Abstract
Inflammation is well established to significantly impact metabolic diseases. The inflammatory protease caspase-1 has been implicated in metabolic dysfunction, however a potential role for the related inflammatory caspases is currently unknown. In this study, we investigated a role for caspase-11 and caspase-12 in obesity and insulin resistance. Loss of caspase-12 in two independently generated mouse strains predisposed mice to develop obesity, metabolic inflammation and insulin resistance, while loss of caspase-11 had no effect. The use of bone marrow chimeras determined that deletion of caspase-12 in the radio-resistant compartment was responsible for this metabolic phenotype. The Nlrp3 inflammasome pathway mediated the metabolic syndrome of caspase-12-deficient mice as ablation of Nlrp3 reversed Casp12−/− mice obesity phenotype. While the majority of people lack a functional caspase-12 due to a T125 single nucleotide polymorphism (SNP) that introduces a premature stop codon, a fraction of African descendents express full-length caspase-12. Expression of caspase-12 was linked to decreased systemic and adipose tissue inflammation in a cohort of African-American obese children. However, analysis of the Dallas Heart Study African American cohort indicated that the coding T125C SNP was not associated with metabolic parameters in humans, suggesting that host specific differences mediate the expressivity of metabolic disease.
Keywords: Caspase-12, caspase-11, inflammasome, Nlrp3, obesity, insulin resistance, adipocytes, inflammation, metabolism
INTRODUCTION
Metabolic diseases such as Type 2 Diabetes (T2D) and the metabolic syndrome have quickly become a major global health concern. The increasing prevalence of obesity has led to the development of these diseases in adults as well as children. While lifestyle is a large contributor to this problem, the underlying genetic factors are less well understood, and their elucidation may thus aid in the identification of therapeutic targets. A rapidly expanding number of studies have implicated the immune system in playing a pivotal role in the development of metabolic diseases.
Obesity results from an energy imbalance, caused by excess caloric intake that exceeds metabolic requirements. This energy surplus is stored as lipids, leading to overexpansion of the adipose tissue, and consequently local inflammation, possibly initiated by hypoxia, ER stress, and/or pattern recognition receptor (PRR) detection of endogenous metabolic 'danger' signals (1). The onset of obesity correlates with chronic inflammation of metabolic tissues, characterized by high pro-inflammatory cytokine secretion and elevated levels of infiltrating immune cells including macrophages, neutrophils, T cells, and B cells (1). Macrophages in particular may constitute up to 40% of the cells found in the adipose tissue of obese patients (2). Genetic studies or antibody depletion of immune cells or chemokines have implicated numerous inflammatory mediators in metabolic disease. Inflammation can lead to insulin resistance by acting directly on insulin responsive cells, partially through the action of the inflammatory kinases JNK and IKK that phosphorylate and inhibit Insulin Receptor Substrate-1 (IRS-1) (3; 4).
The inflammatory caspases are a family of cysteine proteases, comprised of caspases-1, -4, -5 and -12 in humans and -1, -11 and 12 in mice. Caspase-1, along with a PRR and the adaptor molecule ASC, is capable of forming a cytosolic multi-protein complex termed the inflammasome. Detection of exogenous or endogenous danger signals by the PRR promotes the activation of caspase-1, which triggers an inflammatory response that is primarily characterized by IL-1β and IL-18 secretion (5). Mounting evidence suggests a critical role for the Nlrp3 inflammasome as a major regulator of inflammation in metabolic diseases. In response to several metabolic danger signals, including saturated fatty acids, ceramide, Islet Amyloid Polypeptide (IAPP) and hyperglycemia (6–8), Nlrp3 assembles an inflammasome, which activates the pro-inflammatory protease caspase-1. Implementation of the diet-induced obesity (DIO) experimental model to mice deficient in various inflammasome components, such as Nlrp3−/−, Asc−/−, or Ice−/− mice (deficient in caspase-1 and carrying a null mutation in caspase-11 (9; 10)), has demonstrated that inflammasome signalling alters mouse susceptibility to high fat diet (HFD)-induced insulin resistance. Whereas many studies have demonstrated that caspase-1-dependent IL-1β production is the triggering pathological mechanism in insulin resistance, it is debated whether inflammasome signalling regulates obesity per se and how. For instance, while some reported that the weight of Nlrp3−/− mice was comparable to that of WT controls in the DIO model (7; 8), others described Nlrp3−/−, Asc−/−, and Ice−/− mice leaner in phenotype (11; 12), possibly mediated by decreased intestinal lipid absorption (13) or increased lipid oxidation (11).
Furthermore, it was shown that the inflammasome pathway might affect obesity indirectly through effects on the host microbiota (14). While the inflammasome is primarily studied in cells of hematopoietic origin, it may play a role in both immune cells and stromal cells in the context of metabolic disease. The myeloid derived compartment was determined to be important for the insulin sensitivity observed in Asc−/− mice (8). However, bone marrow chimera experiments have also indicated that caspase-1 is active in radio-resistant cells, where it mediates host lipid metabolism (15). The precise roles of the inflammasome and its regulation of obesity and metabolic disease have not yet been fully determined.
Unlike Caspase-1, little is known about how the remaining inflammatory caspases affect metabolic disease. Caspase-11 has recently been established to recognize intracellular LPS and bacterial pathogens, leading to activation of a non-canonical inflammasome (9). A role of caspase-12, as an immunomodulatory factor, has been primarily studied in the context of infections and exposure to microbial ligands (16–19), however little is known regarding its function in sterile inflammation, such as that elicited in obesity. A single nucleotide polymorphism (SNP) in the human CASP12 gene at amino acid position 125 confines its expression to a fraction of African descendants (20). It is currently unknown what evolutionary pressures have resulted in the loss of a functional caspase-12 allele from the majority of the human population or its continued maintenance.
Given the importance of the contribution of innate immunity to metabolic diseases (1), we investigated the role of caspase-11 and caspase-12 in obesity and insulin resistance in mice and humans. In this study we demonstrate that mice ablated for caspase-12 develop spontaneous obesity and insulin resistance. This is dependent on the Nlrp3 inflammasome, though interestingly independent of the radio-sensitive hematopoietic compartment. Analysis of the effect of the CASP12 T125C SNP in the Dallas Heart Study African-American cohort (21) however, suggested that a functional caspase-12 allele does not correlate with improved metabolic parameters in humans, although in a small cohort of African American obese children, expression of caspase-12 was associated with dampened inflammatory markers.
MATERIALS and METHODS
Animal experiments
Mice were housed at room temperature with a 12 hour light/dark cycle with food and water provided ad libitum. The mice were fed either a standard chow diet (LFD) (2020× Teklad Rodent Diet; 16% calories from fat; 3.1 kcal/g) or a HFD (Research Diet D12451; 45% kcal from fat; 4.73 kcal/g). HFD feeding was initiated in mice at 6 weeks of age.
Mouse strains
Casp11−/−, Casp12−/−(129), Ice−/−, Nlrp3−/−, and Ripk2−/− mice have been previously described (9; 10; 18; 22; 23). Casp12−/−Ripk2−/− and Casp12−/−Nlrp3−/− were generated for this study. Casp12−/−(BL6) (Casp12tm1a(KOMP)Wtsi) mice were generated on a BL6 background by the Wellcome Trust Sanger Institute. All experiments were performed under guidelines of the animal ethics committee of McGill University (Canada).
GTT and ITT
Age matched male mice were fasted for 6h before i.p. injection with 2g/kg dextrose (LFD and HFD) or human recombinant insulin (Humulin, Eli Lilly) 0.75 mU/g (LFD) or 1.5 and 2.0 mU/g (HFD). Blood glucose levels were measured from the tail vein using a Onetouch ultra 2 glucometer.
Western Blots
Tissues were lysed in buffer B150 (20 mM Tris-HCl pH 8.0, 150 mM KCl, 10% glycerol, 5 mM MgCl2, and 0.1% NP40) supplemented with Complete-mini protease inhibitors (Roche Applied Science, Cat# 11836153001) and phosphatase inhibitors (Sigma Cat# S7920, 71768, G6376). Protein lysates were separated on SDS-PAGE and transferred to nitrocellulose membrane. Blots were probed with antibodies against Caspase-1 p20 (Genentech), Caspase-12 (Sigma, Cat# C7611), Caspase-11 (Sigma, Cat# C1354), β-actin (Sigma, Cat# A1978), β -tubulin (sc-9104), AKT (Cell signalling #4691), and P473 AKT (Cell signalling #4060). Densitometry was performed using ImageJ (NIH).
ELISAs and serum analysis
Cytokines were determined using the following ELISA kits: IL-6 (R&D, Cat# DY406), IL-18 (MBL International, Cat# 1625), KC (R&D, Cat#DY453), MCP-1 (R&D, Cat#DY479). Serum ALT and cholesterol were determined by a Vitros 250/350 machine.
DEXA Scan
Fat and lean mass were calculated using a GE Lunar PIXImus machine.
Hepatic Triglyceride Analysis
Hepatic lipids were extracted using a modified Bligh-Dyer extraction protocol. Approximately 200 mg of liver tissue was homogenized in a 1/2.5/1.25 (vol/vol) mixture of 0.5M acetic acid/methanol/chloroform. The mixture was shaken and 1.25 volumes of chloroform were added. After overnight shaking, 1.25 volumes of 0.5M acetic acid were added and the samples were spun at 1,500 × g. The organic phase was collected, dried, and resuspended in Isopropanol. Triglycerides were determined using a Serum Triglyceride Determination kit (Sigma TR0100) and normalized to liver weight.
Bone marrow Chimera
Age matched male mice were irradiated and reconstituted with bone marrow cells from donor mice. Genotype was verified by PCR analysis of blood. Mice were placed on antibiotics (Trimethoprim 0.048 g/250 mL, Sulfamethoxazole 0.24g/250 mL), beginning 3 days prior to lethal irradiation, for 3 weeks. Mice were allowed to recover before being fed a HFD.
FACS
Epididymal adipose tissue was excised from 26w old HFD mice, minced, and incubated in 1mg/ml Type 2 Collagenase (Sigma) for 1 hour at 37°C. Folllowing RBC lysis, the stromal vascular fraction cells were counted and stained. Data were acquired on a Canto instrument (BD Biosciences) equipped for the detection of 8 fluorescent parameters. The following antibodies were used for FACS analysis: anti-CD3-PerCPCy5.5 (145-2C11), anti-CD11b eFluor450 (M1/70), anti-B220-APC (RA3-6B2), anti-CD45-PECy7 30-F11) (all from eBioscience); anti-CD19-PECy7 (1D3), anti-GR1-APCCy7 (RB6-8C5), (all from BD Biosciences); anti-F4/80-PerCPCy5.5 (BM8.1) (Tonbo Biosciences).
Indirect Calorimetry
Mice were placed in Oxymax-CLAMS system (Columbus Instruments) metabolic cages housed with a 12 hour light/dark cycle and free access to water and food. Animals were allowed to acclimatize for 48 hours before readings were taken for a 24 hour period.
Yale Pediatric Cohort
The Yale Pediatric Obesity cohort is a multiethnic cohort of obese children and adolescents carefully phenotyped in regard to glucose and lipids metabolism, liver function as well as to fat partitioning. As of today the cohort consists of 1109 obese children and adolescents from New Haven area (New Haven, CT) recruited through the Yale Pediatric Obesity Clinic. For the purpose of this study we genotyped 256 obese African American children and adolescents with a mean age of 14.1+/−3.7 and a mean z-score BMI of 2.33+/−0.52 who underwent an oral glucose tolerance test, and the measure of plasma CRP and IL-6 as described before (24), a subgroup of 15 of them (mean age 14.8+/− 3.4 and mean z-score BMI 2.0 +/−0.4) underwent a subcutaneous fat biopsy (25). All the patients were genotyped for the T125C variant by automatic sequencing using the following primers 5’-ATATAATTCCTATAATATCATAC-3’ and 5’-GTCTAAACTCTCCACCACCT-3’ (TA 55 °C).
Dallas Heart Study Analysis
Dallas Heart Study (DHS) is a longitudinal, multiethnic population-based probability sample of Dallas County residents. African Americans were oversampled to comprise approximately 50% of the population. Details of the study design and recruitment procedures have been previously described (26). The study was approved by the institutional review board of the University of Texas Southwestern Medical Center, and all participants provided written informed consent. The present investigation includes all African American participants of the DHS who provided fasting blood samples during the initial enrollment (2000–2002) or the follow-up examination (2007–2009) (n=2,360). During each examination, participants completed a detailed staff-administered survey, which included questions about demographics, socioeconomic status, medical history, and current medication use, and underwent a health evaluation that involved measurement of blood pressure, anthropometry, blood and urine sample collection, and imaging studies. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Blood lipid and lipoprotein levels were measured using standard enzymatic methods. Insulin resistance was quantified from fasting blood glucose and insulin levels using homeostatic model assessment (HOMA-IR). Diabetes was defined as a self-reported physician diagnosis of diabetes, use of glucose-lowering medication, or fasting glucose ≥ 126 mg/dL. Hepatic triglyceride content was measured using 1H-MRS in a subset of 1,106 African Americans participants, who completed the initial clinic visit (27). Genotyping was performed using Illumina Infinium Human Exome-12 v1_A BeadChip. Genotype calling and quality control have been previously described (28). CASP12 genotypes were in Hardy-Weinberg equilibrium (p = 0.80). The association between CASP12 T125C genotype and clinical phenotypes was tested using linear regression adjusted for age, gender, BMI and type-2 diabetes mellitus, where necessary. We applied a logarithm transformation to BMI, HOMA-IR, and triglycerides (TG) and a power transformation to hepatic TG content prior to analysis to achieve approximate normality of the residuals. Diabetic individuals were excluded from selected analyses as indicated.
RESULTS
Caspase-12 deficient mice develop obesity and insulin resistance on a HFD
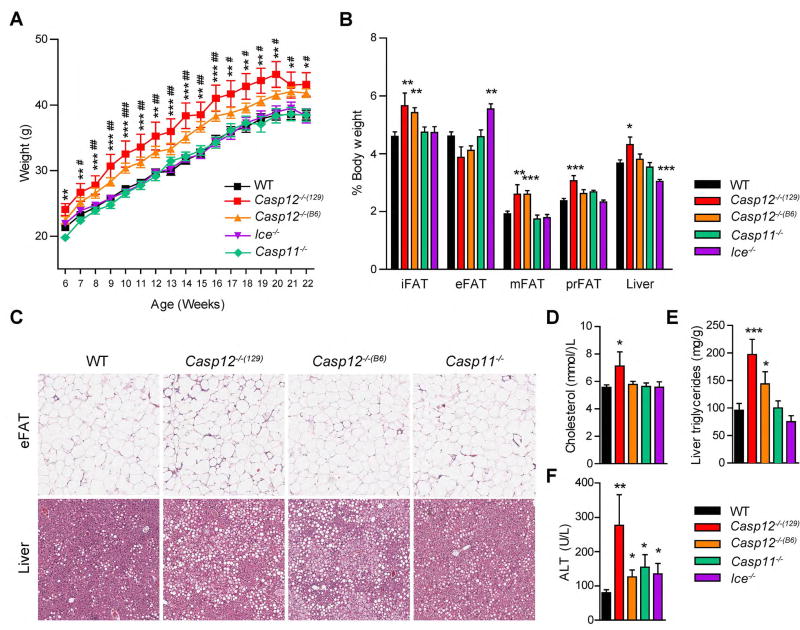
Following the extensive evidence linking innate immunity and the inflammasome-caspase-1 pathway to metabolic disease, we sought to investigate a possible role of the related inflammatory caspases-12 and -11 in metabolic regulation. We examined two independent Casp12−/− mutant mouse lines, the first generated with 129 ES cells then backcrossed to a B6 background (referred to as Casp12−/−(129) mice) and the second generated using B6 ES cells (referred to Casp12−/−(B6)). Kayagaki et al. recently identified that the 129 strain of mice harbored a null mutation in the Casp11 gene (9), raising the possibility that knockout mice of genes neighboring Casp11 generated with 129 ES cells might also be deficient in caspase-11. Indeed, Casp1−/− mice (herein referred to as Ice−/− mice) also carry the 129S-derived Casp11 null mutation (9). Genotyping for the passenger mutation in Casp11 indicated that the Casp12−/−(129) mice in our facility are also deficient in caspase-11, while caspase-11 is expressed in Casp12−/−(B6) mice (Figure S1A). Casp11−/− mice were also examined. Interestingly, when placed on a HFD (45% kcal fat) both Casp12−/−(B6) and Casp12−/−(129) strains both became obese compared to WT mice (Figure 1A). Casp11−/− and Ice−/− mice were similar to controls, suggesting that loss of caspase-11 had little effect on the weight gain of the Casp12−/−(129) mice. After 16 weeks of HFD, caspase-12 deficient strains had increased inguinal (iFAT), mesenteric (mFAT), and perirenal (pFAT) adipose depot weight compared to WT mice (Figure 1B). Ice−/− mice had increased epididymal (eFAT) fat pad weight in relation to all other strains, however in our studies, eFAT weight from DIO mice did not directly correlate with total body weight or the weight of other fat pads (data not shown). Casp11−/− mice were similar to controls. Liver weight was increased in Casp12−/−(129) mice, which correlated with their overall heavier bodyweight, while reduced in Ice−/− mice compared to WT controls. Histological analysis of the eFAT revealed no differences in adipocyte hypertrophy (Figure 1C), while the liver of caspase-12 deficient mice had increased lipid droplet formation (Figure 1C). Casp12−/−(129) mice also had increased serum cholesterol compared to WT mice (Figure 1D) and both caspase-12-deficient strains exhibited increased liver triglycerides (Figure 1E). Interestingly all strains tested had slightly elevated alanine transaminase (ALT) serum levels (Figure 1F), indicative of liver damage, with the highest levels detected in Casp12−/−(129) mice, which correlated with increased liver weight (Figure 1B, C). Overall these results suggest that caspase-12 plays a role in inhibiting obesity in DIO mice.
Figure 1. Caspase-12 deficient mice develop obesity on a HFD.
A) Body weight curve of male mice fed a HFD started at 6 weeks of age, WT n=31, Casp12−/−(129) n= 10, Casp12−/−(B6) n=13, Ice−/− n=15, Casp11−/− n=12. B) Relative weight of adipose depots and liver after 16 weeks of HFD. C) H&E staining of epididymal adipose (eFAT) and liver sections after 16 weeks of HFD. Scale bar is 200 µM. D) Serum cholesterol. E) Liver triglycerides. F) Serum ALT. Data are presented as the mean +/−SEM. Statistical analysis was performed using Student’s t-test. Statistical significance is presented as follows: # P<0.05 ## P < 0.01, ### P<0.001 for Casp12−/−(B6) vs WT; * P<0.05 ** P < .01; *** P <0.001 for Casp12−/−(129) vs WT in A).
Casp12−/−(129) mice develop spontaneous obesity and insulin resistance even on low fat diet
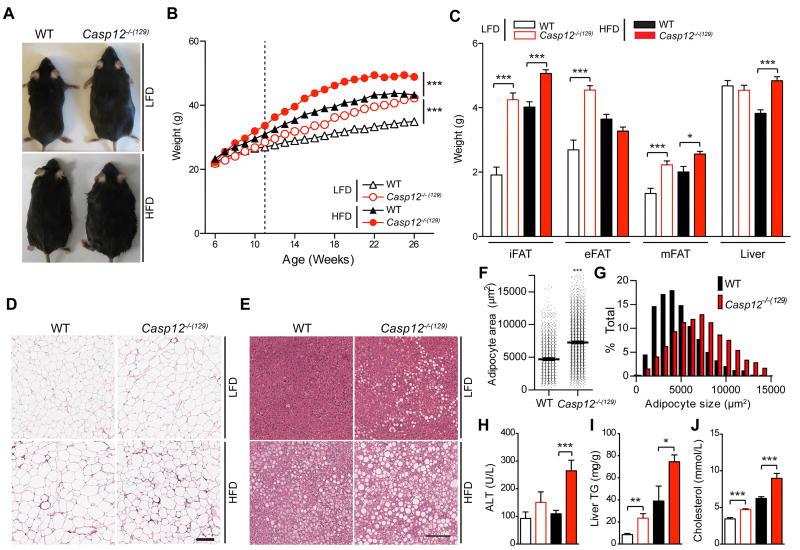
To further characterize the role of caspase-12 in obesity, we assessed the metabolic phenotype of Casp12−/−(129) mice fed a normal chow diet (LFD). Similarly to the HFD phenotype (filled symbols), loss of caspase-12 also led to obesity on LFD (open symbols), however this response was confined to male but not female mice (Figure 2A, B and S1B, C). The difference in body weight gain between LFD fed Casp12−/−(129) mice and WT controls was evident starting at 12 weeks of age (Figure 2A, B). The increased weight gain in Casp12−/−(129) mice corresponded to increased adiposity, as determined by DEXA scan (Figure S1D), increased adipose depot weight of the inguinal (iFAT), epididymal (eFAT), and mesenteric (mFAT) fat pads (Figure 2C), and increased eFAT adipocyte hypertrophy in LFD fed mice (Figure 2D, F, G). Although differences in liver weight and liver injury, as determined by serum ALT levels, were only apparent on HFD (Figure 2C, H), both diets resulted in increased hepatic lipid deposition and triglycerides in Casp12−/−(129) mice compared to WT controls (Figure 2E, I). Additionally, serum cholesterol was more elevated in Casp12−/−(129) mice when fed a HFD (Figure 1D and 2J). To determine if there were metabolic abnormalities associated with caspase-12 ablation, we first determined food intake in LFD-fed mice and found that it was similar between genotypes (Figure S2A). Next, we performed indirect calorimetry experiments on 8 week old WT and Casp12−/−(129) mice fed a LFD. We selected this time point as it is prior to the onset of differential body weight gains in the two genotypes to assess causative rather than consequential effects of obesity (Figure S2B). We observed no significant differences in respiration (VO2 or CO2), energy expenditure, or movement in the cages (Figure S2C–H). However, after HFD, Casp12−/−(129) mice had reduced respiration and movement (Figure S2I–O), suggesting that after the onset of obesity, metabolic abnormalities may begin to contribute to disease. Altogether, these results indicate that loss of caspase-12 in mice results in spontaneous obesity that is exacerbated with HFD feeding leading to fatty liver disease. This phenotype, however, is not a result of drastic intrinsic metabolic abnormalities in Casp12−/−(129) mice.
Figure 2. Casp12−/−(129) mice develop spontaneous obesity on a LFD.
A) Photos of male C57BL/6 WT mice and Casp12−/−(129) at 30 weeks of age, fed a LFD or HFD. B) Body weight curve of male mice. HFD started at 6 weeks of age, n=24–39 mice per group. C) Relative weight of adipose depots and liver at 26 weeks of age (n≥15 mice per group). D) H&E staining of epididymal adipose tissue at 26 weeks of age. Scale bar is 200 µM. E) H&E staining of liver tissue from 26 week old mice. Scale bar is 200 µM. F–G) Average epididymal adipocyte area from LFD fed mice. H) Serum ALT (LFD WT n=13, LFD Casp12−/−(129) n= 12, HFD WT n=12, HFD Casp12−/−(129) n=10). I) Hepatic triglycerides. (LFD WT n=14, LFD Casp12−/−(129) n=13, HFD WT n=6, HFD Casp12−/−(129) n=6). J) Serum cholesterol. Data are presented as the mean +/−SEM. Statistical analysis was performed using Student’s t-test. Statistical significance is presented as follows: ## P < .01, ### P<0.001 vs the WT LFD group; ** P < .01; *** P <0.001 vs the WT HFD group.
Caspase-12 deficient mice develop glucose intolerance and insulin resistance
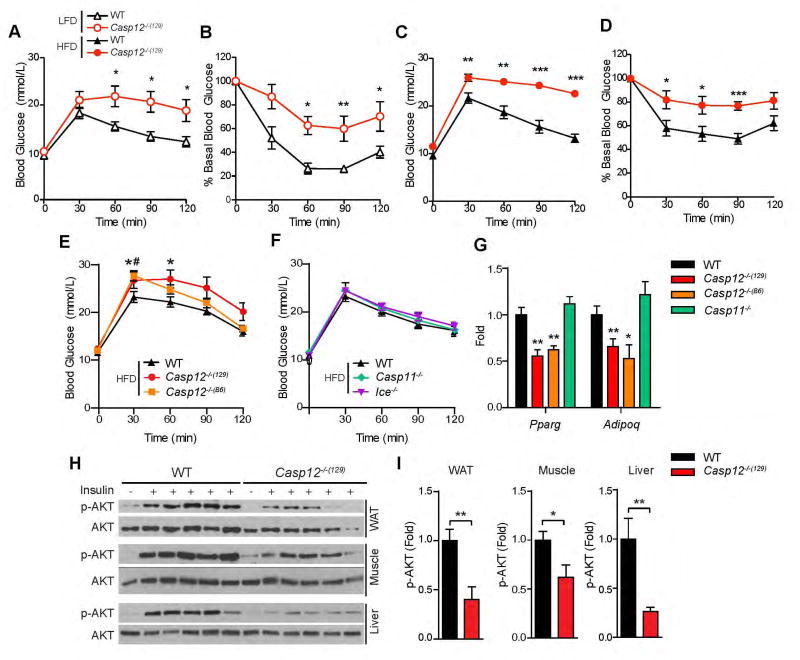
There is a significant correlation between obesity and metabolic diseases in humans and rodent models, particularly between obesity-induced metabolic inflammation and insulin resistance. To determine whether obese Casp12−/−(129) mice became insulin resistant, we performed glucose tolerance tests (GTT) and insulin tolerance tests (ITT). This analysis demonstrated that at approximately 20 weeks of age Casp12−/−(129) mice developed glucose intolerance and insulin resistance both when fed a LFD (Figure 3A, B) or a HFD (Figure 3C, D). Casp12−/−(B6) mice also had impaired tolerance to glucose compared to WT controls (Figure 3E), while loss of caspase-11 or caspase-1 did not have an effect at this time point (Figure 3F). Analysis of the epididymal fat revealed reduced expression of the insulin sensitive genes Adiponectin and Ppar-γ in caspase-12-deficient mouse strains but not in Casp11−/− mice (Figure 3H). Binding of insulin to its receptor leads to a downstream signalling cascade, resulting in phosphorylation of AKT. To assess insulin signalling in metabolic tissues, HFD-fed mice were injected intraperitoneally (i.p.) with a 5.0 U/kg bolus of insulin and levels of phosphorylated AKT in white adipose tissue (WAT), muscle and liver were analyzed 10 minutes later by immunoblot analysis. Figure 3H and 3I show that Casp12−/−(129) mice exhibited reduced insulin signalling in all three metabolic tissues.
Figure 3. Casp12−/− mice are glucose intolerant and insulin resistant.
A) LFD fed C57BL/6 WT and Casp12−/−(129) mice were injected i.p. with 2.0 mg/g dextrose at 20 weeks of age (n=5). B) LFD WT and Casp12−/−(129) mice were injected i.p. with 0.75 U/kg insulin at 20 weeks of age (n=4). C) HFD fed WT and Casp12−/−(129) mice were injected i.p. with 2.0 mg/g dextrose at 20 weeks old (n=6–7). D) HFD fed mice were injected with 2.0 U/kg of insulin at 25 weeks of age (n=6). E) GTT of WT, Casp12−/−(129) and Casp12−/−(B6) mice at 20 weeks of age (n=8). F) GTT of WT, Casp11−/− and Ice−/− mice at 20 weeks of age (n=8). G) qPCR analysis of epididymal adipose tissue in HFD mice (n=8–16). H) WT and Casp12−/−(129) mice on HFD were injected i.p. with 5.0 U/kg insulin and sacrificed 10 minutes later. Tissues were frozen and immunoblotted for Ser-473 phospho-AKT and total AKT. I) Ratio of phospho-AKT to total AKT was calculated using ImageJ. Error bars represent +/− SEM. Determined by student’s t-test. (*p < 0.05, **p < 0.01, ***p < 0.001 vs. WT).
Ablation of Caspase-12 in the radioresistant compartment leads to obesity
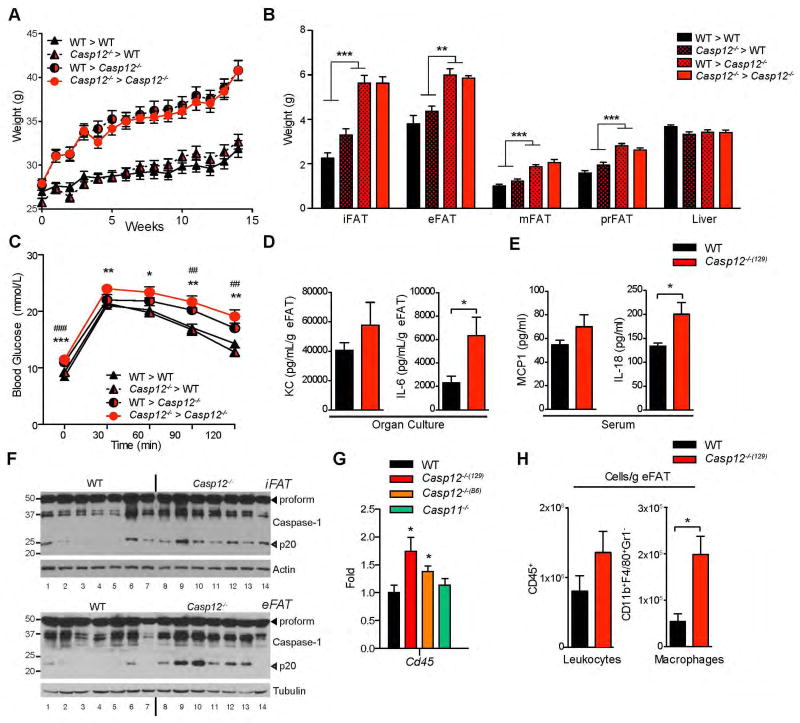
To further delineate how caspase-12 may be contributing to the development of obesity, we generated bone marrow chimeras. Interestingly, HFD-fed Casp12−/−(129) recipient mice developed increased body weight, adiposity, and liver weight irrespective of whether they were transplanted with bone marrow from WT or Casp12−/−(129) donors (Figure 4A–B). Thus, the genotype of the transplanted hematopoietic cells had little bearing on the obesity phenotype of Casp12−/−(129) mice. Similarly loss of caspase-12 in the radioresistant compartment was responsible for mediating the glucose intolerance phenotype (Figure 4C). These results suggested that both obesity and insulin resistance in Casp12−/−(129) mice are likely independent of bone marrow-derived immune cells but instead may be linked to the function of caspase-12 in radioresistant cells.
Figure 4. Loss of caspase-12 in the radioresistant compartment results in obesity and glucose intolerance.
Age matched male C57BL/6 WT and Casp12−/−(129) recipient mice were irradiated and reconstituted with bone marrow from WT and Casp12−/−(129) donor mice. The mice were then fed a HFD. A) Body weight curve. (n=12–13) B) Relative weight of adipose depots and liver after 14 weeks of HFD. C) GTT after 14 weeks of HFD injected i.p. with 2.0 mg/g dextrose. Statistical analysis was performed using Student’s t-test. Statistical significance is presented as follows: * P < 0.05, ** P < 0.01; *** P <0.001 for WT>WT vs Casp12>Casp12. ## P < 0.01, ### P <0.001 for WT>WT vs WT>Casp12. D) Organ culture of epididymal adipose tissue excised from HFD mice and incubated overnight in media. IL-6 and KC levels were analyzed by ELISA and normalized to adipose tissue mass. E) Serum ELISA of HFD mice. F) Epididymal (eFAT) adipose depots were excised from 26 week old HFD mice and immunobloted for Caspase-1. G) qPCR analysis of eFAT from HFD fed mice. (n=7–16 per genotype). H) FACS analysis of eFAT infiltrating immune cells after HFD (n=5 per genotype).
Caspase-12 deficiency leads to increased WAT inflammation
Given our previous findings that caspase-12 can regulate various inflammatory pathways, including caspase-1, NOD and NF-κB Signalling (16–18), we examined adipose tissue inflammation in DIO WT and Casp12−/−(129) mice. Overnight organ culture of epididymal adipose tissue from Casp12−/−(129) mice after 20 weeks of HFD revealed enhanced secretion of IL-6, and to a lesser extent KC (Figure 4D). Similarly, Casp12−/−(129) mice had significantly more serum IL-18 cytokine, but not MCP1, compared to WT mice (Figure 4E). Consistent with increased IL-18 levels, Casp12−/−(129) and Casp12−/−(B6) mice also exhibited increased activation of caspase-1 in the epididymal adipose tissue, evident by elevated levels of the active p20 fragment, as detected by western blot (Figure 4F and S3A). The epididymal adipose tissue of Casp12−/−(129) and Casp12−/−(B6) had increased expression of CD45 suggestive of increased immune cell infiltration (Figure 4G). We next determined levels of immune cell infiltrates in the epididymal adipose tissue stromal vascular fraction (SVF) from DIO mice by FACS analysis. Quantification of the numbers of neutrophils, myeloid cells, NK cells, and T and B lymphocytes revealed a general trend of increased immune cell infiltration in the WAT of Casp12−/−(129) mice compared to WT controls with macrophages being most significantly increased in the absence of caspase-12 (Figure 4H and S3B).
Ablation of Nlrp3 but not Ripk2 reverses the obesity and insulin resistance phenotype of Casp12−/−(129) mice
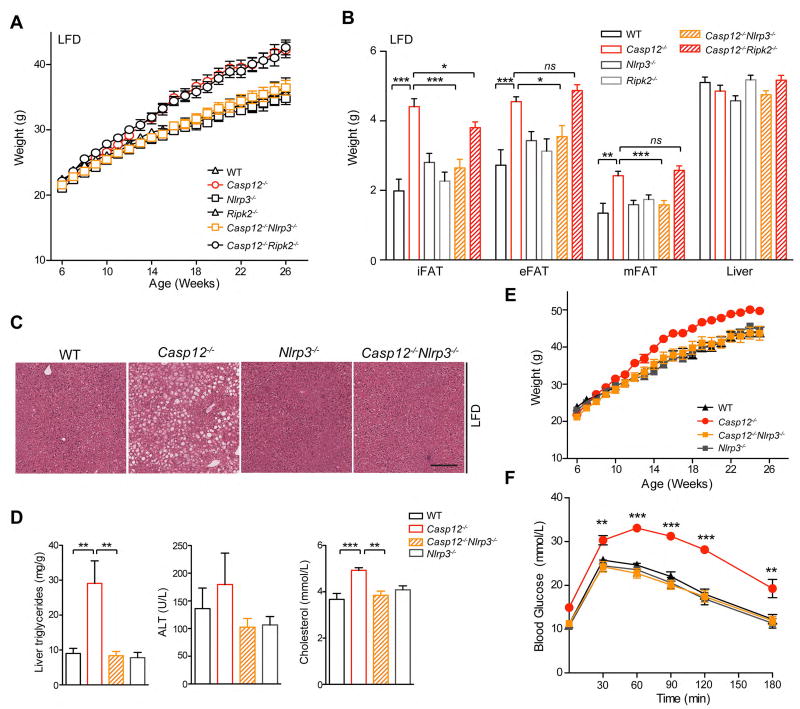
To define the mechanism of caspase-12 action in metabolism and metabolic inflammation and determine whether the inflammasome or Nod1/2 pathways (17; 18) were involved, we bred Casp12−/−(129) mice with Nlrp3−/− mice or mice deficient in the Nod1/2 pathways central adaptor Receptor-interacting protein kinase (Ripk)2 (29) to generate Casp12−/−Nlrp3−/− and Casp12−/− Ripk2−/− double-knockout (DKO) mice. Deletion of Ripk2 did not modify the Casp12−/− mouse obesity phenotype; Casp12−/−Ripk2−/− mice were similar in weight to Casp12−/− mice, both being significantly heavier than Ripk2−/− and WT mice (Figure 5A). Interestingly, in contrast to Casp12−/− and Casp12−/−Ripk2−/− mice, Casp12−/−Nlrp3−/− mice did not develop increased bodyweight gain and were similar to Nlrp3−/− mice and WT controls (Figure 5A). These results suggest that the Nlrp3 inflammasome may promote obesity in Casp12−/− mice. As expected from body weight differences, Casp12−/−Nlrp3−/− mice were similar in adiposity and liver weight to Nlrp3−/− and WT mice, which are significantly reduced than in Casp12−/− mice (Figure 5B). Histopathological examination of the liver revealed that both Casp12−/−Nlrp3−/− and Nlrp3−/− mice had similar levels of steatosis and liver triglycerides compared to WT mice, which is significantly attenuated compared to Casp12−/− mice (Figure 5C–D). Serum cholesterol of the DKO mice was similarly reduced compared to Casp12−/− mice (Figure 5D). Casp12−/−Nlrp3−/− DKO mice were also analyzed on a HFD, and as for the LFD, these mice were leaner and more glucose tolerant compared to Casp12−/− mice (Figure 5E, F).
Figure 5. Casp12−/− Nlrp3−/− mice are protected from obesity and glucose intolerance.
Casp12−/− mice were crossed with Ripk2−/− or Nlrp3−/− mice to generate double knock-out strains Casp12−/−Nlrp3−/− and Casp12−/−Ripk2−/−. A) Body weight curve of male mice fed a LFD. B) Relative weight of adipose depots and liver at 26 weeks of age. Statistical analysis was performed using Student’s t-test. Statistical significance is presented as follows: * P < 0.05, ** P < 0.01; *** P <0.001 vs the Casp12−/− group. C) H&E staining of liver tissue of 26 week old mice. Scale bar is 200 µM. D) Hepatic triglycerides and serum ALT. E) Serum cholesterol. F) Body weight curve of male mice fed a HFD. G) GTT of HFD mice at 22 weeks of age injected i.p. with 2.0 mg/g dextrose. Error bars represent +/− SEM. Determined by student’s t-test. (*p < 0.05, **p < 0.01, ***p < 0.001 vs. WT).
Human caspase-12 expression dampens metabolic inflammation but the human CASP12 T125C functional allele is not associated with metabolic disease
A SNP in exon 4 of the human CASP12 gene at amino acid position 125 (T125) introduces a premature stop codon and precludes expression of caspase-12 from the majority of the human population. In contrast, a fraction of people of African descent carry a functional allele due to a T125C SNP (nucleotide substitution c.373C>T) (20). To assess if the expression of human caspase-12 plays a role in modulating metabolic inflammation, we examined African American obese children of the Yale Pediatric Obesity cohort for inflammatory markers in both the serum and subcutaneous adipose tissue. ELISA measurements revealed decreased levels of C-reactive protein (CRP) (p=0.04) and IL-6 (p=0.002) in the serum of carriers of the function C allele. This was most significant in boys (p=0.02 for CRP and p=0.006 for IL-6) compared to girls (p=0.95 for CRP and p=0.11 for IL-6) (Figure 6A–F). Consistently, qPCR analysis of the subcutaneous adipose tissue indicated decreased levels of macrophages and IL-6 and TNF expression in carriers of the C allele (Figure 6G–I). Next, to determine whether the CASP12 polymorphism is associated with metabolic disease parameters, we examined the African American cohort of the Dallas Heart Study (21). Association of the c.373T>C SNP with BMI, fasting blood glucose, HOMA-IR, hepatic triglycerides, and serum triglycerides revealed no protective effect in carriers of the C allele (Table 1). Together, these results suggest that in humans, a functional caspase-12 may not lead to obesity similarly to the effects observed in mice, but may contribute to suppressing inflammation associated with metabolic disease.
Figure 6. Human caspase-12 dampens metabolic inflammation.
Subjects carrying the risk allele for the T125C SNP showed lower CRP (A–C) and IL-6 (D–F) levels than the non-carriers. This phenomenon seemed to be more marked in males than in females. In the subgroup of subjects who underwent an adipose tissue biopsy, the risk allele was associated with a lower percent of macrophages, and a lower expression of CD68, IL-6 and TNF-alpha (G–I).
Table 1. Analysis of T125C SNP in African American cohort of Dallas Heart Study.
The DHS population was genotyped for the Caspase-12 T125C SNP using the Illumina HumanExome chip, as described in the Methods. The analysis was performed using linear regression, adjusted for age and BMI and stratified by gender.
Demographic, anthropometric and clinical characteristics of African American participants of the Dallas Heart Study stratified by gender and CASP12 R125X (rs497116) genotype.
| Characteristic | Female (N=1,412) | P- value |
Male (N=955) | P- value |
Total (N=2,367) | P- value |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| TT (N=1042) |
TC (N=345) |
CC (N=25) |
TT (N=696) |
TC (N=238) |
CC (N=21) |
TT (N=1738) |
TC (N=583) |
CC (N=46) |
||||
| Age, years (N=2,367) | 46±11 | 44±11 | 45±8 | 0.14 | 45±11 | 44±11 | 47±12 | 0.87 | 45±11 | 44±11 | 46±10 | 0.22 |
| BMI, kg/m2 (N=2,349) | 32.2±8.3 | 32.6±7.7 | 35.3±6.9 | 0.038 | 28.6±6.4 | 29.4±7.1 | 29.6±7.3 | 0.13 | 30.7±7.8 | 31.3±7.6 | 32.6±7.6 | 0.011 |
| Glucose, mg/dLa (N=2,003) | 90±12 | 91±12 | 93±11 | 0.29 | 92±12 | 93±15 | 92±11 | 0.23 | 91±12 | 92±14 | 93±11 | 0.11 |
| HOMA-IR, Ub (N=2,031) | 4.5±4.4 | 4.8±4.1 | 5.2±3.3 | 0.37 | 4.3±5.0 | 4.6±5.8 | 5.7±7.7 | 0.37 | 4.5±4.6 | 4.7±4.9 | 5.4±5.7 | 0.92 |
| TG, mg/dLb (N=2,365) | 83 (61–114) | 78 (61–116) | 73 (63–87) | 0.76 | 91 (65–133) | 92 (66–134) | 85 (60–148) | 0.68 | 86 (63–122) | 84 (63–122) | 75 (60–120) | 0.60 |
| HTGC, % b (N=1,065) | 3.3 (1.9–5.1) | 3.3 (2.0–6.3) | 3.1 (1.6–3.8) | 0.47 | 3.1 (2.0–5.3) | 3.4 (2.0–5.3) | 2.4 (1.4–3.7) | 0.44 | 3.2 (2.0–5.2) | 3.3 (2.0–5.8) | 2.9 (1.1–3.9) | 0.96 |
| CRP (N=1,761) | 4.5 (1.8–9.6) | 5 (2.1–10.4) | 8.9 (5.7–12.8) | 0.056 | 2.2 (1.0–5.5) | 2.4 (1.1–6.7) | 2.0 (1.1–4.0) | 0.46 | 3.5 (1.4–8.1) | 3.9 (1.5–9.2) | 5.5 (2.0–10.0) | 0.058 |
Values are mean ±SD or median (interquartile range). Comparisons were made using linear regression models, adjusted for gender, age, and body-mass index, as necessary. The numbers in parentheses indicate the number of individuals with available data for each phenotype. BMI – body-mass index; TG – triglycerides; HTGC – hepatic triglyceride content.
Diabetic individuals excluded.
Analysis adjusted for diabetes in addition to other covariates.
DISCUSSION
It is now well known that inflammation and metabolism are tightly interwoven processes, and fluctuations in one can have detrimental consequences in the other, potentially leading to disease. Studies in mice and humans has implicated various inflammatory pathways, including caspase-1 and the inflammasome, to obesity and diabetes (30). In this study we investigated the role of the two related inflammatory caspases, caspase-11 and caspase-12 in a DIO mouse model. Interestingly, two mouse strains deficient in caspase-12 developed obesity on a HFD while Casp11−/− or Ice−/− mice did not. The increased weight gain and adiposity of caspase-12-deficient mice was also associated with glucose intolerance and insulin resistance. As Casp11−/− mice were equivalent to WT mice, deficiency in caspase-11 is unlikely to mediate the phenotype of Casp12−/−(129) mice. This was confirmed in Casp12−/−(B6) mice that are sufficient for caspase-11. Caspase-11 is known to recognize intracellular LPS and intracellular bacterial pathogens (31). Obesity has been linked to increased gut permeability, resulting in elevated levels of circulating microbial products and LPS (32). While studies have linked numerous PRRs that detect bacterial motifs, such as Nod1/Nod2 (33) and TLR4 (34) to metabolic disease, caspase-11 may not play a similar role.
Caspase-12 has previously been demonstrated to have anti-inflammatory properties. While the protease is catalytically active, currently its only known substrate is itself (35) and autoproteolysis was determined to not be required for its ability to inhibit both caspase-1 and NF-κB activity (16; 18). This suggests that caspase-12 primarily functions through modulating various signalling pathways through its ability to form CARD-CARD interactions. These include binding to caspase-1 (18), Ripk2 (17), NF-κB (16) and RIG-I (19), stearically hindering protein interactions and leading to attenuation of inflammation. Concordant with these functions, in our DIO mice we observed increased inflammation in the visceral adipose tissue, characterized by increased inflammasome activation and immune cell infiltration. However, it is difficult to determine if the increased inflammation is a consequence of obesity, rather than an underlying cause.
It is unknown how caspase-12 may be affecting host metabolism, leading to the obese state of the mice. Indirect calorimetry and food intake measurements revealed no influence conferred by caspase-12 ablation. However the obesity phenotype was dependent on loss of caspase-12 in the non-myeloid compartment. Caspase-12 is expressed in the muscle, liver and fat (data not shown), tissues that are highly associated with metabolic health. Tissue specific knockouts are required to determine precisely where the enzyme is required to suppress obesity. Interestingly, when Nlrp3, but not Ripk2, was deleted in the caspase-12 null background, the mice were protected from increased adiposity and weight gain. The Nlrp3 inflammasome and caspase-1 have been previously linked to the regulation of metabolic health in humans and animal models, though there is some discrepancy in how it may regulate obesity in rodents. Caspase-1, Nlrp3, and ASC deficient mice have been reported to be leaner than WT controls (11; 12), equivalent in body weight (7; 8) or have increased adiposity (14; 15; 36). Obesity in these mice is also correlated with reduced glucose tolerance, suggesting that any beneficial effects on metabolic health that loss of the inflammasome may have, are not significant enough to counteract other pathways influenced by obesity. Differences in adipogenesis (11), cleavage of SIRT1 (37), and alteration of lipid metabolism (13; 15) are a few of the proposed mechanisms by which caspase-1 may regulated metabolic processes. In the context of obesity, caspase-12 might impact caspase-1 directly or indirectly. Loss of Nlrp3 may antagonize direct effects of caspase-12 on caspase-1, conferring a lean phenotype. Alternatively, caspase-12 may affect a parallel metabolic pathway, that is reversed by loss of Nlrp3. For example, the inflammasome has been linked to gut microbiota dysbiosis, which affects obesity (14). Potential microbial dysbiosis in caspase-12 deficient mice may be altered upon removal of Nlrp3. Further work is required to determine how caspase-12 may function in non-myeloid cells and how these potential interactions may drive metabolic health in mice.
While the majority of people lack a functional caspase-12, the T125C SNP has persisted in a small percent of people of African descent. Many studies have examined why the functional caspase-12 allele has been maintained, including examining a potential role in protection against bacterial sepsis (20; 38), candidemia (39), and rheumatoid arthritis (40). Our previous work has implicated that the T125C SNP may confer susceptibility to sepsis (20), however a second study assessing community-acquired pneumonia, did not report similar findings (38). There is no clear indication what role caspase-12 may play in people and given how the loss of caspase-12 led to obesity in mice, we investigated if it may have similar properties in the human population. In the Yale Pediatric Cohort, the caspase-12 SNP was associated with reduced inflammatory parameters in the serum and adipose tissue. These results correlate with previous findings implicating Caspase-12 playing an anti-inflammatory role. However, genotyping the Dallas Heart Study cohort of African descent for the T125C SNP indicated that there was no association with improved metabolic parameters. These results are at odds with our findings in mice, suggesting that host specific differences are involved. It is possible that caspase-12 is differentially regulated in mice and humans. For example, we have previously reported that human caspase-12 is repressed by estrogen, an effect not observed in the murine system (41). Therefore in humans, loss of caspase-12 appears to recapitulate the pro-inflammatory effects observed in mice during obesity though may not be involved with the development of an altered metabolic state. Further analyses of other populations are required to further clarify its role.
Supplementary Material
Highlights.
Loss of caspase-12 results in spontaneous obesity and insulin resistance;
Expression of caspase-12 in the radio-resistant compartment mediates its activity in metabolic regulation;
The Nlrp3 inflammasome promotes obesity in caspase-12-deficient mice;
Expression of caspase-12 in humans is associated with reduced metabolic inflammation
Acknowledgments
We thank the Wellcome Trust Sanger Institute for providing Casp12−/−(B6) (Casp12tm1a(KOMP)Wtsi) mice; V. Dixit, Genentech, for providing Casp11−/− and Nlrp3−/− mice; R. Flavell, Yale University, for providing Ice−/− and Ripk2−/− mice; Helen Hobbs, University of Texas Southwestern Medical Center, and the Dallas Heart Study investigators; J. Rinz, McGill University, and G. Perrault, McGill University, for animal husbandry. Anna Kinio for technical assistance.
FUNDING
This work was supported by grants from the Canadian Institutes for Health Research (CIHR) and the Burroughs Wellcome Fund to M.S. who is a Fonds de Recherche en Santé du Québec (FRSQ) Senior Investigator and a McGill University William Dawson Scholar. A.M.S. was supported by a doctoral award from the (CIHR), A.M. was supported by a CIHR/CAG/Abbott post-doctoral fellowship and is part of the Mitacs Accelerate program, and T.D. was supported by a FRSQ doctoral award. The Dallas Heart Study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105.
Footnotes
AUTHOR CONTRIBUTIONS
A.M.S. designed and performed experiments, analyzed the data, and wrote and edited the manuscript. A.M. and T.D. performed experiments; J.K. analyzed the DHS data. M.S. designed experiments, analyzed the data, and wrote and edited the manuscript. S.C. recruited the Yale cohort patients, N.S. genotyped the T125C SNP, R.K. collected adipose tissue samples and performed the qPCR, WZM designed the data acquisition and data analysis of the Yale cohort patients.
References
- 1.McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36–48. doi: 10.1016/j.immuni.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 4.Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 5.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 6.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nunez G, Yodoi J, Kahn SE, Lavelle EC, O'Neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 10.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 11.Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, Rensen PC, Voshol PJ, Fantuzzi G, Hijmans A, Kersten S, Muller M, van den Berg WB, van Rooijen N, Wabitsch M, Kullberg BJ, van der Meer JW, Kanneganti T, Tack CJ, Netea MG. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, Hooiveld GJ, Hijmans A, Vroegrijk I, van den Berg S, Romijn J, Rensen PC, Joosten LA, Netea MG, Kanneganti TD. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108:15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Diepen JA, Stienstra R, Vroegrijk IO, van den Berg SA, Salvatori D, Hooiveld GJ, Kersten S, Tack CJ, Netea MG, Smit JW, Joosten LA, Havekes LM, van Dijk KW, Rensen PC. Caspase-1 deficiency in mice reduces intestinal triglyceride absorption and hepatic triglyceride secretion. J Lipid Res. 2013;54:448–456. doi: 10.1194/jlr.M031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotas ME, Jurczak MJ, Annicelli C, Gillum MP, Cline GW, Shulman GI, Medzhitov R. Role of caspase-1 in regulation of triglyceride metabolism. Proc Natl Acad Sci U S A. 2013;110:4810–4815. doi: 10.1073/pnas.1301996110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labbe K, Miu J, Yeretssian G, Serghides L, Tam M, Finney CA, Erdman LK, Goulet ML, Kain KC, Stevenson MM, Saleh M. Caspase-12 dampens the immune response to malaria independently of the inflammasome by targeting NF-kappaB signaling. J Immunol. 2010;185:5495–5502. doi: 10.4049/jimmunol.1002517. [DOI] [PubMed] [Google Scholar]
- 17.LeBlanc PM, Yeretssian G, Rutherford N, Doiron K, Nadiri A, Zhu L, Green DR, Gruenheid S, Saleh M. Caspase-12 modulates NOD signaling and regulates antimicrobial peptide production and mucosal immunity. Cell Host Microbe. 2008;3:146–157. doi: 10.1016/j.chom.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Saleh M, Mathison JC, Wolinski MK, Bensinger SJ, Fitzgerald P, Droin N, Ulevitch RJ, Green DR, Nicholson DW. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature. 2006;440:1064–1068. doi: 10.1038/nature04656. [DOI] [PubMed] [Google Scholar]
- 19.Wang P, Arjona A, Zhang Y, Sultana H, Dai J, Yang L, LeBlanc PM, Doiron K, Saleh M, Fikrig E. Caspase-12 controls West Nile virus infection via the viral RNA receptor RIG-I. Nat Immunol. 2010;11:912–919. doi: 10.1038/ni.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saleh M, Vaillancourt JP, Graham RK, Huyck M, Srinivasula SM, Alnemri ES, Steinberg MH, Nolan V, Baldwin CT, Hotchkiss RS, Buchman TG, Zehnbauer BA, Hayden MR, Farrer LA, Roy S, Nicholson DW. Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature. 2004;429:75–79. doi: 10.1038/nature02451. [DOI] [PubMed] [Google Scholar]
- 21.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 22.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA, Medzhitov R, Flavell RA. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 24.Santoro N, Feldstein AE, Enoksson E, Pierpont B, Kursawe R, Kim G, Caprio S. The association between hepatic fat content and liver injury in obese children and adolescents: effects of ethnicity, insulin resistance, and common gene variants. Diabetes Care. 2013;36:1353–1360. doi: 10.2337/dc12-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kursawe R, Caprio S, Giannini C, Narayan D, Lin A, D'Adamo E, Shaw M, Pierpont B, Cushman SW, Shulman GI. Decreased transcription of ChREBP-alpha/beta isoforms in abdominal subcutaneous adipose tissue of obese adolescents with prediabetes or early type 2 diabetes: associations with insulin resistance and hyperglycemia. Diabetes. 2013;62:837–844. doi: 10.2337/db12-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH Dallas Heart Study I. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. The American journal of cardiology. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 27.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 28.Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjaerg-Hansen A, Vogt TF, Hobbs HH, Cohen JC. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14:9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 30.Haneklaus M, O'Neill LA. NLRP3 at the interface of metabolism and inflammation. Immunol Rev. 2015;265:53–62. doi: 10.1111/imr.12285. [DOI] [PubMed] [Google Scholar]
- 31.Stowe I, Lee B, Kayagaki N. Caspase-11: arming the guards against bacterial infection. Immunol Rev. 2015;265:75–84. doi: 10.1111/imr.12292. [DOI] [PubMed] [Google Scholar]
- 32.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 33.Schertzer JD, Tamrakar AK, Magalhaes JG, Pereira S, Bilan PJ, Fullerton MD, Liu Z, Steinberg GR, Giacca A, Philpott DJ, Klip A. NOD1 activators link innate immunity to insulin resistance. Diabetes. 2011;60:2206–2215. doi: 10.2337/db11-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy S, Sharom JR, Houde C, Loisel TP, Vaillancourt JP, Shao W, Saleh M, Nicholson DW. Confinement of caspase-12 proteolytic activity to autoprocessing. Proc Natl Acad Sci U S A. 2008;105:4133–4138. doi: 10.1073/pnas.0706658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Capell W, Yoon JH, Faubel S, Eckel RH. Obesity development in caspase-1-deficient mice. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.59. [DOI] [PubMed] [Google Scholar]
- 37.Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 2012;16:180–188. doi: 10.1016/j.cmet.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J, Wilson ES, Dahmer MK, Quasney MW, Waterer GW, Feldman C, Wunderink RG. Lack of association of the caspase-12 long allele with community-acquired pneumonia in people of African descent. PLoS One. 2014;9:e89194. doi: 10.1371/journal.pone.0089194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosentul DC, Plantinga TS, Scott WK, Alexander BD, van de Geer NM, Perfect JR, Kullberg BJ, Johnson MD, Netea MG. The impact of caspase-12 on susceptibility to candidemia. Eur J Clin Microbiol Infect Dis. 2012;31:277–280. doi: 10.1007/s10096-011-1307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marshall L, Obaidullah M, Fuchs T, Fineberg NS, Brinkley G, Mikuls TR, Bridges SL, Jr, Hermel E. CASPASE-12 and rheumatoid arthritis in African-Americans. Immunogenetics. 2014;66:281–285. doi: 10.1007/s00251-014-0762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeretssian G, Doiron K, Shao W, Leavitt BR, Hayden MR, Nicholson DW, Saleh M. Gender differences in expression of the human caspase-12 long variant determines susceptibility to Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 2009;106:9016–9020. doi: 10.1073/pnas.0813362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.