Significance
Daily rhythms in animal behavior, physiology, and metabolism are driven by cell-autonomous mechanisms that keep time and control overt rhythms via transcriptional feedback loops, making it fundamental to define the mechanisms driving rhythmic transcription. In mammals, PERIOD and CRYPTOCHROME (CRY) rhythmically repress CLOCK:BMAL1 transcriptional activity, but the mechanisms by which CRY represses CLOCK:BMAL1 activity are not fully understood. Using CRISPR/Cas9 for in vivo genetic manipulations in the monarch, we show that repression of circadian transcription by vertebrate-like CRY is mediated primarily by a BMAL1 transactivation domain (TAD)-independent mechanism involving the CLK-PAS B domain, while repression on the BMAL1 TAD is dispensable for the generation of rhythms but alters circadian phase during the first day of constant darkness by affecting activation levels.
Keywords: circadian clock, CRYPTOCHROME 2, BMAL1 C terminus, CLOCK, CRISPR
Abstract
Circadian repression of CLOCK-BMAL1 by PERIOD and CRYPTOCHROME (CRY) in mammals lies at the core of the circadian timekeeping mechanism. CRY repression of CLOCK-BMAL1 and regulation of circadian period are proposed to rely primarily on competition for binding with coactivators on an α-helix located within the transactivation domain (TAD) of the BMAL1 C terminus. This model has, however, not been tested in vivo. Here, we applied CRISPR/Cas9-mediated mutagenesis in the monarch butterfly (Danaus plexippus), which possesses a vertebrate-like CRY (dpCRY2) and an ortholog of BMAL1, to show that insect CRY2 regulates circadian repression through TAD α-helix–dependent and –independent mechanisms. Monarch mutants lacking the BMAL1 C terminus including the TAD exhibited arrhythmic eclosion behavior. In contrast, mutants lacking the TAD α-helix but retaining the most distal C-terminal residues exhibited robust rhythms during the first day of constant darkness (DD1), albeit with a delayed peak of eclosion. Phase delay in this mutant on DD1 was exacerbated in the presence of a single functional allele of dpCry2, and rhythmicity was abolished in the absence of dpCRY2. Reporter assays in Drosophila S2 cells further revealed that dpCRY2 represses through two distinct mechanisms: a TAD-dependent mechanism that involves the dpBMAL1 TAD α-helix and dpCLK W328 and a TAD-independent mechanism involving dpCLK E333. Together, our results provide evidence for independent mechanisms of vertebrate-like CRY circadian regulation on the BMAL1 C terminus and the CLK PAS-B domain and demonstrate the importance of a BMAL1 TAD-independent mechanism for generating circadian rhythms in vivo.
Circadian timing enables organisms to coordinate their physiology and behavior with the daily cycle by anticipating fluctuating environmental changes (1, 2). At the core of the timekeeping mechanism in animals is a cell-autonomous molecular transcriptional/translational feedback loop that controls the rhythmic expression of clock-controlled genes with a period close to 24 h. In mammals, the heterodimeric basic helix–loop–helix (bHLH) transcription factor CLOCK:BMAL1 initiates feedback loop function by activating transcription of the Period (Per1, Per2) and Cryptochrome (Cry1, Cry2) genes (3–5). Accumulating levels of PER-CRY then form complexes that translocate to the nucleus and interact with CLOCK:BMAL1 to repress the transcription of their own genes. Once the repressors are degraded, CLOCK:BMAL1 activity is restored to initiate a new cycle of transcription. Despite the central importance of circadian repression for generating 24-h rhythms, the molecular mechanisms underlying PERs’ and CRYs’ repressive function are not fully understood.
PERs are essential for the nuclear translocation of PER-CRY complexes (6) and for rhythmic PER-CRY-CLK-BMAL1 interactions (6, 7). CRYs, on the other end, are essential for CLK:BMAL1 transcriptional repression (5, 8, 9). The potent inhibitory effect of CRYs on CLOCK:BMAL1-mediated transcription has been observed in vitro in the absence of PERs (10), suggesting that CRYs can interact directly with CLOCK:BMAL1. Residues that are important for the CRY1-CLOCK-BMAL1 interactions have been identified in both the PAS-B domain of CLOCK and the BMAL1 C terminus (11). The crystal structure of a complex containing the mouse CLOCK:BMAL1 bHLH-PAS domains has revealed that five of these residues localized on the CLOCK PAS-B HI loop form a finger accessible for CRY1 binding (12). However, the CRY1-CLOCK interaction is thought to provide stability only to the ternary complex, while CRY1-BMAL1 C terminus interaction is proposed to mediate repression (13–15). Based on in vitro cell culture and biophysical experiments, CRY1 has recently been found to dock on CLOCK PAS-B and to regulate circadian cycling by competing for binding with coactivators, such as p300, on an α-helix located in the transactivation domain (TAD) of the BMAL1 C terminus (13–17). Despite these important advances, it is unknown if CRY1-BMAL1 C terminus interaction constitutes the main mechanism driving circadian rhythms in vivo. The genetic dissection of this mechanism has been hampered by the lack of a BMAL1 C-terminal mutant mouse that retains transcriptional activity and by the functional redundancy of the two mouse CRY paralogues.
The monarch butterfly, Danaus plexippus, is uniquely suited to determine the importance of the CRY-BMAL1 C terminus for generating circadian rhythms in vivo. Not only does the monarch butterfly possess a single copy of a mammalian-like repressive CRY (designated “CRY2”) (18–20) and an ortholog of mammalian BMAL1 with a highly conserved TAD in the last 30 amino acids of its C terminus, but its genome can also be targeted with CRISPR/Cas9 (21). Here, we generated domain-specific mutations in the monarch (dp)BMAL1 in vivo using CRISPR/Cas9. We found that while the dpBMAL1 C terminus lacking in its Drosophila ortholog (dCYCLE; dCYC) is required for transcriptional activity, the TAD α-helix located on this domain plays a role in regulating circadian phase or period but is not required for the generation of circadian rhythms. Using cell-based reporter assays in Drosophila Schneider 2 (S2) cells, we showed that dpCRY2 represses through two distinct mechanisms: a TAD-dependent mechanism that involves the dpBMAL1 TAD α-helix and dpCLK W328 located in the dpCLK PAS-B domain and a TAD-independent mechanism involving dpCLK E333. These findings provide insights into the mechanisms of repression by vertebrate-like dpCRY2 and demonstrate that a BMAL1 TAD-independent mechanism plays a major role in the generation of circadian rhythms.
Results
Monarch dpCLK:dpBMAL1 Transcriptional Activity Requires the dpBMAL1 C-Terminal Domain Lacking in Drosophila CYCLE.
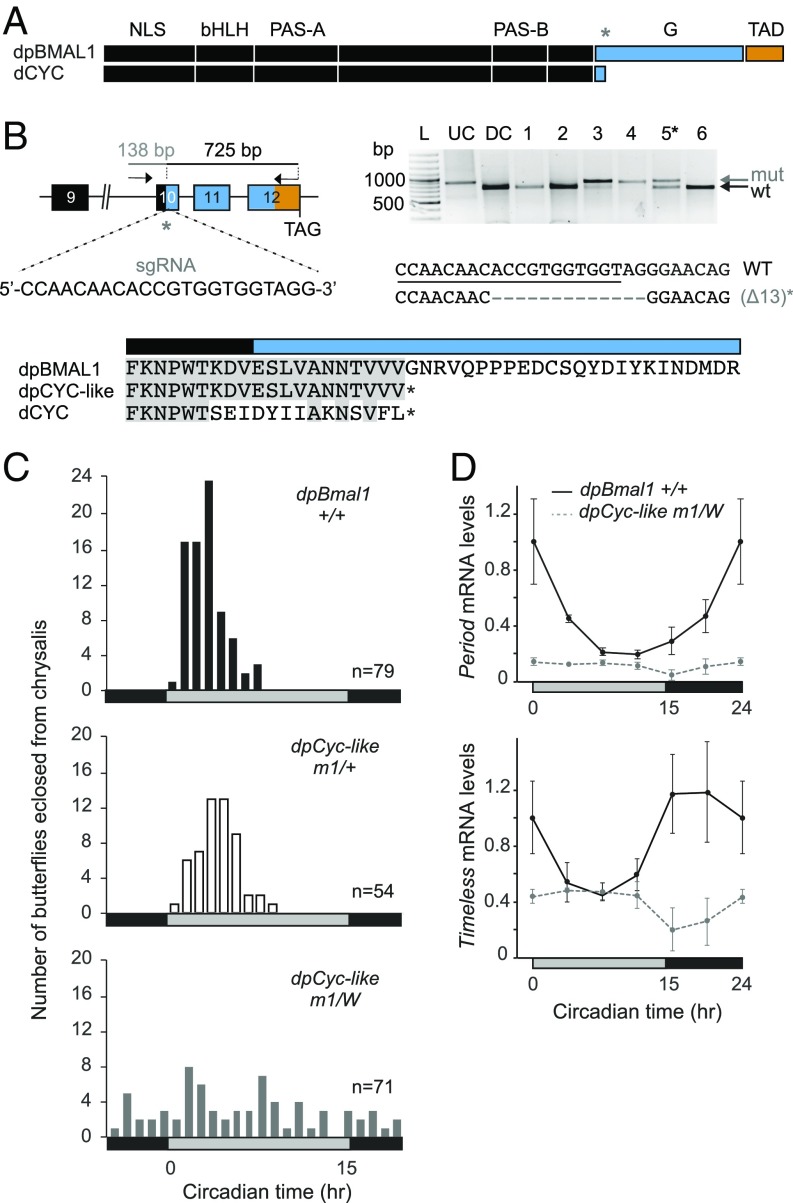
To begin to genetically define the mechanisms by which circadian activation is mediated in the monarch molecular clockwork, we first sought to determine if transactivation function was mediated by monarch dpCLK or the C terminus of dpBMAL1 in vivo. To this end, we generated a monarch dpBMAL1-deletion mutant lacking the C-terminal domain, which is lacking in its Drosophila ortholog CYCLE (dCYC) (Fig. 1A). Using CRISPR/Cas9, we introduced frameshift mutations at a location of the coding region that would result in the production of Drosophila-like dpCYC (Fig. 1B). From 150 embryos injected, 6 of 22 surviving larvae were mosaic at the targeted site based on a Cas9-based in vitro cleavage assay (Fig. 1B and Fig. S1 A and B). We selected an adult male butterfly bearing somatic mutations at about 50% for backcrosses (Fig. 1B and Fig. S1C), and progeny were screened for germline transmission of the targeted lesions. Six of ten genotyped larvae carried a mutated allele, which was a single 13-bp deletion in all germline transformants (Fig. 1B and Fig. S1C). We found sex-based segregation for the mutation in the corresponding adults, with females being hemizygous and males heterozygous, demonstrating that dpBmal1 is located on the Z sex chromosome along with monarch Clk (21) [in lepidopterans, females are heterogametic (ZW), and males are homogametic (ZZ)]. Importantly, the 13-bp deletion germline mutation resulted in a frameshift leading to the truncation of the dpBmal1 C terminus and was designated “dpCyc-like.”
Fig. 1.
Monarch dpCLK:dpBMAL1 transcriptional activity requires the dpBMAL1 C-terminal domain lacking in Drosophila CYC. (A) Schematic representation of monarch dpBMAL1 and its C-terminal domain (G and TAD) conserved with mammalian BMAL1, lacking in its Drosophila ortholog dCYC. The gray star indicates the position of the single-guide RNA (sgRNA) used to introduce indels. (B, Upper Left) DpBmal1 genomic locus with the sgRNA and the primers used to amplify the 863-bp targeted region for analysis of mutagenic lesions. (Upper Right) Detection of mutagenic lesions (mut) in somatic cells of a subset of potential founder G0 butterflies using a Cas9-based in vitro cleavage assay. DC, digested control; L, ladder; UC, undigested control. The black star indicates the somatic mutant selected for backcrossing to generate a monarch dpCYC-like mutant lacking the BMAL1 G and TAD regions. DpCYC-like mutants carry a 13-bp deletion. (Lower) Partial alignment of dpBMAL1, dpCYC-like mutant, and dCYC proteins showing the position of the truncation in dpCYC-like relative to the C terminus of dCYC. (C) Profiles of adult eclosion in DD of wild-type (black bars), heterozygous (white bars), and hemizygous mutant (gray bars) siblings of the dpCyc-like mutant line (designated “m1”) entrained to 15 h light/9 h dark (LD 15:9) throughout the larval and pupal stages. Data from DD1 and DD2 are pooled and binned in 1-h intervals. The horizontal bars at the bottom of the graphs show subjective day (gray) and night (black). P < 0.0001 (one-way ANOVA); dpBmal1+/+ vs. dpCyc-likem1/+, P > 0.05; dpBmal1+/+ vs. dpCyc-likem1/W, P < 0.01; dpCyc-likem1/+ vs. dpCyc-likem1/W, P < 0.01 (Tukey’s post hoc test). (D) Circadian expression of period and timeless in brains of wild-type (solid black lines) and hemizygous mutant (dashed gray lines) siblings of the dpCyc-like mutant line. Values are mean ± SEM of three animals. The horizontal bars at the bottom of the graphs show subjective day (gray) and night (black). Interaction genotype × time: per, P < 0.01; tim, P < 0.05 (two-way ANOVA).
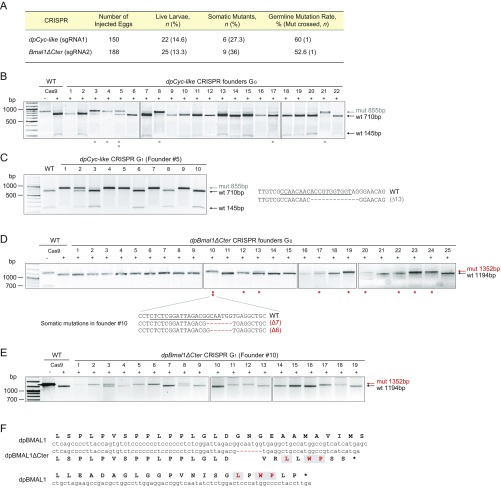
Fig. S1.
CRISPR/Cas9-mediated targeted mutagenesis of dpBMAL1 after mRNA microinjection. (A) Table summarizing results for the generation of two dpBMAL1 mutants. The percentage of live larvae corresponds to the number of larvae that survived to adulthood relative to the number of injected eggs. The percentage of somatic mutants corresponds to the number of live larvae that presented any degree of somatic mosaicism. The germline mutation rate corresponds to the percentage of progeny carrying a mutated allele from the total of progeny tested. The number of mosaic mutants crossed (Mut crossed, n) is shown. (B) Targeted mutagenesis of the dpBmal1 exon 10 for all alive potential founders (G0) validated by PCR and the Cas9-based in vitro cleavage assay. Full cleavage of a wild-type PCR fragment by the sgRNA and Cas9 was validated, and PCR products from somatic tissue of each founder were subjected to the Cas9-based in vitro cleavage assay. The gray arrow indicates genomic amplicons carrying targeted mutations that are resistant to cleavage by Cas9, and the black arrows indicate cleaved wild-type fragments (+) subjected to and (−) not subjected to Cas9 cleavage assay. Gray stars indicate somatic mutants, and two-stacked stars indicate the individual (founder #5) used to establish the dpCyc-like mutant line. (C, Left) Genotyping of founder #5 progeny (G1) with the Cas9-based cleavage assay. Six of ten individuals carry a mutated allele, including one heterozygote and five homozygotes. (Right) All mutants carried a single 13-bp deletion. (D) Targeted mutagenesis of the dpBmal1 exon 12 for all alive potential founders (G0) validated by PCR and the Cas9-based in vitro cleavage assay. The red arrow indicates genomic amplicons carrying targeted mutations that are resistant to cleavage by Cas9, and the black arrow indicates the larger cleaved wild-type fragment. Red stars indicate somatic mutants, and two stacked stars indicate the individual (founder #10) used to establish the dpBmal1ΔCter mutant line. The two mutations identified in somatic tissues of this founder are shown. (E) Genotyping of founder #10 progeny (G1) with the Cas9-based cleavage assay. Ten of nineteen individuals carried a mutated allele, either a 7-bp deletion or a 6-bp deletion. (F) Alignment of nucleotide (center) and amino acid (outward) sequences of the wild-type or mutated (7-bp deletion) dpBMAL1 C-terminal regions. The conserved most distal residues are in red.
To test whether the dpCLK:dpCYC-like heterodimer retained transcriptional activity, we assessed the effect of the dpCYC-like truncation on circadian behavior and the molecular clockwork. We first examined the timing of pupal eclosion (i.e., the emergence of the adult from its pupal case), a robust and quantifiable behavior under the control of the brain circadian clock (18, 22, 23), focusing our analysis on female dpCyc-like knockouts, heterozygous males, and wild-type siblings of both sexes (Materials and Methods). We found that the circadian timing of adult eclosion was abolished in dpCyc-like hemizygous butterflies, while heterozygous and wild-type siblings eclosed rhythmically with a similar peak of eclosion during the early subjective day (P > 0.05, one-way ANOVA followed by Tukey’s post hoc test) (Fig. 1C and Fig. S2A). To assess if arrhythmicity in dpCyc-like mutants resulted from a defect in the transcriptional activity of dpCLK:dpCYC-like, we examined the expression levels of two well-characterized CLK:BMAL1/CYC direct target genes that are core clock components, period (per) and timeless (tim), in the brain of dpCyc-like hemizygous and wild-type siblings by qPCR (Fig. 1D). Consistent with our behavioral data, per and tim circadian rhythms were abolished in brains of dpCYC-like hemizygous mutants, and their expression levels were constitutively low (Fig. 1D). Together, these data demonstrate that the BMAL1 C-terminal domain containing the G and TAD regions described in mammals (14) is required for transcriptional activation in the monarch.
Fig. S2.
Profiles of adult eclosion during the first 2 d of DD. (A) Profiles of wild-type (black bars), heterozygous (white bars), and hemizygous mutant (gray bars) siblings of the dpCyc-like mutant line. (B) Profiles of wild-type (black bars), heterozygous (white bars), and hemizygous mutant (red bars) siblings of the dpBmal1ΔCter mutant line. (C) Profiles of dpBmal1ΔCter mutants in a wild-type background (red bars), in a heterozygous background for dpCry2 (blue bars), and in a dpCry2-null background (brown bars). Butterflies were entrained throughout their larval and pupal stages to LD 15:9. Eclosion is binned at 1-h intervals. The horizontal bars below the graphs indicate subjective night (black) and day (gray).
The dpBMAL1 TAD Helix Is Dispensable for Generating Behavioral Rhythms.
Previous in vitro studies have shown that an α-helix within the last 30 amino acids of the mouse (m)BMAL1 C terminus plays an important role in circadian cycling by acting as the site for coactivator binding and repression by mCRY1 (13, 14). Whether this mechanism is crucial in vivo for generating circadian rhythms has never been tested.
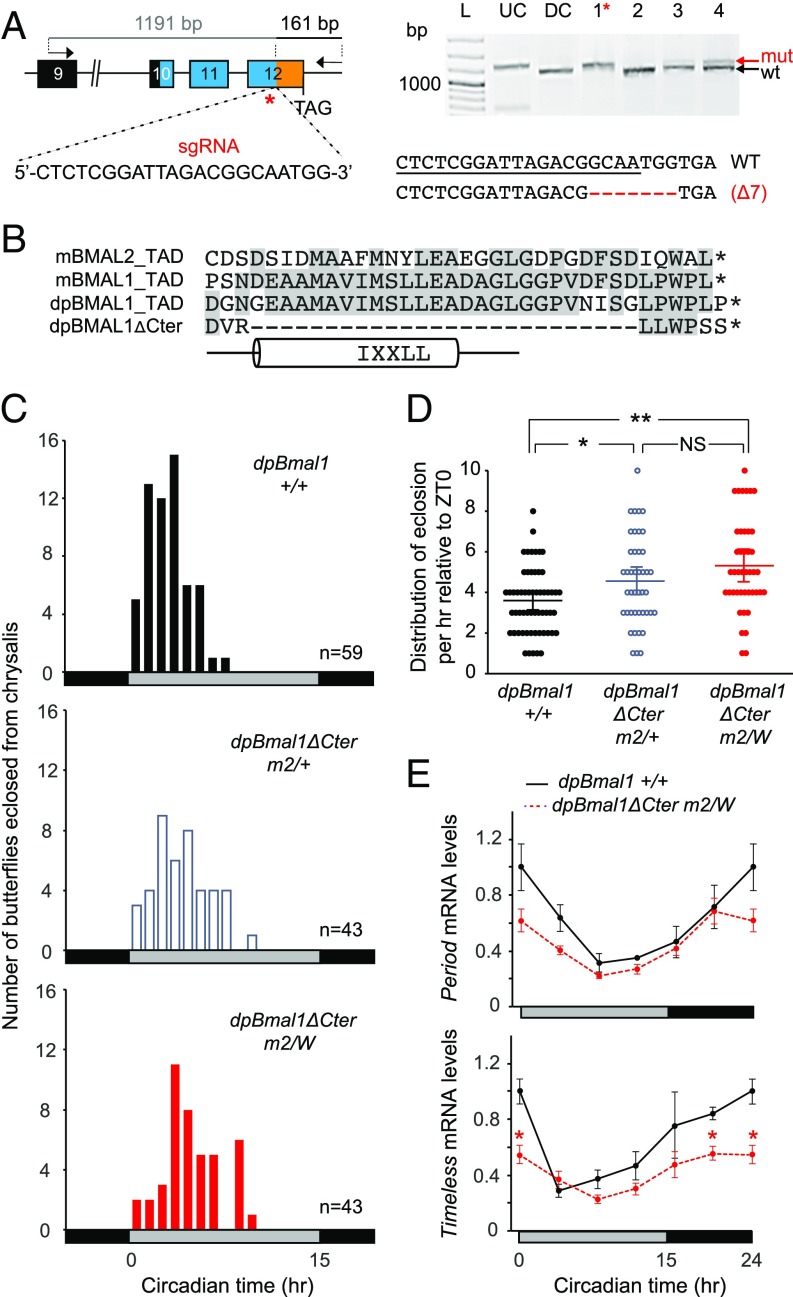
We therefore sought to generate a dpBMAL1 mutant butterfly carrying a partially truncated C-terminal TAD lacking the α-helix using CRISPR/Cas9, by targeting the region upstream of the TAD with a single guide RNA (Fig. 2A). From the 25 larvae surviving the injection (of 188 injected embryos), eight exhibited somatic mosaicism at the targeted site, and we selected the most highly targeted of the ones surviving to adulthood for backcrosses (Fig. S1 A and D). Half of their progeny were either heterozygous or hemizygous for a targeted lesion, and sequencing of the mutated alleles identified two microdeletions of 6 bp or 7 bp, respectively, which were reminiscent of the only two somatic mutations also identified in the founder (Fig. S1 D and E). The 7-bp deletion introduced a frameshift which, by chance, resulted in the coding of three of the last six residues (i.e., LxWPxx) found within the most distal dpBMAL1 C terminus and introduced a stop codon immediately thereafter (Fig. 2B and Fig. S1F). This mutation thus eliminated the TAD α-helix and adjacent sequences but retained some of the most distal C-terminal residues that are conserved in mBMAL1.
Fig. 2.
The dpBMAL1 TAD helix is dispensable for the generation of circadian rhythm in vivo but regulates the phase by maintaining high activation levels. (A, Left) DpBmal1 genomic locus showing positions of the sgRNA used to generate a TAD truncation mutant (dpBMAL1ΔCter) and primers used to amplify the 1,352-bp targeted region for analysis of mutagenic lesions. (Right) Detection of mutagenic lesions (mut) in somatic cells of a subset of potential founder G0 butterflies using a Cas9-based in vitro cleavage assay. DC, digested control; L, ladder; UC, undigested control. The red star indicates the somatic mutant selected for backcrossing to generate a dpBMAL1ΔCter mutant lacking most of the TAD but retaining three of the most distal amino acids. DpBMAL1ΔCter mutants carry a 7-bp deletion. (B) Sequence alignment showing TAD regions of BMAL1 and BMAL2 proteins from mouse (m), dpBMAL1, and the dpBMAL1ΔCter mutant. The TAD helix region is shown below the alignment. (C) Profiles of adult eclosion in DD of wild-type (black bars), heterozygous (white bars), and hemizygous mutant (red bars) siblings of the dpBmal1ΔCter mutant line (designated “m2”) entrained to LD 15:9 throughout the larval and pupal stages. Data from DD1 and DD2 are pooled and binned in 1-h intervals. Horizontal bars at the bottom of the graphs indicate subjective day (gray) and night (black). (D) Distribution of eclosion during the subjective day for each genotype. Dots indicate the number of butterflies eclosed at each time interval relative to subjective lights on. P < 0.0001 (one-way ANOVA); *P < 0.05, **P < 0.01, NS, nonsignificant (Tukey’s post hoc test). (E) Circadian expression of period and timeless in brains of wild-type (solid black lines) and hemizygous mutant (dashed red lines) siblings of the dpBmal1ΔCter mutant line. Values are the mean ± SEM of three animals. Horizontal bars below the graphs show subjective day (gray) and night (black). per, P > 0.05; tim, P < 0.02 (two-way ANOVA, interaction genotype × time); *P < 0.05 (Student’s t test between each genotype at each time point).
To explore the relative contribution of the TAD α-helix and the most distal C-terminal region to the generation of circadian rhythms in vivo, we therefore established a mutant monarch line carrying this 7-bp deletion, hereafter named “dpBmal1ΔCter.” To our surprise, we found that dpBmal1ΔCter hemizygous mutants exhibited a robust circadian rhythm of eclosion (Fig. 2C and Fig. S2B), demonstrating that the TAD α-helix is dispensable for behavioral rhythms in vivo. However, we found that the distribution in eclosion time was significantly affected in a dose-dependent manner, with a mean of eclosion time for hemizygous mutant butterflies occurring ∼1.5 h later than for wild-type siblings (P < 0.05, one-way ANOVA followed by Tukey’s post hoc test) (Fig. 2 C and D). This result demonstrates that lack of the TAD α-helix causes a delay in eclosion behavior on the first day of constant darkness (DD), consistent with its previously reported role in mCRY1 and p300 binding and in regulating circadian rhythms in vitro (14). Although these data show that the mutation alters the phase of the rhythm, it is equally likely that this phase difference results from an altered circadian period.
To determine the effect of the dpBmal1ΔCter deletion on the brain molecular clockwork, we quantified per and tim expression in both wild-type and hemizygous mutants (Fig. 2E). As expected based on our behavioral data, both clock genes were cycling in DD in dpBmal1ΔCter hemizygous mutants, albeit with a decreased amplitude compared with the rhythms observed in wild-type butterflies. Notably, peak levels of tim were significantly reduced, while trough levels were not significantly altered in dpBmal1ΔCter hemizygous mutants [P < 0.05 at circadian time 0 (CT0) and CT20; Student’s t test], and a similar trend was observed for per. This decrease in activation levels is consistent with the delayed eclosion observed in heterozygous and hemizygous dpBmal1ΔCter mutants and with a role of the TAD α-helix in coactivator binding (14). Together, these data demonstrate that the monarch BMAL1 C-terminal TAD α-helix and downstream adjacent sequences are dispensable for circadian rhythms in vivo but contribute to the amplitude of the molecular rhythms.
Repression by dpCRY2 on the dpBMAL1 TAD Helix Regulates Circadian Phase.
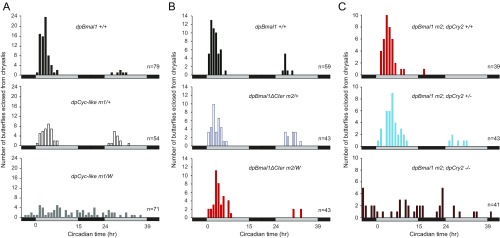
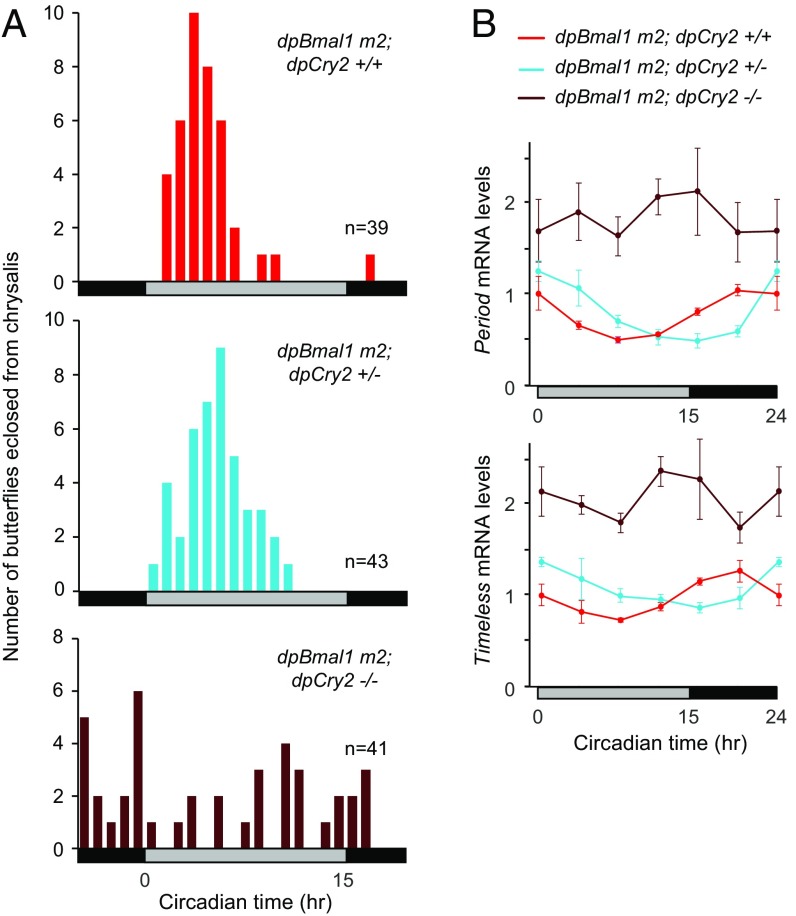
To determine if circadian repression in our dpBmal1ΔCter monarch mutant was mediated by dpCRY2, we generated homozygous dpBmal1ΔCter monarch mutants carrying two, one, or no functional dpCry2 alleles through two subsequent interbreeding crosses (Materials and Methods and Fig. 3). At the behavioral level, we found that dpBmal1ΔCter homozygous mutants with no functional allele of dpCry2 (dpBmal1 m2; dpCry2−/−) lost circadian rhythmicity of eclosion (Fig. 3A and Fig. S2C), as expected based on the previously reported circadian arrhythmicity of dpCry2 knockouts (18). However, to our surprise, dpBmal1ΔCter homozygous mutants carrying a single functional allele of dpCry2 (dpBmal1 m2; dpCry2+/−) eclosed significantly later during the circadian cycle than dpBmal1ΔCter homozygous mutants carrying two functional alleles of dpCry2 (dpBmal1 m2; dpCry2+/+) (P < 0.05, one-way ANOVA followed by Tukey’s post hoc test) (Fig. 3A and Fig. S2C). These results stand in sharp contrast with previous work showing no difference in the timing of eclosion between monarchs carrying either two or a single functional allele of dpCry2 in a wild-type dpBMAL1 background (18).
Fig. 3.
Behavioral and molecular rhythms in dpBmal1ΔCter mutants are driven by dpCRY2 repression. (A) Profiles of adult eclosion in DD of dpBmal1ΔCter mutants (m2, containing both homozygous males and hemizygous females) in a wild-type background for dpCry2 (red bars), in a heterozygous background for dpCry2 (blue bars), and in a dpCry2-null background (brown bars). Data collected in DD1 and DD2 are pooled and binned in 1-h intervals. The horizontal bars below the graphs indicate subjective day (gray) and night (black). dpBmal1 m2; dpCry2+/+ vs. dpBmal1 m2; dpCry2−/−, P < 0.01; dpBmal1 m2; dpCry2+/+ vs. dpBmal1 m2; dpCry2+/−, P < 0.05 (one-way ANOVA followed by Tukey’s post hoc test). (B) Circadian expression of period and timeless in brains of dpBmal1ΔCter mutants in a wild-type background for dpCry2 (red lines), in a heterozygous background for dpCry2 (blue lines), and in a dpCry2-null background (brown lines). Values shown are the mean ± SEM of three animals. The horizontal bars below the graphs indicate subjective day (gray) and night (black). Interaction genotype × time, dpBmal1 m2; dpCry2+/+ vs. dpBmal1 m2; dpCry2−/−: tim and per, P < 0.00001; dpBmal1 m2; dpCry2+/+ vs. dpBmal1 m2; dpCry2+/−: per, P < 0.001; tim, P < 0.005 (two-way ANOVA).
To examine the consequences of this interaction on the molecular clock, we quantified per and tim mRNA levels in the brains of dpBmal1ΔCter homozygous mutants with two, one, or no functional alleles of dpCry2 (Fig. 3B). Consistent with our behavioral analysis, we found that in the absence of dpCRY2 the circadian rhythms of per and tim mRNA in the brains of dpBmal1ΔCter homozygous mutants were abolished, with per and tim mRNA expression being constitutively high over the circadian cycle. In contrast, per and tim mRNA in the brains of dpBmal1ΔCter homozygous mutants with a single functional allele of dpCry2 were rhythmic, but the phase of the rhythm was significantly delayed compared with butterflies with two functional alleles of dpCry2. Per and tim mRNAs have previously been shown to cycle in a circadian manner with the same phase in brains of wild-type monarchs and heterozygous CRY2 mutants (18). Therefore, the dose-dependent effect of dpCRY2 we observed in dpBmal1ΔCter homozygous mutants suggests a dose-dependent effect of dpCRY2 for repression on a domain in dpCLK or outside the dpBMAL1 TAD helix that is masked in the presence of the BMAL1 TAD helix.
Domains on dpCLK:dpBMAL1 Other than the dpBMAL1 C Terminus Contribute to dpCRY2-Dependent Transcriptional Repression.
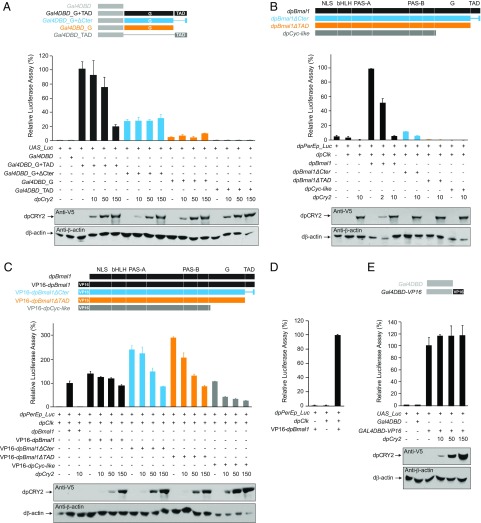
To determine whether circadian repression of dpBmal1ΔCter mutants by dpCRY2 could be mediated through the more distal C-terminal residues of BMAL1 retained in the dpBmal1ΔCter, we used a GAL4/UAS cell-based reporter assay to assess the transactivation capacity of different dpBMAL1 C-terminal domains and their ability to be repressed by dpCRY2. Because both the G and TAD regions in the C terminus of BMAL1 have been implicated in activation and repression by mCRY1, we included the G domain in our analysis. S2 cells were cotransfected with a luciferase reporter under the control of a 10×UAS promoter and either a wild-type dpBMAL1 C-terminal domain or a deletion mutant fused at the C terminus of the DNA-binding domain of the yeast transcription factor GAL4 (GAL4DBD) in the absence or presence of increasing doses of dpCRY2 (Fig. 4A). We found that the G and TAD regions fused to GAL4DBD elicited a large increase in transcriptional activity, while neither the G region nor the TAD region alone elicited substantial transcriptional activity, indicating that both regions are necessary for transcription. Consistent with the idea that dpCRY2 could bind to the TAD helix, transcription mediated by both the G and TAD regions was inhibited by dpCRY2 in a dose-dependent manner (P < 0.05; one-way ANOVA). We also found that the G and truncated TAD domain reminiscent of our dpBmal1ΔCter mutant elicited transcription but with levels reduced by approximately threefold compared with the full-length C-terminal domain, in agreement with a role for the TAD α-helix in enhancing transcriptional activation. However, transcription elicited by the C-terminal domain present in the dpBmal1ΔCter mutant was not inhibited by dpCRY2, even at high doses (P = 0.81; one-way ANOVA), demonstrating that neither the G domain nor the more distal C-terminal residues (i.e., LxWPxx) are sufficient to mediate dpCRY2 repression. These results suggested that the circadian rhythms observed in vivo in dpBmal1ΔCter mutants resulted from transactivation provided by the G and the most distal C-terminal residues of the TAD and repression by dpCRY2 on domains other than the TAD α-helix, on either dpCLK or dpBMAL1.
Fig. 4.
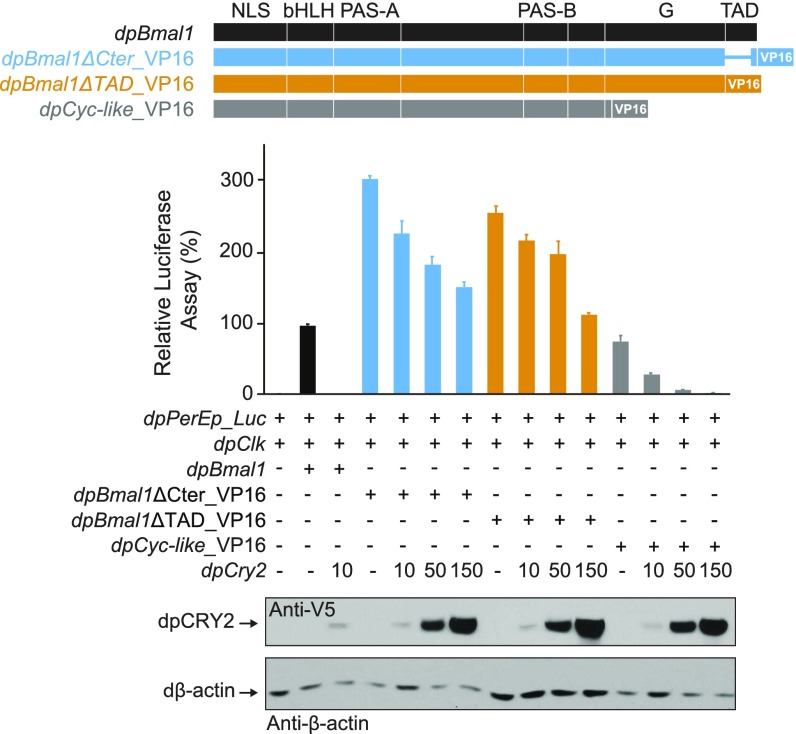
Several domains on dpCLK and dpBMAL1 contribute to transcriptional repression by dpCRY2 in S2 cells. (A, Upper) DpCRY2 does not repress on the BMAL1 G and ΔCter mutant TAD domains. A UAS luciferase reporter (UAS_Luc; 10 ng) was used in the presence (+) or absence (−) of Gal4DBD, Gal4DBD_G+TAD, Gal4DBD_G+ΔCter, Gal4DBD_G, and Gal4DBD_TAD expression plasmids (5 ng each), and increasing doses of dpCRY2 (amounts are given in nanograms). Firefly luciferase activity was computed relative to renilla luciferase activity. Each value is the mean ± SEM of three replicates. (Lower) Western blots of V5-tagged dpCRY2 and Drosophila β-actin protein expression levels. (B) DpCRY2 inhibits dpCLK:dpBMAL1- and dpCLK:dpBMAL1ΔCter-mediated transcription. The monarch per E box luciferase reporter (dpPerEp_Luc; 10 ng) was used in the presence (+) or absence (−) of dpCLK, dpBMAL1, dpBMAL1ΔCter, dpBMAL1ΔTAD, and dpCYC-like expression plasmids (5 ng each) and increasing doses of dpCRY2 (amounts are given in nanograms). Quantification of luciferase activity, values, and Western blot analysis are shown as in A. (C) DpCRY2 dose-dependent inhibition of dpCLK:dpBMAL1 mutants fused to the VP16 transactivation domain in their N termini. The monarch per E box luciferase reporter (dpPerEp_Luc; 10 ng) was used in the presence (+) or absence (−) of dpCLK, dpBMAL1, VP16-dpBMAL1, VP16-dpBMAL1ΔCter, VP16-dpBMAL1ΔTAD, and VP16–dpCYC-like expression plasmids (5 ng each) and increasing doses of dpCRY2 (amounts are given in nanograms). Quantification of luciferase activity, values, and Western blot analysis are depicted as in A. One-way ANOVAs for dose-dependent repression by dpCRY2 on each BMAL1 variant: P < 0.0001 to P < 0.005. (D) DpCLK is required for VP16-dpBMAL1–mediated transcription. The monarch per E box luciferase reporter (dpPerEp_Luc; 10 ng) was used in the presence (+) or absence (−) of dpCLK and VP16-dpBMAL1 expression plasmids (5 ng each). Quantification of luciferase activity and values are depicted as in A. (E) DpCRY2 does not repress the VP16 activation domain. A UAS luciferase reporter (UAS_Luc; 10 ng) was used in the presence (+) or absence (−) of Gal4DBD and Gal4DBD-VP16 expression plasmids (5 ng each) and increasing doses of dpCRY2 (amounts are given in nanograms). Quantification of luciferase activity, values, and Western blot analysis are depicted as in A. P = 0.79 (one-way ANOVA).
To test this hypothesis, we next tested the ability of dpCRY2 to repress dpCLK:dpBMAL1 C-terminal truncation mutants by cotransfecting S2 cells with a luciferase reporter construct containing a tandem repeat of the proximal CACGTG E-box enhancer from the monarch per promoter (20) and with constructs expressing dpCLK and either full-length dpBMAL1 or C-terminal truncation mutants, in the absence or presence of increasing doses of dpCRY2 (Fig. 4B). As previously shown (20), dpCLK:dpBMAL1 elicited an increase in transcriptional activity, which was inhibited by dpCRY2 in a dose-dependent manner (P < 0.0001; one-way ANOVA). No increase in transcriptional activity could be detected when dpCLK was cotransfected with either a truncated dpBMAL1 mutant lacking the TAD domain (dpBmal1ΔTAD) or a truncated dpBMAL1 mutant lacking the G and TAD domains (dpCyc-like), consistent with a transactivation function for these domains. However, cotransfection of dpCLK and dpBMAL1ΔCter elicited only a small but significant increase in transcriptional activity (P < 0.05; Student’s t test), which, despite its low level, was inhibited by dpCRY2 (P < 0.0001; Student’s t test). To unambiguously determine whether dpCRY2 was able to repress different dpCLK:dpBMAL1 deletion variants, we next enhanced transcription by fusing the strong viral transcriptional activator VP16 (24) to dpBMAL1 variants at either the N terminus (Fig. 4C) or the C terminus (Fig. S3). Regardless of the position of VP16 on the fusion proteins, all heterodimers of dpCLK-dpBMAL1 variants, including the dpCLK:dpCYC-like heterodimer, elicited a large increase in transcriptional activity and were inhibited in a dose-dependent manner by dpCRY2. Importantly, we verified that activation of these fusion proteins was not an artifact caused by the VP16 activation domain (Fig. 4D) and that repression by dpCRY2 was not caused by repression on the VP16 domain itself (Fig. 4E). Collectively, these data demonstrate that at least two sites for repression by dpCRY2 exist, one on the TAD α-helix of dpBMAL1 (Fig. 4A) and one on either dpCLK or another region of dpBMAL1 upstream of the G and TAD regions.
Fig. S3.
DpCRY2-mediated inhibition of dpCLK:dpBMAL1 mutants fused to the VP16 transactivation domain in their C termini. The monarch per E box luciferase reporter (dpPerEp_Luc; 10 ng) was used in the presence (+) or absence (−) of dpCLK, dpBMAL1, dpBMAL1ΔCter_VP16, dpBMAL1ΔTAD_VP16, or dpCYC-like_VP16 expression plasmids (5 ng each) and increasing doses of dpCRY2 (amounts are given in nanograms). Firefly luciferase activity was computed relative to renilla luciferase activity. Each value is the mean ± SEM of three replicates. Western blots of V5 epitope-tagged CRY2 and Drosophila β-actin protein expression levels are shown below the graph.
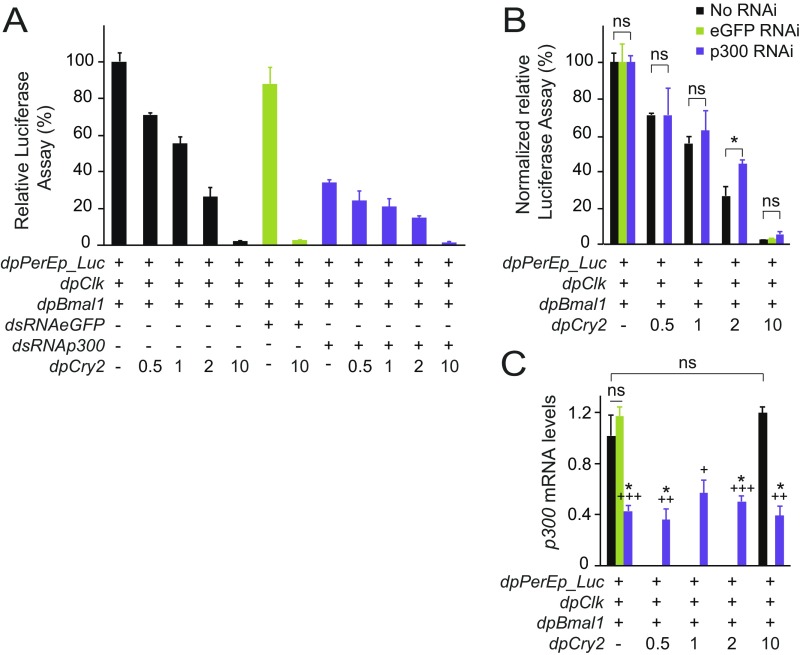
Given that CRY1-mediated circadian repression in mouse has been shown to occur via competition for binding with coactivators such as p300 to the BMAL1 C terminus (13, 14), we predicted that knocking down endogenous Drosophila p300 (i.e., nejire) in S2 cells cotransfected with dpCLK:dpBMAL1 would not only decrease transcriptional activity but also facilitate transcriptional repression by dpCRY2. While dsRNA-mediated knockdown of eGFP had no effect on transcription, we found that knocking down p300 by ∼60% led to a threefold decrease in transcriptional activity of dpCLK:dpBMAL1 (Fig. S4 A and C). In both cases, dpCRY2 inhibited dpCLK:dpBMAL1-mediated transcription in a dose-dependent manner, but we did not observe any difference in the amount of dpCRY2 necessary for transcriptional repression in untreated cells and cells treated with dsRNA against p300 (Fig. S4 A and B). Although we cannot exclude the possibility that p300 has a higher affinity than dpCRY2 for the dpBMAL1 TAD, this finding is consistent with the idea that the dpBMAL1 C terminus is not the only site of repressive action by vertebrate-like CRY2. It may also suggest the intriguing possibility that dpCRY2 competes with other coactivators for dpCLK:dpBMAL1 binding either on the dpBMAL1 TAD or on yet unknown domains of dpCLK and dpBMAL1.
Fig. S4.
Knockdown of p300 in S2 cells cotransfected with dpCLK:dpBMAL1. (A) The monarch per E box luciferase reporter (dpPerEp_Luc; 10 ng) was used in the presence (+) of dpCLK and dpBMAL1 (5 ng each) and either dsRNA against eGFP as a control (7.5 μg) or dsRNA against p300 (7.5 μg), in the absence (−) or presence (+) of increasing doses of dpCRY2 (amounts are given in nanograms). Firefly luciferase activity was computed relative to renilla luciferase activity. Each value is the mean ± SEM of three replicates. P < 0.0001 without dsRNA; P < 0.0002 with dsRNA (one-way ANOVA). (B) Normalized relative luciferase activities from A. *P < 0.05; ns, not significant; P > 0.05 (Student’s t test). (C) mRNA levels of p300 from samples subjected or not to dsRNA-mediated knockdown of p300 and of eGFP as a control. Legend is as in B. Asterisks denote significant differences at P < 0.05 between each condition treated with dsRNA against endogenous p300 and the untreated control in absence of dpCRY2; crosses denote significant differences between each condition treated with dsRNA against endogenous p300 and the untreated control in presence of 10 ng of plasmid expressing dpCRY2. +P < 0.005; ++P < 0.001; +++P < 0.0005; ns, not significant (Student’s t test).
Both the CLK PAS-B Domain and the BMAL1 C Terminus Contribute to the Repressive Potency of Vertebrate-Like CRY2.
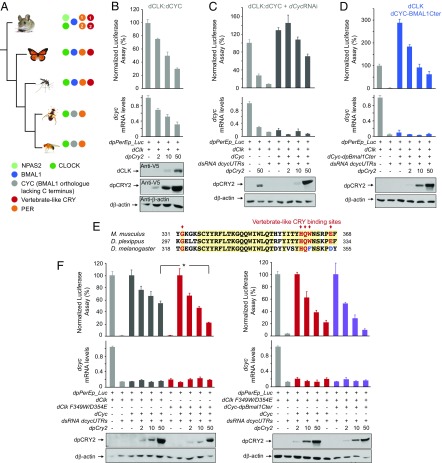
To assess the relative contribution of the BMAL1 C terminus versus other domains of CLK and BMAL1 to repression by insect CRY2, we took advantage of Drosophila circadian transcriptional activators. Like many other dipterans, Drosophila has lost both CRY2 and the BMAL1 C terminus including the G and TAD regions on dCYC (Fig. 5A). However, dCLK has evolved a TAD domain that mediates dCLK:dCYC transcriptional activity in the absence of the BMAL1 C terminus (25), thereby allowing us to compare the strength of repression by insect CRY2 in the absence or presence of the BMAL1 C terminus by using a wild-type dCLK:dCYC or a dCLK:dCYC chimeric protein bearing the dpBMAL1 C terminus.
Fig. 5.
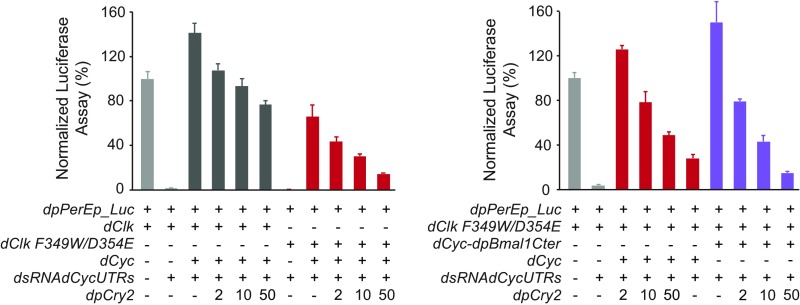
Fusing the dpBMAL1 C terminus to Drosophila dCLK:dCYC or mutating dCLK F349W/D354E independently restores strong repression by dpCRY2 in S2 cells. (A) Evolutionary relationship of insect species representative of lepidopterans and dipterans (Left) with their respective core clock components (Right). Dipterans shown comprise the Nematoceran (mosquitoes) and the Brachyceran (flies) lineages with the melon fly and the fruit fly. The mouse is shown as a representation of vertebrate clocks and as an outgroup of the tree. (B, Top) dpCRY2 affects Drosophila dCLK:dCYC transcription by decreasing dcyc mRNA levels. The monarch per E box luciferase reporter (dpPerEp_Luc; 10 ng) was used in presence of a dpCLK expression plasmid (5 ng each) and increasing doses of dpCRY2 (amounts are given in nanograms). Firefly luciferase activity was computed relative to renilla luciferase activity. P < 0.0001 (one-way ANOVA). (Middle) Endogenous dcyc mRNA levels were quantified using qPCR. P < 0.0005 (one-way ANOVA). Each value is the mean ± SEM of three replicates. (Bottom) Western blots of V5-tagged dCLK and dpCRY2 and Drosophila β-actin protein expression levels. (C) DpCRY2 weakly represses transcription by acting directly on Drosophila dCLK:dCYC proteins. The monarch per E box luciferase reporter (dpPerEp_Luc; 10 ng) was used in the presence of dCLK with (+) or without (−) exogenous dCYC expression plasmids (5 ng each) in the presence of dsRNA against the 5′ and 3′ UTR of endogenous dcyc (7.5 μg each). Increasing doses of dpCRY2 were provided; amounts are given in nanograms. Quantification of luciferase activity and endogenous dcyc, values, and Western blot analysis are depicted as in B. (D) Fusing a dpBMAL1 C terminus to dCYC rescues dpCRY2’s strong repressive capability. The monarch per E box luciferase reporter (dpPerEp_Luc; 10 ng) was used in the presence of dCLK alone or in the presence of dCLK and dCYC-dpBMAL1 C terminus expression plasmid (5 ng each) and dsRNA against the 5′ and 3′ UTR of endogenous dcyc (7.5 μg each). Increasing doses of dpCRY2 were provided; amounts are given in nanograms. Quantification of luciferase activity and endogenous dcyc, values, and Western blot analysis are depicted as in B. (E) Alignment of partial CLK proteins from the mouse (Mus musculus), the monarch butterfly (Danaus plexippus), and the fruit fly (Drosophila melanogaster). The red arrows indicate the conservation of the five previously described mouse CRY1-binding amino acids (11, 12). (F) Mutating dCLK F349W/D354E increases dpCRY2’s repressive capability on dCLK:dCYC-mediated transcription (Left), but fusing the dpBMAL1 C terminus to the mutant protein has no additional effect (Right). The monarch per E box luciferase reporter (dpPerEp_Luc; 10 ng) was used in the presence of either wild-type dCLK or dCLK F349W/D354E expression plasmids (5 ng each) with (+) or without (−) exogenous dCYC or dCYC-dpBMAL1 C terminus expression plasmids (5 ng each) in the presence of dsRNA against the 5′ and 3′ UTR of endogenous dcyc (7.5 μg each). Increasing doses of dpCRY2 were provided; amounts are given in nanograms. Quantification of luciferase activity is presented as values relative to 100% for each set of conditions, and endogenous dcyc values and Western blot analysis are depicted as in B. *P < 0.05 (two-way ANOVA followed by Tukey’s post hoc test).
We first examined the ability of dpCRY2 to repress dCLK:dCYC in S2 cells and found that dpCRY2 strongly repressed dCLK:dCYC in a dose-dependent manner (Fig. 5B). However, to our surprise, this inhibition was accompanied by a dose-dependent decrease in dcyc expression levels, an effect that has not been observed with the Drosophila circadian repressor dPER (26) and which was not due to CLK destabilization (Fig. 5B). To eliminate a possible confounding effect of dpCRY2 on dcyc transcription or RNA stability, we knocked down endogenous dcyc mRNA levels via RNAi and expressed dCYC exogenously (Fig. 5C) and showed that overexpression of both dCLK:dCYC fully rescued the transcriptional activity that was otherwise efficiently reduced by the knock down of dcyc mRNA. In this condition, we found that dpCRY2 inhibited dCLK:dCYC in a dose-dependent manner, reaching ∼50% inhibition at the maximal dose tested (P < 0.005; one-way ANOVA), thereby demonstrating that dpCRY2 is able to repress dCLK:dCYC. In addition, fusing the dpBMAL1 C terminus to dCYC further enhanced transcriptional activity by approximately threefold as well as dose-dependent repression by dpCRY2 (P < 0.0001; one-way ANOVA), which repressed activation levels at maximum doses tested by ∼80% compared with the 50% observed in the absence of the BMAL1 C terminus (Fig. 5D). Together, these results show that both the BMAL1 C terminus and other domains on CLK or CYC/BMAL1 contribute to repression potency by vertebrate-like CRY2.
Because of its known role in mammalian CRY1 binding (11, 27), the CLOCK PAS-B domain, and in particular five of its residues (12), appeared to be an ideal candidate region for repression by vertebrate-like CRY2. While the five residues are fully conserved in monarch dpCLK (Fig. 5E), two of them are changed to conserved residues in Drosophila dCLK (W349F and E354D) (Fig. 5E). We thus reasoned that if dpCRY2 represses dCLK:dCYC on these five residues, mutating F349 and D354 on dCLK to vertebrate-like residues (i.e., F349W/D354E) should increase the potency of its repression. As predicted, we found that dpCRY2 represses dCLK F349W/D354E in a dose-dependent manner with higher potency than wild-type dCLK (Fig. 5F). However, the levels of activation of dCLK F349W/D354E:dCYC were reduced by ∼50% compared with dCLK:dCYC (Fig. S5), suggesting that the changes in residues 349 and 354 of dCLK may have arisen to maintain high activation levels. However, we did not observe a significant increase in dpCRY2 repression potency of the dCYC:dCLK F349W/D354E transcription factor when the BMAL1 C terminus was fused to dCYC (Fig. 5F and Fig. S5). Taken together, our results suggest that both the BMAL1 C terminus and the CLK PAS-B domain contribute to the repressive potency of vertebrate-like CRY2 and identify the amino acids tryptophan and glutamic acid in the CLK HI loop as residues important for this function.
Fig. S5.
Absolute levels of firefly luciferase activity computed relative to renilla luciferase activity presented in Fig. 5F. The monarch per E box luciferase reporter (dpPerEp_Luc; 10 ng) was used in the presence of either wild-type dCLK or dCLK F349W/D354E expression plasmids (5 ng each) with (+) or without (−) exogenous dCYC or dCYC-dpBMAL1 C terminus expression plasmids (5 ng each) in presence of dsRNA against the 5′ and 3′ UTR of endogenous dcyc (7.5 μg each). Increasing doses of dpCRY2 were provided in the amounts indicated in nanograms. Each value is the mean ± SEM of three replicates.
DpCLK W328 and E333 Residues Independently Contribute to a TAD-Dependent and a TAD-Independent Repression by dpCRY2.
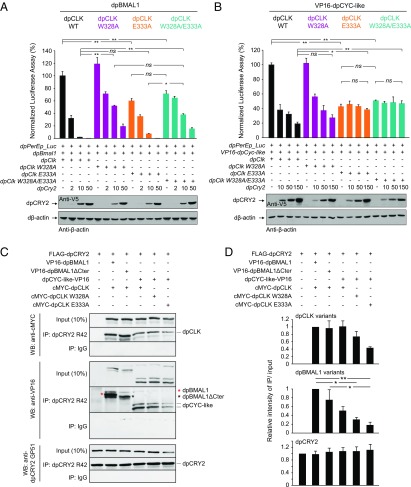
To determine the relative contribution of the corresponding tryptophan and glutamic acid residues on dpCLK for dpCRY2-dependent repression in the monarch clock, we tested the effect of mutations on dpCLK (W328A/E333A, W328A, and E333A) in S2 cells (Fig. 6 and Fig. S6). Consistent with our hypothesis that residues in the dpCLK PAS-B domain contribute to dpCRY2-dependent repression, we found that W328A/E333A and W328A mutations in dpCLK significantly weakened dpCRY2-dependent repression of dpCLK:dpBMAL1 (Fig. 6A). However, the single E333A mutation, which significantly decreased activation levels, did not affect the potency of repression by dpCRY2 (Fig. 6A). These results suggest that dpCLK W328, but not dpCLK E333, plays an important role in dpCRY2-dependent repression when the dpBMAL1 C terminus is present, similar to results shown in mammals (15). Because our in vivo and in vitro data supported the existence of a TAD-independent mechanism of repression by dpCRY2, we also tested whether it could be mediated through dpCLK by assessing the effect of the same dpCLK mutations on the dpCLK:VP16-dpCYC-like transcription factor in which the dpBMAL1 C terminus was lacking. Surprisingly, in this context, we found that while the W328A mutation had no significant effect on the ability of dpCRY2 to repress the transcription factor, both W328A/E333A and E333A abolished dpCRY2-dependent repression (Fig. 6B). Together, these results demonstrate the existence of two independent mechanisms of repression by dpCRY2: a TAD-dependent mechanism that involves dpCLK W328 and the C terminus of dpBMAL1 and a TAD-independent mechanism involving the E333 residue on dpCLK that may be responsible for the persistence of rhythms observed in the dpBmal1ΔCter mutants.
Fig. 6.
The dpCLK W328 and E333 residues independently contribute to TAD-dependent and TAD-independent repression by dpCRY2. (A and B) Effects of dpCLK mutations (dpCLK W328A, dpCLK E333A, and dpCLK W328A/E333A) in the presence (A) or absence (B) of the dpBMAL1 C terminus. The monarch per E box luciferase reporter (dpPerEp_Luc; 10 ng) was used in the presence (+) of dpBMAL1 (A) or VP16–dpCYC-like (B) and dpCLK variants expression plasmids (5 ng each) with increasing doses of dpCRY2; amounts are given in nanograms. Firefly luciferase activity was computed relative to renilla luciferase activity. Each value is the mean ± SEM of three replicates. Western blots of V5-tagged dpCRY2 and Drosophila β-actin protein expression levels are shown below the graphs. *P < 0.05; **P < 0.01; ns, not significant (two-way ANOVA followed by Tukey’s post hoc test). (C) Coimmunoprecipitations (IP) of c-Myc–dpCLK and either VP16-dpBMAL1 or VP16-dpBMAL1ΔCter and of dpCYC-like–VP16 and c-Myc–dpCLK WT, c-Myc–dpCLK W328A, or c-Myc-dpCLK E333A by FLAG-dpCRY2 with anti-dpCRY2 R42 antibody from transfected S2 cells. Western blots (WB) were performed using the indicated antibodies. The double band for dpCYC-like–VP16 likely corresponds to alternatively used translation initiation sites. The top band was quantified in D. (D) Quantification of C. For each protein, the relative intensity corresponds to the intensity of IP over input signal measured using Image J in each condition, relative to the intensity of IP over input signal of dpCRY2, WT dpCLK, and WT dpBMAL1. Each value is the mean ± SEM of three independent experiments. dpCLK variants, P < 0.05; dpBMAL1 variants, P < 0.005; dpCRY2, P = 0.96 (one-way ANOVA). *P < 0.05; **P < 0.01 (Tukey’s post hoc test).
Fig. S6.
Similar to dpCLK, dpCLK W328A, E333A, and W328A/E333A do not activate transcription in absence of dpBMAL1. The monarch per E box luciferase reporter (dpPerEp_Luc; 10 ng) was used in the presence (+) of dpCLK variants (5 ng each) with (+) or without (−) dpBMAL1 expression plasmids (5 ng). Firefly luciferase activity was computed relative to renilla luciferase activity. Each value is the mean ± SEM of three replicates.
To determine whether deletion of the dpBMAL1 TAD α-helix impairs the interaction between dpBMAL1 and dpCRY2, we coimmunoprecipitated dpCRY2 with WT c-Myc-dpCLK and either WT VP16-dpBMAL1 or the VP16-dpBMAL1ΔCter mutant. Consistent with our finding that the dpBMAL1 TAD α-helix is dispensable for dpCRY2-dependent repression, dpBMAL1ΔCter and WT dpCLK coimmunoprecipitated with dpCRY2 to a similar extent as WT dpBMAL1 and dpCLK (Fig. 6 C and D). Because the E333A mutation in the dpCLK HI loop abolished TAD-independent repression by dpCRY2 (Fig. 6B), we also sought to determine whether this mutation also disrupts the dpCYC-like/dpCLK/dpCRY2 complex by coimmunoprecipitating dpCRY2 with dpCYC-like–VP16 and the c-Myc–dpCLK E333A mutant, the c-Myc–dpCLK W328A mutant, or WT c-Myc–dpCLK as a control. We showed that dpCYC-like and all dpCLK variants tested (WT, W328A, and E333A) coimmunoprecipitated with dpCRY2, demonstrating that none of these mutations disrupted the stable interaction of dpCLK and dpCYC-like with dpCRY2 (Fig. 6 C and D). However, we observed a decrease in the amount of coimmunoprecipitated dpCYC-like and dpCLK in the presence of the E333A mutation. Given the lack of repression by dpCRY2 on the E333A mutant but not the W328A mutant (Fig. 6B), our results suggest that dpCLK E333 supports either dpCYC-like–dpCRY2 binding or dpCLK-dpCRY2 binding, which is likely required for TAD-independent repression by dpCRY2.
Discussion
The mechanisms by which CRYs regulate the circadian activity of CLOCK-BMAL1 in mammals have been proposed, based on biophysical and cell-based assays, to primarily occur through dynamic interactions between CRY1 and the BMAL1 TAD α-helix (13, 14). In contrast, the CRY-interacting interface on the CLOCK PAS-B HI loop (12) is thought to play a role only in docking CRY onto CLOCK-BMAL1 (14). However, the relative importance of these two sites on CLOCK:BMAL1 for CRY1 repression has not been firmly established through in vivo experiments, because the only existing mouse mutant lacking the BMAL1 C-terminal TAD harbors compromised transcriptional activity (28). In this work, we leveraged the monarch butterfly as an alternative system to directly test in vivo the importance of the BMAL1 TAD for vertebrate-like CRY repressive function because it possesses mammalian-like clock components and is readily amenable to CRISPR-mediated targeted mutagenesis (21). Through the generation of a mutant lacking the BMAL1 TAD α-helix but retaining the most distal C-terminal residues sufficient to provide transcriptional activity, we present in vivo genetic evidence showing that, despite regulating the circadian phase or period, the BMAL1 TAD α-helix is not necessary for repression of CLOCK-BMAL1 transcriptional activity by insect CRY2. Using cell-based reporter assays, we show that monarch dpCRY2 can repress dpCLK:dpCYC (a BMAL1 mutant lacking the C terminus lost in Drosophila) in the presence of a VP16-activation domain as well as the Drosophila dCLK:dCYC heterodimer, which is transcriptionally active through the glutamine-rich region of dCLK (29). DpCRY2 repression of dCLK:dCYC can be enhanced by either fusing a dpBMAL1 C terminus to dCYC or mutating two of the vertebrate-like CRY1-binding sites on the dCLK PAS-B domain to mammalian-like residues. Conversely, mutating the corresponding residues on the monarch dpCLK PAS-B domain not only weakened dpCRY2-dependent repression of dpCLK:dpBMAL1 but also abolished that of the transcriptionally active dpCLK:VP16–dpCYC-like. Together, these results demonstrate that vertebrate-like CRY regulation of circadian rhythms occurs through two independent mechanisms on CLK and the BMAL1 C terminus.
Our discovery that mutant monarchs lacking the BMAL1 TAD α-helix but retaining transcriptional activity maintain robust behavioral and molecular rhythms (Fig. 2) challenges the idea that the BMAL1 TAD is key to circadian repression (13, 14, 30) and shows that, at least in the butterfly, the BMAL1 TAD α-helix is not required for circadian repression by vertebrate-like CRY. The delayed phase of adult eclosion (by ∼1 h on DD day 1) observed in butterfly mutants lacking the TAD α-helix is nevertheless consistent with the TAD α-helix having a role in phase or period determination, similar to its function in mammals (13, 14). Our results showed that both the G and TAD C-terminal regions of monarch BMAL1 are necessary for strong activation and that the TAD α-helix plays a dual role in activation and repression by insect CRY2 in vitro (Fig. 4A). The decreased activation levels of per and tim mRNA in the brain of the monarch mutant lacking the BMAL1 TAD α-helix are consistent with its function as an activation domain and may explain the behavioral phase delay observed in vivo, as activation levels have previously been correlated to phase or period determination (31). In mammals, transcriptional activation occurs through the recruitment of coactivators such as CBP/p300 to BMAL1 (14, 32, 33), and repression by CRY1 is thought to occur through competition for binding with CBP/p300 on the BMAL1 TAD α-helix and the most distal C-terminal residues (14). As expected if dpCLK:dpBMAL1 activates transcription through a conserved mechanism involving recruitment of p300, knocking down endogenous Drosophila p300 in S2 cells substantially reduced dpCLK:dpBMAL1-mediated activation (Fig. S4). However, in contrast to what would be expected if repression by dpCRY2 is mediated solely through competition with p300 on the dpBMAL1 TAD, no decrease in the amount of dpCRY2 necessary for transcriptional repression was observed when p300 levels were reduced. This finding does not necessarily contradict a model in which dpCRY2 and p300 compete for dpBMAL1 TAD binding in the monarch. Because the reduction of p300 was only partial (∼60%) (Fig. S4) in our reporter assay, we cannot exclude the possibility that the remaining p300 could efficiently outcompete dpCRY2 for dpBMAL1 TAD binding, as previously observed for the mammalian p300 kinase-inducible domain interacting (KIX) domain (14). Alternatively, given that our results were obtained in a cellular context rather than in vitro chemical shift perturbation studies (14), it is possible that additional coactivators bound to dpCLK:dpBMAL1 also compete with dpCRY2 for binding. Histone-modifying enzyme orthologs of MLL and JARID1a, which are recruited at CLOCK:BMAL1, or proteins that recruit the transcriptional machinery are all potential candidates (34–37).
Regardless of the exact molecules with which dpCRY2 might compete on the dpBMAL1 TAD, our results provide strong evidence that this interaction is not sufficient for repression by dpCRY2. Generating monarch mutants lacking the dpBMAL1 TAD α-helix and harboring no functional allele or a single functional allele of dpCry2 has allowed us to unambiguously demonstrate that repression in these mutants was mediated by dpCRY2 and not by other negative regulators (Fig. 3). Furthermore, using in vitro reporter assays, we have excluded the possibility that dpCRY2 repression could occur through the last, most distal amino acids (LxWPxx) of the dpBMAL1 TAD retained in our mutant. Importantly, we showed that dpCRY2 has the ability to repress a dpBMAL1 protein lacking the C terminus (i.e., the G and TAD regions lost in Drosophila dCYC) when transcriptional activity is provided by fusing a VP16 activation domain (Fig. 4). Together, these results demonstrate that a dpBMAL1 TAD-independent mechanism is critical for repression and rhythm generation by dpCRY2. They are also consistent with previous findings in mice showing that the C-terminal domain of CRY1, which interacts with the BMAL1 C terminus, regulates clock function but is not necessary for transcriptional repression (38, 39).
The CLOCK PAS-B domain HI loop has been shown to play a central role in the establishment of complexes with mammalian CRY1, where a single W at position 362 on CLOCK directly interacts with the photolyase homology region (PHR) of vertebrate-like CRY (15). Here, we identify a dual role for the monarch CLK PAS-B domain HI loop in both TAD-dependent and TAD-independent repressive mechanisms by dpCRY2 (Fig. 6). Similar to mammalian CLOCK W362 (15), dpCLK W328 plays a role in the TAD-dependent repression of dpCLK:dpBMAL1 by dpCRY2, contributing to strong repression presumably by facilitating sequestration of the BMAL1 TAD by dpCRY2, as proposed in mammals (14, 15). We also show that dpCLK E333, another residue of the CLK PAS-B domain HI loop, is necessary for both strong activation and TAD-independent repression by dpCRY2. Based on both our in vivo and in vitro results, we propose that dpCRY2 represses dpCLK:dpBMAL1 primarily through a BMAL1 TAD-independent mechanism involving dpCLK E333, with the BMAL1 TAD modulating circadian rhythms only by modulating activation levels. We speculate that these two repressive mechanisms could represent two consecutive phases of repression, and, given the conservation of the BMAL1 TAD and the vertebrate-like CRY-binding sites on the CLK PAS-B between mammals and monarchs, could also apply to mammalian CRYs.
Our results underscore the relevance of the monarch butterfly, in which clock proteins are not duplicated, as a system for the in vivo genetic dissection of clockwork mechanisms that could have far-reaching implications for our understanding of how the mammalian clock works. Given the crucial role that the circadian clock plays in the remarkable navigational capabilities of the migratory monarch butterfly (40), understanding the intricate mechanisms by which the monarch circadian clock keeps time will also provide a molecular foundation for the identification of the neural clock circuits involved in flight orientation and migratory behavior.
Materials and Methods
For details on CRISPR/Cas9 targeted mutagenesis, genetic crosses, eclosion behavior assays, real-time PCR, S2 cell assays, and coimmunoprecipitations, see SI Materials and Methods. All primers and templates used for generating constructs are listed in Dataset S1.
SI Materials and Methods
Monarch Butterfly Husbandry.
Monarch butterflies were raised in the laboratory on semiartificial diet under LD 15:9 conditions in Percival incubators at 25 °C and 70% humidity, as previously described (18). Adults were housed in glassine envelopes in the same lighting and temperature conditions and were manually fed a 25% honey solution daily.
Guide RNA Design and Construction.
The guide RNA (gRNA) target sites were selected within the 3′ end of the dpBmal1 coding sequence using the Jack Lin’s CRISPR/Cas9 gRNA finder (spot.colorado.edu/∼slin/cas9.html). The gRNA expression vectors were constructed by inserting annealed synthetic oligomers into the DR274 plasmid from Addgene (41) at the BsaI cleavage site. Oligomer sequences were as follow (F, forward primer; R, reverse primer): dpCyc-like F, 5′-TAGGCCAACAACACCGTGGTGGT-3′, R: 5′-AAACACCACCACGGTGTTGTTGG-3′; dpBmal1ΔCter F: 5′-TAGGCTCTCGGATTAGACGGCAA-3′, R: 5′-AAACTTGCCGTCTAATCCGAGAG-3′.
Synthesis of Cas9 mRNA and sgRNA.
In vitro transcription of Streptococcus pyogenes Cas9 mRNA was performed using the mMESSAGE mMACHINE T3 Transcription Kit (Ambion), using the pCS2-nCas9n expression plasmid from Addgene (42) linearized with XbaI and purified with phenol-chloroform. The resulting capped poly(A) mRNAs were purified by acid-phenol-chloroform extraction and resuspended in RNase-free water following isopropanol precipitation. sgRNAs were transcribed in vitro using T7 RNA polymerase (Promega) from purified PCR products containing the T7 promoter, gRNA, and gRNA scaffold amplified from the DR274 vectors using the primers F: 5′-ATTTCGGATTTTATAGGAAGAGG-3′ and R: 5′-GGCCATGGGAGTCCAGAGATAT-3′. sgRNAs were treated with RQ1 DNase and purified by acid-phenol-chloroform extraction. Cas9 mRNAs and each sgRNA were quantitated by spectrophotometry (NanoDrop 1000) and diluted in RNase-free water to a final concentration of 0.5 μg/μL.
Egg Microinjection.
Eggs were collected and microinjected as previously described (21) with a mix of Cas9 mRNA and sgRNA (at 0.45 μg/μL and 0.5 μg/μL, respectively, for generating dpCyc-like targeted mutations, and at 0.5 μg/μL and 0.1 μg/μL, respectively, for generating dpBmal1ΔCter targeted mutations), to which RNase-free blue food coloring was added to visually track the injection. After injection, embryos were placed in an incubator at 25 °C and 70% relative humidity. Developing embryos were transferred into individual small Petri dishes containing milkweed leaves until larvae hatched, and second-instar surviving larvae were transferred onto a semiartificial diet.
Analysis of CRISPR/Cas9-Induced Mutations and Generation of Monarch Mutant Lines.
PCR fragments flanking the targeted regions were amplified from genomic DNA of larval sensors of potential founders with the following primers: dpCyc-like F: 5′-ATTTCGGATTTTATAGGAAGAGG-3′ and R: 5′-GGCCATGGGAGTCCAGAGATAT-3′; dpBmal1ΔCter F: 5′-ACAAAGCGGCGTTACTGAGA-3′ and R: 5′-CCTTAGATGTCTTCGGATTGG-3′. PCR products were purified using 1.5× modified Sera-Mag Magnetic Speed-beads (Thermo Fisher Scientific) as previously described (21, 43) and were resuspended in 10 µL of RNase-free water. Cas9-based cleavage assays of PCR products were performed using a recombinant Cas9 protein and either sgRNA, as previously described (21), purified using 1.5× modified Sera-Mag Magnetic Speed-beads before being resolved with agarose gel electrophoresis and EtBr staining. Uncleaved fragments corresponding to mutated alleles were gel purified and sequenced using one of the primers used for PCR amplification. Larvae presenting a high degree of targeting in somatic cells, estimated based on the relative abundance of uncleaved fragments, were selected and reared to adulthood. Surviving adults presenting the highest level of somaticism were hand-paired in individual cages with virgin wild-type monarchs of the opposite sex to establish lines. Eggs were collected from each cross, and the hatched larvae were screened for the presence of mutated alleles as described above. Frameshift deletions mutants were selected to establish mutant lines.
Eclosion Behavior.
Eclosion behavior was monitored in DD as previously described (21) in butterflies raised in LD 15:9 in a Percival incubator at 25 °C and 70% humidity.
Real-Time qPCR.
Brains from adult monarch butterflies entrained to seven LD 15:9 cycles after eclosion were dissected under red light on the first day of transfer into DD. Dissections were performed in 0.5× RNAlater (Ambion) to prevent RNA degradation, and brains free of eye photoreceptors were stored at −80 °C until use. Total RNA was extracted using an RNeasy Mini kit (Qiagen) and was treated with RQ1 DNase (Promega), and random hexamers (Promega) were used to prime reverse transcription with SuperScript II Reverse Transcriptase (Thermo Fisher Scientific), all according to the manufacturers’ instructions. Quantifications of gene expression were performed on a QuantStudio 6 Flex Real-Time PCR System (Thermo Fisher Scientific) using iTaq Universal SYBR Green Supermix (Bio-Rad) and monarch per, tim, and control rp49 primers, as previously described (21).
For quantifying the levels of Drosophila melanogaster p300 and cyc in RNAi experiments by qPCR, cells were lysed in 100 mM Tris⋅HCl (pH 7.0), 100 mM LiCl, 20 mM DTT, and 10% SDS, and total RNA was extracted by two rounds of acid phenol/chloroform (Ambion). cDNA synthesis and quantification of p300 and dcyc expression levels were performed as described above using the following primers: p300 F, 5′-TCGTTATCCGTCTGCATTCG-3′ and R, 5′-GCAGCGACGAGAACTCAAAGT-3′; d-5′UTRcyc F, 5′-TTCGTCGGAAAGGGCTTAATT-3′ and R, 5′-CGCAGAACTCCTGAACTTCCA-3′; control d-rp49 F, 5′-GCTAAGCTGTCGCACAAATGG-3′ and R, 5′-CGGCGACGCACTCTGTT-3′. The nearly 100% efficiency of each primer set was validated by determining the slope of the cycle threshold (Ct) versus dilution plot on a 3 × 104 dilution series. Individual reactions were used to quantify each RNA level in a given cDNA sample, and the average Ct from duplicated reactions within the same run was used for quantification. The data for each gene in any given condition were normalized to rp49 as an internal control and were normalized to the mean of one sample within a set for statistics.
Genetic Crosses.
For behavioral and molecular analyses, dpCyc-like and dpBmal1ΔCter heterozygous and hemizygous mutants and wild-type siblings were obtained by crossing heterozygous dpCyc-like and dpBmal1ΔCter males to wild-type females, as dpBmal1 is located on the Z chromosome in monarchs. To generate dpBmal1ΔCter m2; dpCry2−/− double mutants, heterozygous dpCry2 monarch females (18) carrying a null allele were crossed with heterozygous dpBmal1ΔCter males to produce dpBmal1ΔCter m2/+; dpCry2+/− males and dpBmal1ΔCter m2/W; dpCry2+/− females. Intercrossing of these males and females produced homozygous and hemizygous dpBmal1ΔCter mutants (male: m2/m2, female: m2/W) in a dpCry2 wild-type (+/+), heterozygous (+/−), and homozygous (−/−) background.
Plasmids for Expression in S2 Cells.
The pGL-dpPer4Ep, pAC-dpBmal1, pAC-dpClk-V5, pAC-dpCry2-V5, and pAC5.1V5/HisA plasmids were provided by Steven Reppert, University of Massachusetts Medical School, Worcester, MA (20), and pAC-Renilla-Luc control plasmid was provided by Michael Rosbash, Brandeis University, Waltham, MA (44).
pAC plasmids containing dpBmal1 C-terminal deletion variants were generated by PCR amplification of dpBmal1 truncated at the C terminus from either pAC-dpBmal1 (for dpBmal1ΔTAD and dpCyc-like variants) or from cDNA generated from the dpBmal1ΔCter monarch mutant line. The different dpBmal1 variants were subcloned into pAC5.1V5/HisA between NotI and XbaI sites. pAC plasmids containing dpClk W328A, dpClk E333A, and dpClk W328A/E333A mutants were generated by overlapping PCRs of dpClk fragments amplified from pAC-dpClk-V5 with primers containing the desired mutations and were subcloned into pAC5.1V5/HisA between NotI and XhoI sites.
Overlapping PCRs were used to fuse a VP16 transactivation domain either at the C terminus or at the N terminus of all V5-tagged dpBmal1 variants. C-terminal VP16-tagged dpBmal1 variants were produced by overlap extension PCR of dpBmal1, dpBmal1ΔCter, dpBmal1ΔTAD, and dpCyc-like fragments with a V5-tagged VP16 domain amplified by PCR from a pAC-ClkJrk-VP16-V5 plasmid provided by Michael Rosbash, Brandeis University, Waltham, MA. Overlapping PCR products were digested with NotI and PmeI and were subcloned into pAC5.1V5/HisA at these cleavage sites. To construct N-terminal VP16-tagged dpBmal1 variants, the VP16 domain was first PCR amplified and subcloned in the N terminus of dpBmal1 into the pAC-Bmal1 plasmid between EcoRI and NotI cleavage sites. The different N-terminal VP16-tagged dpBmal1 variants were then produced by PCR amplification of each variant that was subsequently subcloned into the pAC-VP16-dpBmal1 vector between NotI and XbaI, which restores the frame of the C-terminal V5 tag. To construct pAC plasmids containing c-Myc–tagged dpClk variants (wild-type, W328A, and E333A) for use in coimmunoprecipitations, a pAC plasmid containing the myc epitope was first generated by annealing two single-stranded complementary oligos and the resulting dsDNA was inserted into pAC5.1V5/HisA using KpnI and EcoRI. pAC plasmids containing c-Myc–tagged dpClk variants (wild-type, W328A, and E333A) were then generated by subcloning into this plasmid PCR-amplified full-length coding regions of dClk variants lacking the start codon but containing a stop codon, using XhoI. To construct a pAC plasmid containing 3×FLAG-tagged dpCry2, the 3×FLAG was first amplified from a pCCG-C-Gly::3×FLAG plasmid and was subcloned into pAC5.1V5/HisA between KpnI and EcoRI, and the PCR-amplified full-length coding region of dpCry2 lacking the start codon but containing a stop codon was inserted in frame with the 3×FLAG using EcoRI and XhoI.
pAC plasmids containing Drosophila melanogaster dClk and dcyc were generated by subcloning PCR-amplified full-length coding regions of dClk and dcyc into pAC5.15/HisB between KpnI and XbaI and between KpnI and NotI, respectively. The pAC plasmid containing the monarch dpBmal1 C-terminal G and TAD regions fused to dcyc (pAC-dcyc-Bmal1Cter) was generated by overlapping PCRs of the full-length ORF of dcyc amplified from pAC-dcyc and the C-terminal G and TAD regions of dpBmal1 amplified from pAC-dpBmal1 and was subcloned into the pAC-HisB vector between KpnI and XhoI to obtain a functional V5 tag. The pAC plasmid containing the dClkF349W/D354E mutant was generated by overlapping PCRs of dClk fragments amplified from pAC-dClk with primers containing the desired mutations and was subcloned into pAC-dClk between KpnI and BmgBI.
For use in a GAL4/UAS luciferase system suitable for S2 cells, the UAS_Luc reporter plasmid was constructed by digesting the 10×UAS promoter from p5E_UAS (Tol2kit) (45) and subcloning it into pGL3-Basic plasmid (20) using KpnI and XmaI. Plasmids containing the Gal4DBD were constructed by inserting the Gal4DBD amplified by PCR from pGBKT7 (Clontech) into pAC5.1V5/HisA using EcoRI and NotI. Activation domains (VP16, dpBmal1 G and TAD, dpBmal1 G and ΔCter mutant TAD, dpBmal1 G and dpBmal1 TAD) amplified by PCR were then fused to the Gal4DBD by subcloning into pAC-Gal4DBD-V5 using NotI and XbaI.
All primers and templates used for generating the constructs described above are listed in Dataset S1, and all plasmids were sequenced.
S2 Cell Culture, Transfections, Transcription Assays, and Western Blotting.
S2 cells were maintained at 25 °C in Schneider’s Drosophila medium (Gibco) supplemented with 10% heat-inactivated FBS (Gibco) and 100 U/mL penicillin and streptomycin (Gibco). Transient transfections were performed as previously described (46) in 12-well plates using 10 ng per well of dpPer4Ep-Luc as reporter and 30 ng per well of pAC-Renilla-Luc as a normalization control. S2 cells were cotransfected with 5 ng per well of pAC plasmids expressing wild-type, mutant variants, or single domains of circadian activators (see plasmids section above), with or without varying amounts of a pAC-dpCry2-V5 plasmid. Plasmids were mixed with 5 μL Cellfectin II (Invitrogen), incubated for 15 min at room temperature, and mixed with serum-free Schneider’s Drosophila medium before being added to S2 cells. An equal volume of Schneider’s Drosophila medium supplemented with 20% heat-inactivated FBS and 2% penicillin and streptomycin was added after ∼6 h of incubation at 25 °C. Cells were incubated for 2 d before harvesting for luciferase assay, quantification of gene expression, and Western blot analysis.
For the luciferase assay and Western blot analysis, cells were lysed with 50 μL of 1× Passive Lysis Buffer (Promega). Firefly and renilla luciferase activities were quantified with a Dual-Luciferase Reporter Assay system (Promega) using 5 μL of cell protein lysate on a VICTOR3 V Multilabel Plate Counter (PerkinElmer).
For RNAi of endogenous D. melanogaster p300 in S2 cells, a 485-bp cDNA fragment was amplified using Phusion Taq polymerase (New England Biolabs) with the primers p300i F, 5′-GTGGAATTCAAGCCCGGCAGTGTGCTGAATAATATG-3′ and R, 5′-CAGGTACCGGTGGCCATCGTGGCCGATGTAG-3′ and was subcloned into p5E_UAS plasmid between KpnI and EcoRI sites, which are flanked by T7 promoters in opposite directions. Large amounts of template for in vitro transcription were generated by PCR amplification of the plasmid using the primer 5′-GTTGGTAATACGACTCACTATAGGG-3′. For RNAi of endogenous dcyc in S2 cells, 164-bp and 216-bp fragments corresponding to the 3′ and 5′ UTRs of dcyc, respectively, were amplified from S2 cell cDNA using the following primers containing a T7 promoter: dcyc 3′ UTR F, TAATACGACTCACTATAGGGATACGAACGTTCCGATGGGG and R, TAATACGACTCACTATAGGGCTTATCTAAGCGAATCTCTTTTCAAGTTG; dcyc 5′ UTR F, TAATACGACTCACTATAGGGTGAAGATGCAGGAAAAGCTTGCAAC and R, TAATACGACTCACTATAGGGCGCCAATCGGTGACGTTTGC.
The amplicons were purified by phenol-chloroform extraction and subjected to in vitro transcription using T7 RNA polymerase (Promega) according to the manufacturer’s instructions. After RQ1 Dnase treatment, the two ssRNA strands of each target gene were purified by acid phenol/chloroform extraction and annealed by incubation at 65 °C for 30 min followed by slow cooling to room temperature. dsRNA of eGFP, used as a control, was generated as previously described (47). For transfections of dsRNA-treated cells, S2 cells were treated with either 7.5 μg of dsRNA against p300 or a mix of 7.5 μg of dsRNA against the 3′ UTR of dcyc and 7.5 μg of dsRNA against the 5′ UTR of dcyc in 500 μL of serum-free Schneider’s medium for 6 h at 25 °C and were incubated for 2 d in supplemented medium before plasmid DNA transfection, performed as described above. Cells were harvested 2 d later by scraping, resuspended in 1× PBS, and divided into two aliquots of equal volume for the luciferase assay and qPCR, respectively.
For Western blotting, triplicate S2 cell protein extracts lysed with 1× Passive Lysis Buffer (Promega) were combined in equal volumes, and protein concentrations were measured using the Pierce Coomassie Plus (Bradford) Assay kit (Thermo Fisher Scientific) and BSA as a standard on a VICTOR3 V Multilabel Plate Counter (PerkinElmer). For each sample, 10–20 μg of protein were loaded per lane onto a 7.5% SDS PAGE. V5-tagged dpCRY2 and dCLK were detected using a mouse anti-V5 monoclonal primary antibody (1:5,000) (R96025; Novex); Drosophila actin was detected using a mouse anti–β-actin monoclonal antibody (1:5,000) (8224; Abcam); and a goat anti-mouse IgG HRP secondary antibody was used for both (1:10,000) (170-6516; Bio-Rad).
Coimmunoprecipitations.
Plasmids were transfected using Cellfectin II (Invitrogen) in S2 cells cultured in six-well plates with the following plasmid ratios: 150 ng of pAC-FLAG-dpCry2 and 600 ng each of pAC-VP16-dpBmal1-V5 and pAC–c-Myc–dpClk, or pAC-VP16-dpBmal1ΔCter-V5 and pAC–c-Myc–dpClk, or pAC–dpCyc-like–VP16-V5 and pAC–c-Myc–dpClk, or pAC–dpCyc-like–VP16–V5 and pAC–c-Myc–dpClkW328A, or pAC–dpCyc-like–VP16–V5 and pAC–c-Myc–dpClkE333A. Cells were harvested 48 h after transfection, lysed on ice for 10 min in 300 μL of extraction buffer [20 mM Hepes (pH 7.5), 100 mM NaCl, 0.5% Nonidet P-40, 1× Complete Protease Inhibitor (Pierce)], and after centrifugation 50 μL was retained as the input sample. Protein G Plus/Protein A agarose beads (50 μL; EMD Millipore) were prepared for immunoprecipitation by washing three times in 1 mL of extraction buffer. The beads were then incubated with a rat anti-dpCRY2 polyclonal antibody (R42) (23) for 1 h at room temperature. Normalized rat IgG (Abcam) was used as control. The unbound antibodies were removed by an additional washing with 300 μL of extraction buffer. Protein extracts were added to the beads and incubated overnight at 4 °C on a rotator. The beads were washed two times with ice-cold extraction buffer, and proteins were eluted by adding 40 μL of 5× protein sample SDS buffer followed by boiling for 6 min. Complexes were resolved by 6% SDS/PAGE, and proteins were detected by immunoblotting with the following primary antibodies: affinity-purified anti-monarch CRY2 GP51 for dpCRY2 (1:2,000) (23), mouse anti-V5 monoclonal primary antibody (1:5,000) (R96025; Novex), and mouse anti–c-Myc monoclonal primary antibody (1:1,000) (9E10, sc-40; Santa Cruz). Secondary antibodies used were either goat anti-guinea pig-HRP (1:5,000) (A7289; Sigma) or goat anti-mouse IgG-HRP (1:5,000) (170-6,516; Bio-Rad).
Statistical Analysis.
P values were calculated using Student’s t test and one-way or two-way ANOVAs using the online calculator from vassarstats.net and the QI Macros Statistical software for Excel.
Supplementary Material
Acknowledgments
We thank Steve Reppert for the generous gift of the anti-dpCRY2 R42 and anti-dpCRY2 GP51 antibodies and for providing the pAC plasmids containing dpClk, dpBmal1, and dpCry2; Michael Rosbash for providing the pAC-Renilla-Luc– and VP16-containing plasmids and for discussions; Courtney Caster for information that neither CRY2 nor the BMAL1 C terminus is present in the genome of flies belonging to the Brachyceran lineage and for help in generating Fig. 5A; Justin Vann, Sarah Kenny, and Kendall Bowen for help with monarch husbandry; Aldrin Lugena and Samantha Iiams for help with the generation of a construct used in the study; Jerome Menet for discussions and suggestions; and Deborah Bell-Pedersen for comments on the manuscript. This work was supported by startup funds from Texas A&M University and by National Science Foundation Grant IOS-1456985 (to C.M.) and NIH Grant NS052854 (to P.E.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702014114/-/DCSupplemental.
References
- 1.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 2.Young MW, Kay SA. Time zones: A comparative genetics of circadian clocks. Nat Rev Genet. 2001;2:702–715. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]
- 3.Gekakis N, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 4.King DP, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kume K, et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 6.Chen R, et al. Rhythmic PER abundance defines a critical nodal point for negative feedback within the circadian clock mechanism. Mol Cell. 2009;36:417–430. doi: 10.1016/j.molcel.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nangle SN, et al. Molecular assembly of the period-cryptochrome circadian transcriptional repressor complex. eLife. 2014;3:e03674. doi: 10.7554/eLife.03674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffin EA, Jr, Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286:768–771. doi: 10.1126/science.286.5440.768. [DOI] [PubMed] [Google Scholar]
- 9.van der Horst GT, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 10.Ye R, et al. Dual modes of CLOCK:BMAL1 inhibition mediated by cryptochrome and period proteins in the mammalian circadian clock. Genes Dev. 2014;28:1989–1998. doi: 10.1101/gad.249417.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato TK, et al. Feedback repression is required for mammalian circadian clock function. Nat Genet. 2006;38:312–319. doi: 10.1038/ng1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang N, et al. Crystal structure of the heterodimeric CLOCK:BMAL1 transcriptional activator complex. Science. 2012;337:189–194. doi: 10.1126/science.1222804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiyohara YB, et al. The BMAL1 C terminus regulates the circadian transcription feedback loop. Proc Natl Acad Sci USA. 2006;103:10074–10079. doi: 10.1073/pnas.0601416103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu H, et al. Cryptochrome 1 regulates the circadian clock through dynamic interactions with the BMAL1 C terminus. Nat Struct Mol Biol. 2015;22:476–484. doi: 10.1038/nsmb.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michael AK, et al. Formation of a repressive complex in the mammalian circadian clock is mediated by the secondary pocket of CRY1. Proc Natl Acad Sci USA. 2017;114:1560–1565. doi: 10.1073/pnas.1615310114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czarna A, et al. Quantitative analyses of cryptochrome-mBMAL1 interactions: Mechanistic insights into the transcriptional regulation of the mammalian circadian clock. J Biol Chem. 2011;286:22414–22425. doi: 10.1074/jbc.M111.244749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahata S, et al. Transactivation mechanisms of mouse clock transcription factors, mClock and mArnt3. Genes Cells. 2000;5:739–747. doi: 10.1046/j.1365-2443.2000.00363.x. [DOI] [PubMed] [Google Scholar]
- 18.Merlin C, Beaver LE, Taylor OR, Wolfe SA, Reppert SM. Efficient targeted mutagenesis in the monarch butterfly using zinc-finger nucleases. Genome Res. 2013;23:159–168. doi: 10.1101/gr.145599.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan Q, Metterville D, Briscoe AD, Reppert SM. Insect cryptochromes: Gene duplication and loss define diverse ways to construct insect circadian clocks. Mol Biol Evol. 2007;24:948–955. doi: 10.1093/molbev/msm011. [DOI] [PubMed] [Google Scholar]
- 20.Zhu H, et al. The two CRYs of the butterfly. Curr Biol. 2005;15:R953–R954, and erratum (2006) 16:730. doi: 10.1016/j.cub.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 21.Markert MJ, et al. Genomic access to monarch migration using TALEN and CRISPR/Cas9-mediated targeted mutagenesis. G3 (Bethesda) 2016;6:905–915. doi: 10.1534/g3.116.027029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Froy O, Gotter AL, Casselman AL, Reppert SM. Illuminating the circadian clock in monarch butterfly migration. Science. 2003;300:1303–1305. doi: 10.1126/science.1084874. [DOI] [PubMed] [Google Scholar]
- 23.Zhu H, et al. Cryptochromes define a novel circadian clock mechanism in monarch butterflies that may underlie sun compass navigation. PLoS Biol. 2008;6:e4. doi: 10.1371/journal.pbio.0060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wysocka J, Herr W. The herpes simplex virus VP16-induced complex: The makings of a regulatory switch. Trends Biochem Sci. 2003;28:294–304. doi: 10.1016/S0968-0004(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 25.Allada R, White NE, So WV, Hall JC, Rosbash M. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell. 1998;93:791–804. doi: 10.1016/s0092-8674(00)81440-3. [DOI] [PubMed] [Google Scholar]
- 26.Bae K, Lee C, Hardin PE, Edery I. dCLOCK is present in limiting amounts and likely mediates daily interactions between the dCLOCK-CYC transcription factor and the PER-TIM complex. J Neurosci. 2000;20:1746–1753. doi: 10.1523/JNEUROSCI.20-05-01746.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao WN, et al. CIPC is a mammalian circadian clock protein without invertebrate homologues. Nat Cell Biol. 2007;9:268–275. doi: 10.1038/ncb1539. [DOI] [PubMed] [Google Scholar]
- 28.Park N, et al. A novel Bmal1 mutant mouse reveals essential roles of the C-terminal domain on circadian rhythms. PLoS One. 2015;10:e0138661. doi: 10.1371/journal.pone.0138661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darlington TK, et al. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science. 1998;280:1599–1603. doi: 10.1126/science.280.5369.1599. [DOI] [PubMed] [Google Scholar]
- 30.Gustafson CL, et al. A slow conformational switch in the BMAL1 transactivation domain modulates circadian rhythms. Mol Cell. 2017;66:447–457. doi: 10.1016/j.molcel.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadener S, Menet JS, Schoer R, Rosbash M. Circadian transcription contributes to core period determination in Drosophila. PLoS Biol. 2008;6:e119. doi: 10.1371/journal.pbio.0060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosoda H, et al. CBP/p300 is a cell type-specific modulator of CLOCK/BMAL1-mediated transcription. Mol Brain. 2009;2:34. doi: 10.1186/1756-6606-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee Y, et al. Coactivation of the CLOCK-BMAL1 complex by CBP mediates resetting of the circadian clock. J Cell Sci. 2010;123:3547–3557. doi: 10.1242/jcs.070300. [DOI] [PubMed] [Google Scholar]
- 34.DiTacchio L, et al. Histone lysine demethylase JARID1a activates CLOCK-BMAL1 and influences the circadian clock. Science. 2011;333:1881–1885. doi: 10.1126/science.1206022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katada S, Sassone-Corsi P. The histone methyltransferase MLL1 permits the oscillation of circadian gene expression. Nat Struct Mol Biol. 2010;17:1414–1421. doi: 10.1038/nsmb.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lande-Diner L, Boyault C, Kim JY, Weitz CJ. A positive feedback loop links circadian clock factor CLOCK-BMAL1 to the basic transcriptional machinery. Proc Natl Acad Sci USA. 2013;110:16021–16026. doi: 10.1073/pnas.1305980110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valekunja UK, et al. Histone methyltransferase MLL3 contributes to genome-scale circadian transcription. Proc Natl Acad Sci USA. 2013;110:1554–1559. doi: 10.1073/pnas.1214168110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Czarna A, et al. Structures of Drosophila cryptochrome and mouse cryptochrome1 provide insight into circadian function. Cell. 2013;153:1394–1405. doi: 10.1016/j.cell.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Khan SK, et al. Identification of a novel cryptochrome differentiating domain required for feedback repression in circadian clock function. J Biol Chem. 2012;287:25917–25926. doi: 10.1074/jbc.M112.368001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reppert SM, Guerra PA, Merlin C. Neurobiology of monarch butterfly migration. Annu Rev Entomol. 2016;61:25–42. doi: 10.1146/annurev-ento-010814-020855. [DOI] [PubMed] [Google Scholar]
- 41.Hwang WY, et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol. 2013;31:227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jao LE, Wente SR, Chen W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc Natl Acad Sci USA. 2013;110:13904–13909. doi: 10.1073/pnas.1308335110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohland N, Reich D. Cost-effective, high-throughput DNA sequencing libraries for multiplexed target capture. Genome Res. 2012;22:939–946. doi: 10.1101/gr.128124.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDonald MJ, Rosbash M, Emery P. Wild-type circadian rhythmicity is dependent on closely spaced E boxes in the Drosophila timeless promoter. Mol Cell Biol. 2001;21:1207–1217. doi: 10.1128/MCB.21.4.1207-1217.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwan KM, et al. The Tol2kit: A multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- 46.Chang DC, Reppert SM. A novel C-terminal domain of drosophila PERIOD inhibits dCLOCK:CYCLE-mediated transcription. Curr Biol. 2003;13:758–762. doi: 10.1016/s0960-9822(03)00286-0. [DOI] [PubMed] [Google Scholar]
- 47.Hung HC, Maurer C, Kay SA, Weber F. Circadian transcription depends on limiting amounts of the transcription co-activator nejire/CBP. J Biol Chem. 2007;282:31349–31357. doi: 10.1074/jbc.M702319200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.