Abstract
Background
Poor access to essential medicines is common in many low- and middle-income countries, partly due to an insufficient and inadequately trained workforce to manage the medicines supply chain. We conducted a prospective impact evaluation of the training and deployment of pharmacy assistants (PAs) to rural health centers in Malawi.
Methods
A quasi-experimental design was used to compare access to medicines in two districts where newly trained PAs were deployed to health centers (intervention) and two districts with no trained PAs at health centers (comparison). A baseline household survey and two annual post-intervention household surveys were conducted. We studied children under five years with a history of fever, cough and difficulty in breathing, and diarrhea in the previous two weeks. We collected data on access to antimalarials, antibiotics and oral rehydration salts (ORS) during the childrens’ symptomatic periods. We used difference-in-differences regression models to estimate the impact of PA training and deployment on access to medicines.
Results
We included 3974 children across the three rounds of annual surveys: 1840 (46%) in the districts with PAs deployed at health centers and 2096 (53%) in districts with no PAs deployed at health centers. Approximately 80% of children had a fever, nearly 30% had a cough, and 43% had diarrhea in the previous two weeks. In the first year of the program, the presence of a PA led to a significant 74% increase in the odds of access to any antimalarial, and a significant 49% increase in the odds of access to artemisinin combination therapies. This effect was restricted to the first year post-intervention. There was no effect of presence of a PA on access to antibiotics or ORS.
Conclusion
The training and deployment of pharmacy assistants to rural health centers in Malawi increased access to antimalarial medications over the first year, but the effect was attenuated over the second year. Pharmacy assistants training and deployment demonstrated no impact on access to antibiotics for pneumonia or oral rehydration salts for diarrhea.
Keywords: Health workforce, Supply chain, Quasi-experiment, Impact evaluation, Malawi, Essential medicines, Malaria, Pneumonia, Diarrhea
Background
The UN Sustainable Development Goals called for improved access to safe, effective, quality and affordable essential medicines and vaccines for all [1]. However, access to the most life-saving medicines and medical supplies in low- and middle-income countries (LMICs) remains unacceptably low. For example, it is estimated that only 46% of adults and 49% of children living with HIV in LMICs had access to treatment with antiretroviral medications at the end of 2015 [2]. The public sector availability of essential medicines in 23 LMICs in 2012 was estimated at only a mean value of 40% [3]. In sub-Saharan Africa, children bear the brunt of the adverse morbidity and mortality impact of lack of access to essential medicines, particularly as it applies to medicines for the treatment of malaria, pneumonia and diarrhea [4, 5].
The reasons for persistently low access to essential medicines in LMICs include inadequate financing, regulatory issues, lengthy procurement processes, poor logistics management, and a lack of qualified health workers to manage the medicines supply chain [6–9]. Malawi reflects many of these constraints — poor health systems infrastructure and working conditions [10]; an ongoing health worker crisis [11]; and limitations in product availability, supply chain knowledge, and transportation [12]. Additionally, the 2011–2013 Malawi Health Sector Strategic Plan identified only five pharmacists in the country’s public health system and estimated that only 24% of the established positions for pharmacy technicians were filled [13]. And despite substantial improvements between 1990 and 2013, Malawi still has one of the highest under-five mortality rates in the world [14].
Task-shifting, the delegation of tasks from more- to less-sophisticated health workers, is the reality in sub-Saharan Africa, driven by severe health workforce shortages. Task-shifting has the potential to increase the effective health workforce and to be cost-effective [15, 16]. In Malawi, the logistics management and supply chain functions of health center pharmacies rely on task-shifting from pharmacists and pharmacy technicians to clinical health workers such as medical assistants and nursing aides [17, 18], and, in our experience, even to grounds laborers and security guards. The lack of training in medicines logistics management of these lower-level cadres leads to inefficiencies in supply chain management and clinical care, which may lead to shortages in essential medicines resulting in adverse health consequences for patients [11, 17, 19].
To address the shortage of well-trained pharmacy workers, VillageReach, a Seattle-based international non-governmental organization with expertise in supply chain management, initiated an innovative pharmacy assistant (PA) training program, in conjunction with the Malawi College of Health Sciences (MCHS) and the Malawi Ministry of Health. The training program, initiated in 2013, provides two years of training towards a Certificate in Pharmacy, i.e., pharmacy assistant. Malawi previously trained and deployed pharmacy assistants at district and central hospitals, but the program was discontinued nearly two decades ago, resulting in a shortage of pharmacy workers at the primary level [13, 20]. The primary level of health service delivery in Malawi is the frontline of a three-tier health system, operating at the village level. It consists of village clinics, health posts, dispensaries, maternities, health centers and community and rural hospitals. The secondary level operates at the district level and the tertiary level operates nationally.
There is limited evidence on the impact of strengthening human resources capacity for medicines supply on access to medicines and on health outcomes [18]. To bridge this knowledge gap, we designed a prospective evaluation of the impact of pharmacy worker training and deployment on access to essential medicines for malaria, pneumonia, and diarrhea, i.e., artemisinin-based combination therapies (ACTs), antibiotics, and oral rehydration salts (ORS), respectively, the leading causes of childhood mortality in Malawi [5]. The pre-specified hypothesis was that pharmacy assistant training and deployment, as implemented by VillageReach and partners, would improve access to these essential medicines through improved management, logistics information flow for essential medicines, and supply chain functions at the primary health centers.
Methods
Study design
We used a quasi-experimental design (Fig. 1), with data derived from three population-based cluster household surveys: one pre-implementation sample conducted in 2014 and two post-implementation samples conducted in 2015 and 2016. The primary outcome as measured in the survey was access to ACTs, antibiotics, and ORS. Access to medicines was defined as the percentage of children with non-severe malaria, pneumonia, or diarrhea in the last two weeks who obtained a full course of ACT, antibiotics, or ORS, respectively. This definition was adopted from the Malawi Demographic and Health Survey (DHS), 2010 [21]. The details of the study design have been reported elsewhere [22] and are summarized below.
Fig. 1.
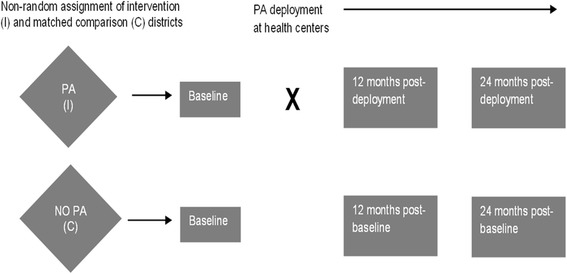
Quasi-experimental design of the impact evaluation of pharmacy assistant training and deployment in Malawi. PA—pharmacy assistant; I—Intervention; C—comparison; X—timing of intervention. Adapted from Lubinga et al. [22]
Study setting
The study was conducted in four districts of Malawi: Ntchisi and Machinga (intervention) and Dedza and Chikwawa (comparison). The comparison districts were matched on region of the country; presence of high burden of malaria, pneumonia, and diarrhea; access to basic services; geographic location; and socioeconomic status of residents. Within each intervention district, a single health center was the site of PA student deployment: Khuwi health center in Ntchisi and Ntaja health center in Machinga. The health centers of Dolo in Chikwawa and Lobi in Dedza were chosen as comparison sites.
The intervention
The intervention was a newly developed and implemented pharmacy assistant training program. Briefly, the training program is composed of both didactic and an extended practicum training [23]. During the first year, students underwent ten weeks of class-based instruction at the MCHS. After this period, half of the students were deployed for a five-month period of field training supervised by a pharmacy technician at a district hospital, while the other half underwent additional didactic training. The students then switched places for the next five months, i.e., the students who underwent field training undergo didactic training, and vice versa. During the second year, half the students were deployed to rural health centers for five months while the other half underwent additional didactic training, before switching places. Therefore, during the second year, student trainees were present at participating rural health centers for ten of twelve months. The potential benefits of the practicum were two-fold: PA students trained in an environment that is characteristic of their future workplace, and PA students filled an immediate human resources gap at the primary health center.
Study procedures
Following a baseline household survey conducted in March 2014, we conducted annual household surveys in March 2015 and March 2016. A two-stage cluster sampling design was used. In the first stage, enumeration areas (EAs) close to intervention and comparison health centers were identified. In the second stage, households were randomly selected within EAs by identifying a central location and going from house-to-house guided by a random number generator.
We identified households, using self-report by parent or caregiver, with at least one child under five years of age with symptoms of non-severe malaria, pneumonia, or diarrhea in the previous two weeks (i.e., fever; cough, difficulty breathing or a rapid respiratory rate; or diarrhea, respectively). Eligible children were included upon consent from the parent or caregiver and excluded if they had severe disease. The questionnaire, adapted from the 2010 Malawi Demographic and Health Survey and Women’s questionnaires [21], included details about household characteristics, care-seeking behavior, and medicine-use. The questionnaire was translated into Chichewa and back-translated into English to confirm reliability and validity of the survey instrument. The questionnaires were piloted and refined by local field teams for clarity and ease of understanding among the target population. Individuals who conducted data collection underwent two-day trainings before each round of data collection.
Sample size and power
The study was powered to detect increases in access to ACTs, antibiotics, and ORS of 50%, 40% and 80% respectively, with 80% power and 5% precision. Given the prevalence of fever, pneumonia symptoms, and diarrhea of 35%, 7%, and 18%, respectively [21], and assuming 1.5 children per household, a non-response rate of 10%, and a design effect of 1.5 to account for within-cluster homogeneity, in the primary outcome [21, 24], we estimated needing to sample 4150 households, divided equally between intervention and comparison sites, to enroll the required number of children with the symptoms of interest.
Statistical analyses
Data from the questionnaires were double-entered and cross-validated using the Census and Survey Processing System, CSPro (US Census Bureau, USA). All analyses were conducted using R.
Methods described by the Malawi DHS were used to calculate household wealth index scores from data on ownership of goods and housing characteristics [21]. Participant characteristics and the overall illness distribution were summarized over the three independent annual samples.
Using a logit link function, three empiric specifications of the mean were estimated:
All post-intervention data for 2015 and 2016 were grouped together and the simple difference-in-differences effect was estimated. The coefficient is the difference in the difference in log-odds of access to medicines in the pre-intervention versus the post-intervention periods, when comparing the intervention group with the comparison group.
Dummy variables were created for the year. The coefficient is the difference in the difference in log-odds of access to medicine in the pre-intervention versus the post-intervention periods, when comparing the intervention group with the comparison group, assuming the intervention effect is the same in both post-intervention periods (2015 and 2016).
Dummy variables for year were interacted with the intervention indicator, permitting the estimation of differential effects (trends) by year. The coefficient is the difference in the difference in log-odds of access to medicine in the pre-intervention versus the first year (2015) post-intervention and the difference in the pre-intervention versus the second year (2016) post-intervention when comparing the intervention group with the comparison group.
All models were estimated using maximum likelihood, and standard errors were adjusted for heteroscedasticity and clustering at enumeration area level and family level. In all cases unadjusted and adjusted models were estimated. Models were adjusted for child’s age and sex, care giver’s age and sex, level of education and marital status, and the household socioeconomic status (wealth index).
Results
Participant demographic and clinical characteristics
We included 3974 children in the study across the three rounds of annual surveys: 1840 (46%) in the districts to which PAs were deployed (intervention) and 2096 (53%) in the districts to which no PAs were deployed (comparison). The demographic and clinical characteristics of the children and their caregivers are summarized in Table 1. The average age of children in the study was two years, and approximately half of study children were male. Caregivers were, on average, 29 years old, predominantly female (98%), and a majority had some primary education. Half the families were in the middle of five quantiles of socioeconomic status. Nearly 80% of children included in the study were reported to have had a fever, nearly 30% were reported to have had a cough, and 43% were reported to have had diarrhea in the previous two weeks.
Table 1.
Demographic and clinical characteristics
| Intervention (n = 2096, 52.74%) |
Comparison (n = 1840, 46.3%) |
Total (n = 3947) |
|
|---|---|---|---|
| Age of child, years, mean (SD) | 2.34 (1.3) | 2.15 (1.3) | 2.25 (1.3) |
| Male child, n (%) | 843 (48.9%) | 991 (50.7%) | 1834 (49.9%) |
| Number of persons in household, mean (SD) | 5.39 (1.8) | 5.24 (1.8) | 5.32 (1.8) |
| Age of primary caregiver, mean (SD) | 29.11 (7.9) | 29.15 (8.6) | 29.13 (8.3) |
| Female primary caregiver, n (%) | 1765 (97.8%) | 2008 (98.7%) | 3810 (98.3%) |
| Education level of primary caregiver | |||
| None, n (%) | 348 (20.2%) | 187 (9.6%) | 535 (14.6%) |
| Primary, n (%) | 1263 (73.5%) | 1487 (76.4%) | 2750 (75.1%) |
| Above primary, n (%) | 106 (6.1%) | 270 (13.8%) | 376 (10.2%) |
| Socioeconomic status quantile | |||
| Highest, n (%) | - | - | - |
| Second Highest, n (%) | 421 (24.4%) | 292 (14.9%) | 713 (19.4%) |
| Middle, n (%) | 927 (53.8%) | 884 (45.2%) | 1811 (49.2%) |
| Second Lowest, n (%) | 324 (18.8%) | 561 (28.7%) | 885 (24.0%) |
| Lowest, n (%) | 49 (2.8%) | 217 (11.1%) | 266 (7.2%) |
| Child’s symptoms in last two weeks | |||
| Fever, n (%) | 1390 (76.3%) | 1676 (80.8%) | 3096 (78.7%) |
| Cough, n (%) | 515 (28.3%) | 578 (27.8%) | 1101 (28.0%) |
| Diarrhea, n (%) | 783 (43.0%) | 898 (43.3%) | 1697 (43.2%) |
Impact of pharmacy assistant training and deployment
The results of having a trained PA at health centers on access to medicines are shown in Table 2 and Fig. 2. With regard to the impact of PA training and deployment at health centers on access to any antimalarial, model 1 shows a significant effect of the program on the odds of access to any antimalarial. This effect is maintained after adjusting for year fixed effects (model 2). However, model 3 shows that the effect of PA training and deployment on access to any antimalarial was restricted to the first year post intervention.
Table 2.
Regression analyses
| Any antimalarial | AL | Antibiotic | ORS | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |||||||||||||
| Unadj | Adj | Unadj | Adj | Unadj | Adj | Unadj | Adj | Unadj | Adj | Unadj | Adj | Unadj | Adj | Unadj | Adj | Unadj | Adj | Unadj | Adj | Unadj | Adj | Unadj | Adj | |
| Constant | −0.285** | −0.624** | −0.285** | −0.625** | −0.285** | −0.611** | −0.587*** | −1.097*** | −0.587*** | −1.100*** | −0.587*** | −1.088*** | 0.869*** | 0.951** | 0.869*** | 0.947** | 0.869*** | 0.949** | −0.613*** | −0.975** | −0.613*** | −0.929** | −0.613*** | −0.925** |
| (0.116) | (0.255) | (0.116) | (0.254) | (0.116) | (0.253) | (0.092) | (0.247) | (0.092) | (0.244) | (0.092) | (0.244) | (0.160) | (0.445) | (0.160) | (0.446) | (0.160) | (0.446) | (0.175) | (0.417) | (0.175) | (0.424) | (0.175) | (0.424) | |
| Intervention | −0.222 | −0.188 | −0.222 | −0.187 | −0.222 | −0.188 | −0.010 | 0.026 | −0.010 | 0.029 | −0.010 | 0.028 | −0.199 | −0.513** | −0.199 | −0.513** | −0.199 | −0.513** | 0.208 | 0.121 | 0.208 | 0.116 | 0.208 | 0.113 |
| (0.180) | (0.169) | (0.180) | (0.169) | (0.180) | (0.169) | (0.170) | (0.163) | (0.170) | (0.163) | (0.170) | (0.163) | (0.234) | (0.245) | (0.234) | (0.245) | (0.234) | (0.245) | (0.261) | (0.268) | (0.261) | (0.268) | (0.261) | (0.269) | |
| Post Intervention | 0.045 | 0.019 | 0.252* | 0.230* | −0.420** | −0.453** | 0.315 | 0.339 | ||||||||||||||||
| (0.156) | (0.156) | (0.133) | (0.138) | (0.211) | (0.213) | (0.219) | (0.230) | |||||||||||||||||
| Intervention* Post-Intervention | 0.590** | 0.565** | 0.588** | 0.560** | 0.374* | 0.335 | 0.364 | 0.323 | −0.123 | −0.078 | −0.133 | −0.087 | −0.339 | −0.297 | −0.298 | −0.251 | ||||||||
| (0.234) | (0.224) | (0.235) | (0.225) | (0.222) | (0.216) | (0.223) | (0.217) | (0.306) | (0.307) | (0.308) | (0.308) | (0.298) | (0.302) | (0.305) | (0.309) | |||||||||
| year 2015 | 0.026 | −0.014 | −0.085 | −0.123 | 0.172 | 0.132 | 0.082 | 0.042 | −0.329 | −0.362 | −0.379 | −0.395 | 0.543** | 0.582** | 0.392 | 0.422* | ||||||||
| (0.176) | (0.177) | (0.206) | (0.209) | (0.146) | (0.152) | (0.166) | (0.173) | (0.249) | (0.246) | (0.270) | (0.274) | (0.228) | (0.236) | (0.243) | (0.253) | |||||||||
| year 2016 | 0.062 | 0.051 | 0.169 | 0.157 | 0.328** | 0.324** | 0.413** | 0.410** | −0.488** | −0.524** | −0.451* | −0.500** | 0.046 | 0.051 | 0.227 | 0.251 | ||||||||
| (0.164) | (0.163) | (0.176) | (0.175) | (0.151) | (0.155) | (0.170) | (0.174) | (0.221) | (0.227) | (0.233) | (0.233) | (0.244) | (0.254) | (0.253) | (0.267) | |||||||||
| year 2015* Intervention | 0.794*** | 0.762*** | 0.529** | 0.488* | −0.054 | −0.036 | −0.002 | 0.070 | ||||||||||||||||
| (0.282) | (0.281) | (0.251) | (0.252) | (0.361) | (0.375) | (0.350) | (0.353) | |||||||||||||||||
| year 2016* Intervention | 0.409 | 0.384 | 0.223 | 0.179 | −0.197 | −0.130 | −0.612* | −0.597* | ||||||||||||||||
| (0.264) | (0.251) | (0.262) | (0.253) | (0.374) | (0.359) | (0.332) | (0.341) | |||||||||||||||||
| N | 2873 | 2873 | 2873 | 2873 | 2873 | 2873 | 2873 | 2873 | 2873 | 2873 | 2873 | 2873 | 1036 | 1036 | 1036 | 1036 | 1036 | 1036 | 1552 | 1552 | 1552 | 1552 | 1552 | 1552 |
| Log-Likelihood | −1963 | −1922 | −1963 | −1921 | −1961 | −1919 | −1939 | −1893 | −1938 | −1891 | −1937 | −1889 | −679 | −653 | −678 | −652 | −678 | −652 | −1041 | −1022 | −1033 | −1014 | −1031 | −1010 |
| AIC | 3935 | 3871 | 3937 | 3873 | 3935 | 3871 | 3887 | 3813 | 3886 | 3811 | 3885 | 3810 | 1365 | 1333 | 1366 | 1335 | 1368 | 1337 | 2090 | 2072 | 2077 | 2057 | 2073 | 2053 |
AL Artemether Lumefantrine, ORS Oral Rehydration Salts, Unadj unadjusted models, Adj models adjusted for child’s age and sex, care giver’s age and sex, level of education and marital status, and the household socioeconomic status (wealth index)
* p < 0.1; ** p < 0.05; *** p < 0.01
Fig. 2.
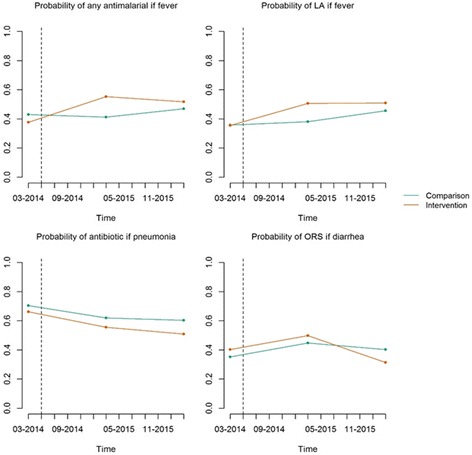
Impact of pharmacy assistant training and deployment. The dotted line represents the timing of the intervention
The results of the impact of PA training and deployment on access specifically to ACTs mirror those of the impact of having trained PAs at health centers on access to any antimalarial. Model 1 shows a significant effect of the program on the odds of access to Artemether-Llumefantrine, which is maintained after adjustment for year fixed effects (model 2), and restricted to the first year post-intervention (model 3). The analysis showed no effect of PA training and deployment on access to antibiotics or ORS (Table 2 and Fig. 2).
Discussion
During the study, we tracked access to tracer essential medicines at intervention and comparison primary health centers to assess possible differential access to medicines. In this three-year prospective quasi-experimental impact evaluation of pharmacy assistant training and deployment to rural health centers in Malawi, we found mixed results: an increase in access to antimalarials over the first year that was attenuated during the second year. Additionally, there was no impact on access to antibiotics for pneumonia and oral rehydration salts for diarrhea. Therefore, our pre-specified hypothesis held for antimalarials over the first year but did not hold at all for antibiotics and ORS.
The differential impact of PA training and deployment may be explained in part by the difference in funding structure and supply chain dynamics for malaria, antibiotics, and ORS in Malawi during the study period [25]. Antimalarials are frequently donor-supported and channeled to tertiary health facilities through an informed “push” system that, in turn, distribute to lower level health facilities. Other essential medicines including antibiotics and ORS are more often supplied through the government central medical stores with limited donor support and channeled through a traditional “pull” system. Donor-supported commodities are also subject to interruptions in supply that are not necessarily amenable to improvements with interventions at the various levels of health centers. However, interventions at tertiary health centers are more likely to increase access to the donor-supported antimalarials than access to other essential medicines [25]. While the reasons for the attenuation in the effect of pharmacy worker training and deployment on access to antimalarials in the second year were unclear, it is not uncommon for low-income countries such as Malawi to experience supply chain shocks leading to interruptions in supply of medicines, including donor-funded or donor-supplied medicines [26].
Previous studies have evaluated the impact of health worker training on knowledge and clinical practice behavior in LMICs and reported mixed results, with didactic training appearing to be less successful than interactive and in-practice training [27]. The PA training model included both didactic and in-practice training, although the outcome of interest was access to tracer commodities and not clinical practice behavior. Impact evaluations of task-shifting have also been performed and generally demonstrate that health workers with less training are successful at performing a variety of clinical tasks [28–33]. However, we found no prospective evaluations of the impact of training and deploying a new cadre of health workers that are trained at a level comparable to the PAs.
Pharmacy workers are critical to health service delivery in LMICs, particularly in rural areas, where medicines are the mainstay of primary health care. The VillageReach PA training program expanded the health workforce in Malawi and the deployment of PAs appeared to increase community access to antimalarials in the short-term. In addition, monitoring data collected by VillageReach during supervision visits to students showed that PA students led to increased data quality (from 55 to 73% report accuracy) and improved adherence to storeroom management (from 72 to 79% adherence) and dispensing standards (from 41 to 60% adherence) (Jessica Crawford, personal communication). However, the intervention neither had sustained impact on access to antimalarials over two years, nor had impact on access to other medicines evaluated in this study.
Other possible reasons for our mixed results include the fact that poor access to medicines in LMICs is a multi-faceted problem with multiple contributing factors over-and-beyond the lack of sufficient and well-trained personnel. Other contributing factors include: poor infrastructure and bottlenecks at higher levels of the health system including poor financing, regulatory barriers, long procurement process, mismanagement, and corruption [6–9, 34]. It is unlikely that training and deploying PAs alone would have been enough to increase community access to medicines within the study timeframe without concurrent interventions also targeting the other factors that limit access to essential medicines.
In Malawi, healthcare in general and essential medicines in particular are intended to be free-of-charge, but patients often have to pay out-of-pocket in private pharmacies and drug shops due to frequent stock-outs [34]. Therefore, care seeking often involves multiple facilities, usually starting with the government facility, and progressing to the private sector — private clinics, drug shops, non-drug-shop retail outlets — in search of medicines. In navigating this system of facilities, patients may have sought essential medicines outside of our radius of PA deployment and impact evaluation. This would make it difficult to demonstrate an impact of PA training and deployment on access to medicines within the immediate vicinity of health facilities.
The impact evaluation was conducted over a relatively short time period given the effort to demonstrate change at the population level and was conducted with students (rather than graduated and deployed PAs). We would expect that the bulk of the benefits of this ongoing PA training program would accrue in the future as the newly trained PAs complete the learning curve and establish themselves in their practice settings. In addition, it is anticipated that the facility level improvements observed in the logistics management and information system reporting and ordering of medicines will, overtime, improve forecasting and supply planning at higher levels. As such, this study may have underestimated the impact of PA training and deployment.
Training programs are likely to succeed if there is ready employment for the trained individuals, and the trained individuals are retained in the health care system. In Malawi, there are challenges in recruitment of health workers, with complex recruitment processes and a poorly-funded recruitment agency within the Ministry of Health, leading to a health worker vacancy rate of over 50% in the public sector [35]. Retention is also a major problem because health workers in Malawi work in challenging environments and are poorly remunerated and can be under-motivated [36, 37]. Retention of trained health workers requires the deployment of remuneration and non-remuneration strategies such as provision of housing, improved practice conditions, and opportunities for career advancement and further training [38, 39]. While the long term success of the PA training program will depend on such efforts, it is important to note that at the time of the conclusion of this impact evaluation, 100% of the graduates of the PA training program were employed by the Ministry of Health.
One limitation of this study is that we were unable to randomize PA deployment to intervention and comparison sites due to practical limitations, such as logistical constraints and political influences [22]. This means that, despite the use of the differences-in-differences method, the observed effect of PA training and deployment, or lack thereof, may be explained in part by differences in baseline characteristics between the intervention and control groups. Additionally, we are only able to assess the combined impact of training and deployment and are unable to differentiate between the two [22]. Another limitation that could have influenced the results is that we used a single pre-intervention survey and two post-intervention surveys. As such, we are unable to establish a pre-intervention trend in access to medicines. This means that we had to make an assumption that the pre-intervention one-time access estimate amounted to a trend in access. Given the timing of the decision to perform the evaluation, it was the best possible design despite this limitation. The lack of two pre-intervention time points also means that a selection threat remains, and precludes our ability to test empirically whether this threat was present, or not, in the evaluation. Finally, the outcomes in this study were based exclusively on data from household surveys. This precluded a more holistic view of the impact of pharmacy worker training and deployment on access to medicines by excluding system-level effects. The overall project included health-facility-based experiential and time-motion surveys and data from these studies will be reported separately.
Conclusions
Pharmacy assistant training and deployment to rural health centers in Malawi increased access to antimalarial medications for children under five years in the short-term but this effect was attenuated over the subsequent year. The intervention had no effect on access to antibiotics for pneumonia and oral rehydration salts for diarrhea. While pharmacy assistants are viewed as valuable additions to the health workforce in Malawi and will likely contribute to long-term systemic benefits, their short-term impact on access to essential medicines was limited. This study points to the challenges of expanding the health workforce in low- and middle-income countries in general and illustrates the importance of performing impact evaluations of health workforce interventions despite difficulties in isolating the effects of one intervention from other complex health system issues.
Acknowledgements
We appreciate the cooperation of the study participants. We thank Invest In Knowledge (IKI) which was responsible for data collection. We thank the members of staff of the Pharmacy Department at the Malawi College of Health Sciences for their input into the design and implementation of the PA program—John Chipula, Phillip Chinula and John Kandaya; and the staff of VillageReach in Lilongwe, Malawi who are responsible for the routine implementation and monitoring of the PA program—Shallnever Tambala, Mathew Ziba, Fanny Mulwafu, Gertrude Nowa and Godfrey Dzoole.
Funding
The research reported in this publication is supported by a grant from the Barr Foundation, Grant Number 4471. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Barr Foundation.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- ACTs
Artemisinin-Based Combination Therapies
- DHS
Demographic and Health Surveys
- EA
Enumeration Area
- LMICs
Low- and Middle-Income Countries
- MCHS
Malawi College of Health Sciences
- ORS
Oral Rehydration Salts
- PA
Pharmacy Assistant
Authors’ contributions
JBB, JC, and AS conceived the study. All authors participated in designing the study and finalizing the protocol. JBB and SJL developed the analytic plan. JBB, SJL, and AJ performed the analysis. JBB and SJL produced the first version of the manuscript. All authors participated in writing the manuscript. JBB drafted the final version of the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Ethical clearance was obtained from the University of Washington Institutional Review Board and the Malawi National Health Sciences Research Committee. All study participants provided informed consent prior to participating in the study.
Consent for publication
Not applicable
Competing interests
All authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joseph B. Babigumira, Email: babijo@uw.edu
Solomon J. Lubinga, Email: slubinga@uw.edu
Alisa M. Jenny, Email: ajenny@uw.edu
Erin Larsen-Cooper, Email: erin.larsen-cooper@villagereach.org.
Jessica Crawford, Email: jessica.crawford@villagereach.org.
Charles Matemba, Email: charles.matemba@villagereach.org.
Andy Stergachis, Email: stergach@uw.edu.
References
- 1.United Nations Departement of Economic and Social Affairs. Sustainable Development Knowledge Platform. Susatainable Development Goals. https://sustainabledevelopment.un.org/sdg3. Accessed on 25 Oct 2016.
- 2.UNAIDS . Fact sheet. 2016. [Google Scholar]
- 3.Bazargani YT, Ewen M, de Boer A, Leufkens HGM, Mantel-Teeuwisse AK. Essential medicines are more available than other medicines around the globe. PLoS One. 2014;9(2):e87576. doi: 10.1371/journal.pone.0087576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson J, Forte G, Trapsida JM, Hill S. What essential medicines for children are on the shelf? Bull World Health Organ. 2009;87(3):231–237. doi: 10.2471/BLT.08.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 6.Quick JD, Boohene NA, Rankin J, Mbwasi RJ. Medicines supply in Africa. BMJ (Clinical research ed) 2005;331(7519):709–710. doi: 10.1136/bmj.331.7519.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quick JD. Ensuring access to essential medicines in the developing countries: a framework for action. Clin Pharmacol Ther. 2003;73:279. doi: 10.1016/S0009-9236(03)00002-X. [DOI] [PubMed] [Google Scholar]
- 8.Prescription for Healthy Development: Increasing Access to Medicine . Report of the task force on HIV/AIDS, malaria, TB, and access to essential medicines, working group on access to essential medicines. Sterling: Earthcscan; 2005. [Google Scholar]
- 9.Dickens T. The world medicines situation. Geneva: World Health Organization; 2011. Procurement of medicines. [Google Scholar]
- 10.Mueller DH, Lungu D, Acharya A, Palmer N. Constraints to implementing the essential health package in Malawi. PLoS One. 2011;6(6):e20741. doi: 10.1371/journal.pone.0020741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer D. Tackling Malawi's human resources crisis. Reprod Health Matters. 2006;14(27):27–39. doi: 10.1016/S0968-8080(06)27244-6. [DOI] [PubMed] [Google Scholar]
- 12.Chandani Y, Noel M, Pomeroy A, Andersson S, Pahl MK, Williams T. Factors affecting availability of essential medicines among community health workers in Ethiopia, Malawi, and Rwanda: solving the last mile puzzle. Am J Trop Med Hyg. 2012;87(5 Suppl):120–126. doi: 10.4269/ajtmh.2012.11-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Government of Malawi, Ministry of Health: Malawi health sector strategic plan 2011–2016; 2011. Available from: http://www.medcol.mw/commhealth/publications/3%20Malawi%20HSSP%20Final%20Document%20(3).pdf. Accessed 5 Jan 2013.
- 14.Kanyuka M, Ndawala J, Mleme T, Chisesa L, Makwemba M, Amouzou A, Borghi J, Daire J, Ferrabee R, Hazel E, et al. Malawi and millennium development goal 4: a countdown to 2015 country case study. Lancet Glob Health. 2016;4(3):e201–e214. doi: 10.1016/S2214-109X(15)00294-6. [DOI] [PubMed] [Google Scholar]
- 15.Mdege ND, Chindove S, Ali S. The effectiveness and cost implications of task-shifting in the delivery of antiretroviral therapy to HIV-infected patients: a systematic review. Health Policy Plan. 2013;28(3):223–236. doi: 10.1093/heapol/czs058. [DOI] [PubMed] [Google Scholar]
- 16.Babigumira JB, Castelnuovo B, Lamorde M, Kambugu A, Stergachis A, Easterbrook P, Garrison LP. Potential impact of task-shifting on costs of antiretroviral therapy and physician supply in Uganda. BMC Health Serv Res. 2009;9:192. doi: 10.1186/1472-6963-9-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King RC, Fomundam HN. Remodeling pharmaceutical care in Sub-Saharan Africa (SSA) amidst human resources challenges and the HIV/AIDS pandemic. Int J Health Plann Manag. 2010;25:30. doi: 10.1002/hpm.997. [DOI] [PubMed] [Google Scholar]
- 18.O'Connell KA, Gatakaa H, Poyer S, Njogu J, Evance I, Munroe E, Solomon T, Goodman C, Hanson K, Zinsou C, et al. Got ACTs? Availability, price, market share and provider knowledge of anti-malarial medicines in public and private sector outlets in six malaria-endemic countries. Malar J. 2011;10:326. doi: 10.1186/1475-2875-10-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.People that deliver: Workforce excellence in health supply chain management: literature review. Available from: http://peoplethatdeliver.org/sites/peoplethatdeliver.org/files/People%20that%20Deliver/files/Literature%20Review%20EN.pdf. Accessed 25 Oct 2016.
- 20.Muula AS, Chipeta J, Siziya S, Rudatsikira E, Mataya RH, Kataika E. Human resources requirements for highly active antiretroviral therapy scale-up in Malawi. BMC Health Serv Res. 2007;7:208. doi: 10.1186/1472-6963-7-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malawi Demographic and Health Survey 2010 . National Statistics Office (NSO), ICF Macro, Zomba, Malawi, and Calverton, Maryland, USA. 2011. [Google Scholar]
- 22.Lubinga SJ, Jenny AM, Larsen-Cooper E, Crawford J, Matemba C, Stergachis A, Babigumira JB. Impact of pharmacy worker training and deployment on access to essential medicines and health outcomes in Malawi: protocol for a cluster quasi-experimental evaluation. Implement Sci. 2014;9(1):156. doi: 10.1186/s13012-014-0156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziba M, Babigumira J, Crawford J, Kandaya J, Chimenya C, Jenny A, Lubinga S, Matemba C, Larsen-Cooper E, Stergachis A. Introducing an enhanced cadre of pharmacy assistants to improve dispensing, management, and availability of medicines at the health centre level in Malawi. J Pharm Policy Prac. 2014;7(1):O23. doi: 10.1186/2052-3211-7-S1-O23. [DOI] [Google Scholar]
- 24.Bennett S, Woods T, Liyanage WM, Smith DL. A simplified general method for cluster-sample surveys of health in developing countries. World Health Stat Q. 1991;44(3):98–106. [PubMed] [Google Scholar]
- 25.Khuluza F, Heide L. Availability and affordability of antimalarial and antibiotic medicines in Malawi. PLoS One. 2017;12(4):e0175399. doi: 10.1371/journal.pone.0175399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamal-Yanni MM, Potet J, Saunders PM. Scaling-up malaria treatment: a review of the performance of different providers. Malar J. 2012;11(1):414. doi: 10.1186/1475-2875-11-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith LA, Jones C, Meek S, Webster J. Review: provider practice and user behavior interventions to improve prompt and effective treatment of malaria: do we know what works? Am J Trop Med Hyg. 2009;80(3):326–335. [PubMed] [Google Scholar]
- 28.McCollum ED, Preidis GA, Kabue MM, Singogo EB, Mwansambo C, Kazembe PN, Kline MW. Task shifting routine inpatient pediatric HIV testing improves program outcomes in urban Malawi: a retrospective observational study. PLoS One. 2010;5(3):e9626. doi: 10.1371/journal.pone.0009626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris MB, Chapula BT, Chi BH, Mwango A, Chi HF, Mwanza J, Manda H, Bolton C, Pankratz DS, Stringer JS, et al. Use of task-shifting to rapidly scale-up HIV treatment services: experiences from Lusaka, Zambia. BMC Health Serv Res. 2009;9:5. doi: 10.1186/1472-6963-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanjana P, Torpey K, Schwarzwalder A, Simumba C, Kasonde P, Nyirenda L, Kapanda P, Kakungu-Simpungwe M, Kabaso M, Thompson C. Task-shifting HIV counselling and testing services in Zambia: the role of lay counsellors. Hum Resour Health. 2009;7:44. doi: 10.1186/1478-4491-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selke HM, Kimaiyo S, Sidle JE, Vedanthan R, Tierney WM, Shen C, Denski CD, Katschke AR, Wools-Kaloustian K. Task-shifting of antiretroviral delivery from health care workers to persons living with HIV/AIDS: clinical outcomes of a community-based program in Kenya. J Acquir Immune Defic Syndr. 2010;55(4):483–490. doi: 10.1097/QAI.0b013e3181eb5edb. [DOI] [PubMed] [Google Scholar]
- 32.Shumbusho F, van Griensven J, Lowrance D, Turate I, Weaver MA, Price J, Binagwaho A. Task shifting for scale-up of HIV care: evaluation of nurse-centered antiretroviral treatment at rural health centers in Rwanda. PLoS Med. 2009;6(10):e1000163. doi: 10.1371/journal.pmed.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torpey KE, Kabaso ME, Mutale LN, Kamanga MK, Mwango AJ, Simpungwe J, Suzuki C, Mukadi YD. Adherence support workers: a way to address human resource constraints in antiretroviral treatment programs in the public health setting in Zambia. PLoS One. 2008;3(5):e2204. doi: 10.1371/journal.pone.0002204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shenard Masengera. Missing Medicines in Malawi: Campaigning against stock-outs of essential drugs (Case Study) Oxfam Programme Insights. Oxfam GB; 2012.http://policy-practice.oxfam.org.uk/publications/missing-medicines-in-malawi-campaigning-against-stock-outs-of-essential-drugs-226732. Accessed 27 Oct 2016.
- 35.Khunga S. Half health sector posts vacant, worsens health worker to patient ratio, the nation. 2016. [Google Scholar]
- 36.Muula AS, Maseko FC. How are health professionals earning their living in Malawi? BMC Health Serv Res. 2006;6(1):97. doi: 10.1186/1472-6963-6-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manafa O, McAuliffe E, Maseko F, Bowie C, MacLachlan M, Normand C. Retention of health workers in Malawi: perspectives of health workers and district management. Hum Resour Health. 2009;7(1):65. doi: 10.1186/1478-4491-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmiedeknecht K, Perera M, Schell E, Jere J, Geoffroy E, Rankin S. Predictors of workforce retention among Malawian nurse graduates of a scholarship program: a mixed-methods study. Glob Health Sci Pract. 2015;3(1):85–96. doi: 10.9745/GHSP-D-14-00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chimwaza W, Chipeta E, Ngwira A, Kamwendo F, Taulo F, Bradley S, McAuliffe E. What makes staff consider leaving the health service in Malawi? Hum Resour Health. 2014;12:17. doi: 10.1186/1478-4491-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.


