Significance
Abnormal blood vessel growth occurs in many common diseases, from cancers and cardiovascular diseases to ocular conditions like age-related macular degeneration (AMD) and is thus a major target of many recent treatment approaches. Long-chain polyunsaturated fatty acids are of significant interest in this context, since bioactive lipid metabolites derived from this pathway have been shown to be potent regulators of inflammation and angiogenesis. Our study has identified key compounds of the cytochrome P450 metabolic pathway that are responsible for resolving abnormal vascular growth in AMD, which work in part by modulating the recruitment of inflammatory immune cells. We believe these findings have significant therapeutic implications not only for AMD but also for other inflammatory disorders.
Keywords: P450, choroidal neovascularization, oxylipin, omega-3 fatty acids, lipid metabolites
Abstract
Age-related macular degeneration (AMD) is the most common cause of blindness for individuals age 50 and above in the developed world. Abnormal growth of choroidal blood vessels, or choroidal neovascularization (CNV), is a hallmark of the neovascular (wet) form of advanced AMD and leads to significant vision loss. A growing body of evidence supports a strong link between neovascular disease and inflammation. Metabolites of long-chain polyunsaturated fatty acids derived from the cytochrome P450 (CYP) monooxygenase pathway serve as vital second messengers that regulate a number of hormones and growth factors involved in inflammation and vascular function. Using transgenic mice with altered CYP lipid biosynthetic pathways in a mouse model of laser-induced CNV, we characterized the role of these lipid metabolites in regulating neovascular disease. We discovered that the CYP-derived lipid metabolites epoxydocosapentaenoic acids (EDPs) and epoxyeicosatetraenoic acids (EEQs) are vital in dampening CNV severity. Specifically, overexpression of the monooxygenase CYP2C8 or genetic ablation or inhibition of the soluble epoxide hydrolase (sEH) enzyme led to increased levels of EDP and EEQ with attenuated CNV development. In contrast, when we promoted the degradation of these CYP-derived metabolites by transgenic overexpression of sEH, the protective effect against CNV was lost. We found that these molecules work in part through their ability to regulate the expression of key leukocyte adhesion molecules, on both leukocytes and endothelial cells, thereby mediating leukocyte recruitment. These results suggest that CYP lipid signaling molecules and their regulators are potential therapeutic targets in neovascular diseases.
Age-related macular degeneration (AMD) is a progressive chronic disease and a leading cause of irreversible visual impairment in developed countries (1). AMD is generally classified into two forms: exudative (“wet”) and nonexudative (“dry”). Advanced exudative AMD is characterized by neovascularization arising from the choroid and infiltrating into the subretinal space. This choroidal neovascularization (CNV) is immature and leaky in nature, resulting in subretinal and intraretinal edema and hemorrhage that can lead to severe vision loss (2, 3). Current standard therapy for patients with CNV involves inhibiting vascular endothelial growth factor (VEGF), a molecule that promotes angiogenesis and vascular permeability (3–6). Despite their therapeutic value, intravitreal injections of anti-VEGF agents act primarily to prevent vascular permeability and resultant edema; thus, constant anti-VEGF injections are necessary to keep these abnormal vessels at bay (4, 5, 7, 8). Anti-VEGF therapy in patients has also been shown to lead to progressive vision loss with long-term use (9). Substantial vision improvement occurs in only one-third of patients treated with VEGF antagonists, and one-sixth of treated patients still progress to legal blindness (4, 5). Moreover, anti-VEGF therapy minimally affects the contribution of immune cells, known to play a vital role in CNV disease progression and regression (10–13). Given these limitations, there is an urgent need for additional pharmacological interventions for the early treatment of exudative AMD.
Omega (ω)-3 and ω-6 fatty acids are two functionally distinct subsets of long-chain polyunsaturated fatty acids (LCPUFAs) (14, 15). Endogenous lipid bioactive metabolites derived from ω-3 and ω-6 LCPUFAs, termed oxylipins, are known to function in inflammation and overall tissue homeostasis (16). Mechanistically, the metabolism of arachidonic acid (AA), docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) into biologically active lipid mediators by cyclooxygenase (COX) and lipoxygenase (LOX) enzymes is relatively well established (17, 18). However, these LCPUFAs are also substrates for the cytochrome P450 (CYP) enzymes, which either hydroxylate AA to hydroxyeicosatetraenoic acids (HETEs) or epoxygenate AA to epoxyeicosatrienoic acids (EETs) (19, 20). Of the three main pathways involved in oxylipin biosynthesis (i.e., COX, LOX, and CYP), the lipid metabolites of the CYP branch are likely the most susceptible to changes in dietary fatty acid composition (20, 21). Importantly, the ω-3 double bond that distinguishes DHA and EPA from their ω-6 counterparts provides a preferred epoxidation site for specific CYP family members. Among the major CYP metabolites derived from DHA are epoxydocosapentaenoic acids (EDPs), while those derived from EPA are epoxyeicosatetraenoic acids (EEQs) (19). Interestingly, specific CYP family members can metabolize DHA and EPA up to 10 times faster than AA (20, 22). EETs, EDPs, and EEQs are known to generally oppose the inflammatory and vascular functions of their ω-hydroxylase counterparts such as 20-HETE, and are therefore of significant interest (19, 20). These fatty acid metabolites are produced primarily in the endothelium by CYP2C and CYP2J enzymes and are further metabolized principally by soluble epoxide hydrolase (sEH) into less active dihydroxyeicosatrienoic acids (DHETs), dihydroxydocosapentaenoic acids (DHDPs), and dihydroxyeicosatetraenoic acids (DHEQs) (derived from AA, DHA, and EPA, respectively), thereby conferring tight regulation of these bioactive lipid metabolites (19, 20, 23).
ω-3 LCPUFAs have recently been implicated in neovascular AMD (9, 24, 25). Moreover, we have previously reported that ω-3 LCPUFA-derived CYP metabolites, 17,18-EEQ and 19,20-EDP, are involved in regulating CNV (13). However, the precise role and mechanism of the CYP monooxygenases and the CYP-dependent metabolites in regulating neovascular ocular disease still remains unclear. In this study, we focused on defining the contribution of the CYP monooxygenases and their metabolites on CNV and elucidating the effect of CYP-dependent metabolites on leukocyte recruitment during CNV formation. We demonstrated that ω-3 CYP metabolites modulate CNV disease progression and severity using transgenic and knockout mice designed to increase or decrease the endogenous levels of epoxide metabolites (CYP2C8-transgenic and sEH-knockout versus sEH-transgenic mice). Moreover, we showed that ω-3–derived CYP metabolites regulate the leukocyte rolling velocity by modulating the expression of adhesion molecules both on the surface of circulating leukocytes and in the CNV lesions. These results indicate that CYP monooxygenases and the epoxides produced by these enzymes from EPA and DHA could be potent mediators of intraocular inflammation and neovascular disease regression.
Results
CYP-Derived Epoxide Metabolites of EPA and DHA Reduce CNV.
We have previously shown that a diet enriched in EPA and DHA or administration of ω-3–derived CYP metabolites (i.e., 17,18-EEQ and 19,20-EDP) reduces the size of CNV (13). However, the relative importance of the ω-3–derived CYP metabolites and their downstream metabolites (e.g., 17,18-DHEQ and 19,20-DHDP) has not been characterized. To investigate whether the CYP metabolic pathway is a critical pathway regulating CNV size, we used transgenic mice that modulate CYP pathway-selective enzymes. Tie2-CYP2C8-Tg mice overexpress the monooxygenase CYP2C8 in endothelial cells (and to a lesser extent immune cells), which promotes the metabolism of the primary LCPUFAs to their active downstream metabolites. The size of CNV lesions 7 d after laser induction was significantly decreased in Tie2-CYP2C8-Tg mice fed a diet enriched with EPA and DHA compared with mice fed a control diet, as assessed in choroidal flat mounts (Fig. 1A) and optical coherence tomography (OCT) cross-sectional images (Fig. 1B). Vascular leakage in CNV lesions, assessed by fluorescein angiography (FA), was also significantly decreased in Tie2-CYP2C8-Tg mice fed a diet enriched with EPA and DHA compared with mice on a control diet (Fig. 1C). sEH, an enzyme that functions downstream of the CYP pathway, metabolizes bioactive epoxy metabolites of the primary LCPUFAs into less bioactive vicinal diols (vic-diols). The loss of sEH had a similar effect as the overexpression of the monooxygenase CYP2C8 in endothelial cells. The size of CNV lesions 7 d after laser induction was significantly decreased in sEH null mice fed a diet enriched with EPA and DHA compared with mice on a control diet, as assessed in choroidal flat mounts (Fig. 2A) and OCT cross-sectional images (Fig. 2B). The severity of vascular leakage in CNV lesions was also significantly decreased in sEH null mice fed a diet enriched with EPA and DHA compared with mice on a control diet (Fig. 2C). These results suggest that the increase in CYP-derived epoxide metabolites of EPA and DHA by overexpression of CYP2C8 or by loss of sEH play an important role in suppression of CNV development.
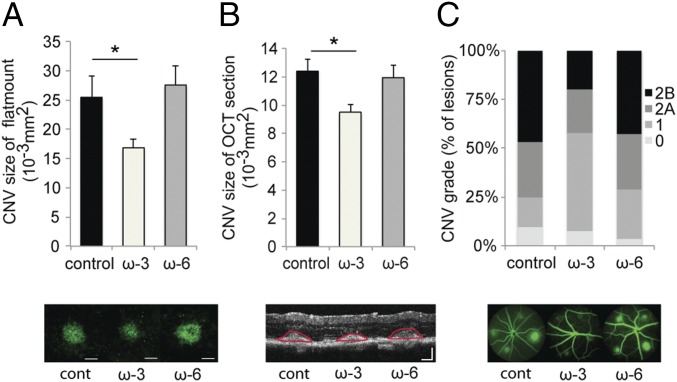
Fig. 1.
Dietary intake of ω-3 LCPUFAs in Tie2-CYP2C8-Tg mice attenuates CNV. (A and B) Lesion size at 7 days (d) after CNV induction was assessed by staining of choroidal flat-mount preparations with fluorescent isolectin B4 (A), and a cross-sectional area of lesions was quantified by SD-OCT (demarcated by red lines) (B), for Tie2-CYP2C8-Tg mice fed a control diet (n = 25 lesions, respectively), ω-3 LCPUFA-enriched diet (n = 38 lesions, respectively), or ω-6 LCPUFA-enriched diet (n = 30 lesions, respectively) beginning 2 weeks (wk) before laser photocoagulation. Tie2-CYP2C8-Tg mice overexpress the monooxygenase CYP2C8 in endothelial cells, which promotes the metabolism of the primary LCPUFAs to their active downstream fatty acid metabolites. ω-3 LCPUFAs decreased CNV size in choroidal flat-mount and OCT section compared with the control diet and ω-6 LCPUFA-diet groups. Data are presented as means ± SEM. *P < 0.05. (Scale bar: 100 μm.) (C) Fluorescein leakage in CNV lesions was graded at 7 d after CNV induction in Tie2-CYP2C8-Tg mice fed a control diet (n = 25 lesions), ω-3 LCPUFA-enriched diet (n = 38 lesions), or ω-6 LCPUFA-enriched diet (n = 30 lesions) beginning 2 wk before laser photocoagulation. ω-3 LCPUFAs also attenuated fluorescein leakage from the CNV lesions compared with the control diet and ω-6 diet groups. The grade of the hyperfluorescent lesions is as follows: score 0 (i.e., no leakage); score 1 (i.e., debatable leakage); score 2A (i.e., definite leakage); score 2B (i.e., clinically significant leakage).
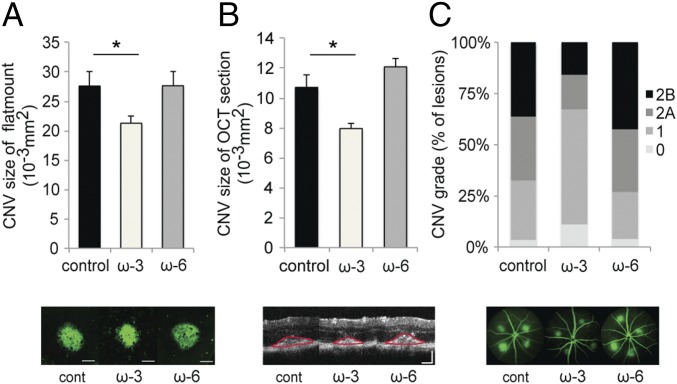
Fig. 2.
Dietary intake of ω-3 LCPUFAs in sEH null mice attenuates CNV. (A and B) Lesion size at 7 d after CNV induction was assessed by staining of choroidal flat-mount preparations with fluorescent isolectin B4 (A) and a cross-sectional area of lesions was quantified by SD-OCT (demarcated by red lines) (B), for sEH null mice fed a control diet (n = 49 lesions, respectively), ω-3 LCPUFA-enriched diet (n = 57 lesions, respectively), or ω-6 LCPUFA-enriched diet (n = 50 lesions, respectively) beginning 2 wk before laser photocoagulation. sEH null mice lack expression of sEH, an enzyme that degrades CYP-dependent epoxynoid fatty acid metabolites into less bioactive vic-diols. ω-3 LCPUFAs decreased CNV size in choroidal flat-mount and OCT section compared with the control diet and ω-6 LCPUFA-diet groups. Data are presented as means ± SEM. *P < 0.05. (Scale bar: 100 μm.) (C) Fluorescein leakage in CNV lesions was graded at 7 d after CNV induction in sEH null mice fed a control diet (n = 49 lesions), ω-3 LCPUFA-enriched diet (n = 57 lesions), or ω-6 LCPUFA-enriched diet (n = 50 lesions) beginning 2 wk before laser photocoagulation. ω-3 LCPUFAs also attenuated fluorescein leakage from the CNV lesions compared with the control diet and ω-6 diet groups. The grade of the hyperfluorescent lesions is as follows: score 0 (i.e., no leakage); score 1 (i.e., debatable leakage); score 2A (i.e., definite leakage); score 2B (i.e., clinically significant leakage).
The Protective Effect Observed from CYP-Derived Epoxide Metabolites of EPA and DHA Is Lost by Their Metabolic Degradation.
To confirm that CYP-derived epoxide metabolites of EPA and DHA are the primary mediators of protection against CNV, we examined Tie2-sEH mice (26). This strain overexpresses the sEH enzyme in endothelial cells and immune cells, which rapidly breakdown CYP epoxide metabolites into less bioactive vic-diols (27). When Tie2-sEH mice were fed a diet enriched with EPA and DHA, the protective effects conferred by a diet enriched in DHA and EPA on the CNV lesion was abolished (Fig. 3 A–C). Littermate control animals, on a C57BL/6 background, confirmed that a diet enriched with EPA and DHA was still protective against increased CNV disease severity (Fig. S1). These results suggest that the epoxide metabolites produced from EPA and DHA via the cytochrome P450s are conferring significant protection against CNV. In light of these findings, we speculated that CYP-derived eicosanoids of AA (i.e., EETs) promote CNV pathogenesis. However, contrary to our hypothesis, feeding a diet enriched with AA to CYP-Tg mice and administration of EETs to C57BL/6 mice did not alter CNV size or severity compared with their respective base line controls (Figs. 1–3 and Fig. S2). These data suggest that CYP-derived EEQs and EDPs confer protection rather than the AA-derived EETs as drivers of disease severity.
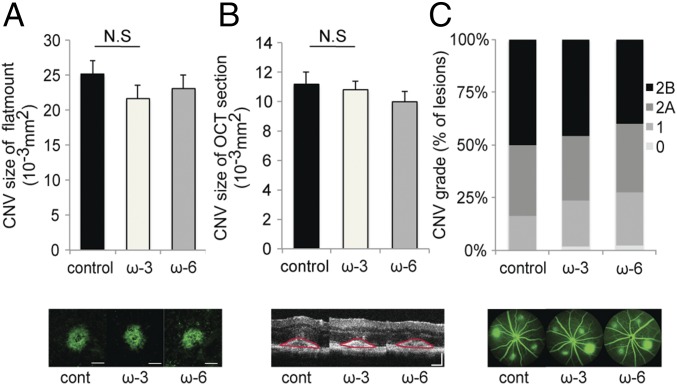
Fig. 3.
Dietary intake of ω-3 LCPUFAs in Tie2-sEH-Tg mice does not affect CNV. (A and B) Lesion size at 7 d after CNV induction was assessed by staining of choroidal flat-mount preparations with fluorescent isolectin B4 (A), and a cross-sectional area of lesions was quantified by SD-OCT (demarcated by red lines) (B), for Tie2-sEH-Tg mice fed a control diet (n = 21 lesions, respectively), ω-3 LCPUFA-enriched diet (n = 44 lesions, respectively), or ω-6 LCPUFA-enriched diet (n = 39 lesions, respectively) beginning 2 wk before laser photocoagulation. sEH overexpression in Tie2-sEH-Tg mice resulted in no significant differences in CNV size in choroidal flat-mount and OCT section among the three dietary groups. Data are presented as means ± SEM. N.S., not significant. (Scale bar: 100 μm.) (C) Fluorescein leakage in CNV lesions was graded at 7 d after CNV induction in Tie2-sEH-Tg mice fed a control diet (n = 21 lesions), ω-3 LCPUFA-enriched diet (n = 44 lesions), or ω-6 LCPUFA-enriched diet (n = 39 lesions) beginning 2 wk before laser photocoagulation. The severity of fluorescein leakage did not change among the three groups. The grade of the hyperfluorescent lesions is as follows: score 0 (i.e., no leakage); score 1 (i.e., debatable leakage); score 2A (i.e., definite leakage); score 2B (i.e., clinically significant leakage).
Fig. S1.
Dietary intake of ω-3 LCPUFAs in C57BL/6 background mice attenuates CNV. (A and B) Lesion size at 7 d after CNV induction was assessed by staining of choroidal flat-mount preparations with fluorescent isolectin B4 (A), and cross-sectional area of lesions was quantified by SD-OCT (demarcated by red lines) (B), for C57BL/6J background mice fed a control diet (n = 20 lesions, respectively), ω-3 LCPUFA-enriched diet (n = 26 lesions, respectively), or ω-6 LCPUFA-enriched diet (n = 40 lesions, respectively) beginning 2 wk before laser photocoagulation. The ω-3 LCPUFAs decreased CNV size in choroidal flat mounts and OCT sections compared with control diet and ω-6 LCPUFA-diet groups. Data are presented as means ± SEM *P < 0.05. (Scale bar: 100 μm.) (C) Fluorescein leakage in CNV lesions was graded at 7 d after CNV induction in C57BL/6 background mice fed a control diet (n = 20 lesions), ω-3 LCPUFA-enriched diet (n = 26 lesions), or ω-6 LCPUFA-enriched diet (n = 40 lesions) beginning 2 wk before laser photocoagulation. The ω-3 LCPUFAs also attenuated fluorescein leakage from the CNV lesions compared with the control diet and ω-6 diet groups. The grade of the hyperfluorescent lesions is as follows: score 0 (i.e., no leakage); score 1 (i.e., debatable leakage); score 2A (i.e., definite leakage); score 2B (i.e., clinically significant leakage).
Fig. S2.
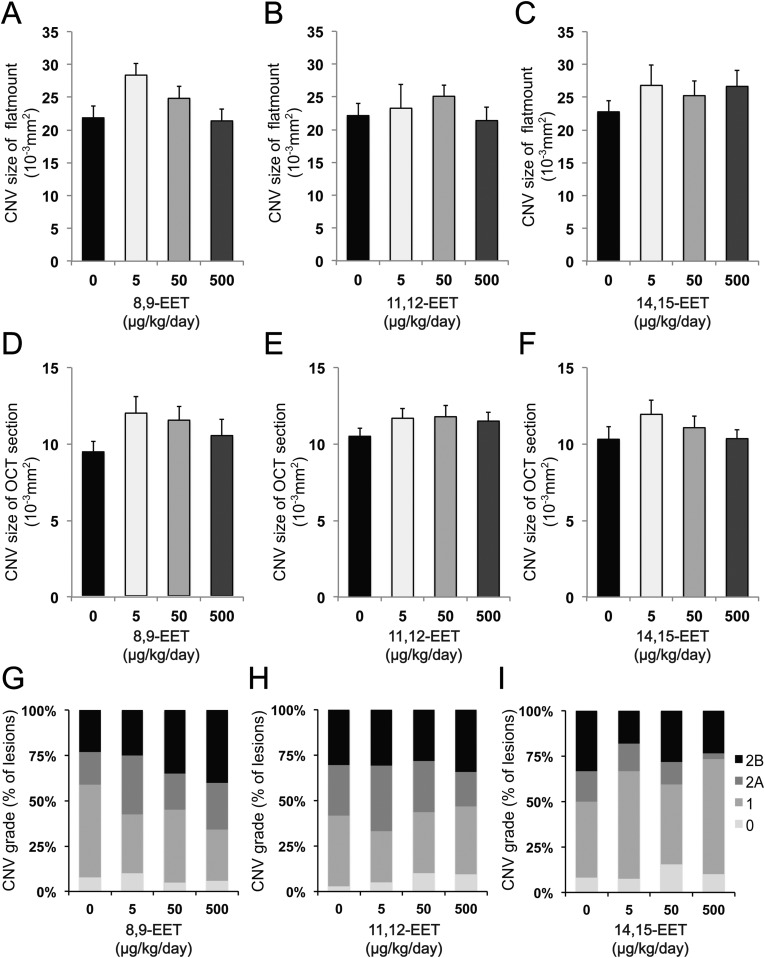
CYP-dependent ω-6 epoxides do not affect CNV. (A–F) Lesion size at 7 d after CNV induction was assessed by staining of choroidal flat-mount preparations with fluorescent isolectin B4 (A–C), and cross-sectional area of lesions was quantified by SD-OCT (D–F), for C57BL/6 mice administered 8,9-EET (A and D), 11,12-EET (B and E), and 14,15-EET (C and F) once a day immediately after laser photocoagulation. Data are presented as means ± SEM. (G–I) Fluorescein leakage in CNV lesions was graded at 7 d after CNV induction in C57BL/6 mice administered 8,9-EET (G), 11,12-EET (H), and 14,15-EET (I). n = 55–63 lesions per experimental group. The grade of the hyperfluorescent lesions is as follows: score 0 (i.e., no leakage); score 1 (i.e., debatable leakage); score 2A (i.e., definite leakage); score 2B (i.e., clinically significant leakage).
Derivatives of CYP Monooxygenases Are Significantly Regulated in Our CYP-Tg Mice.
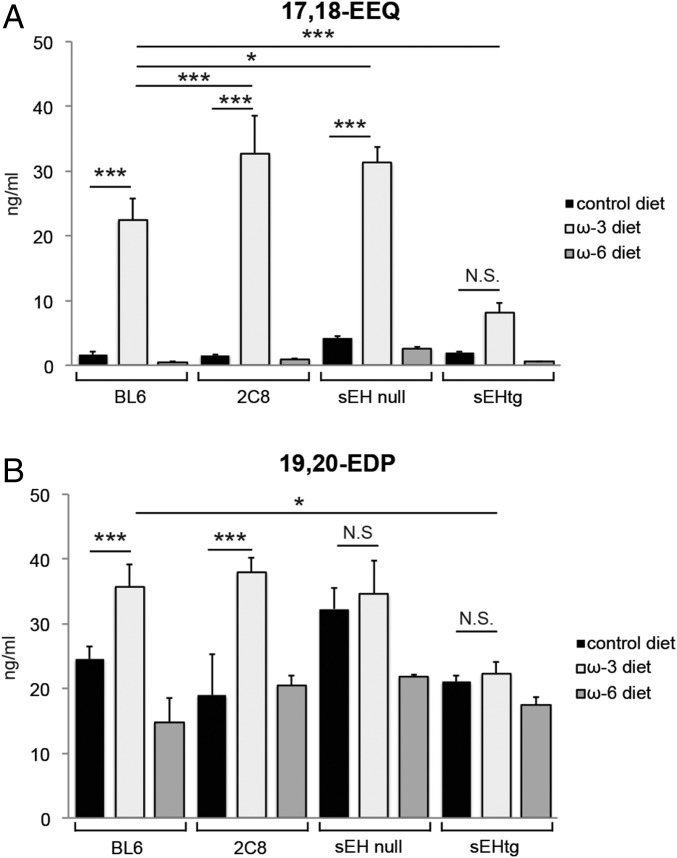
We previously identified 17,18-EEQ and 19,20-EDP, two metabolites synthesized by CYP monooxygenases, as potential mediators in CNV disease resolution (13). To assess if these lipid metabolites are significantly modulated in our transgenic mice, we measured the plasma concentrations of 17,18-EEQ and 19,20-EDP in these animals maintained on our experimental diets 7 d after laser induction. LC-MS/MS revealed that EPA-derived 17,18-EEQ was significantly increased in both Tie2-CYP2C8-Tg and sEH null mice fed an EPA- and DHA-enriched diet compared with their respective strain-matched mice fed a control diet (Fig. 4A). Additionally, DHA-derived 19,20-EDP was significantly increased in Tie2-CYP2C8-Tg mice fed an EPA- and DHA-enriched diet compared with Tie2-CYP2C8-Tg mice fed a control diet (Fig. 4B). The plasma levels of 17,18-EEQ in Tie2-CYP2C8-Tg mice and sEH null mice on an EPA- and DHA-enriched diet were significantly up-regulated compared with C57BL/6 mice on an EPA- and DHA-enriched diet. In contrast, Tie2-sEH-Tg mice on an EPA- and DHA-enriched diet did not exhibit a significant increase in either 17,18-EEQ or 19,20-EDP compared with Tie2-sEH-Tg mice on a control diet (Fig. 4 A and B). Taken together these results implicate 17,18-EEQ and 19,20-EDP as important effector metabolites in CNV disease resolution. However, to date, the role of additional EDP and EEQ family members remains unknown. These data are supported by our observation that the protective effect of an enriched EPA and DHA diet is lost with overexpression of sEH, an enzyme that degrades these bioactive fatty acid metabolites (27).
Fig. 4.
The plasma profile of CYP-dependent metabolites in CYP enzyme Tg mice. The plasma levels of 17,18-EEQ (A) and 19,20-EDP (B) in CYP-Tg mice [C57BL/6 background (abbreviated as BL6), Tie2-CYP2C8-Tg (abbreviated as 2C8), sEH null, and Tie2-sEH-Tg (abbreviated as sEHtg), respectively] were determined at 7 d after CNV induction by LC-MS/MS. Tie2-CYP2C8-Tg mice overexpress the monooxygenase CYP2C8 in endothelial cells, which promotes the metabolism of the primary LCPUFAs to their active downstream fatty acid metabolites (e.g., 17,18-EEQ and 19,20-EDP). sEH null mice lack expression of sEH, an enzyme that degrades these CYP-derived fatty acid metabolites into less bioactive vic-diols. Conversely, Tie2-sEH-Tg mice overexpress sEH. Mice were fed either a ω-3 LCPUFA-enriched diet, ω-6 LCPUFA-enriched diet, or control diet. Data are presented as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. N.S., not significant. n = 3–4 mice per experimental group.
Blockade of the Metabolic Degradation of CYP Epoxide Metabolites of EPA and DHA Enhances the Protective Effect Against CNV.
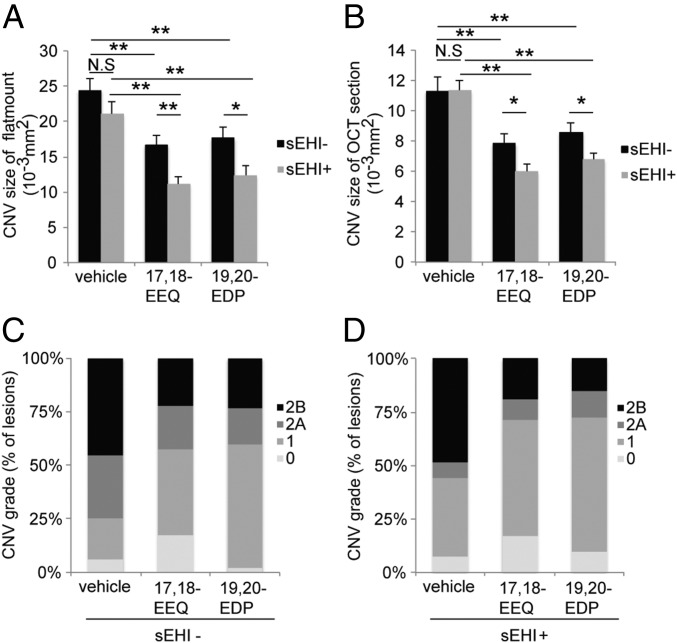
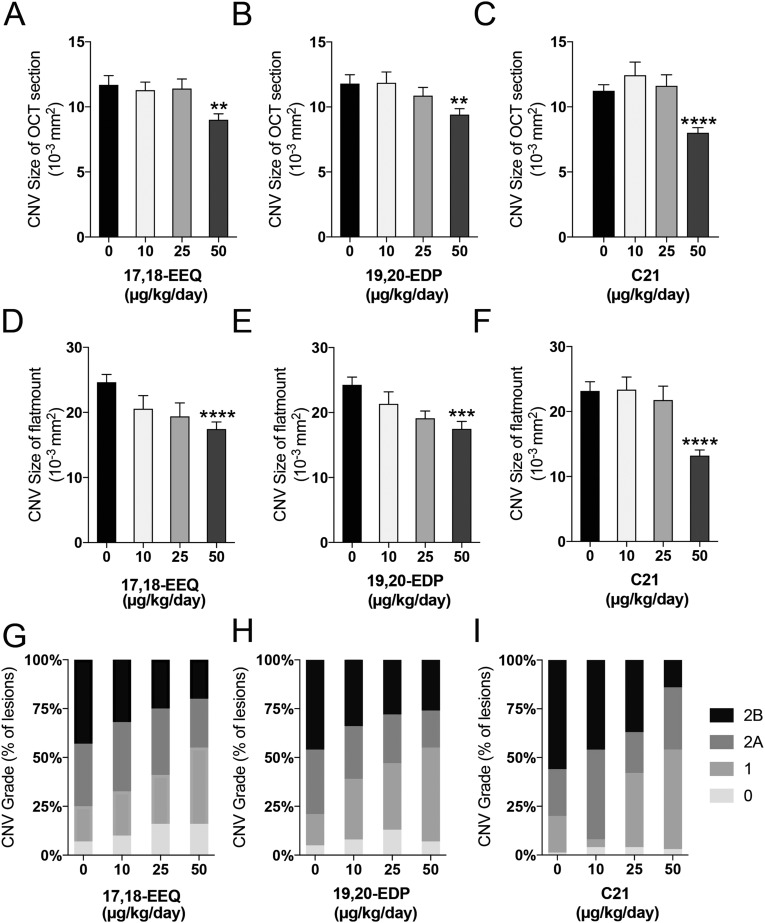
To further elucidate the effect of CYP epoxide metabolites of EPA and DHA on CNV, we used a sEH inhibitor, which can block the hydrolysis of 17,18-EEQ and 19,20-EDP by sEH (28). We administered daily i.p. injections (13) of 17,18-EEQ and 19,20-EDP into mice on a control diet with or without sEH inhibitor administered orally in the drinking water. The administration of both 17,18-EEQ and 19,20-EDP alone significantly attenuated CNV size in choroidal flat mounts (Fig. 5A, black bars) and in cross-sectional OCT images compared with the administration of vehicle control (Fig. 5B, black bars), as previously shown (13). Moreover, the administration of both 17,18-EEQ and 19,20-EDP alone also significantly reduced the severity of vascular leakage in CNV lesions (Fig. 5C). Coadministration of the sEH inhibitor with either 17,18-EEQ or 19,20-EDP further attenuated CNV size (Fig. 5 A and B, gray bars and Fig. S3 A and B) and also decreased the severity of vascular leakage in CNV lesions (Fig. 5D and Fig. S3C) compared with 17,18-EEQ or 19,20-EDP administration alone. Of note, our previous study demonstrated the effective dose for both 17,18-EEQ and 19,20-EDP (50 μg/kg/d) (13); however, we had not assessed the minimal effective dose for these compounds. To assess the minimum effective dose of 17,18-EEQ and 19,20-EDP, we administered daily i.p. injections of each eicosanoid at concentrations of 0, 15, 25, or 50 μg/kg/d to mice on a control diet. A concentration of 50 μg/kg/d significantly attenuated CNV size compared with the control vehicle, shown using choroidal flat-mount preparations (Fig. S4 A–C) and spectral domain OCT (SD-OCT) (Fig. S4 D–F), and reduced the severity of vascular leakage in CNV lesions (Fig. S4 G–I). However, administration of 15 or 25 μg/kg/d did not show significant protective effects on CNV size (Fig. S4 A–F), indicating that 50 μg/kg/d is the minimum effective dose for both 17,18-EEQ and 19,20-EDP.
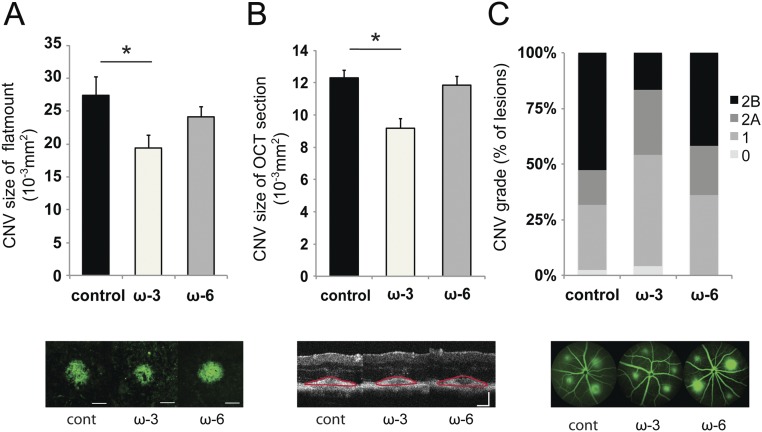
Fig. 5.
sEH inhibition enhances the protective effect of CYP-derived ω-3 fatty acids on CNV. (A and B) Lesion size at 7 d after CNV induction was assessed by staining of choroidal flat-mount preparations with fluorescent isolectin B4 (A), and a cross-sectional area of lesions was quantified by SD-OCT (B), for C57BL/6 mice administered control vehicle, 17,18-EEQ (50 μg/kg/d), or 19,20-EDP (50 μg/kg/d) once a day without sEH inhibitor (black bars) or with sEH inhibitor (1 mg/kg/d, gray bars). Mice were fed a control diet over the course of the experiment. Data are presented as means ± SEM. *P < 0.05, **P < 0.01. n = 35–43 lesions per experimental group. (C and D) Fluorescein leakage in CNV lesions was graded at 7 d after CNV induction in C57BL/6 mice administered control vehicle, 17,18-EEQ (50 μg/kg/d), or 19,20-EDP (50 μg/kg/d) once a day without sEH inhibitor (C) or with sEH inhibitor (1 mg/kg/d) (D). The grade of the hyperfluorescent lesions is as follows: score 0 (i.e., no leakage); score 1 (i.e., debatable leakage); score 2A (i.e., definite leakage); score 2B (i.e., clinically significant leakage). Mice were fed a control diet over the course of the experiment. n = 35–43 lesions per experimental group.
Fig. S3.
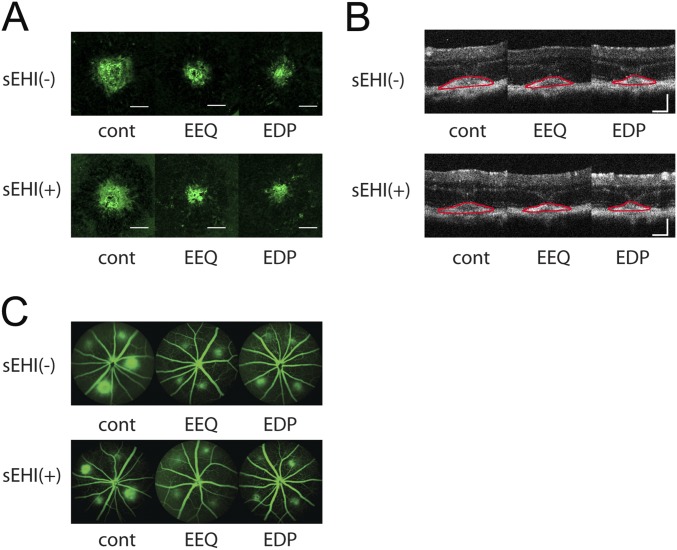
Representative images of fundus, OCT, and FA images of CNV experiment using sEH inhibitor (sEHI) in Fig. 5. (A–C) CNV lesions at 7 d after laser photocoagulation were assessed by staining of choroidal flat-mounts with fluorescent isolectin B4 (A), cross-sectional area of lesions quantified by SD-OCT (demarcated by red lines) (B), and fluorescein angiography (C), for C57BL/6J mice administered control vehicle, 17,18-EEQ (50 μg/kg/d), or 19,20-EDP (50 μg/kg/d) once a day with or without sEH Inhibitor (1 mg/kg/d). Mice were fed a control diet over the course of the experiment. (Scale bar: 100 μm.)
Fig. S4.
The dose-dependent effect of CYP-derived ω-3 epoxides and C21 on CNV. (A–F) Lesion size at 7 d after CNV induction was assessed by staining of choroidal flat-mount preparations with fluorescent isolectin B4 (A–C), and cross-sectional area of lesions was quantified by SD-OCT (D–F), for C57BL/6 mice administered control vehicle, 17,18-EEQ (10, 25 or 50 μg/kg/d), 19,20-EDP (10, 25 or 50 μg/kg/d) or C21 (10, 25 or 50 μg/kg/d) once a d immediately after laser photocoagulation. Data are presented as means ± SEM **P < 0.01, ***P < 0.001, ****P < 0.0001. n = 20–30 lesions per experimental group (G–I) Fluorescein leakage in CNV lesions was graded at 7 d after CNV induction in C57BL/6 mice administered 17,18-EEQ, 19,20-EDP or C21. n = 24–32 lesions per experimental group. The grade of the hyperfluorescent lesions is as follows: score 0 (i.e., no leakage); score 1 (i.e., debatable leakage); score 2A (i.e., definite leakage); score 2B (i.e., clinically significant leakage).
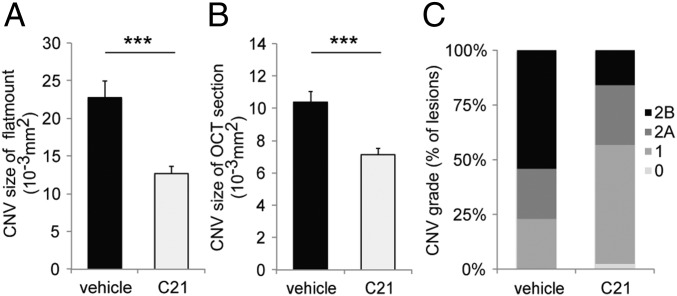
We next assessed a stable 17,18-EEQ analog, termed C21, which maintains its potent bioactive functions, but is unable to be degraded by sEH (29). Administration of C21 significantly reduced CNV size compared to vehicle control as assessed in both choroidal flat mounts and SD-OCT (Fig. 6 A and B and Fig. S5 A and B). In addition, C21 significantly reduced the extent of vascular leakage observed in these lesions (Fig. 6C and Fig. S5C). The minimum effective dose was also assessed for C21 and found to be 50 μg/kg/d (Fig. S4). These results are consistent with the results from our aforementioned experiments using CYP-Tg mice, which demonstrate that CYP epoxide metabolites of EPA and DHA are the critical mediators in protection against CNV. Furthermore, our results suggest that increasing resistance to degradation of these metabolites as well as increasing their corresponding bioavailability may be useful approaches to enhance protection against CNV.
Fig. 6.
Administration of C21 in C57BL/6 mice attenuates CNV. (A and B) Lesion size at 7 d after CNV induction was assessed by staining of choroidal flat-mount preparations with fluorescent isolectin B4 (A), and a cross-sectional area of lesions was quantified by SD-OCT (B), for C57BL/6 mice administered control vehicle or C21 (50 μg/kg/d) once a day immediately after laser photocoagulation. Data are presented as means ± SEM. ***P < 0.001. n = 37–48 lesions per experimental group. (C) Fluorescein leakage in CNV lesions was graded at 7 d after CNV induction in C57BL/6 mice administered control vehicle or C21 (50 μg/kg/d) once a day immediately after laser photocoagulation. The grade of the hyperfluorescent lesions is as follows: score 0 (i.e., no leakage); score 1 (i.e., debatable leakage); score 2A (i.e., definite leakage); score 2B (i.e., clinically significant leakage). n = 37–48 lesions per experimental group.
Fig. S5.
Representative images of fundus, OCT, and FA images of CNV experiment using C21 in Fig. 6. (A–C) CNV lesions at 7 d after laser photocoagulation were assessed by staining of choroidal flat mount with fluorescent isolectin B4 (A), cross-sectional area of lesions quantified by SD-OCT (demarcated by red lines) (B), and fluorescein angiography (C), for C57BL/6J mice administered control vehicle or C21 (50 μg/kg/d) once a day immediately after laser photocoagulation. Mice were fed a control diet over the course of the experiment. (Scale bar: 100 μm.)
Pharmacokinetics of CYP-Derived Eicosanoids of EPA and DHA.
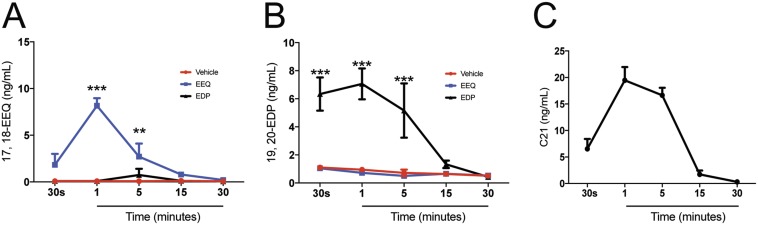
To assess if the protective effect of 17,18-EEQ, 19,20-EDP, and C21 is due to an increase of these lipid metabolites in plasma, we measured their concentration in the plasma following i.p. injections at 30 s (immediately following injection), 1, 5, 15, and 30 min after injection by LC-MS/MS. The plasma concentration of 17,18-EEQ was significantly increased 1 and 5 min following injection compared with mice injected with the control vehicle (Fig. S6A). The 19,20-EDP levels were also significantly increased 30 s and 1 and 5 min after injection (Fig. S6B). The peak plasma concentration of all metabolites occurred 1 min after i.p. injection, indicating a relatively short half-life for these metabolites postadministration (Fig. S6 A–C).
Fig. S6.
The time course of plasma concentration of CYP eicosanoids and C21. The plasma samples were obtained at 30 s and 1, 5, 15, and 30 min after i.p. injections of control vehicle, 17,18-EEQ (50 μg/kg), 19,20-EDP (50 μg/kg), or C21 (50 μg/kg). The plasma levels of 17,18-EEQ (A), 19,20-EDP (B), and C21 (C) were determined by LC-MS/MS. Mice were fed a control diet beginning 2 wk before the experiments. Data are presented as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. n = 3–4.
Leukocyte Recruitment Is Modulated by CYP Epoxide Metabolites.
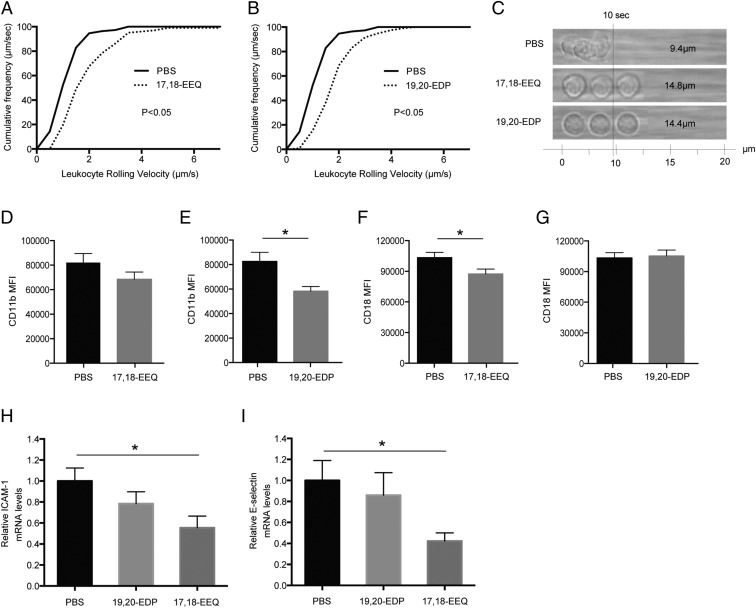
Systemic leukocyte recruitment to extravascular areas is thought to play a crucial role in the inflammatory process and the pathophysiology of several diseases, including AMD (10). Modulation of key adhesion molecules is known to recruit different populations of immune cells to sites of injury. Leukocytes in the blood stream attach and roll on the surface of endothelial cells via interactions between adherent molecules and their ligands, such as intercellular cell-adhesion molecules (ICAM) or selectins, leading to leukocyte crawling and transendothelial migration (10). In particular, proinflammatory leukocyte recruitment to CNV lesions has been shown to be a critical step in increasing the severity of exudative AMD (11–13). We have recently shown that consuming an EPA- and DHA-enriched diet down-regulates CNV by modulating leukocyte infiltration into the CNV lesions (13). These findings have led us to presume that CYP metabolites are the critical mediators of intraocular inflammation and angiogenesis by modulating leukocyte recruitment. To address this hypothesis, we used the autoperfused microflow chamber assay to assess the impact of 17,18-EEQ and 19,20-EDP on systemic leukocyte recruitment during CNV development (30). The autoperfused microflow chamber allows us to assess leukocyte-adhesion molecule interactions by connecting a coated chamber (which mimics endothelium) to the mouse circulatory system, where we are able to assess leukocyte rolling velocity without introducing extraneous manipulation, more closely mimicking the natural microenvironment (30). The rolling velocity of peripheral blood leukocytes (PBLs) in the circulating blood was measured 3 d post-CNV induction, as this reflects the peak of immune cell infiltration in this model (12, 31). The rolling velocity of PBLs on the chamber, which is coated with adherent molecules ICAM-1 and P-selectin, was significantly faster for mice administered 17,18-EEQ (1.48 ± 0.16 μm/s) than for those administered PBS (0.94 ± 0.06 μm/s) (Fig. 7 A and C). The rolling velocity of PBLs along the chamber was also significantly faster in mice administered 19,20-EDP (1.44 ± 0.12 μm/s) than in mice administered PBS (0.94 ± 0.06 μm/s) (Fig. 7 B and C). These data suggest that the functional down-regulation of ICAM-1 and P-selectin ligands on the surface of leukocytes in mice administered 17,18-EEQ and 19,20-EDP results in increased rolling velocity and decreased recruitment. To further investigate the increase in ICAM-1–dependent rolling velocity of PBLs in mice administered 17,18-EEQ and 19,20-EDP, we measured the expression of the ICAM-1 ligand, CD11b/CD18, on circulating leukocytes. Flow cytometry analysis revealed that the surface expression level of CD11b on PBLs was significantly lower for mice administered 19,20-EDP, but not 17,18-EEQ, than for those administered PBS (Fig. 7 D and E). On the contrary, the surface expression level of CD18 on PBLs was significantly lower for mice administered 17,18-EEQ, but not 19,20-EDP, than for those administered PBS (Fig. 7 F and G). Next, we examined the effects of 17,18-EEQ and 19,20-EDP on the expression of adhesion molecules in CNV lesions. CNV lesions were collected using laser-capture microdissection. Mice administered 17,18-EEQ had significantly reduced mRNA levels of ICAM-1 and E-selectin in the CNV lesions 7 d post-CNV induction compared with PBS-injected controls (Fig. 7 H and I). These data suggest that the increased rolling velocity of leukocytes from mice administered 17,18-EEQ and 19,20-EDP is attributable to the down-regulation of the expression of adhesion molecules; furthermore, each lipid metabolite has differential regulation on leukocytes or endothelial cells in CNV lesions. Modulation of specific adhesion molecules likely allows for the recruitment of individual leukocyte populations to the site of injury that can be proresolving or proinflammatory depending on the individual's lipidomic profile.
Fig. 7.
Administration of CYP-dependent ω-3 LCPUFA metabolites modulates leukocyte rolling velocity and adhesion molecule expression. (A and B) Cumulative frequency of leukocyte rolling velocity in a chamber coated with both P-selectin and ICAM-1 was assessed at day 3 after CNV induction. Mice were injected i.p. with (A) 17,18-EEQ (50 μg/kg/d), (B) 19,20-EDP (50 μg/kg/d), or PBS daily beginning immediately after CNV induction. Administration of both EEQ and EDP increased leukocyte rolling velocity compared with PBS groups (n = 3 mice per group). (C) Representative rendering depicting images of individual leukocytes tracked over 10 s. (D–G) Flow cytometric analysis of (D and E) CD11b and (F and G) CD18 expression on peripheral blood leukocytes 3 d after CNV induction in C57BL/6 mice injected i.p. with (D and F) 17,18-EEQ (50 μg/kg/d) or (E and G) 19,20-EDP (50 μg/kg/d). Administration of EEQ decreased CD18 expression and EDP decreased CD11b expression on leukocytes compared with PBS-injected groups. Data are presented as means ± SEM. n = 10 mice per group. MFI, mean fluorescence intensity values. *P < 0.05. (H and I) Real-time PCR analysis of (H) ICAM-1 and (I) E-selectin mRNAs in laser-captured CNV lesions at 7 d after CNV induction in C57BL/6 mice injected i.p. with 17,18-EEQ (50 μg/kg/d) and 19,20-EDP (50 μg/kg/d). Administration of 17,18-EEQ significantly decreased ICAM-1 and E-selectin expression compared with PBS-injected groups. Data are presented as means ± SEM. n = 6 mice per group. *P < 0.05.
Leukocyte Adhesion Is Up-Regulated by Administration of CYP Epoxide Metabolites of AA.
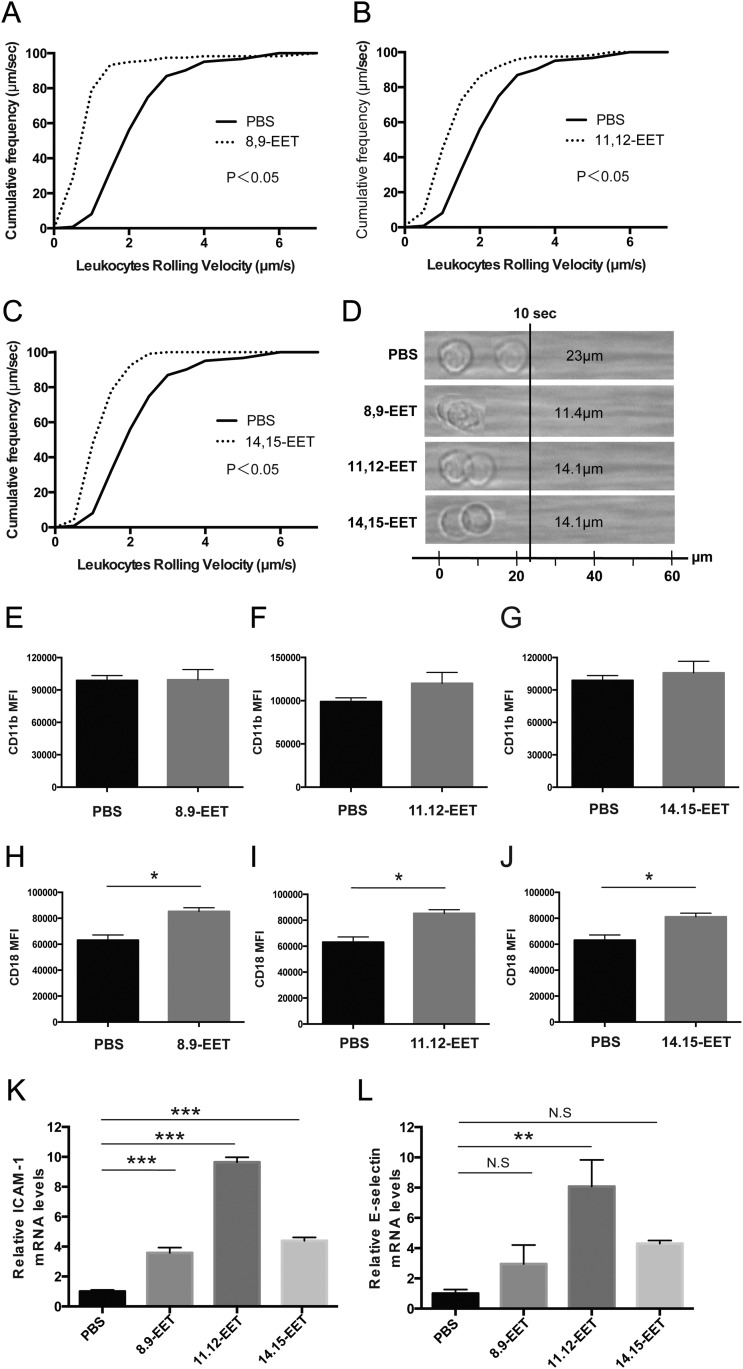
We also assessed the effect of CYP-generated eicosanoids of AA, EETs, on leukocyte recruitment. The rolling velocity of PBLs along the chamber was significantly decreased for mice administered 8,9-EET (1.14 ± 0.15 μm/s) than those administered PBS (2.30 ± 0.13 μm/s) (Fig. S7 A and D). The rolling velocity of PBLs on the chamber was also significantly decreased in mice administered other regioisomeric EETs, 11,12-EET (1.41 ± 0.45 μm/s) and 14,15-EET (1.41 ± 0.11 μm/s), than in mice administered PBS (2.30 ± 0.13 μm/s) (Fig. S7 B–D). The surface expression levels of CD11b on PBLs were unchanged for mice administered each EET compared with those administered PBS (Fig. S7 E–G). Conversely, the surface expression levels of CD18 on PBLs were significantly higher for mice administered EETs compared with those administered PBS (Fig. S7 H–J). Mice administered EETs had significantly increased levels of ICAM-1 mRNAs in the CNV lesions 7 d post-CNV induction compared with PBS-injected controls (Fig. S7K). In contrast, only 11,12-EET showed a significant increase in the mRNA level of E-selectin compared with mice administered PBS (Fig. S7L). These data indicate that the decreased rolling velocity of leukocytes from mice administered EETs are attributable to the up-regulation of the expression of adhesion molecules both on the surface of leukocytes and on endothelial cells in CNV lesions. The cell types and their ability to confer either a pro- or antiinflammatory actions is as yet to be determined.
Fig. S7.
Administration of CYP-dependent ω-6 epoxides modulates leukocyte rolling velocity and adhesion molecule expression. (A–C) Cumulative frequency of leukocyte rolling velocity in a chamber coated with both P-selectin and ICAM-1 were assessed at day 3 after CNV induction. Mice were injected i.p. with (A) 8.9-EET (50 μg/kg/d), (B) 11.12-EET (50 μg/kg/d), (C) 14.15-EET (50 μg/kg/d), or PBS daily beginning immediately after CNV induction. Administration of EETs decreased leukocyte rolling velocity compared with PBS groups (n = 3 mice per group). (D) Representative rendering depicting images of individual leukocytes tracked over 10 s. (E–J) Flow cytometric analysis of (E–G) CD11b and (H–J) CD18 expression on peripheral blood leukocytes 3 d after CNV induction in mice injected i.p. with (E and H) 8,9-EET (50 μg/kg/d), (F and I) 11,12-EET (50 μg/kg/d), or (G and J) 14,15-EET (50 μg/kg/d). Administration of EETs significantly increased CD18 expression on leukocytes compared with PBS-injected groups. Data are presented as means ± SEM. n = 10 mice per experimental group. MFI, mean fluorescence intensity values. *P < 0.05. (K and L) Real-time PCR analysis of (K) ICAM-1 and (L) E-selectin mRNAs in laser-captured CNV lesions at 7 d after CNV induction in injected i.p. with EETs (50 μg/kg/d for each group). Administration of EETs significantly increased ICAM-1 expressions and administration of 11,12-EET increased E-selectin expressions compared with PBS-injected group. Data are presented as means ± SEM. n = 6 mice per experimental group. **P < 0.01, ***P < 0.001. N.S., not significant.
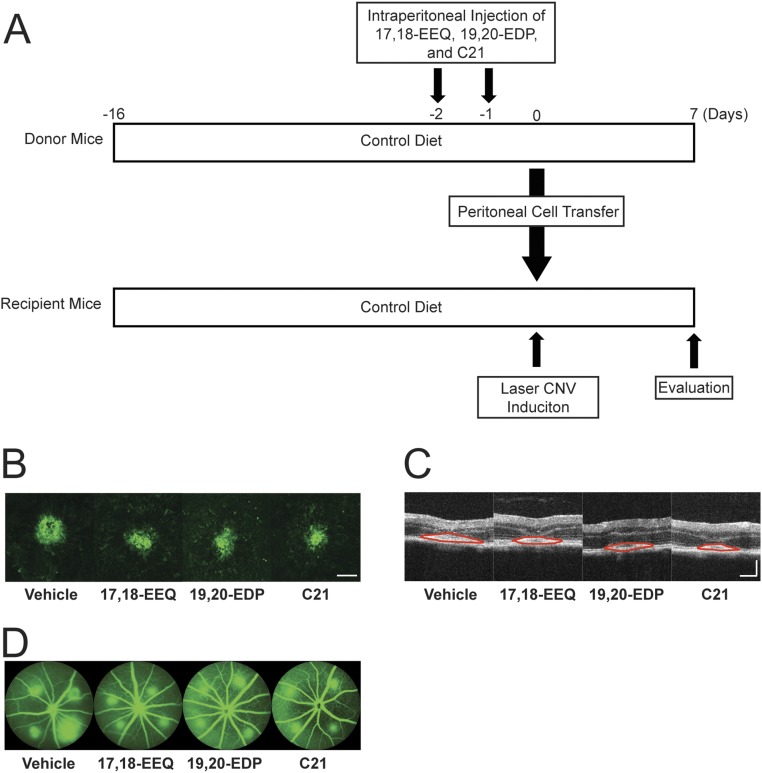
Adoptive Transfer of Peritoneal Cells from Omega-3 Epoxide Metabolite-Treated Mice Reduces CNV.
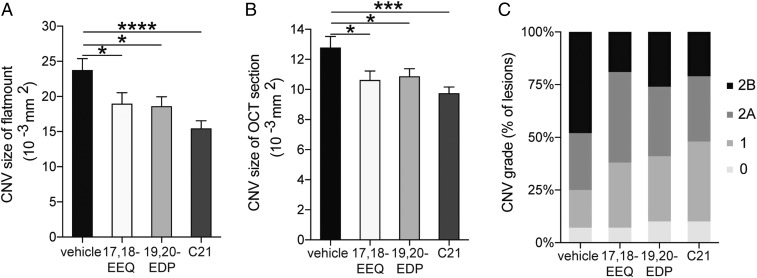
Given the relatively short half-life for 17,18-EEQ, 19,20-EDP, and C21 in the serum of mice receiving i.p. injections, we sought to identify if their protective effect was systemic or local (retina). To begin to investigate the systemic contribution of these bioactive lipids, we wanted to address the involvement of peritoneal cells (at the sight of administration) on CNV disease regulation. Peritoneal cells were transferred from donor mice that received i.p. injections of either control vehicle, 17,18-EEQ, 19,20-EDP, or C21 to untreated recipient mice and then CNV disease severity was assessed (Fig. S8A). Choroidal flat mounts and SD-OCT (Fig. 8 A and B and Fig. S8 B and C) showed that recipient mice, which received peritoneal cells from donor mice treated with 17,18-EEQ, 19,20-EDP, and C21, had significantly smaller CNV lesions compared with control donor mice. Vascular leakage in CNV lesions was also significantly decreased in recipient mice that received peritoneal cells from donor mice treated with 17,18-EEQ, 19,20-EDP, and C21 (Fig. 8C and Fig. S8D). These results suggest peritoneal cells functionally modified by 17,18-EEQ, 19,20-EDP, or C21 administration, play an important systemic role in CNV resolution.
Fig. S8.
Representative images of fundus, OCT, and FA images of adoptive transfer experiments in Fig. 8. (A) Mice were placed on a control diet 2 wk prior (day −16) to the induction of CNV and for the rest of the experiment. At 2 and 1 d before laser photocoagulation (days −2 and −1), donor mice were injected i.p. with control vehicle, 17,18-EEQ, 19,20-EDP, or C21 (50 μg/kg/d). At day 0, peritoneal cells of the donor mice were transferred into the recipient mice and laser CNV was induced. The CNV size was evaluated at 7 d after laser treatment (day 7). (B–D) CNV lesions at 7 d after laser photocoagulation were assessed by staining of choroidal flat mounts with fluorescent isolectin B4 (B), cross-sectional area of lesions quantified by SD-OCT (demarcated by red lines) (C), and fluorescein angiography (D), for the recipient mice receiving peritoneal cells from the donor animals injected with either vehicle control, 17,18-EEQ, 19,20-EDP, or C21. (Scale bar: 100 μm.)
Fig. 8.
Adoptive transfer of CYP-dependent ω-3 epoxides and C21-treated peritoneal cells attenuate CNV. Lesion size at 7 d after CNV induction was assessed by staining of choroidal flat-mount preparations with fluorescent isolectin B4 (A), and a cross-sectional area of lesions was quantified by SD-OCT (B), for the recipient mice receiving transferred peritoneal cells from the donor mice injected with 17,18-EEQ, 19,20-EDP, or C21. Data are presented as means ± SEM. *P < 0.05, n = 28–42 lesions per experimental group. (C) Fluorescein leakage in CNV lesions was graded at 7 d after CNV induction in recipient mice. The grade of the hyperfluorescent lesions is as follows: score 0 (i.e., no leakage); score 1 (i.e., debatable leakage); score 2A (i.e., definite leakage); score 2B (i.e., clinically significant leakage). n = 42–48 lesions per experimental group.
Discussion
The metabolism of LCPUFAs is initiated by three distinct enzyme classes: COX, LOX, and CYP. COX and LOX pathways are well known to produce many structurally different fatty acid metabolites from LCPUFAs (17, 18). The COX enzymes are responsible for prostaglandin and thromboxane synthesis (17, 18, 32), while LOXs are responsible for leukotriene and lipoxin synthesis (17, 18, 33). In addition to these two enzyme types, the CYP enzyme family also has the capacity to synthesize fatty acid metabolites. Of these three main pathways, the cytochrome P450 branch appears most susceptible to changes in dietary fatty acid composition. Moreover, the ω-3 double bond that distinguishes DHA and EPA from their ω-6 counterparts provides a preferred epoxidation site for specific CYP family members leading to the formation of 17,18-EEQ and 19,20-EDP as predominant epoxide metabolites upon dietary EPA/DHA supplementation (20–22, 34, 35). Compared with EETs, which are CYP epoxide metabolites of AA widely investigated for their important roles in cardiovascular physiology and cancer (19, 34, 35), EDPs and EEQs are not well defined in terms of their bioactivity. We have recently reported that intake of EPA- and DHA-enriched diets reduces disease severity in a mouse model of neovascular AMD (13). Our subsequent interest was to elucidate which bioactive lipid effector molecules of EPA and DHA might prevent or dampen neovascular AMD disease severity. We identified that the plasma levels of EEQs and EDPs are increased in mice fed an EPA- and DHA-enriched diet and found that direct administration of 17,18-EEQ and 19,20-EDP can reduce CNV (13). Expanding on this work, our current study clearly demonstrates that CYP epoxide metabolites of EPA and DHA are key modulators in the attenuation of inflammation and CNV. CYP2C subfamily members, such as CYP2C8, are considered potent monooxygenases of EPA and DHA, as well as AA (20, 22). Specifically, CYP2C8 is the predominant isoform that converts EPA to 17,18-EEQ and DHA to 19,20-EDP (19). Several recent studies have demonstrated the involvement of these CYP enzymes and their metabolites in arrhythmia, cardiac hypertrophy, hypertension, and other cardiovascular diseases (36, 37). In our CNV model, overexpression of human CYP2C8, coupled with an EPA- and DHA-enriched diet, markedly attenuated CNV lesions with a corresponding increase in the plasma levels of 17,18-EEQ and 19,20-EDP (Figs. 1 and 4). Data from our pharmacokinetic study (illustrating the short half-life of i.p.-administered compounds in plasma, Fig. S6) and adoptive transfer (17,18-EEQ and 19,20-EDP primed immune cells) studies point to a major role for circulating immune cells as the major effectors in CNV resolution and not necessarily a predominantly local retinal effect. Of note, to date the contribution of the additional EEQ and EDP family members (7,8-EDP, 10,11-EDP, 13,14-EDP, and 16,17-EDP; 8,9-EEQ, 11,12-EEQ, and 14,15-EEQ, respectively) on CNV disease progression and regression is as yet unknown.
In the current study, the total plasma levels of 17,18-EEQ were slightly higher and levels of 19,20-EDP were lower than those from our previous analyses (13). One possible explanation for this divergence could involve a difference in the composition of the EPA-and DHA-enriched diets between these studies. The current study used an EPA- and DHA-enriched diet containing 2.5% (wt/wt) of an oil with a higher percentage of EPA (EPA:DHA = 478.5 mg:193.5 mg/g oil) compared with the previous diet (EPA:DHA = 465 mg:375 mg/g oil). Thus, we can infer that increased plasma levels of 17,18-EEQ and 19,20-EDP can improve the efficiency of protection against CNV development. We tried two different strategies based on this idea to increase the biological activity of these metabolites: pharmacological inhibition of sEH (Fig. 5) and treatment of a stable analog of 17,18-EEQ (Fig. 6). These chemicals have been shown to have significant effects on antiinflammatory activity (38), lowering of blood pressure (39), attenuation of hepatic fibrosis (40), and antiarrhythmic activity (29). We demonstrate that both strategies are effective in attenuating CNV development in our model. Although further analyses are needed, we believe these tools can be applied to the clinical setting.
Leukocyte recruitment and transmigration from circulating blood into tissues are essential for various inflammatory and autoimmune disorders, including CNV progression in exudative AMD (10–13). In the current study, we demonstrate that 17,18-EEQ and 19,20-EDP down-regulate peripheral blood leukocyte adhesion ability (Fig. 7). The interaction between β2 integrins on leukocytes and their endothelial ligands, such as ICAM-1 and E-selectin, is required for modulating adhesion, migration, and infiltration of leukocytes into inflammatory sites (41). Integrin CD11b/CD18, which consists of a heterodimer of the αM (CD11b) and β2 (CD18) subunits, is the most crucial β2 integrin for leukocyte recruitment (41). In our intraocular model of inflammation, we show that 17,18-EEQ and 19,20-EDP reduced CD11b/CD18 expression in PBLs, and that 17,18-EEQ reduced ICAM-1 and E-selectin expression in endothelial cells in CNV lesions (Fig. 7). These findings indicate that the inhibitory effect of 17,18-EEQ and 19,20-EDP on CNV is due to their inhibitory effect on leukocyte recruitment by reducing the expression of adherent molecules on both leukocytes and endothelial cells. Significant down-regulation of CNV by gene disruption of CD18 and ICAM-1 (42) supports our theory. However, it is still unclear how CYP-dependent fatty acids modulate the expression of these adherent molecules. On one end of the LCPUFA spectrum, the major dietary ω-6 LCPUFA is AA, which has attracted significant attention due to increasing evidence of its role in cancer biology (34, 43), vascular injury, and arteriosclerosis (44, 45). Several studies have shown the proangiogenic effect of EETs, which are CYP-dependent eicosanoids derived from AA (46, 47). Contrary to our expectations, dietary intake of AA (13) and direct administration of EETs did not have a significant effect on CNV development (Fig. S2). One potential reason is that the EETs, as relatively weak angiogenic agents, might not have reached sufficient levels in the local environment to exacerbate CNV development at the administered dosages. Another possibility is that the CNV in this model may have reached peak size because of the severe intraocular inflammation already caused by laser administration. In contrast to our findings in CNV development, EETs promoted PBL adhesion, increased CD18 expression in PBLs, and increased ICAM-1 and E-selectin expression in endothelial cells in CNV lesions (Fig. S7). However, it is more than likely that different subsets of immune cells, either proinflammatory or proresolving, are brought to the CNV lesion based on the bioactive lipid microenvironment. In regards to the effect of EETs on leukocyte recruitment, 14,15-EET has been shown to promote monocyte adherence to endothelial cells (48). However, several recent studies have shown that some EETs have antiinflammatory effects and can attenuate vascular inflammatory responses (49–51). Node et al. (49) demonstrated that 11,12-EET significantly attenuated cytokine-induced NF-κB activation and adhesion molecule expression in endothelial cells, and exogenous 11,12-EET administration attenuated leukocyte adhesion to murine carotid arteries. Compared with their in vitro experiments that stimulated potent proinflammatory cytokines (e.g., TNF-α, IL-1, and LPS) to activate endothelial cells, our injury conditions were in an in vivo local microenvironment and, thus, may have a different pathology from prior studies that can account for the difference in results.
Earlier work has illustrated that an imbalance in retinal lipids leads to photoreceptor degradation and the accumulation of lipid and lipoprotein debris in the retinal pigment epithelium layer (14). Because the continuous renewal of retinal membranes requires a constant supply of ω-3s, supplied by retinal pigment epithelium cells, diets rich in ω-3s may improve retinal function and may delay the development of neovascular AMD (52, 53). In this study, we demonstrate the role of ω-3 CYP-dependent metabolites in the resolution of CNV. However, this is not to say that our study is without its limitations. To date, two major clinical studies assessing the role of ω-3 supplementation on AMD disease progression have yielded conflicting results. The NIH-initiated age-related eye disease study 2 (AREDS2) found no protective effects from dietary supplementation with ω-3s on disease progression [based on both geographic atrophy (GA) and CNV] (54). In contrast, the nutritional AMD treatment 2 (NAT2) study identified a significant protective effect of ω-3 supplementation when CNV development alone was assessed (24, 55). In the NAT2 study, patients with neovascular AMD in one eye were given oral ω-3s or a placebo over 3 y, and the study eye was assessed for CNV occurrence (24, 55). It was observed that elevated EPA serum status or a high red-blood cell membrane (RBCM) EPA+DHA index (i.e., ω-3 index) was significantly associated with a reduction in the odds of developing neovascular AMD (24, 55). In particular, patients with the highest EPA/DHA levels in their RBCMs had a significant decrease (68% reduction) in CNV development over the 3-y period (24). These data indicate that ω-3 compounds have potent antiangiogenic properties. Conversely, in AREDS2, the dose of supplemented DHA, the primary ω-3 LCPUFA in the retina, was significantly lower than that in the NAT2 study (350 mg from ethyl esters versus 840 mg from fish oil, respectively), suggesting that the supplementation dose is crucial to CNV outcome (24, 54). In addition, AREDS2 only measured the serum levels of the primary ω-3 LCPUFAs in patients, but not the incorporation of these lipids into cellular membranes (i.e., RBCM). Thus, AREDS2’s assessment is only reflective of the dietary levels and not the tissue levels of these lipids. Moreover, the placebo in AREDS2 was not a true placebo, as it was also supplemented with the AREDS1 formulation that contained a number of antioxidant compounds (54, 56). Lastly, in AREDS2, GA and CNV were used as diagnostic markers of disease progression; the effect of these lipids on CNV was not directly studied (54). The NAT2 study is the first study reporting associations of elevated EPA serum status or a high RBCM EPA+DHA index with a reduction in the odds of developing neovascular AMD (55, 57). The strength of this study was the combined use of biological data with dietary assessment of ω-3 LCPUFA status in the same groups of individuals affected or unaffected with AMD. It is important to note that potential protection against neovascular AMD may not be consistently achieved with dietary intake of ω-3 LCPUFAs alone, especially since there is significant variation among individuals in the tissue levels of EPA/DHA. Thus, for patients who may not have improvements in AMD progression with ω-3 supplementation, it would be beneficial to identify bioactive lipid metabolites of these compounds that possess antiangiogenic and antiinflammatory functions. In contrast to the aforementioned clinical studies, our current work in animal models incorporated stricter regulations on the level of ω-3 LCPUFAs in the diets, thus allowing us to make more robust conclusions on the specific role of these ω-3 CYP-derived fatty acids. Although there are inherent limitations in translating our findings from animal models to human patients that warrant further investigation, our work clearly begins to elucidate the therapeutic potential of specific ω-3 CYP-dependent downstream lipid metabolites in CNV resolution.
In conclusion, we have clearly identified CYP-derived lipid biometabolites, 17,18-EEQ and 19,20-EDP, as important mediators of the protection against CNV, determined their ability to regulate leukocyte recruitment and local inflammation in the microenvironment of CNV lesions, and demonstrated their potential as a pharmacologic target for neovascular AMD disease progression. Given the systemic nature of this metabolic pathway, we feel that the findings from this study are not only relevant to AMD, but also to many other salient conditions involving angiogenesis and inflammation, such as diabetic retinopathy, atherosclerosis, and cancer.
Materials and Methods
All animal procedures adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. The Animal Care and Use Committee of Massachusetts Eye and Ear Infirmary approved the protocol for the experiments outlined herein. Tie2-CYP2C8-Tg mice, which overexpress human CYP2C8 in endothelial cells (58); Tie2-sEH-Tg mice, which overexpress human sEH in endothelial cells (26); and sEH null mice, which have systemic knockout of sEH (59), were kindly provided by Darryl C. Zeldin, NIH/National Institute of Environmental Health Sciences, Bethesda. All mice were on the C57BL/6 (area R16) genetic background from Charles River Laboratories. These C57BL/6 mice were used as control mice for CNV experiments involving the aforementioned CYP-Tg mice. Male C57BL/6 mice (stock no. 000664) at 6–8 wk of age obtained from The Jackson Laboratory were used for all other experiments.
Detailed descriptions of the animal studies, reagents, diets, lipid analysis, pharmacokinetics, autoperfused flow chamber assay, immune cell analysis, and laser-capture microdissection can be found in SI Materials and Methods.
SI Materials and Methods
Diet and Bioactive Lipid Metabolites.
Mice were fed one of three experimental diets: a diet containing high ω-3 LCPUFAs by supplementing the control diet with 2.5% of an oil containing 478.5 mg EPA and 193.5 mg DHA per gram of oil without any AA; a diet devoid of ω-3 LCPUFAs, but containing 5% sunflower oil, which is rich in linoleic acid, a precursor of AA; and a standard control diet, which was completely devoid of AA, EPA, and DHA. The polyunsaturated fatty acids in the diets originated from 5% soybean oil. The ω-3 LCPUFAs were obtained from Pronova Biopharma (500:200 EE; PronovaPure) and the experimental diets were formulated by ssniff Spezialdiäten. Mice were placed on these diets 2 wk before the induction of CNV and for the rest of the experiment duration. The control diet was used for the experiments involving CYP-dependent metabolites to avoid the influence of fatty acids from the diet. CYP-dependent metabolites, 17,18-epoxyeicosatetraenoic acid (EEQ), 19,20-epoxydocosapentaenoic acid (EDP), and 8,9-, 11,12-, 14,15-epoxyeicosatrienoic acid (EET), were obtained from Cayman Chemical. Mice were injected intraperitoneally (i.p.) daily with 17,18-EEQ, 19,20-EDP, 8,9-EETs, 11,12-EETs, 14,15-EETs, or PBS as a vehicle control beginning immediately after CNV induction. Based on our earlier work, a dose of 50 μg/kg/d for these agents was used to assess the role of each of the aforementioned bioactive metabolites in the laser-induced CNV model (13). The stable 17,18-EEQ analog, named C21, was synthesized as previously described (29). This analog was also injected 50 μg/kg/d i.p. daily at the same dose as the other fatty acid metabolites. The 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea, a potent sEH inhibitor, was synthesized as previously described (28), dissolved in polyethylene glycol 400 (PEG400), and administered in the drinking water at a dose of 1 mg/kg/d 3 d before the induction of CNV and through the duration of the experiment. Drinking water containing PEG400 alone served as the vehicle control group.
Laser-Induced CNV Model.
A 532-nm laser (Oculight GLx Laser System; IRIDEX) attached to a slit lamp was used for photocoagulation. To view the posterior pole of the eye, a coverslip with a drop of Goniosol was applied to applanate the cornea. For size and leakage studies, four laser-induced lesions were made around the optic nerve in a clockwise manner (i.e., 3, 6, 9, and 12 o’clock). For fluorescence-activated cell sorting (FACS) and laser-capture microdissection (LCM) analysis, 10 lesions were placed. The laser was set as follows: 100-mW power, 100-μm spot size, and 0.1-s pulse duration. The appearance of a bubble at the site of photocoagulation signified a disruption in Bruch’s membrane, and so any laser injury without a corresponding bubble was excluded from analysis. Seven days after laser treatment, mouse eyes were enucleated and subsequently fixed in 4% paraformaldehyde. Whole retinas were then separated from the underlying choroid and sclera (i.e., eyecup). At a dilution of 1:100, Alexa Fluor 488 conjugated to isolectin B4 (Invitrogen) was used to stain the eyecup overnight at room temperature. PermaFluor aqueous mount (Thermo Scientific) was then used to flat mount the eyecup with the choroid facing upward and the sclera facing downward. Flat-mount images were captured using a Zeiss AxioCam MRm camera and Zeiss AxioObserver (Z1) microscope, and ImageJ software (NIH) was used to assess fluorescent CNV lesions (13, 31).
Fluorescein Angiography.
A Μicron IV Retinal Imaging Microscope (Phoenix Research Laboratories) was used to take fluorescein images. First, mice were anesthetized with Avertin (Sigma) and kept on a heating pad (37 °C) to maintain body temperature. The 5% phenylephrine and 0.5% tropicamide were used for pupil dilation. The corneas were covered with 2.5% Goniovisc (HUB Pharmaceuticals) and then placed in contact with the Μicron IV camera lens. Images were obtained 3–5 and 7–10 min after i.p. injection of 0.1 mL of 2% fluorescein sodium (Akorn) using StreamPix software (Phoenix Research Laboratories). A previously established scheme was used to grade the hyperfluorescent lesions: faint or mottled fluorescence without leakage was scored as 0 (i.e., no leakage); hyperfluorescence without any increase in size or intensity was scored as 1 (i.e., debatable leakage); hyperfluorescence with constant size but increasing intensity was scored as 2A (i.e., definite leakage); and hyperfluorescence with increasing size and intensity was scored as 2B (i.e., clinically significant leakage) (13).
Defining the Minimum Effective Dose for 17,18-EEQ, 19,20-EDP, and C21.
Male C57BL/6 mice (stock no. 000664) at 6 wk of age were obtained from The Jackson Laboratory. Mice were fed a standard control diet 2 wk before the induction of CNV and for the rest of the experiment duration. Doses of 0, 15, 25, or 50 μg/kg/d of 17,18-EEQ, 19,20-EDP, or C21were injected intraperitoneally daily beginning immediately after laser photocoagulation. Seven days after laser treatment, the size of CNV lesions were evaluated by staining of choroidal flat-mount preparations with fluorescent isolectin B4 and cross-sectional area of lesions was quantified by SD-OCT. Vascular leakage in CNV lesions were graded by fluorescein angiography.
Spectral Domain-Optical Coherence Tomography.
Seven days after laser photocoagulation, a Bioptigen system was used to conduct spectral domain-optical coherence tomography (SD-OCT) as previously described (13). Mice were first anesthetized and placed on a freely rotating cassette customized for optimal eye alignment. AB scan with an area size of 1.4 × 1.4 mm was obtained using 100 horizontal, raster, and consecutive B-scan lines, each composed of 1,000 A scans. Cross-sectional CNV lesion size from these OCT images was measured in ImageJ using sections through the center (i.e., midline through the ruptured area of Bruch’s membrane and the retinal pigment epithelium) of each CNV lesion.
Liquid Chromatography-Tandem Mass Spectrometry.
Seven days after CNV induction, plasma samples were obtained from mice fed ω-3, ω-6, or control diets for analysis of the CYP-mediated metabolite profile via liquid chromatography-tandem mass spectrometry (LC-MS/MS) as previously described (20). Plasma samples were combined with an internal standard solution (i.e., 10 ng each of 14,15-epoxyeicosatrienoic acid-d8, 20-hydroxyeicosatetraenoic acid-d6, and 14,15-dihydroxyeicosatrienoic acid-d11; Cayman Chemical). After alkaline hydrolysis, Agilent Bond Elute Certify II columns were used for the solid-phase extraction of metabolites. Metabolite analysis was performed in a solvent system (acetonitrile and 0.1% aqueous formic acid) with an Agilent 1200 HPLC system and Phenomenex Kinetex-C18 column (2.6 μm; 2.1 Å–150 mm). Starting at a 5% acetonitrile concentration, gradient elution was conducted by increasing the acetonitrile concentration to 90% in 10 min and then held at 90% for 10 min, with a flow rate of 0.3 mL/min. The HPLC system was linked to a mass spectrometer (Agilent 6460 triple quadrupole) with a source of electrospray ionization. As previously described (20), multiple reaction monitoring (negative mode) was used to analyze the CYP-mediated metabolites, and the results were calculated via Agilent Mass Hunter software. The concentrations of metabolites are expressed in nanograms per milliliter of plasma, which were obtained with the Lowry assay.
Pharmacokinetic Analysis of Plasma 17,18-EEQ, 19,20-EDP, and C21 Levels by LC-MS/MS Following i.p. Injection.
To assess the pharmacokinetics of CYP eicosanoids and C21, the plasma levels of 17,18-EEQ, 19,20-EDP, and C21 were determined by LC-MS/MS. C57BL/6 mice at 6 wk of age were fed a standard control diet beginning 2 wk before the experiment. The plasma samples were obtained at 30 s [immediately following injection (20–30 s)], and 1, 5, 15, and 30 min after i.p. injections of control vehicle, 17,18-EEQ (50 μg/kg), 19,20-EDP (50 μg/kg), or C21 (50 μg/kg), and were prepared and analyzed for LC-MS/MS analysis, described above, however, without including alkaline hydrolysis to access the free-circulating levels of the compounds after i.p. injection.
Autoperfused Microflow Chamber Microscopy.
An autoperfused microflow chamber assay was used to assess leukocyte rolling velocity as previously described (30). A combination of intercellular adhesion molecule 1 (ICAM-1) and P-selectin (R&D Systems), each at a concentration of 5 μg/mL, was used to coat translucent microchambers (0.4 × 0.04 × 50 mm; VitroCom) overnight at 4 °C. Biocompatible polyester tubing (PE10, Becton Dickinson) was attached to both ends of each microchamber, and the apparatus was subsequently incubated for 1 h with 0.1% BSA (Sigma-Aldrich) to prevent nonspecific leukocyte interaction with the inner surface of the apparatus. The left jugular vein and right carotid artery in an anesthetized mouse were surgically connected to the free ends of both tubes, thus allowing blood to flow from the right carotid artery and into the microchamber (via the inlet tube), and then into the left jugular vein (via the outlet tube) for recirculation. Blood flow rate through the microchamber was controlled via a screw valve on the inlet tube that could change the tube diameter. For continuous blood pressure measurements, three-way connectors were used to embed microtransducers (Harvard Apparatus) before and after the microchamber. Digitizing the analog output from these microtransducers required an A/D converter (ML785 PowerLab/8SP, ADInstruments) linked to a computer running CHART V7 software (ADInstruments). In terms of the hemodynamic conditions in the chamber, the pressure drop (ΔP) was calculated as the difference between the recorded pressure readings before (inlet) and after (outlet) the microchamber, and ΔP was used to calculate shear stress (τ) as previously described (60, 61). A fixed-stage intravital microscope (Leica) with a 63× objective and a monochromic camera (DFC 360 FX, Leica) were used to observe and record leukocyte rolling along immobilized adhesion molecules. Recordings lasted at least 30 s and incorporated 10 different fields of view. Using ImageJ software, leukocyte rolling velocity was calculated based on individual cell displacement along the surface of the chamber over time (i.e., Δd/Δt). Flux was calculated as the mean number of leukocytes interacting with the chamber surface per field of view (i.e., average of 10 independent fields of view). There were no significant differences in the flux of peripheral blood leukocytes (PBLs) from mice on either ω-3 or ω-6 fatty acid diets.
Isolation of PBLs for Flow Cytometric Analysis of CD11b and CD18.
An EDTA-treated syringe was used to obtain blood from mouse hearts. The blood was then incubated on ice for 30 min with phycoerythrin conjugated to rat monoclonal antibodies CD18 (C71/16, BD Biosciences) or CD11b (M1/70, BD Biosciences), or with isotype control antibodies (concentration of 1 μg per 1 × 106 total cells). After lysing red blood cells (Easy-Lyse, Leinco Technologies), the solution was centrifuged and the remaining cells were resuspended for flow cytometry (Cytomics FC500 instrument, Beckman Coulter). PBLs were gated based on their characteristic forward and side scatter pattern, and subsequent analysis was conducted with FlowJo 10.0 software (Beckman Coulter).
Laser-Capture Microdissection.
Immediately after enucleation, mouse eyes were placed in optimum cutting temperature compound and swiftly frozen. Under RNase-free conditions, eyes were cryosectioned (10-μm thickness per section) and mounted on polyethylene naphthalate glass slides (Leica). After dehydrating with washes of 50%, 75%, and 100% ethanol, the slides were stained with isolectin B4 at a dilution of 1:50 in 1 mM CaCl2. Laser-capture microdissection (LMD 7000 system, Leica) was used to isolate chorioretinal vessels in CNV lesions, which were then placed in RNA-stabilizing buffer (RNeasy Micro Kit, Qiagen). Reverse transcription of the extracted RNA was achieved with SuperScript III reverse transcriptase and random hexamer primers (Invitrogen). Real-time PCR was conducted on a StepOne real-time PCR System (Applied Biosystems) with mouse TaqMan expression assays for the following genes (Applied Biosystems): E-selectin (Mm00441278_m1), Icam-1 (Mm00516023_m1), and β-actin (Mm00607939_s1). All data were normalized to β-actin cDNA.
Adoptive Transfer Experiment.
Donor mice and recipient mice were placed on the control diet 2 wk before the induction of CNV. Donor mice received i.p. injections of control vehicle, 17,18-EEQ (50 µg/kg), 19,20-EDP (50 µg/kg), or C21 (50 µg/kg) daily at day −2 and day −1. At day 0, donor mice were killed. Peritoneal cells were collected by peritoneal lavage with ice-cold PBS. The cells were treated with a red blood cell lysis buffer (BioLegend). Adoptive transfer was performed from the vehicle, 17,18-EEQ, 19,20-EDP, or C21-treated donor mice to the recipient mice. A total of 3 × 106 cells were injected intraperitoneally and then laser CNV was induced in the recipient mice on the same day of transfer. CNV lesions at day 7 were assessed by staining of choroidal flat mounts with fluorescent isolectin B4, cross-sectional area of lesions quantified by SD-OCT, and fluorescein angiography.
Statistical Analysis.
Results are shown as means ± SEM. The Student’s t test was used to compare two groups while Dunnett’s test was used to compare more than two groups. Statistical significance was defined as a P value of <0.05.
Acknowledgments
This study was supported by a Special Scholar Award (to K.M.C.), a Medical Student Fellowship Grant (to C.B.K.), and an unrestricted grant (to J.W.M.) from Research to Prevent Blindness. Special thanks go to Department of Ophthalmology, Harvard University, and Massachusetts Eye and Ear Infirmary for supporting this research (K.M.C.); the Massachusetts Lions Eye Research Fund (K.M.C.); the BrightFocus Foundation (K.M.C.); Grant R01EY022084/S1 (to K.M.C.); partial support (to K.M.C.) from the West Coast Metabolomics Center (NIH 1U24DK097154); a fellowship from the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad (to E.H.); the Intramural Program of the NIH, National Institute of Environmental Health Sciences (NIEHS) (Z01 ES025034 to D.C.Z.); the Robert A. Welch Foundation (I-0011 to J.R.F.); and NIH (HL111392 to J.R.F.). Partial support was supplied by NIEHS (R01ES002710 to B.D.H.), NIEHS/Superfund Research Program (P42 ES004699 to B.D.H.), and the NIH (K99ES024806 and R00ES024806 to K.S.S.L.).
Footnotes
Conflict of interest statement: Massachusetts Eye and Ear Infirmary holds a patent application on anticomplement therapeutics in ocular cell death titled Cyp450 lipid metabolites reduce inflammation and angiogenesis (MEEI WO 2014110261 A1: PCT/US2014/010880) (of which K.M.C. and R.Y. are inventors) and has a pending application of the use of sEH inhibitors in inflammation and angiogenesis (of which K.M.C., E.H., and B.D.H. are inventors). Additionally, Massachusetts Eye and Ear has a proprietary interest in photodynamic therapy for conditions involving unwanted ocular neovascularization and has received financial remuneration related to this technology. J.W.M. receives a share of the same in accordance with institutional guidelines. Max Delbruck Center for Molecular Medicine and University of Texas Southwestern hold a patent family on novel eicosanoid derivatives, also encompassing C21 and its use (WO 2010/081683, PCT/EP2010/000140) (of which W.-H.S., J.R.F., and N.P. are inventors). Max Delbruck Center for Molecular Medicine holds a pending patent application of the use of novel eicosanoid derivatives in indications, associated with inflammation and neovascularization (of which W.-H.S. is inventor).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620898114/-/DCSupplemental.
References
- 1.Miller JW. Age-related macular degeneration revisited–piecing the puzzle: The LXIX Edward Jackson memorial lecture. Am J Ophthalmol. 2013;155:1–35. e13. doi: 10.1016/j.ajo.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379:1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 3.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 4.Ng EW, Adamis AP. Anti-VEGF aptamer (pegaptanib) therapy for ocular vascular diseases. Ann N Y Acad Sci. 2006;1082:151–171. doi: 10.1196/annals.1348.062. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld PJ, et al. MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 6.Miller JW. Beyond VEGF—The Weisenfeld lecture. Invest Ophthalmol Vis Sci. 2016;57:6911–6918. doi: 10.1167/iovs.16-21201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang D, Jia Y, Rispoli M, Tan O, Lumbroso B. Optical coherence tomography angiography of time course of choroidal neovascularization in response to anti-angiogenic treatment. Retina. 2015;35:2260–2264. doi: 10.1097/IAE.0000000000000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuehlewein L, Sadda SR, Sarraf D. OCT angiography and sequential quantitative analysis of type 2 neovascularization after ranibizumab therapy. Eye (Lond) 2015;29:932–935. doi: 10.1038/eye.2015.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SanGiovanni JP, et al. DNA sequence variants in PPARGC1A, a gene encoding a coactivator of the ω-3 LCPUFA sensing PPAR-RXR transcription complex, are associated with NV AMD and AMD-associated loci in genes of complement and VEGF signaling pathways. PLoS One. 2013;8:e53155. doi: 10.1371/journal.pone.0053155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitroulis I, et al. Leukocyte integrins: Role in leukocyte recruitment and as therapeutic targets in inflammatory disease. Pharmacol Ther. 2015;147:123–135. doi: 10.1016/j.pharmthera.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noda K, et al. Vascular adhesion protein-1 blockade suppresses choroidal neovascularization. FASEB J. 2008;22:2928–2935. doi: 10.1096/fj.07-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanai R, et al. Cytochrome P450-generated metabolites derived from ω-3 fatty acids attenuate neovascularization. Proc Natl Acad Sci USA. 2014;111:9603–9608. doi: 10.1073/pnas.1401191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Serhan CN, Savill J. Resolution of inflammation: The beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 17.Funk CD. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 18.Capra V, et al. Transcellular biosynthesis of eicosanoid lipid mediators. Biochim Biophys Acta. 2015;1851:377–382. doi: 10.1016/j.bbalip.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Arnold C, Konkel A, Fischer R, Schunck WH. Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol Rep. 2010;62:536–547. doi: 10.1016/s1734-1140(10)70311-x. [DOI] [PubMed] [Google Scholar]
- 20.Arnold C, et al. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of omega-3 fatty acids. J Biol Chem. 2010;285:32720–32733. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer R, et al. Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J Lipid Res. 2014;55:1150–1164. doi: 10.1194/jlr.M047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fer M, et al. Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Arch Biochem Biophys. 2008;471:116–125. doi: 10.1016/j.abb.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Askari A, Thomson SJ, Edin ML, Zeldin DC, Bishop-Bailey D. Roles of the epoxygenase CYP2J2 in the endothelium. Prostaglandins Other Lipid Mediat. 2013;107:56–63. doi: 10.1016/j.prostaglandins.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Souied EH, et al. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: The Nutritional AMD Treatment 2 Study. Ophthalmology. 2013;120:1619–1631. doi: 10.1016/j.ophtha.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds R, Rosner B, Seddon JM. Dietary omega-3 fatty acids, other fat intake, genetic susceptibility, and progression to incident geographic atrophy. Ophthalmology. 2013;120:1020–1028. doi: 10.1016/j.ophtha.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edin ML, et al. Endothelial expression of human cytochrome P450 epoxygenase CYP2C8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart. FASEB J. 2011;25:3436–3447. doi: 10.1096/fj.11-188300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner K, Vito S, Inceoglu B, Hammock BD. The role of long chain fatty acids and their epoxide metabolites in nociceptive signaling. Prostaglandins Other Lipid Mediat. 2014;113-115:2–12. doi: 10.1016/j.prostaglandins.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose TE, et al. 1-Aryl-3-(1-acylpiperidin-4-yl)urea inhibitors of human and murine soluble epoxide hydrolase: Structure-activity relationships, pharmacokinetics, and reduction of inflammatory pain. J Med Chem. 2010;53:7067–7075. doi: 10.1021/jm100691c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falck JR, et al. 17(R),18(S)-epoxyeicosatetraenoic acid, a potent eicosapentaenoic acid (EPA) derived regulator of cardiomyocyte contraction: Structure-activity relationships and stable analogues. J Med Chem. 2011;54:4109–4118. doi: 10.1021/jm200132q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulki L, Sweigard JH, Connor KM. Assessing leukocyte-endothelial interactions under flow conditions in an ex vivo autoperfused microflow chamber assay. J Vis Exp. 2014:e52130. doi: 10.3791/52130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giani A, et al. In vivo evaluation of laser-induced choroidal neovascularization using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:3880–3887. doi: 10.1167/iovs.10-6266. [DOI] [PubMed] [Google Scholar]
- 32.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 33.Rinaldo-Matthis A, Haeggström JZ. Structures and mechanisms of enzymes in the leukotriene cascade. Biochimie. 2010;92:676–681. doi: 10.1016/j.biochi.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Panigrahy D, Greene ER, Pozzi A, Wang DW, Zeldin DC. EET signaling in cancer. Cancer Metastasis Rev. 2011;30:525–540. doi: 10.1007/s10555-011-9315-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Wang D, Dubois RN. Epoxyeicosatrienoic acids: A double-edged sword in cardiovascular diseases and cancer. J Clin Invest. 2012;122:19–22. doi: 10.1172/JCI61453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westphal C, et al. CYP2J2 overexpression protects against arrhythmia susceptibility in cardiac hypertrophy. PLoS One. 2013;8:e73490. doi: 10.1371/journal.pone.0073490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westphal C, Konkel A, Schunck WH. CYP-eicosanoids: A new link between omega-3 fatty acids and cardiac disease? Prostaglandins Other Lipid Mediat. 2011;96:99–108. doi: 10.1016/j.prostaglandins.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Liu JY, et al. Substituted phenyl groups improve the pharmacokinetic profile and anti-inflammatory effect of urea-based soluble epoxide hydrolase inhibitors in murine models. Eur J Pharm Sci. 2013;48:619–627. doi: 10.1016/j.ejps.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulu A, et al. An omega-3 epoxide of docosahexaenoic acid lowers blood pressure in angiotensin-II-dependent hypertension. J Cardiovasc Pharmacol. 2014;64:87–99. doi: 10.1097/FJC.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris TR, et al. Inhibition of soluble epoxide hydrolase attenuates hepatic fibrosis and endoplasmic reticulum stress induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol. 2015;286:102–111. doi: 10.1016/j.taap.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishijima K, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakurai E, et al. Targeted disruption of the CD18 or ICAM-1 gene inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:2743–2749. doi: 10.1167/iovs.02-1246. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, et al. Abundant lipid and protein components of drusen. PLoS One. 2010;5:e10329. doi: 10.1371/journal.pone.0010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hui Y, et al. Targeted deletions of cyclooxygenase-2 and atherogenesis in mice. Circulation. 2010;121:2654–2660. doi: 10.1161/CIRCULATIONAHA.109.910687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang M, et al. Microsomal prostaglandin e2 synthase-1 modulates the response to vascular injury. Circulation. 2011;123:631–639. doi: 10.1161/CIRCULATIONAHA.110.973685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, et al. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J Pharmacol Exp Ther. 2005;314:522–532. doi: 10.1124/jpet.105.083477. [DOI] [PubMed] [Google Scholar]
- 47.Webler AC, et al. Epoxyeicosatrienoic acids are part of the VEGF-activated signaling cascade leading to angiogenesis. Am J Physiol Cell Physiol. 2008;295:C1292–C1301. doi: 10.1152/ajpcell.00230.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pritchard KA, Jr, Tota RR, Stemerman MB, Wong PY. 14, 15-Epoxyeicosatrienoic acid promotes endothelial cell dependent adhesion of human monocytic tumor U937 cells. Biochem Biophys Res Commun. 1990;167:137–142. doi: 10.1016/0006-291x(90)91741-a. [DOI] [PubMed] [Google Scholar]
- 49.Node K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng Y, et al. Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB J. 2011;25:703–713. doi: 10.1096/fj.10-171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Falck JR, et al. 11,12-epoxyeicosatrienoic acid (11,12-EET): Structural determinants for inhibition of TNF-alpha-induced VCAM-1 expression. Bioorg Med Chem Lett. 2003;13:4011–4014. doi: 10.1016/j.bmcl.2003.08.060. [DOI] [PubMed] [Google Scholar]
- 52.Seddon JM, et al. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001;119:1191–1199. doi: 10.1001/archopht.119.8.1191. [DOI] [PubMed] [Google Scholar]
- 53.Smith W, Mitchell P, Leeder SR. Dietary fat and fish intake and age-related maculopathy. Arch Ophthalmol. 2000;118:401–404. doi: 10.1001/archopht.118.3.401. [DOI] [PubMed] [Google Scholar]
- 54.Age-Related Eye Disease Study 2 Research Group Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 55.Merle BM, et al. Nutritional AMD Treatment 2 Study Group Circulating omega-3 fatty acids and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:2010–2019. doi: 10.1167/iovs.14-13916. [DOI] [PubMed] [Google Scholar]
- 56.SanGiovanni JP, et al. Age-Related Eye Disease Study Research Group The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch Ophthalmol. 2007;125:671–679. doi: 10.1001/archopht.125.5.671. [DOI] [PubMed] [Google Scholar]
- 57.Souied EH, et al. Nutritional AMD Treatment 2 Study Group Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: The Nutritional AMD Treatment 2 study. Ophthalmology. 2013;120:1619–1631. doi: 10.1016/j.ophtha.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Lee CR, et al. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J. 2010;24:3770–3781. doi: 10.1096/fj.10-160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sinal CJ, et al. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275:40504–40510. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- 60.Almulki L, et al. Surprising up-regulation of P-selectin glycoprotein ligand-1 (PSGL-1) in endotoxin-induced uveitis. FASEB J. 2009;23:929–939. doi: 10.1096/fj.08-118760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hafezi-Moghadam A, Thomas KL, Cornelssen C. A novel mouse-driven ex vivo flow chamber for the study of leukocyte and platelet function. Am J Physiol Cell Physiol. 2004;286:C876–C892. doi: 10.1152/ajpcell.00500.2003. [DOI] [PubMed] [Google Scholar]