes that are up-regulated in 24 h imbibed after-ripened (AR; non-dormant) seeds of the same lines. One thousand eight hundred ninety-six genes (P < 0.0001) were differentially expressed when performing within-genotype comparisons for the two stages analysed (dormant versus AR). In dormant seeds 63, 670, 198, 348, and 441 genes were up regulated and 49, 609, 148, 475 and 688 genes were up-regulated in AR seeds for Ler, NILDOG1, NILDOG2, NILDOG3 and NILDOG6, respectively (Fig. 1b).
A large proportion of the differentially expressed genes is specific for the genotypes analysed at P < 0.0001; however, these genes were differentially expressed in the other genotypes at lower significances. This has been visualised for the genes that are specific for NILDOG1 (Fig. 1 c,d). Most of the 245 NILDOG1 D-up and 159 NILDOG1 AR-up genes are differentially expressed (P < 0.01) in the other genotypes. This indicates that the genes that are specifically differentially expressed are based on quantitative expression differences rather than qualitative.
Genes that are important for dormancy and AR are expected to be differentially expressed between these stages in all genotypes tested (intersections in the Venn diagrams of Fig. 1b). This led to the identification of 45 up-regulated genes in all dormant genotypes (Dormancy-up; D-up; Table 1) and 25 genes that were up-regulated in all after-ripened genotypes (After-ripened–up; AR-up; Table 2). Further investigation of the expression patterns using the Seed EFP browser (http://www.bioinformatics.nl/efp/cgi-bin/efpWeb.cgi) revealed that, in general, all the genes that were up-regulated in dormant seeds at 24 HAI, were highly expressed in dry seeds, remained high during the imbibition of dormant seeds but were down regulated during the germination of AR seeds. Vice versa, genes that were up-regulated in AR seeds, had a low expression in dry seeds that increased with imbibition time (Additional file 2: Figure S2). Furthermore, the relation with dormancy becomes clear when the expression of the individual genes is investigated in imbibed seeds of Ler, dog1-1 and NILDOG1-Cvi that have very clear dormancy differences (Fig. 1e). D-up genes are highly expressed in dormant Ler and NILDOG1-Cvi seeds, whereas AR-up genes are highly expressed in the non-dormant dog1-1 mutant (Fig. 1 f,g).
Table 1.
Mutants isolated from D-up genes
| SALK/SAIL entry | AGI code | Knock out # | Encoded protein |
|---|---|---|---|
| SALK_073011C | AT2G29300 | KO 1 | NAD(P)-binding Rossmann-fold superfamily protein (RFSP?) |
| SALK_028749.55.25.x | AT2G31350 | KO 2 | Mitochondrial glyoxalase 2 (GLX2-5) |
| SALK_054451.53.45.x | AT2G33830 | KO 3 | Dormancy/auxin associated family protein(ATDRM2) |
| SALK_025507C | AT2G38800 | KO 4 | Plant calmodulin-binding protein-related (PCBP) |
| SALK_082639C | AT3G14880 | KO 5 | Transcription factor-related |
| SALK_150592C | AT5G01670 | KO 6 | NAD(P)-linked oxidoreductase superfamily protein |
| SALK_059351 | AT5G64210 | KO 7 | Alternative oxidase2 (AOX2) |
| SALK_104275C | AT1G01240 | KO 8 | Unknown protein |
| SALK_110011C | AT1G05840 | KO 9 | Eukaryotic aspartyl protease family protein |
| SALK_027164C | AT1G27990 | KO 10 | Unknown protein |
| SALK_036898C | AT2G19900 | KO 11 | The malic enzyme1(ATNADP-ME1) |
| SALK_037108.56.00.x | AT1G13640 | KO 12 | Phosphatidylinositol 3- and 4-kinase family protein |
| SALK_101144 | AT1G56600 | KO 13 | Galactinol synthase(GOLS2) |
| SALK_138905.29.65.x | AT2G27940 | KO 14 | RING/U-box superfamily protein |
| SALK_094895 | AT3G02990 | KO 15 | Member of Heat Stress Transcription Factor family (HSFA1E) |
| SALK_025488.38.10 | AT3G03310 | KO 16 | Lecithin:cholesterol acyltransferase 3 (LCAT3) |
| SALK_038352 | AT3G22490 | KO 17 | Seed maturation protein |
| SALK_082777C | AT3G53410 | KO 18 | Paralog of ubiquitin E3 ligase (LUL2) |
| SALK_090239C | AT3G62090 | KO 19 | Phytochrome-Interacting Factors (PIF6) |
| SAIL_512_E03 | AT4G19390 | KO 20 | Uncharacterised protein family |
| SALK_137617.43.90.x | AT5G02840 | KO 21 | LHY/CCA1-LIKE 1 (LCL1) |
| SALK_101433C | AT1G13340 | KO 22 | Regulator of Vps4 activity in the MVB pathway protein |
| SALK_025893C | AT1G20650 | KO 23 | Altered Seed Germination 5 (ASG5) |
| SALK_087702C | AT1G77450 | KO 24 | NAC domain-containing protein 32 (NAC032) |
| SALK_003223C | AT1G79440 | KO 25 | Succinate-semialdehyde dehydrogenase 1 (SSADH1) |
| SAIL_563_D10 | AT1G80090 | KO 26 | Cystathionine beta-synthase family protein (CBSX4) |
| SALK_078702 | AT3G50740 | KO 27 | UDP-glucosyl transferase 72E1 (UGT72E1) |
| SALK_116062C | AT3G53040 | KO 28 | Late embryogenesis abundant (LEA)protein |
| SALK_082087C | AT4G09600 | KO 29 | Gibberellin-regulated gene family(GASA3) |
| SALK_112631 | AT4G20070 | KO 30 | Allantoate Amidohydrolase (AtAAH) |
| SALK_105045 | AT4G25580 | KO 31 | CAP160 protein |
| SALK_043547C | AT4G36700 | KO 32 | RmlC-like cupins superfamily protein |
| SALK_135551C | AT5G65280 | KO 33 | GCR2-like 1 (GCL1) |
| SAIL_1256_F11 | AT5G58650 | KO 34 | Plant peptide containing sulfated tyrosine 1(PSY1) |
The table includes information about the affected genes (according to TAIR10a)
aTAIR database website: www.arabidopsis.org
Table 2.
Mutants isolated from AR-up genes
| SALK entry | AGI code | Knock out # | Encoded protein |
|---|---|---|---|
| SALK_043889 | AT4G34135 | KO 35 | UDP-Glucosyltransferase 73B2 (UGT73B2) |
| SALK_070860C | AT3G26060 | KO 36 | PEROXIREDOXIN Q (PRXQ) |
| SALK_094069C | AT3G26570 | KO 37 | Phosphate transporter 2;1 (PHT2;1) |
| SALK_091600.51.00.x | AT5G49910 | KO 38 | Chloroplast heat shock protein 70–2 (CPHSC70-2) |
| SALK_097487C | AT4G34131 | KO 39 | UDP-glucosyl transferase 73B3 (UGT73B3) |
| SALK_086616C | AT3G20210 | KO 40 | Delta vacuolar processing enzyme (DELTA-VPE) |
| SAIL_547_D05 | AT4G31330 | KO 41 | Protein of unknown function |
| SALK_007230.56.00.x | AT5G13400 | KO 42 | Peptide transporter 5 |
| SALK_017818.55.50.x | AT2G45180 | KO 43 | Lipid-transfer protein/seed storage 2S albumin superfamily protein |
| SALK_095678 | AT1G07890 | KO 44 | Ascorbate peroxidase 1 (APX1) |
| SALK_090550.52.85.x | AT1G47128 | KO 45 | Responsive to dehydration 21 (RD21) |
| SALK_015756 | AT3G45010 | KO 46 | Serine carboxypeptidase-like 48 (scpl48) |
| SALK_132995.40.05.x | AT4G34260 | KO 47 | Altered Xyloglucan 8 (AXY8) |
The table includes information about the affected gene (according to TAIR10a)a
TAIR database website: www.arabidopsis.org
Among the identified genes there were several that had been related to seed dormancy or germination before, including PHYTOCHROME-INTERACTING FACTOR 6 (PIF6, KO19, AT3G62090)) [47], GIBBERELLIN 3-OXIDASE 2 (GA3OX2) [59] and ALTERED SEED GERMINATION 5 (ASG5, KO23, AT1G20650) [4]. In addition, we found genes encoding for late embryogenesis abundant (LEA) proteins which are known to accumulate during seed desiccation and in response to water deficit induced by drought, low temperature, or salinity [30, 43]. The identified genes cover various GO molecular function categories among which by far the largest proportion is enzyme-related, including transferase activity, kinase activity and hydrolase activity, next to nucleotide binding proteins, including transcription factors.
Isolation of T-DNA mutants for genes involved in seed dormancy and germination
To investigate whether the identified genes indeed affect dormancy and AR we have analysed their T-DNA knock-out lines for seed performance phenotypes. For most of the identified genes, T-DNA mutants could be selected from the SALK and SAIL collections (NASC, http://arabidopsis.info/), but for eight genes no T-DNA insertion mutants were available (Additional file 1: Table S1). In all cases, homozygous lines were generated and confirmed using a PCR-based approach. For 47 genes a homozygous KO mutant could be selected. For nine genes (mostly in the AR-up set) no insertion was found in any of the plants genotyped (described as ‘all wild type’ in Additional file 1: Table S1). Moreover, for two genes FRUCTOSE-BISPHOSPHATE ALDOLASE (FBA2; AT4G38970) and DELTA-9 DESATURASE1 (ADS1 AT1G06080) T-DNA insertions were identified, but no homozygous mutants could be selected. Likely, the mutants homozygous for these genes are lethal; therefore siliques of these lines were dissected to investigate possible seed abortion. This confirmed that homozygous mutant seeds of these lines were aborted (around a quarter) at an early stage of seed development (Fig. 2). Complete genotyping information is given in Additional file 1: Table S1.
Fig. 2.
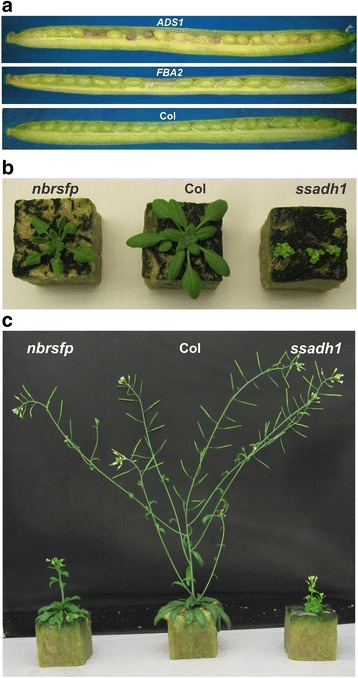
Plant phenotypes of T-DNA knock-out lines in comparison with wild type Columbia (Col). a Aborted seeds in siliques from heterozygous T-DNA lines with insertions in FBA2 (AT1G06080) and ADS1 (AT4G38970). b Four-week old plants of the NAD(P)-BINDING ROSSMANN-FOLD SUPER FAMILY PROTEIN (nbrsfp; KO1, AT2G29300) and SUCCINATE-SEMIALDEHYDE DEHYDROGENASE mutant (ssadh1, KO25, At1G79440) (c) nbrsfp, Col and ssadh1 6 weeks after germination
All the homozygous T-DNA lines were grown together with wild type Columbia (Col) for phenotypic analysis. This revealed normal plant phenotypes for most of the mutants; however, for the NAD(P)-BINDING ROSSMANN-FOLD SUPER FAMILY PROTEIN (NBRSFP; KO1, AT2G29300) and SUCCINATE-SEMIALDEHYDE DEHYDROGENASE (SSADH1, KO25, At1G79440) mutants the phenotype was dramatically altered (Fig. 2). After seed harvest seeds were tested for their seed performance phenotype.
Altered seed dormancy for knock-out mutants in the dormancy and after-ripened up gene sets
Initially only seed dormancy levels were examined by assessing the number of days of seed dry storage that were required to reach 50% of germination (Days of Seed Dry Storage to reach 50% of germination; DSDS50); Fig. 3a). Thereafter, the fully after-ripened seeds were tested for seed longevity (Fig. 3b) and germination in salt (Fig. 3c), mannitol (Fig. 3d) and ABA (Fig. 3e). Thirteen lines showed a dormancy level (DSDS50) that was significantly distinct from the wild type, of which seven were less dormant (KO11, 14, 16, 17, 20, 36, 41 and 43) and six were more dormant (KO5, 16, 19, 23, 25 and 30) than the wild type. Several of the mutants were specifically affected in their seed dormancy levels, so no other seed performance phenotypes were detected. Among these mutants were a transcription factor related gene which is known to respond to karrikin (KO5, At3G14880), LECITHIN:CHOLESTEROL ACYLTRANSFERASE 3 (LCAT3; KO16, At3G03310) which is involved in lipid metabolism, a seed maturation protein (KO17; AT3G22490), SSADH1, PIF6, the antioxidant gene PEROXIREDOXIN Q (PRXQ; KO36, AT3G26060), and a SEED STORAGE 2S ALBUMIN SUPER FAMILY MEMBER (KO43, AT2G455180). Pif6 displayed a more than two times higher DSDS50 (27.7 days) than the wild type (11.67). PIF6 has been previously found to negatively regulate seed dormancy [47]. The other mutants were affected for at least one other seed performance trait, as well. Lines with mutations in MALATE ENZYME 1 (ME1, KO11, At2G19900), ASG5 and an unknown protein (PUF, KO41, At4G31330) displayed both a dormancy and a seed longevity phenotype. Interestingly, atnadp-me1 and puf had reduced dormancy and longevity and puf was also less sensitive to salt stress. Asg5 showed increased dormancy and longevity. A KO in ALLANTOATE AMIDOHYDROLASE (AAH; KO30, AT4G20070) displayed a phenotype for all investigated seeds traits except for seed longevity. This gene encodes an enzyme that hydrolyses ureide allantoate to ureidoglycolate, CO2, and two molecules of ammonium. The aah mutant was more dormant, and more sensitive to salt and mannitol, but less sensitive to ABA. A KO in a U-BOX SUPER FAMILY PROTEIN (KO14, At2G27940) appeared slightly less dormant than wild type but was far more sensitive to ABA. A KO in the UNCHARACTERISED PROTEIN FAMILY gene (UPF; At4G19390, KO20) showed a very strong non-dormant phenotype, and was rather insensitive to mannitol and salt.
Fig. 3.
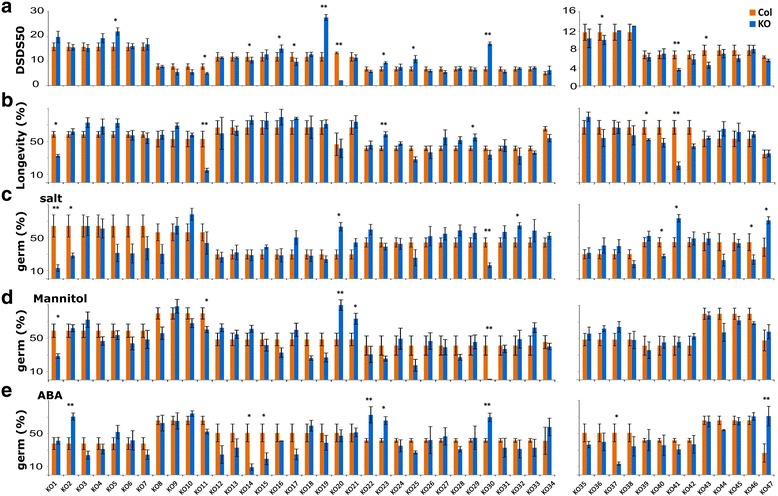
Germination behaviour of knock-out mutants (KO) in dormancy (left) and after –ripened (right) upregulated genes: (a) Average DSDS50 (Days of Seed Dry Storage until 50% germination) values. b germination after accelerated aging. c germination in salt 130 mM; d) in mannitol (−1 MPa) and e) in ABA(0.15 μM) solutions. Significant differences are indicated (*P < 0.05 and **P < 0.01). There are differences in Col-0 values between the different experiments, however, every knock-out line has been compared to the Col-0 that was grown in the same experiment
Other seed performance phenotypes for D-up and AR-up genes
Among the selected mutants were also genotypes that were not affected in their seed dormancy levels but displayed altered phenotypes for other seed performance traits.
Mutants with altered seed longevity phenotype
The nbrsfp mutant (KO1, AT2G29300) is besides its reduced seed longevity, also more sensitive to germination in salt and mannitol. Lines mutated in GIBBERELLIN-REGULATED GENE FAMILY (GASA3, AT4G09600, KO29) and UDP-GLUCOSYL TRANSFERASE 73B3 (UGT73B3, AT4G34131, KO39) showed a longevity phenotype. A role for these genes in seed longevity has not been reported previously.
Mutants with altered response to NaCl and/or osmotic stress
Lines mutated in mitochondrial GLYOXALASE 2 (GLX2; KO2, AT2G31350), DELTA VACUOLAR PROCESSING ENZYME (DELTA-VPE, KO40, At3G20210) and SERINE CARBOXYPEPTIDASE-LIKE 48 (SCPL48,KO46, AT3G45010) showed reduced germination in salt but tolerated low osmotic potentials caused by high concentrations of mannitol. Although more sensitive to salt, glx2 was more resistant to ABA than wild type. The KO in MYB TRANSCRIPTION FACTOR LHY-CCA1-LIKE1 (LCL1, KO21, AT5G02840) was more resistant to germination in mannitol compared to wild type. A similar trend was seen after germination in salt but this effect was not significant. Lines mutated in CUPINS SUPER FAMILY PROTEIN (KO32, AT4G36700) and ALTERED XYLOGLUCAN 8 (AXY8; KO47, AT4G34260) showed a salt resistance phenotype but their germination in mannitol was similar to wild type. Besides lower sensitivity to salt, line axy8 also showed reduced sensitivity to ABA.
Responses of dormancy related genes to ABA
Several lines that showed an altered response to ABA have already been mentioned above because they had at least also one other phenotype. However, there are three lines that showed a phenotype only for germination in the presence of ABA. Two lines, KOs in HEAT SHOCK TRANSCRIPTION FACTOR A1E (HSFA1E; KO15, AT3G02990) and LOW AFFINITY PHOSPHATE TRANSPORTER (PHT2;1, KO37,AT3G26570) were more sensitive, whereas the line mutated in REGULATOR OF VPS4 ACTIVITY in the MVB pathway protein (KO22, AT1G13340) was more resistant to ABA.
Discussion
In our search for novel players in the regulation of Arabidopsis seed dormancy we employed a comparative transcriptomics approach for 24 h imbibed dormant and after-ripened DOGNILs and Ler seeds. The same genotypes were earlier used to investigate the transcriptome of dormant dry seeds [6], which revealed that seed dormancy in the DOGNILs is mainly controlled by different additive genetic and molecular pathways. In dry seeds hardly any differences are found between dormant and after-ripened seeds, however as soon as seeds are being exposed to water, differences in the transcriptomes are evident. Based on these results we hypothesize that dormancy induction in the DOGNILs during seed maturation, for which dry seeds are the readout, is largely regulated by distinct molecular pathways, however dormancy maintenance during seed imbibition and the start of germination are likely very conserved processes. This conservation allowed us to identify a robust set of genes which are expressed at 24 h imbibed dormant and AR seeds. The genes identified depend a lot on the time-points chosen. From our earlier work we know that already at early imbibition (3 h after the start of imbibition) the first differences between dormant and after-ripened seeds can be identified [17]. However, we also know that most of changes in gene expression are related to seed rehydration itself and that those changes are similar between dormant and after-ripened seeds. To exclusively identify differences that are related to dormancy maintenance and germination we have chosen to investigate the transcriptome at 24 h after imbibition. This robustness of the identified genes was confirmed by comparison with previously published expression analysis that were performed with seeds of the Cvi accession at a range of physiological states [10, 17]. Of the dormancy and AR-up genes 63% and 32%, respectively, overlapped with genes that were identified by Cadman et al. [10] (Additional file 1: Table S1). Moreover, the D-up genes are also clearly higher expressed in dormant Cvi seeds as compared to AR Cvi seeds, as well as that the AR-up genes are on average higher expressed in AR seeds when compared to dormant seeds (Additional file 2: Figure S2). Some of the identified genes had previously been shown to play a role during germination and/or priming in several plant species. Among these are GA3OX2 (AT1G80340) a key gene in the gibberellin biosynthesis pathway, PIF6, involved in the phytochrome signalling pathway and ASG5 which is involved in protein and amino acid phosphorylation. The identification of these known dormancy mutants was the incentive to investigate the other dormancy and after-ripening specific genes. We took a reverse genetics approach by using T-DNA insertion lines for the differentially expressed genes and, indeed, we identified genes that had not been related to seed dormancy or germination before. Out of our target list of 66 genes, eight do not currently have any confirmed knock-out line available. This is consistent with a recent report that 12% of Arabidopsis genes do not have insertion lines available in previously generated collections [44]. The fact that a majority of the mutants showed near-wild type dormancy phenotypes, can be explained in several ways. The location of the T-DNA insertion may be decisive, e.g. whether in an intron, an exon or in untranscribed regions, such as promoters. Also, T-DNA–induced mutations do not always result in highly effective mutagenesis. Insertion in the protein-coding region of a gene generates a knockout in 86% of the cases and only 41% of the cases if the insertion is in front of the start codon [56]. Furthermore, gene redundancy may mask any phenotypic difference in plants in which the expression of only one homologue is disrupted [28]. In addition, in our experiments we used mutants with a Columbia-0 background that normally has low dormancy, which consequently does not allow the visualisation of small effects towards a decreasing dormancy level. Seed dormancy can be regulated by either inhibitory or promoting gene expression, considering the fact that in D-up genes we found both mutants that are less dormant (Fig. 3; KO11, 14 and 17) and more dormant (KO5, 16, 19, 23, 25 and 30). These examples demonstrate the inability to predict phenotypes based on expression pattern alone.
Many seed performance characteristics (i.e. seed desiccation tolerance, seed longevity and seed dormancy) are acquired during seed maturation. If genes affect seed maturation in general, it is likely that pleiotropic effects occur. In our study, some of the mutants showed a phenotype for more than one germination trait. The aah mutant for example displayed a phenotype for all investigated seed traits, except longevity. This enzyme degrades allantoate which is required to recycle purine-ring nitrogen in plants. The aah T-DNA mutant is unable to grow on allantoin as sole nitrogen source [57]. Furthermore, it is well known that conditions favouring nitrate accumulation in mother plant may lead to lower seed dormancy levels [1]. Since AAH is a key gene in the purine pathway [57], we speculate that defects in this gene block the pathway and, hence, availability of ammonium, resulting in increased primary dormancy and also affecting other seed performance traits of the mutant. Atnadh-me1, asg5 and ufp mutants affected both dormancy and longevity and one additional trait. It is known that seed longevity can be a pleotropic effect of genes that regulate other traits, such as seed maturation [53], response to temperature [37], oxidative stress [14] and dormancy [7, 39]. Previous studies, using mutant analysis, have shown that the seed dormancy mutants dog1 and rdo4 also have a reduced seed longevity phenotype [7, 39].
Loss of dormancy is expressed as opening of the germination window (permissive range of environments) [19]. It is because of this that germination under stress (i.e. salt or osmotic stress) often correlates with initial seed dormancy levels. We revealed two cases for which reduced dormancy indeed coincided with reduced sensitivity to salt stress (upf (KO20) and puf (KO41)). For some mutants, nbrsfp (KO1), upf (KO20) and aah (KO30), germination patterns on both NaCl and mannitol correlated positively, probably because both treatments confer osmotic stress. Two of these mutants (nbrsfp and aah) displayed enhanced sensitivity to salinity and osmotic stress. The NAD(P)-binding Rossmann-fold superfamily protein has oxidoreductase activity, binding, catalytic activity and, based on TAIR annotation, it is located in the endomembrane system. Upf was the only mutant to be clearly more insensitive to both salt and osmotic stress, which indicates that this mutant is primarily osmotolerant. Furthermore, for some of the salt-tolerant mutants RmlC-like cupins super family protein (KO32), unknown protein KO41 and axy8 (KO47) the germination rates on mannitol were similar to that of wild type. Likely, for the salt-tolerant lines, genes were mutated whose products are elements of stress signalling and inhibit germination under conditions of saline stress. The salt sensitive glx2 mutant was more tolerant to the application of ABA. The glyoxalase pathway consists of the two enzymes GLX1 and GLX2 and has a vital role in chemical detoxification. In Arabidopsis thaliana, GLX2 is required during abiotic stress, as was concluded from the higher sensitive of glx2-1to salt stress and anoxia seeds compared to wild type seeds. Moreover, GLX2-1-OE seeds are more resistant to anoxic stress than wild type [18].
Interestingly, axy8 (KO47) showed both a higher tolerance to ABA and to salt. AXY8 encodes an α-fucosidase acting on hemicellulose xyloglucan (XyG) that occurs in the primary cell wall of all vascular plants. Due to its high levels in elongating tissues [11] and structural alterations during cell elongation [46], XyG has been proposed to be a major player in extension growth [15]. This was confirmed by the induction of genes involved in XyG metabolism during cell elongation and upon the addition of the growth hormone auxin [50]. Overall these findings emphasize the importance of cell wall remodelling in the germination process, especially in response to stress conditions.
In both the dormancy and after-ripened sets we identified many genes encoding enzymes. This result might be linked to the fact that we looked at 24-h imbibed seeds. At this stage most of the cells in the embryo are potentially metabolically active. This also activates hydrolytic and synthetic enzymes and growth hormones to mobilize nutrients and synthesize ingredients for growth. These include the genes encoding ABA- and GA-biosynthesis- and -deactivation enzymes that play critical roles in determining the ABA-GA balance in seeds, and hence, dormancy and germination [42, 58].
Among the identified genes are also nucleotide binding proteins and transcription factors, such as members of the heat stress transcription factor family (HSFA1E, KO15), members of the ERF transcription factor family, transcription factor-related protein (KO5), TRANSCRIPTION FACTOR HOMOLOGOUS TO ABI5 and PIF6 (KO19). Interestingly all are found in the D-up state and for the ones that mutants were analysed they showed either more dormancy (transcription factor-related protein and pif6) or more sensitivity to ABA (hsfa1a).
Conclusion
We identified seed dormancy and germination phenotypes for genes that had not been associated with seed dormancy before. We tested only one T-DNA allele per line which may not be a definitive prove that the insertional mutation causes the observed phenotype, as many as 50% of the lines may contain additional inserts at unknown loci [22, 44]. We identified germination related phenotypes for nearly 50% of the investigated genes, which is far higher compared to what can be expected from a random selection of genes. It is also far higher compared to the genotypes that we identified for genes identified in earlier transcriptome analyses. Nevertheless, it is possible that a second locus may cause the phenotype of interest, or may alter the phenotypic effect of a knockout mutation. This work therefor represented an inventory of genes that are likely involved in the control of seed dormancy or germination. However, in depth studies are required to reveal the molecular mechanism by which these genes affect these important seed traits.
Additional files
Microarray quality and reproducibility. All 28 ATH1 arrays used showed after hybridization similar patterns of intensity (A and B). Slide hybridization patterns were inspected manually without detecting artefacts. The RNAs used as templates for cRNA synthesis were shown to be intact based on Bio-analyzer 2001 analysis of both RNA template and biotinylated cRNA. In agreement with this were the hybridization patterns of control genes on the slide showing a near-identical pattern of hybridization (c). The uniformity of normalized unscaled SE (NUSE) and relative log expression (RLE) indicate high quality and uniformity of the hybridization data (d and e) (1). Raw intensity data were subjected to RMA normalization (2), which kept the uniformity of general levels between the different slides (f and g). Between replicate reproducibility of the experiment was high, exemplified by the high correlation between the data of two biological replicates (h). Array 1 and array 2 are hybridized with cRNA from different replicates of Ler seeds. Figure S2. Spatial and temporal expression patterns of the selected dormancy and after-ripening up-regulated genes. Mean relative expression of (a) D-up and (b) AR-up genes across the Arabidopsis germination time course in the micropylar and chalazal endosperm (MCE) and radicle and hypocotyl (RAD) in dry, 1, 3, 7, 12, 16, 20, 25, 31 and 38 hours after imbibition. Data was taken from Seed EFP Browser (http://www.bioinformatics.nl/efp/cgi-bin/efpWeb.cgi). Figure S3. Log2 expression differences for the D-up and AR-up genes that are presented in Fig. 1c and d. (a) Heat map showing Log2 expression differences of the 245 NILDOG1 D-up genes (P < 0.0001) in NILDOG1 and the other genotypes. (b) Log2 expression differences of the 159 NILDOG1 AR-up genes (P < 0.0001) in NILDOG1 and the other genotypes. (XLS 81 kb)
T-DNA selection of the 46 D-up and 25 AR-up genes. Details like, T-DNA identification, genotype, primers used for genotyping, knock-out # in the analyses, where the T-DNA is inserted and whether the genes overlap with the study of Cadman et al. [10] are indicated. (DOCX 803 kb)
Acknowledgements
Not applicable
Funding
This work was supported by the Dutch Technology Foundation (STW), which is the applied science division of The Netherlands Organization for Scientific Research and the Technology Program of the Ministry of Economic Affairs (to LB) and Bio4Energy, a Strategic Research Environment appointed by the Swedish government (to JH). The funding agencies were not involved in the data collection, analysis, and interpretation neither in writing the manuscript.
Availability of data and materials
The microarray datasets generated during the current study are available in deposited in NCBI’s Gene Expression Omnibus (GEO number GSE90162). All other datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AAH
ALLANTOATE AMIDOHYDROLASE
- ABA
Abscisic acid
- ADS1
DELTA-9 DESATURASE1
- AR
After-ripened
- AR-up
After-ripening up-regulated genes
- ASG5
ALTERED SEED GERMINATION 5
- AXY8
ALTERED XYLOGLUCAN 8
- Col
Columbia
- DELTA-VPE
DELTA VACUOLAR PROCESSING ENZYME
- DOG
DELAY OF GERMINATION
- DOGNILs
DELAY OF GERMINATION near isogenic lines
- DSDS50
Days of seed dry storage to reach 50% of germination
- D-up
Dormancy up-regulated genes
- FBA2
FRUCTOSE-BISPHOSPHATE ALDOLASE
- GA3OX2
GIBBERELLIN 3-OXIDASE 2
- GAs
Gibberellins
- GASA3
GIBBERELLIN-REGULATED GENE FAMILY
- GLX2
GLYOXALASE 2
- HAS
Hours after sowing
- HSFA1E
HEAT SHOCK TRANSCRIPTION FACTOR A1E
- KO
Knock-out
- LCAT3
LECITHIN:CHOLESTEROL ACYLTRANSFERASE 3
- LCL1
MYB TRANSCRIPTION FACTOR LHY-CCA1-LIKE1
- LEA
Late embryogenesis abundant
- Ler
Landsberg erecta
- ME1
MALATE ENZYME
- NBRSFP
NAD(P)-BINDING ROSSMANN-FOLD SUPER FAMILY PROTEIN
- PHT2;1
LOW AFFINITY PHOSPHATE TRANSPORTER
- PIF6
PHYTOCHROME-INTERACTING FACTOR 6
- PRXQ
PEROXIREDOXIN Q
- QTL
Quantitative trait loci
- RIL
Recombinant inbred line
- SCPL48
SERINE CARBOXYPEPTIDASE-LIKE 48
- SSADH1
SUCCINATE-SEMIALDEHYDE DEHYDROGENASE
- UGT73B3
UDP-GLUCOSYL TRANSFERASE 73B3
- UPF
UNCHARACTERISED PROTEIN FAMILY
Authors’ contributions
LB and JH designed, performed and analysed the transcriptome analyses. FY analysed the T-DNA KO lines for their germination performance. FY, HH and LB wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12870-017-1098-z) contains supplementary material, which is available to authorized users.
Contributor Information
Farzaneh Yazdanpanah, Email: farzanehyazdanpanah@gmail.com.
Johannes Hanson, Email: johannes.hanson@umu.se.
Henk W.M. Hilhorst, Email: henk.hilhorst@wur.nl
Leónie Bentsink, Phone: +31 317 48 1325, Email: leonie.bentsink@wur.nl.
References
- 1.Alboresi A, Gestin C, Leydecker MT, Bedu M, Meyer C, Truong HN. Nitrate, a signal relieving seed dormancy in Arabidopsis. Plant Cell Environ. 2005;28:500–512. doi: 10.1111/j.1365-3040.2005.01292.x. [DOI] [PubMed] [Google Scholar]
- 2.Alonso-Blanco C, Aarts MGM, Bentsink L, Keurentjes JJB, Reymond M, Vreugdenhil D, Koornneef M. What has natural variation taught us about plant development, physiology, and adaptation? Plant Cell. 2009;21:1877–1896. doi: 10.1105/tpc.109.068114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai B, Novák O, Ljung K, Hanson J, Bentsink L. Combined transcriptome and translatome analyses reveal a role for transcriptional inhibition of tryptophan dependent auxin biosynthesis in the control of DOG1 dependent seed dormancy. 2017. [DOI] [PubMed] [Google Scholar]
- 4.Bassel GW, Glaab E, Marquez J, Holdsworth MJ, Bacardit J. Functional network construction in Arabidopsis using rule-based machine learning on large-scale data sets. Plant Cell. 2011;23:3101–3116. doi: 10.1105/tpc.111.088153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc B Mec. 1995;57:289–300.
- 6.Bentsink L, Hanson J, Hanhart CJ, Blankestijn-de Vries H, Coltrane C, Keizer P, El-Lithy M, Alonso-Blanco C, de Andrés MT, Reymond M, van Eeuwijk F, Smeekens S, Koornneef M. Natural variation for seed dormancy in Arabidopsis is regulated by additive genetic and molecular pathways. Proc Natl Acad Sci U S A. 2010;107:4264–4269. doi: 10.1073/pnas.1000410107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bentsink L, Jowett J, Hanhart CJ, Koornneef M. Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proc Natl Acad Sci U S A. 2006;103:17042–17047. doi: 10.1073/pnas.0607877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bewley JD, Bradford K, Hilhorst H. Seeds: physiology of development, germination and dormancy. New York: Springer; 2012.
- 10.Cadman CS, Toorop PE, Hilhorst HW, Finch-Savage WE. Gene expression profiles of Arabidopsis Cvi seeds during dormancy cycling indicate a common underlying dormancy control mechanism. Plant J. 2006;46:805–822. doi: 10.1111/j.1365-313X.2006.02738.x. [DOI] [PubMed] [Google Scholar]
- 11.Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313X.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 12.Carrera E, Holman T, Medhurst A, Dietrich D, Footitt S, Theodoulou FL, Holdsworth MJ. Seed after-ripening is a discrete developmental pathway associated with specific gene networks in Arabidopsis. Plant J. 2008;53:214–224. doi: 10.1111/j.1365-313X.2007.03331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung W, Hubert N, Landry B. A simple and rapid DNA microextraction method for plant, animal, and insect suitable for RAPD and other PCR analyses. Genome Res. 1993;3:69–70. doi: 10.1101/gr.3.1.69. [DOI] [PubMed] [Google Scholar]
- 14.Clerkx EJ, Vries BD, Ruys GJ, Groot SP, Koornneef M. Genetic differences in seed longevity of various Arabidopsis mutants. Physiol Plant. 2004;121:448–461. doi: 10.1111/j.0031-9317.2004.00339.x. [DOI] [Google Scholar]
- 15.Cosgrove DJ. Enzymes and other agents that enhance cell wall extensibility. Annu Rev Plant Biol. 1999;50:391–417. doi: 10.1146/annurev.arplant.50.1.391. [DOI] [PubMed] [Google Scholar]
- 16.Dekkers BJW, Pearce SP, van Bolderen-Veldkamp RPM, Holdsworth MJ, Bentsink L. Dormant and after-ripened Arabidopsis thaliana seeds are distinguished by early transcriptional differences in the imbibed state. Front Plant Sci. 2016;7:1323–1338. doi: 10.3389/fpls.2016.01323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dekkers BJ, He H, Hanson J, Willems LA, Jamar DC, Cueff G, Rajjou L, Hilhorst HW, Bentsink L. The Arabidopsis DELAY OF GERMINATION 1 gene affects ABSCISIC ACID INSENSITIVE 5 (ABI5) expression and genetically interacts with ABI3 during Arabidopsis seed development. Plant J. 2016;85:451–465. doi: 10.1111/tpj.13118. [DOI] [PubMed] [Google Scholar]
- 18.Devanathan S, Erban A, Perez-Torres R, Jr, Kopka J, Makaroff CA. (2014) Arabidopsis thaliana Glyoxalase 2–1 is required during abiotic stress but is not essential under normal plant growth. PLoS One. 2014;9:e95971. doi: 10.1371/journal.pone.0095971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytol. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 20.Fu Q, Wang B, Jin X, Li H, Han P, Wei K-H, Zhang X-M, Zhu Y. Proteomic analysis and extensive protein identification from dry, germinating Arabidopsis seeds and young seedlings. J Biochem Mol Biol. 2005;38:650. doi: 10.5483/bmbrep.2005.38.6.650. [DOI] [PubMed] [Google Scholar]
- 21.Gallardo K, Job C, Groot SPC, Puype M, Demol H, Vandekerckhove J, Job D. Proteomic analysis of arabidopsis seed germination and priming. Plant Physiol. 2001;126:835–848. doi: 10.1104/pp.126.2.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gase K, Weinhold A, Bozorov T, Schuck S, Baldwin IT. Efficient screening of transgenic plant lines for ecological research. Mol Ecol Resour. 2011;11:890–902. doi: 10.1111/j.1755-0998.2011.03017.x. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths J, Murase K, Rieu I, Zentella R, Zhang ZL, Powers SJ, Gong F, Phillips AL, Hedden P, Sun TP, Thomas SG. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell. 2006;18:3399–3414. doi: 10.1105/tpc.106.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gubler F, Millar AA, Jacobsen JV. Dormancy release, ABA and pre-harvest sprouting. Curr Opin Plant Biol. 2005;8:183–187. doi: 10.1016/j.pbi.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Holdsworth MJ, Bentsink L, Soppe WJJ. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008;179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 26.Holdsworth MJ, Finch-Savage WE, Grappin P, Job D. Post-genomics dissection of seed dormancy and germination. Trends Plant Sci. 2008;13:7–13. doi: 10.1016/j.tplants.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Howell KA, Narsai R, Carroll A, Ivanova A, Lohse M, Usadel B, Millar AH, Whelan J. Mapping metabolic and transcript temporal switches during germination in rice highlights specific transcription factors and the role of RNA instability in the germination process. Plant Physiol. 2009;149:961–980. doi: 10.1104/pp.108.129874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hua J, Meyerowitz EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell. 1998;94:261–271. doi: 10.1016/S0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- 29.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 30.Ismail AM, Hall AE, Close TJ. Allelic variation of a dehydrin gene cosegregates with chilling tolerance during seedling emergence. Proc Natl Acad Sci U S A. 1999;96:13566–13570. doi: 10.1073/pnas.96.23.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iuchi S, Suzuki H, Kim YC, Iuchi A, Kuromori T, Ueguchi-Tanaka M, Asami T, Yamaguchi I, Matsuoka M, Kobayashi M, Nakajima M. Multiple loss-of-function of Arabidopsis gibberellin receptor AtGID1s completely shuts down a gibberellin signal. Plant J. 2007;50:958–966. doi: 10.1111/j.1365-313X.2007.03098.x. [DOI] [PubMed] [Google Scholar]
- 32.Joosen RV, Arends D, Li Y, Willems LA, Keurentjes JJ, Ligterink W, Jansen RC, Hilhorst HW. Identifying genotype-by-environment interactions in the metabolism of germinating arabidopsis seeds using generalized genetical genomics. Plant Physiol. 2013;162:553–566. doi: 10.1104/pp.113.216176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koornneef M, Van der Veen JH. Induction and analysis of gibberellin-sensitive mutants in Arabidopsis thaliana (L.) Heynh. Theor Appl Genet. 1980;58:257–263. doi: 10.1007/BF00265176. [DOI] [PubMed] [Google Scholar]
- 34.Koornneef M, Jorna M, Brinkhorst-Van der Swan D, Karssen C. The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) Heynh. Theor Appl Genet. 1982;61:385–393. doi: 10.1007/BF00272861. [DOI] [PubMed] [Google Scholar]
- 35.Koornneef M, Reuling G, Karssen C. The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant. 1984;61:377–383. doi: 10.1111/j.1399-3054.1984.tb06343.x. [DOI] [Google Scholar]
- 36.Le BH, Cheng C, Bui AQ, Wagmaister JA, Henry KF, Pelletier J, Kwong L, Belmonte M, Kirkbride R, Horvath S. Global analysis of gene activity during Arabidopsis seed development and identification of seed-specific transcription factors. Proc Natl Acad Sci U S A. 2010;107:8063–8070. doi: 10.1073/pnas.1003530107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee B-h, Lee H, Xiong L, Zhu J-K. A mitochondrial complex I defect impairs cold-regulated nuclear gene expression. Plant Cell. 2002;14:1235–1251. doi: 10.1105/tpc.010433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Zhang H, Zhao Y, Feng Z, Li Q, Yang H-Q, Luan S, Li J, He Z-H. (2013) Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc Natl Acad Sci U S A. 2013;110:15485–15490. doi: 10.1073/pnas.1304651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Koornneef M, Soppe WJ. (2007) The absence of histone H2B monoubiquitination in the Arabidopsis hub1 (rdo4) mutant reveals a role for chromatin remodeling in seed dormancy. Plant Cell. 2007;19:433–444. doi: 10.1105/tpc.106.049221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matakiadis T, Albores A, Jikumaru Y, Tatematsu K, Pichon O, Renou J-P, Kamiya Y, Nambara E, Truong H-N. The Arabidopsis abscisic acid catabolic gene CYP707A2 plays a key role in nitrate control of seed dormancy. Plant Physiol. 2009;149:949–960. doi: 10.1104/pp.108.126938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakabayashi K, Okamoto M, Koshiba T, Kamiya Y, Nambara E. Genome-wide profiling of stored mRNA in Arabidopsis Thaliana seed germination: epigenetic and genetic regulation of transcription in seed. Plant J. 2005;41:697–709. doi: 10.1111/j.1365-313X.2005.02337.x. [DOI] [PubMed] [Google Scholar]
- 42.Nambara E, Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- 43.Nylander M, Svensson J, Palva ET, Welin BV. (2001) Stress-induced accumulation and tissue-specific localization of dehydrins in Arabidopsis thaliana. Plant Mol Biol. 2001;45:263–279. doi: 10.1023/A:1006469128280. [DOI] [PubMed] [Google Scholar]
- 44.O’Malley RC, Ecker JR. Linking genotype to phenotype using the Arabidopsis unimutant collection. Plant J. 2010;61:928–940. doi: 10.1111/j.1365-313X.2010.04119.x. [DOI] [PubMed] [Google Scholar]
- 45.Oh E, Kang H, Yamaguchi S, Park J, Lee D, Kamiya Y, Choi G. Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell. 2009;21:403–419. doi: 10.1105/tpc.108.064691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pauly M, Qin Q, Greene H, Albersheim P, Darvill A, York WS. Changes in the structure of xyloglucan during cell elongation. Planta. 2001;212:842–850. doi: 10.1007/s004250000448. [DOI] [PubMed] [Google Scholar]
- 47.Penfield S, Josse E-M, Halliday KJ. A role for an alternative splice variant of PIF6 in the control of Arabidopsis primary seed dormancy. Plant Mol Biol. 2010;73:89–95. doi: 10.1007/s11103-009-9571-1. [DOI] [PubMed] [Google Scholar]
- 48.Preston J, Tatematsu K, Kanno Y, Hobo T, Kimura M, Jikumaru Y, Yano R, Kamiya Y, Nambara E. (2009) Temporal expression patterns of hormone metabolism genes during imbibition of Arabidopsis thaliana seeds: a comparative study on dormant and non-dormant accessions. Plant Cell Physiol. 2009;50:1786–1800. doi: 10.1093/pcp/pcp121. [DOI] [PubMed] [Google Scholar]
- 49.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sánchez M, Gianzo C, Sampedro J, Revilla G, Zarra I. Changes in α-Xylosidase during intact and Auxin-induced growth of pine hypocotyls. Plant Cell Physiol. 2003;44:132–138. doi: 10.1093/pcp/pcg016. [DOI] [PubMed] [Google Scholar]
- 51.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol. 2004;3:Article 3. [DOI] [PubMed]
- 52.Sreenivasulu N, Usadel B, Winter A, Radchuk V, Scholz U, Stein N, Weschke W, Strickert M, Close TJ, Stitt M. Barley grain maturation and germination: metabolic pathway and regulatory network commonalities and differences highlighted by new MapMan/PageMan profiling tools. Plant Physiol. 2008;146:1738–1758. doi: 10.1104/pp.107.111781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugliani M, Brambilla V, Clerkx EJ, Koornneef M, Soppe WJ. The conserved splicing factor SUA controls alternative splicing of the developmental regulator ABI3 in Arabidopsis. Plant Cell. 2010;22:1936–1946. doi: 10.1105/tpc.110.074674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Team, RC R . A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 55.Toh S, Imamura A, Watanabe A, Nakabayashi K, Okamoto M, Jikumaru Y, Hanada A, Aso Y, Ishiyama K, Tamura N. High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol. 2008;146:1368–1385. doi: 10.1104/pp.107.113738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang YH. How effective is T-DNA insertional mutagenesis in Arabidopsis? J Biochem Technol. 2008;1:11–20. [Google Scholar]
- 57.Werner AK, Sparkes IA, Romeis T, Witte C-P. Identification, biochemical characterization, and subcellular localization of allantoate amidohydrolases from Arabidopsis and soybean. Plant Physiol. 2008;46:418–430. doi: 10.1104/pp.107.110809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamaguchi S. Gibberellin metabolism and its regulation. Annu Rev Plant Biol. 2008;59:225–251. doi: 10.1146/annurev.arplant.59.032607.092804. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi S, Smith MW, Brown RG, Kamiya Y, Sun T-P. Phytochrome regulation and differential expression of gibberellin 3β-hydroxylase genes in germinating Arabidopsis seeds. Plant Cell. 1998;10:2115–2126. doi: 10.1105/tpc.10.12.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamauchi Y, Ogawa M, Kuwahara A, Hanada A, Kamiya Y, Yamaguchi S. Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell. 2004;16:367–378. doi: 10.1105/tpc.018143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Microarray quality and reproducibility. All 28 ATH1 arrays used showed after hybridization similar patterns of intensity (A and B). Slide hybridization patterns were inspected manually without detecting artefacts. The RNAs used as templates for cRNA synthesis were shown to be intact based on Bio-analyzer 2001 analysis of both RNA template and biotinylated cRNA. In agreement with this were the hybridization patterns of control genes on the slide showing a near-identical pattern of hybridization (c). The uniformity of normalized unscaled SE (NUSE) and relative log expression (RLE) indicate high quality and uniformity of the hybridization data (d and e) (1). Raw intensity data were subjected to RMA normalization (2), which kept the uniformity of general levels between the different slides (f and g). Between replicate reproducibility of the experiment was high, exemplified by the high correlation between the data of two biological replicates (h). Array 1 and array 2 are hybridized with cRNA from different replicates of Ler seeds. Figure S2. Spatial and temporal expression patterns of the selected dormancy and after-ripening up-regulated genes. Mean relative expression of (a) D-up and (b) AR-up genes across the Arabidopsis germination time course in the micropylar and chalazal endosperm (MCE) and radicle and hypocotyl (RAD) in dry, 1, 3, 7, 12, 16, 20, 25, 31 and 38 hours after imbibition. Data was taken from Seed EFP Browser (http://www.bioinformatics.nl/efp/cgi-bin/efpWeb.cgi). Figure S3. Log2 expression differences for the D-up and AR-up genes that are presented in Fig. 1c and d. (a) Heat map showing Log2 expression differences of the 245 NILDOG1 D-up genes (P < 0.0001) in NILDOG1 and the other genotypes. (b) Log2 expression differences of the 159 NILDOG1 AR-up genes (P < 0.0001) in NILDOG1 and the other genotypes. (XLS 81 kb)
T-DNA selection of the 46 D-up and 25 AR-up genes. Details like, T-DNA identification, genotype, primers used for genotyping, knock-out # in the analyses, where the T-DNA is inserted and whether the genes overlap with the study of Cadman et al. [10] are indicated. (DOCX 803 kb)
Data Availability Statement
The microarray datasets generated during the current study are available in deposited in NCBI’s Gene Expression Omnibus (GEO number GSE90162). All other datasets used during the current study are available from the corresponding author on reasonable request.


