Significance
A hallmark of effective T cell responses against invading pathogens is the production of effector molecules. Effector molecules such as cytokines not only kill infected cells, but they also recruit innate immune cells and alter the environment to optimally respond to microbial infections. However, because effector molecules are very potent, they can pose a health risk. When produced excessively or when generated at the wrong time, this aberrant production can induce immunopathology. Here, we uncover that each cytokine employs a private regulation of transcriptional and posttranscriptional events to guarantee appropriate production kinetics.
Keywords: T cells, cytokine production, PKC, mRNA stability, translation efficiency
Abstract
Effective T cell responses against invading pathogens require the concerted production of three key cytokines: TNF-α, IFN-γ, and IL-2. The cytokines functionally synergize, but their production kinetics widely differ. How the differential timing of expression is regulated remains, however, poorly understood. We compared the relative contribution of transcription, mRNA stability, and translation efficiency on cytokine production in murine effector and memory CD8+ T cells. We show that the immediate and ample production of TNF-α is primarily mediated by translation of preformed mRNA through protein kinase C (PKC)-induced recruitment of mRNA to polyribosomes. Also, the initial production of IFN-γ uses translation of preformed mRNA. However, the magnitude and subsequent expression of IFN-γ, and of IL-2, depends on calcium-induced de novo transcription and PKC-dependent mRNA stabilization. In conclusion, PKC signaling modulates translation efficiency and mRNA stability in a transcript-specific manner. These cytokine-specific regulatory mechanisms guarantee that T cells produce ample amounts of cytokines shortly upon activation and for a limited time.
Effective CD8+ T cell responses require the production of three key cytokines: tumor necrosis factor alpha (TNF-α), IFN gamma (IFN-γ), and interleukin-2 (IL-2). Intriguingly, T cells release these cytokines in a serial fashion and not simultaneously (1, 2), reflecting their consequential functions. The immediate production of TNF-α induces apoptosis of infected cells and promotes leukocyte infiltration by regulating chemokine release and vascular permeability (3, 4). The slightly later-produced IFN-γ blocks microbial replication and potentiates innate immune responses by directly activating macrophages (5) and recruiting neutrophils (6). The survival and proliferation signals provided by IL-2 are required at later stages of activation. Importantly, all three cytokines have adverse effects when aberrantly produced, which results in immunopathology for TNF-α and IFN-γ (7, 8), and in activation-induced cell death of T cells for IL-2 (9).
Several mechanisms drive cytokine production upon T cell activation. The transcription factors NFAT, AP-1, and NFκB are engaged downstream of Ca2+ signaling, protein kinase C (PKC), and MAP-kinase signaling (10–13). PKC, MAPK, and PI3K/Akt signaling can also control the turnover of cytokine mRNAs (14–16), whereas mTOR is critical to drive the translation of mRNA into protein (17, 18). How these regulatory networks feed into sequential cytokine production, however, is yet to be determined. We hypothesized that each cytokine requires custom-made regulatory pathways to obtain appropriate antimicrobial responses without inducing severe side effects. Here, we show that the timing of these three cytokines is determined by differential control of transcription, mRNA stability, and translation. Importantly, PKC signaling orchestrates this timing in a cytokine-specific manner.
Results
Sequential Production of Cytokines in T Cells.
To determine the kinetics of cytokine release, we activated OT-I T cells for 20 h with OVA257–264 peptide/CD80 expressing cells (17). Activated T cells were removed from the antigen after 20 h and rested for 3–13 d in the presence of IL-7. We then reactivated these effector T cells with OVA257–264 peptide in the presence of brefeldin A (BrfA) to block protein secretion and to measure the intracellular cytokine levels of TNF-α, IFN-γ, and IL-2.
TNF-α was detectable within 30 min of stimulation and reached its plateau within the first 2 h, whereas IFN-γ and IL-2 were measured from 1 h and 2 h of activation onwards (Fig. 1A). Protein levels of all cytokines steadily increased until 6 h (Fig. 1A). Whereas this experimental setup displays the maximal capacity of cytokine production, it cannot distinguish whether cytokines are continuously or transiently generated. Therefore, we added BrfA to activated T cells at later time points, for a maximum of 2 h (Fig. 1B). More than 90% of T cells produced TNF-α during the first hours, which dropped to 20% in the last 2 h of stimulation (Fig. 1B). IL-2 production also declined in the last 2 h, whereas IFN-γ production was robust over time (Fig. 1B) (1, 2). Of note, the kinetics of cytokine production was independent of culture conditions with different cytokines, i.e., IL-7, IL-2, or IL-15 (Fig. S1A). Furthermore, the low-affinity T4 variant of OVA257–264 peptide (aa: SIITFEKL) (19) only altered the magnitude of response but not the kinetics (Fig. S1A).
Fig. 1.
TNF-α, IFN-γ, and IL-2 follow individual production kinetics. (A and B) Cytokine production of resting CD8+ OT-I T cells stimulated with 100 nM OVA peptide. Brefeldin A (BrfA) was present throughout the stimulation (A) or added for the last 0.5, 1, or 2 h of activation (B). (C) Cytokine profile analysis of T cells activated for 2, 4, or 6 h from B. (Upper) Percentage of T cells that produce at least one cytokine. (Lower) Cytokine production of responding T cells. Pooled data from three independently performed experiments (mean ± SD; n = 7 mice). (D) FACS-sorted CD44hi memory-like OT-I T cells activated with peptide-loaded bone marrow-derived dendritic cells. N4, SIINFEKL; T4, SIITFEKL (n = 5 mice). (E) Effector memory (EM, Left) and central memory (CM; Right) T cells from LCMV-infected mice (d60) were reactivated with GP33 and NP396 peptide (n = 5 mice).
Fig. S1.
Cytokine production kinetics in T cells. (A) T cells that were rested for 3 d in the presence of IL-7 (Top), IL-2 (Center), or IL-15 (Bottom) were activated with 100 nM SIINFEKL (N4) or SIITFEKL (T4) OVA peptide for indicated time points (n = 3 mice). Brefeldin A (BrfA) was added during the last 2 h of culture. (B) Representative dot plots for TNF-α and IFN-γ (Upper) and IFN-γ and IL-2 (Lower) production of T cells that were activated for indicated time with 100 nM OVA257–264 peptide (N4) in the presence of BrfA during the last 2 h of stimulation. Numbers indicate percentage of cytokine-producing T cells. (C) mRNA levels of Tnfa, Ifng, and Il2 of T cells that were rested for 3 d in the presence of IL-7, IL-2, or IL-15.
When T cells produce two or more cytokines, they represent the most potent effector cells against pathogens (20, 21). We found that the overall percentage of cytokine-producing T cells was constant, but the production profile evolved over time (Fig. 1C and Fig. S1B). TNF-α dominated the first 2 h (91 ± 4%, of which 54 ± 5% produced TNF-α alone, and 37 ± 6% in combination with IFN-γ and/or IL-2). At 4 h, most T cells (90 ± 2%) produced at least two cytokines. At 6 h, the T cell response shifted toward IFN-γ production (90 ± 5%, of which 48 ± 6% produced IFN-γ alone, and 51 ± 5% together with TNF-α and/or IL-2; Fig. 1C and Fig. S1B).
Also, memory T cells rapidly produce cytokines upon reinfections (22). Indeed, naturally occurring CD44hi memory-like OT-I T cells that can protect from reinfections (23) showed similar production kinetics as in vitro-generated effector T cells, irrespective of antigen affinity (Fig. 1D). Importantly, effector memory (EM) and central memory (CM) T cells from lymphochoriomeningitis virus (LCMV)-infected mice (d60) also rapidly produced TNF-α and IFN-γ upon stimulation with the cognate GP33 and NP396 peptides, and IL-2 production was delayed and of short nature (Fig. 1E). Different cytokine production kinetics is thus a general feature of effector and of memory T cells.
Preformed Tnfa and Ifng mRNA Drives Rapid Protein Production.
We next interrogated what determines the differential onset of cytokine production. Rapid protein synthesis can be initiated from preformed mRNA. Interestingly, at all culture conditions tested, resting CD8+ T cells contained more Tnfa mRNA than Ifng mRNA, and Il2 mRNA was almost undetectable (Fig. S1C and Fig. S2A). To examine whether preformed mRNA can drive the rapid TNF-α production, we pretreated T cells with actinomycin D (ActD) to block de novo transcription, or with cycloheximide (CHX) to block de novo translation before activation. CHX significantly blocked the production of TNF-α, but ActD failed to do so. The percentage of TNF-α–producing T cells remained 76 ± 15% of the control at 30 min of stimulation (Fig. S2B). Thus, CD8+ T cells primarily use translation of preformed mRNA to produce TNF-α. Interestingly, preformed Ifng mRNA also allows for rapid protein production. After 1 h of activation, 42 ± 4% of IFN-γ–producing T cells remained in the presence of ActD (Fig. S2C). IL-2, however, depended almost exclusively on de novo transcription. Only 13 ± 4% T cells remained IL-2+ with ActD (Fig. S2D).
Fig. S2.
Preformed cytokine mRNA promotes early T cell responsiveness. (A) Tnfa, Ifng, and Il2 mRNA levels of resting T cells were measured by RT-PCR. (B–D) T cells were pretreated for 30 min with 1 μg/mL ActD or 10 μg/mL CHX and then stimulated for indicated time points with 100 nM OVA peptide in the presence of BrfA. Graphs depict the relative percentage of TNF-α (B), IFN-γ (C), and IL-2 (D)-producing T cells compared with T cells activated in the absence of inhibitors. Data are presented as mean ± SD of three independently performed experiments. (One-way ANOVA corrected for multiple comparisons with a Tukey test; n = 7 mice; *P < 0.05; ***P < 0.0005.)
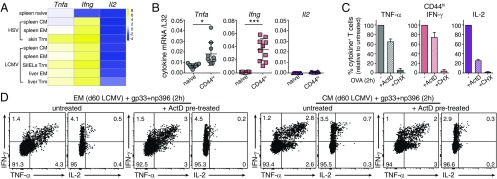
Bona fide memory T cells specific for herpes simplex virus (HSV) and LCMV-specific memory T cells (24) also contain preformed Tnfa and Ifng mRNA, but not of Il2, in almost all memory T cell subsets (Fig. 2A). Similarly, CD44hi memory-like T cells express significant Tnfa and Ifng levels (Fig. 2B). Importantly, both CD44hi T cells and LCMV-specific EM and CM T cells use preformed Tnfa and Ifng mRNA for cytokine production, as shown by the negligible effect of ActD on their protein levels (Fig. 2 C and D). Of note, the levels of preformed Ifng mRNA were higher in memory than in effector T cells (compare Fig. 2 A and B with Fig. S2A), which corresponds with higher use of preformed mRNA (Fig. 2 C and D) and a more similar onset of production with TNF-α (Fig. 1 D and E). Again, IL-2 production primarily depended on de novo transcription (Fig. 2 C and D). Combined, our data demonstrate that TNF-α, IFN-γ, and IL-2 follow different production kinetics in effector and in memory CD8+ T cells, which is at least in part determined by the levels of preformed mRNA available for rapid translation.
Fig. 2.
Preformed cytokine mRNA promotes early T cell responsiveness. (A) Heatmap of the relative expression (Z scores) of Tnfa, Ifng, and Il2 mRNA (ref. 24; GSE70813). (B) Tnfa, Ifng, and Il2 mRNA levels of CD8+ CD44low naive T cells and CD8+ CD44hi memory-like T cells (n = 10 mice) (paired Student’s t test; **P < 0.005; ***P = 0.0001). (C) Relative percentage of TNF-α, IFN-γ, and IL-2 producing CD8+ CD44hi T cells pretreated with 1 μg/mL ActD or 10 μg/mL CHX for 30 min before 2 h stimulation with OVA peptide (n = 6 mice). (D) Cytokine production of EM and CM T cells from LCMV-infected mice pretreated with ActD before 2 h GP33 and NP396 peptide stimulation. Representative of five mice from two independently performed experiments.
PKC Signaling Contributes to Translation Initiation.
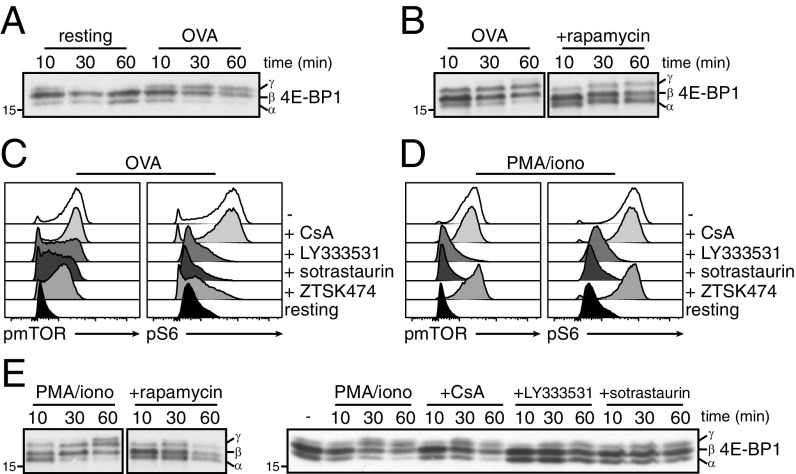
Translation of preformed Tnfa and Ifng mRNA into protein suggests a swift engagement of cytokine mRNA into polyribosomes, a process that is determined by the availability of the eukaryote initiation factor 4E (eIF4E) (25, 26). eIF4E is captured by hypophosphorylated eIF4E-binding proteins (4E-BPs). Phosphorylation of 4E-BP by mTOR releases eIF4E to bind mRNA 5′-cap structures to facilitate the formation and scanning of the preinitiation scanning complex, which allows ribosomes to assemble at start codons (26). Indeed, resting T cells primarily expressed the unphosphorylated α-isoform and the partially phosphorylated β-isoform of 4E-BP1 that both repress translation (Fig. 3A) (25). Stimulation with OVA peptide rapidly decreased the unphosphorylated α-isoform and induced the fully phosphorylated, inactive γ-isoform of 4E-BP1 (Fig. 3A).
Fig. 3.
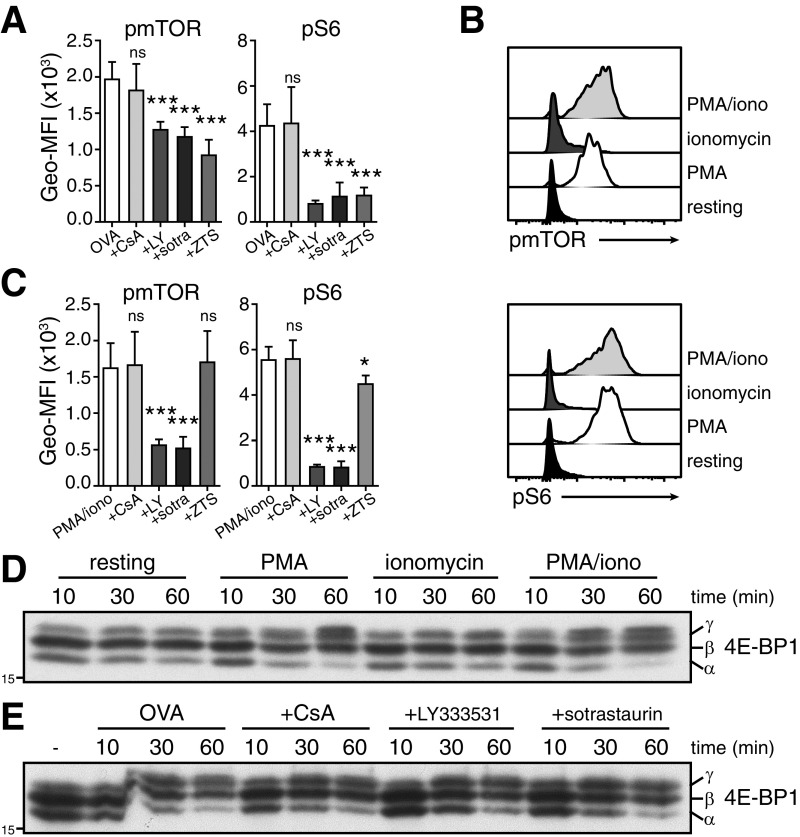
PKC signaling promotes translation initiation. (A and B) 4E-BP1 expression in resting and OVA peptide-stimulated T cells (A), and in OVA-stimulated T cells pretreated for 30 min with rapamycin (B). (C and D) pmTOR and pS6 levels 30 min after T cell activation with OVA peptide (C), or with PMA/ionomycin (D). Indicated inhibitors were added 30 min before T cell activation. (E) 4E-BP1 expression of PMA/ionomycin activated T cells that were pretreated or not with indicated inhibitors. Representative of two independently performed experiments (A, B, and E) and 10 mice from three experiments (C and D).
We and others showed that mTOR activity drives the translation of cytokines (17, 27). In line with that, preincubating T cells with rapamycin to inhibit mTOR blocks 4E-BP1 phosphorylation (Fig. 3B). TCR-mediated PI3K/Akt signaling phosphorylates mTOR and its downstream target, the ribosomal protein S6 (Fig. 3C and Fig. S3A). This was effectively blocked when T cells were pretreated with the pan-PI3K inhibitor ZTSK474 (Fig. 3C and Fig. S3A). TCR triggering, however, also engages other crucial signaling pathways, such as the Ca2+ flux that activates calcineurin (28), and PKC (29). We therefore questioned whether Ca2+ flux and/or PKC signaling also mediate mTOR activity and, thus, translation. Inhibiting calcineurin with cyclosporine A (CsA) did not affect mTOR and S6 phosphorylation (Fig. 3C and Fig. S3A). In contrast, blocking PKC with LY-333531 (specific inhibitor of PKC-β isoform) or sotrastaurin (pan-PKC inhibitor) before activation significantly impaired mTOR and S6 phosphorylation (Fig. 3C and Fig. S3A). Thus, TCR-dependent activation of PKC can enhance mTOR activity.
Fig. S3.
mTOR and 4E-BP phosphorylation in activated T cells. (A and C) mTOR and S6 phosphorylation 30 min after T cell activation with 100 nM OVA257–264 peptide (A), or 10 ng/mL PMA and 1 µM ionomycin (C). Cells were also pretreated for 30 min with 500 ng/mL CsA, 10 μM LY-333531 (LY), 5 μM sotrastaurin (sotra), or 10 μM ZTSK474 (ZTS). Data shown derive from three independently performed experiments (n = 10 mice; mean ± SD) (one-way ANOVA with Dunnett’s multiple comparison. ns, nonsignificant; *P < 0.05; ***P < 0.0001). (B) Histograms represent mTOR and S6 phosphorylation of T cells activated for 30 min with indicated stimuli, or of resting cells (n = 4 mice), (D and E) 4E-BP1 expression upon activation with indicated stimuli (D), and when pretreated for 30 min with indicated inhibitor before activation (E). Representative of three (D) and two (E) independently performed experiments.
PKC promoted mTOR and S6 phosphorylation independently from PI3K signaling. Stimulating T cells with phorbol 12-myristate 13-acetate (PMA) alone to directly engage PKC sufficed for mTOR and S6 phosphorylation, whereas promoting the intracellular Ca2+ influx with ionomycin had no effect (Fig. S3B). Furthermore, pretreating cells with PKC inhibitors LY-333531 and sotrastaurin effectively blocked mTOR and S6 phosphorylation upon PMA/ionomycin stimulation (Fig. 3D and Fig. S3C). Importantly, the PI3K inhibitor ZTSK474 and the calcineurin inhibitor CsA failed to do so, demonstrating that PKC-mediated activation of mTOR was independent from PI3K and Ca2+ influx (Fig. 3D). T cell activation with PMA alone was also sufficient to induce the hyperphosphorylated inactive γ-isoform of 4E-BP1 (Fig. S3D). Again, this shift was blocked by rapamycin, and by LY-333531 or sotrastaurin, but not by CsA (Fig. 3E). In accordance with a more diverse signal upon TCR triggering and a possible redundancy between the PI3K and the PKC pathway, inhibiting PKC upon OVA peptide stimulation did not reduce the migration of 4E-BP1 toward its γ-isoform (Fig. S3E). Altogether, our data show that PKC signaling converges with the PI3K pathway to regulate mTOR activity and to initiate mRNA translation of activated T cells.
Differential Role of PKC and Ca2+ in Cytokine Production.
We next questioned how PKC and Ca2+ signaling contribute to cytokine production. PMA/ionomycin activation induced cytokine production with similar kinetics as antigen stimulation (Fig. 4A and Fig. S4 A and B). Intriguingly, however, PKC and Ca2+ signaling differentially contributed to the individual cytokine production. Ionomycin only marginally supported TNF-α, while IFN-γ largely depended on Ca2+ signaling. In sharp contrast, PMA stimulation alone had limited effects on IFN-γ, but greatly promoted TNF-α production (Fig. 4A and Fig. S4A). This finding was confirmed with PKC and calcineurin inhibitors in PMA/ionomycin-stimulated and antigen-stimulated T cells (Fig. 4 B and C). This indicates that PKC and Ca2+ signaling have divergent roles for cytokine production also when all TCR-mediated signaling nodes are present (Fig. 4C and Fig. S4D).
Fig. 4.
Role of PKC and Ca2+-dependent signaling in cytokine production. (A) Resting T cells were stimulated or not for 4 h with PMA, ionomycin, or with both (PMA/iono). Numbers in dot plots indicate percentage of TNF-α+ and/or IFN-γ+ T cells. Histogram depicts IL-2 production of the same sample. (B and C) T cells were pretreated or not with indicated inhibitor before activation with PMA/ionomycin (B), or with OVA peptide (C). Representative of three independently performed experiments. (D–F) TNF-α (D), IFN-γ (E), and IL-2 (F) protein levels (Left) and mRNA levels (Right) of CD44hi memory-like T cells were determined after 4 h and 2 h of indicated stimulation, respectively (n = 4 mice) (one-way ANOVA with Tukey’s comparison; *P < 0.05; **P < 0.005; ***P = 0.0005; ****P < 0.0001). (G–I) Polysome fractionation of T cells activated for 2 h with indicated stimuli. Tnfa (G), Ifng (H), and Il2 (I) mRNA distribution through all 20 collected fractions. Representative of two independently performed experiments.
Fig. S4.
Regulation of cytokine production through PKC and Ca2+-dependent signaling. (A) TNF-α and IFN-γ (Upper), and IL-2 and IFN-γ (Lower) production of CD44hi memory-like T cells activated for 4 h with indicated stimuli. Unstimulated T cells were used as negative control. Representative of four mice from two independently performed experiments. (B) T cells were stimulated with PMA/ionomycin for indicated time points. The percentage of TNF-α, IFN-γ, and IL-2 protein producing T cells was determined by intracellular cytokine staining (mean ± SD; n = 7 mice). (C and D) IL-2 production of PMA/ionomycin-stimulated T cells (C), and IFN-γ and IL-2 production of OVA257–264-stimulated T cells (D) that were pretreated with the indicated inhibitor. Representative of six mice from three independently performed experiments. (E) Tnfa, Ifng, and Il2 mRNA levels (mean ± SD) of resting T cells that were stimulated for 2 h as indicated (one-way ANOVA with Tukey’s multiple comparison; n = 7 mice; ns, nonsignificant; *P < 0.05; ***P = 0.0005; ****P < 0.0001) (F) Total mRNA distribution in collected sucrose fractions of activated T cells as determined by RNAnano Chip assay.
Strikingly, cytokine production did not necessarily correlate with mRNA levels. PMA alone induced substantial production of TNF-α protein in effector and CD44hi memory-like T cells (Fig. 4 A and D and Fig. S4A), but this did not correspond with significant changes in Tnfa mRNA (Fig. 4D and Fig. S4E). Increases of transcript levels became apparent only upon combined PMA/ionomycin activation (Fig. 4D). In contrast, Ifng mRNA already increased upon single stimulation with ionomycin and was further boosted by the combination of PMA/ionomycin (Fig. 4E and Fig. S4E). Thus, Ifng but not Tnfa transcript levels match with the protein levels.
We next questioned how PKC and Ca2+ signaling influenced the translation efficiency of Tnfa and Ifng mRNA. We fractionated polyribosomes from cytoplasmic extracts of activated T cells by sucrose gradient centrifugation, and determined the distribution of Tnfa and Ifng transcripts between free and polyribosome-bound fractions of increasing density (Fig. 4 G and H and Fig. S4F). A large fraction of Tnfa mRNA remained as free-mRNA upon T cell activation with ionomycin alone (Fig. 4G). PMA activation, however, shifted Tnfa from free-mRNA toward polyribosome-bound fractions. These findings are fully compatible with the capacity of PKC to phosphorylate 4E-BP1 (Fig. 3E) and to produce TNF-α protein (Fig. 4 A–D). Combined PMA/ionomycin activation resulted in further shifts of Tnfa mRNA from light to heavy polyribosomes (Fig. 4G), indicating that Ca2+ signaling—although not sufficient to initiate Tnfa translation—can contribute to protein production by increasing Tnfa mRNA levels, and by enhancing polysomal recruitment.
In contrast to Tnfa, we found Ifng already in polyribosomes in ionomycin-activated cells (Fig. 4H). PMA stimulation, however, was more effective at evoking high-density ribosome loading of Ifng mRNA (Fig. 4H). Because ionomycin also induced de novo transcription of Ifng (Fig. 4E and Fig. S4E), we postulate that newly synthesized Ifng mRNA is immediately engaged by ribosomes, resulting in the observed protein production.
IL-2 production required both PMA and ionomycin stimulation for measurable increases of mRNA levels and cytokine production (Fig. 4 A and F). As with Ifng mRNA, Il2 mRNA always associated with polyribosomes, which was further enhanced by combined PMA/ionomycin stimulation (Fig. 4I). Nevertheless, Il2 mRNA levels at ionomycin activation alone are possibly too low to allow for detectable protein production (Fig. 4 A and F). Thus, IL-2 requires both PKC and Ca2+ signaling for detectable protein levels.
Signal Strength Dictates Protein Production in a Cytokine-Specific Manner.
The levels of cytokines produced by T cells depend on costimulatory signals, on the affinity, and the amount of antigen (30, 31). We stimulated T cells with low (0.1 nM), intermediate (1 nM), and high (100 nM) OVA peptide concentration to determine the effect on cytokine mRNA expression and protein levels. OVAlow did not drive protein production of any of the three cytokines. Upon activation with OVAint, a substantial fraction of T cells produced TNF-α (48 ± 14%, Fig. 5A), and a minor but significant fraction produced IFN-γ (21 ± 3%; Fig. 5B). IL-2 was almost undetectable (2 ± 1%; Fig. 5C), and T cells only produced significant IL-2 levels when activated with OVAhi.
Fig. 5.
Signal strength determines cytokine specific pathway for production. (A–C) TNF-α (A), IFN-γ (B), and IL-2 (C) protein production upon 6 h of stimulation with 0.1 nM (low), 1 nM (intermediate), or 100 nM (high) OVA peptide. Representative of four independently performed experiments (n = 6 mice). (D–F, Left) Tnfa (D), Ifng (E), and Il2 (F) mRNA levels after 2 h of stimulation. Mean ± SD of three (D and F) and four (E) independently performed experiments [n = 5 (D and F); n = 6 (E) mice] (one-way ANOVA with Tukey’s comparison; *P < 0.05; ***P < 0.0005). (Right) Polysome fractionation of cytoplasmic Tnfa (D), Ifng (E), and Il2 (F) mRNA after 2 h stimulation with OVA peptide. Results are representative of two independently performed experiments.
Strikingly, again only activation with OVAhi resulted in elevated Tnfa transcript levels, but OVAint failed to induce Tnfa mRNA despite the substantial TNF-α protein production (Fig. 5D). We therefore interrogated how antigen load affected the recruitment of mRNA to polysomes. In T cells activated with OVAlow, Tnfa was primarily found as free-RNA (Fig. 5D). Importantly, activation with OVAint totally shifted Tnfa toward polyribosome-bound fractions. This was not further amplified with OVAhi, suggesting that the translation efficiency could not increase anymore (Fig. 5D). Thus, TNF-α production depends on recruitment of preformed Tnfa mRNA to polyribosomes, which can be further amplified by de novo transcription at high antigen levels.
In sharp contrast, Ifng and Il2 transcript levels directly correlated with protein levels: OVAint and OVAhi significantly increased Ifng, as did OVAhi for Il2 (Fig. 5 E and F). Furthermore, Ifng and Il2 almost completely associated with polyribosomes at any condition tested (Fig. 5 E and F), again indicating that cytoplasmic Ifng and Il2 mRNA immediately engages with ribosomes. Altogether, we show that mRNA transcription and mRNA translation differentially contribute to the production of TNF-α, IFN-γ, and IL-2.
PKC-Signaling Stabilizes Ifng and Il2 Transcripts for Optimal Protein Production.
In resting T cells, Tnfa mRNA levels are higher than those of Ifng and Il2 (Fig. S4A). However, after 2 h activation with OVAhi, Ifng and Il2 mRNA levels steeply increase and outnumber those of Tnfa mRNA (Fig. 6A), independently of the culture conditions or the antigen affinity (Fig. S5A). Rapid accumulation of transcripts can be promoted by de novo transcription and mRNA stabilization. mRNA stability was associated with increased translation efficiency, including transcripts that contain the regulatory AU-rich elements within their 3′UTR, like Tnfa, Ifng, and Il2 (16, 32). We thus questioned whether optimal cytokine production correlated with mRNA stability. T cells activated for 2 h with OVAhi or PMA/ionomycin were treated with ActD over the course of the next 2 h to measure the mRNA decay rate. The half-life of Tnfa was unaffected by T cell stimulation irrespective of the stimulus used (t1/2 = ∼60 min; Fig. 6B and Fig. S5B), which was in line with its moderate increase in mRNA levels (Fig. 6A). In sharp contrast, Ifng and Il2 mRNA transcripts were substantially stabilized upon T cell activation to t1/2 > 2 h (Fig. 6 C and D and Fig. S5B).
Fig. 6.
PKC activation regulates the stability of Ifng and Il2 mRNA. (A) Tnfa, Ifng, and Il2 mRNA levels upon 2 h stimulation with OVAhi; data are extracted from Fig. 5 D–F (one-way ANOVA with Tukey’s comparison; *P < 0.05). (B–F) T cells stimulated with OVAhi for 2 h (B–D), or for 2 h and 4 h (E and F) and then were incubated with ActD alone (OVA), or in combination with CsA, LY-333531, or sotrastaurin. Cells were harvested at indicated time points to determine the mRNA decay of Tnfa (B), Ifng (C and E), and Il2 (D and F) (n = 5 mice from two independently performed experiments).
Fig. S5.
Role of PKC signaling in cytokine mRNA stability. (A) Tnfa, Ifng, and Il2 mRNA levels (mean ± SD) 2 h after activation with 100 nM SIINFEKL (N4) or SIITFEKL (T4) peptide of T cells that were cultured for 3 d with IL-7, IL-2, or with IL-15 (n = 3 mice) (unpaired Student t test; n = 3 mice; ns, nonsignificant; *P < 0.05; **P = 0.005). (B) T cells were stimulated for 2 h with PMA/iono, and then treated with ActD alone, or in combination with 500 ng/mL CsA, 10 μM LY333531, or 5 μM sotrastaurin for indicated time points (n = 5 mice). (C) mRNA decay of T cells stimulated with indicated stimuli for 2 h before ActD treatment (n = 5 mice). (D) mRNA decay of PMA-stimulated T cells for 2 h or 4 h) before treatment with ActD (n = 3 mice). (B–D) Data are presented as mean ± SD of three (B and C) or two (D) independently performed experiments.
We next studied which signaling pathways drive the stabilization of Ifng and Il2 mRNA. We added PKC or Ca2+-flux inhibitors combined with ActD to T cells that were activated for 2 h with OVAhi or PMA/ionomycin. Again, Tnfa half-life was not affected by any inhibitor (Fig. 6B and Fig. S5B). CsA also did not alter the stability of Ifng, and only slightly reduced the half-life of Il2 mRNA to ∼115 min (Fig. 6 C and D and Fig. S5B). However, the half-life of both Ifng and Il2 dropped back to levels of resting T cells when with PKC inhibitor LY-333531 or sotrastaurin (Fig. 6 C and D and Fig. S5B). Furthermore, activating PKC with PMA alone was already sufficient to fully stabilize Ifng and Il2 mRNA, while ionomycin failed to do so (Fig. S4C). Thus, PKC is the main driver for Ifng and Il2 stabilization.
Of note, Ifng mRNA maintained its stability also at later time points, i.e., at 4 h after T cell activation (Fig. 6E and Fig. S5D). In contrast, the half-life of Il2 at this time point dropped back to levels of resting T cells (Fig. 6F), and Tnfa maintained its low t1/2 of ∼60 min at any time point measured (Fig. S5D). The rapid turnover of Tnfa and Il2 mRNA between 4 and 6 h after activation was in line with the profound decrease of protein production at this time point (Fig. 1 and Fig. S4B). Combined, we here show that mRNA stability is indicative for the duration of cytokine production and that PKC signaling herein is key.
Discussion
A potent T cell response requires the coordinated production of TNF-α, IFN-γ, and IL-2. We show that effector and memory T cells use an individual regulatory program for each cytokine to drive production with distinct kinetics. TNF-α production was fast and intense, but transient, and depended on immediate polyribosome recruitment of preformed mRNA. T cells also use preformed mRNA for the early production of IFN-γ. In contrast, the delayed IL-2 production depended almost exclusively on de novo gene transcription. In addition, stabilization of newly synthesized mRNA defined the duration of cytokine production. Prolonged mRNA half-life supported the sustained production of IFN-γ, whereas Il2 mRNA lost its stability within 4 h concomitant with a gradual loss of protein production.
Although TNF-α, IFN-γ, and IL-2 use private regulatory networks, PKC signaling is crucial for all three cytokines. However, its mode of action is cytokine-specific. PKC drives Tnfa translation without stabilizing its mRNA. Conversely, PKC mediates mRNA stability of Ifng and Il2 and enhances the shift of mRNA from light to heavy polyribosomes.
PKC activation promotes mRNA translation through mTOR, and the two mTOR targets S6 and 4E-BP1 that drive protein synthesis (27). Whether PKC acts directly or indirectly on these targets remains to be determined. Direct phosphorylation of S6 and 4E-BP1 by PKC has been reported (33, 34), but PKC could also regulate mTOR and 4E-BP activity through i.e., ERK-dependent mechanisms (35, 36). Irrespective of its mode of action, PKC-induced phosphorylation of 4E-BPs releases eIF4E, allowing the preinitiation scanning complex to assemble at the mRNA cap (25, 26). The predominant role of polysome binding and mRNA translation in regulating TNF-α, and its addiction to 4E-BP1 phosphorylation, may in part explain the success of rapamycin and its analogs as immune suppressants, since those inhibitors are capable to directly block 4E-BP phosphorylation (37).
How PKC stabilizes cytokine mRNA is yet to be determined. In Jurkat cells, PKC phosphorylates the RNA-binding protein NF-90, which allows NF-90 to bind and stabilize Il2 mRNA (15). Similarly, HuR can stabilize Ifng mRNA (38), and in vitro data show that PKC can phosphorylate HuR (39). Thus, it is tempting to speculate that HuR and NF-90 promote Ifng and Il2 mRNA stability in primary CD8+ T cells in a PKC-mediated manner.
T cells that produce two or more cytokines are the most effective against viruses (20, 21, 40), and their capacity to induce multiflavored responses defines the efficacy of T cell vaccines (41). Here, we show that the kinetics of cytokine production is a general feature of effector and memory T cells and that it is cell-intrinsic. PKC orchestrates the timing of production of each cytokine by providing a custom-made posttranscriptional regulation. Understanding the mechanisms that drive optimal cytokine production should further help to unravel how effective, multifunctional T cell responses can be generated.
Materials and Methods
Activation and Expansion of Murine Effector and Memory CD8+ T Cells.
Mice were housed and bred at the Netherlands Cancer Institute (NCI). Experiments are approved by the Experimental Animal Committee of the NCI. OT-I T cells were activated as described (17). Activated T cells were rested for 3–13 d in 10 ng/mL recombinant murine (rm)IL-7, 120 U rmIL-2, or 10 ng/mL rmIL-15 (Peprotech). Splenic CD44lo and CD44hi OT-I T cells, and splenic CD44hiCD62L+ and CD44hiCD62L− memory T cells from LCMV-Armstrong–infected mice (d60; 105 pfu per mouse, i.p. injection) were FACS-sorted. T cells were stimulated with 100 nM OVA257–264 peptide, or its low-affinity T4 variant, with 10 ng/mL PMA, 1 µM ionomycin (Sigma-Aldrich), or with peptide-loaded bone marrow-derived DCs. Brefeldin A was added as indicated. DCs were incubated for 1.5 h with 100 nM peptide and washed twice. LCMV-specific memory T cells were stimulated with 5 μg/mL GP33 and 5 μg/mL NP396 peptide (kind gift of R. Arens, Leiden University Medical Center, Leiden, The Netherlands). Inhibitors were added to T cells 30 min before activation (Table S1).
Table S1.
Inhibitors used in T cell activation essays
| Inhibitor | Concentration | Manufacturer |
| Actinomycin D (ActD) | 1 μg/mL | Sigma-Aldrich |
| Cycloheximide (CHX) | 10 μg/mL | Sigma-Aldrich |
| Rapamycin | 5 μg/mL | Sigma-Aldrich |
| Cyclosporin A (CsA) | 500 ng/mL | Calbiochem |
| LY-333531 (LY) | 10 μM | Selleckchem |
| Sotrastaurin (sotra) | 5 μM | Selleckchem |
| ZTSK474 (ZTS) | 10 μM | Selleckchem |
Flow Cytometry.
Intracellular cytokine staining was performed with BD Cytofix/Cytoperm kit (BD Biosciences). pmTOR and pS6 was measured upon 4% PFA fixation and methanol permeabilization. For antibodies, see Table S2. Cells were acquired with LSR Fortessa (BD Biosciences) and analyzed by using FlowJo software (Tree Star, version 10).
Table S2.
Antibodies used in flow cytometry
| Antibody | Clone | Manufacturer |
| Anti-CD8α | 53–6.7 | eBioscience |
| Anti-CD44 | IM7 | eBioscience |
| Anti-l-selectin (CD62L) | MEL-14 | eBioscience |
| Anti-TNF-α | MP-6XT22 | eBioscience |
| Anti-IFN-γ | XMG1.2 | eBioscience |
| Anti-IL-2 | JES6-5H4 | eBioscience |
| Anti-phospho mTOR (S2448) | MRRBY | eBioscience |
| Anti-phospho S6 (S235/S236) | cupk43k | eBioscience |
| Near-IR (live-dead marker) | n.a. | Life Technology |
n.a., not applicable.
Polysome Fractionation.
Activated T cells (40 × 106) were washed with PBS containing 100 µg/mL cycloheximide. The cytoplasmic extract was layered on a 17–50% sucrose gradient (42) and centrifuged for 2 h at 178,305 × g (38,000 rpm) at 4 °C in a SW41Ti rotor (Optima L100XP ultracentrifuge; Beckman Coulter). RNA was isolated by Phenol:Chloroform extraction. Total polyribosome-associated mRNA levels were quantified by RNAnano Chip assay (Agilent). Relative mRNA distribution was calculated as percent of the sum of mRNA from all obtained fractions. Total mRNA levels were put at 100% for each condition.
Quantitative PCR Analysis.
cDNA was synthesized by using SuperScript III Reverse Transcriptase (Invitrogen). Quantitative RT-PCR was performed with SYBR Green on StepOne Plus RT-PCR (Applied Biosystems) (17). Gene expression levels were calculated by the 2-ΔCt analysis. Cytokine mRNA half-life was determined with GraphPad Prism 6 by calculating the one-phase exponential decay curve.
Western Blot.
Cell lysates (1 × 106 cells/sample) were prepared by standard procedures. Proteins were separated on a 15% SDS/PAGE and transferred onto a nitrocellulose membrane by iBlot (Thermo). Anti-4E-BP1 (Cell Signaling Technology) (43), followed by goat anti-rabbit-HRP secondary antibody (Dako), was used.
Statistical Analysis.
Results are expressed as mean ± SD. Data were analyzed with GraphPad Prism6. Unpaired or paired two-tailed Student’s t test was used when comparing two groups, one-way ANOVA test with Tukey’s multiple comparison, or Dunnett’s multiple comparison with control group was used when comparing >2 groups. P values of <0.05 were considered statistically significant.
Acknowledgments
We thank the animal caretakers from the NCI; K. Moore and A. Guislain for technical support; K. van Gisbergen for the RNA-seq data; and S. Libregts, D. Amsen, and B. van Steensel for critical reading of the manuscript. This research was supported by the Dutch Science Foundation VIDI Grant 917.14.214 (to M.C.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704227114/-/DCSupplemental.
References
- 1.Han Q, et al. Polyfunctional responses by human T cells result from sequential release of cytokines. Proc Natl Acad Sci USA. 2012;109:1607–1612. doi: 10.1073/pnas.1117194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicolet BP, Guislain A, Wolkers MC. Combined single-cell measurement of cytokine mRNA and protein identifies T cells with persistent effector function. J Immunol. 2017;198:962–970. doi: 10.4049/jimmunol.1601531. [DOI] [PubMed] [Google Scholar]
- 3.Tessier PA, et al. Chemokine networks in vivo: Involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J Immunol. 1997;159:3595–3602. [PubMed] [Google Scholar]
- 4.Griffin GK, et al. IL-17 and TNF-α sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol. 2012;188:6287–6299. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soudja SM, et al. Memory-T-cell-derived interferon-γ instructs potent innate cell activation for protective immunity. Immunity. 2014;40:974–988. doi: 10.1016/j.immuni.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beutler BA. The role of tumor necrosis factor in health and disease. J Rheumatol Suppl. 1999;57:16–21. [PubMed] [Google Scholar]
- 8.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: Implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Souza WN, Schluns KS, Masopust D, Lefrançois L. Essential role for IL-2 in the regulation of antiviral extralymphoid CD8 T cell responses. J Immunol. 2002;168:5566–5572. doi: 10.4049/jimmunol.168.11.5566. [DOI] [PubMed] [Google Scholar]
- 10.McCaffrey PG, Goldfeld AE, Rao A. The role of NFATp in cyclosporin A-sensitive tumor necrosis factor-alpha gene transcription. J Biol Chem. 1994;269:30445–30450. [PubMed] [Google Scholar]
- 11.Teixeira LK, et al. IFN-gamma production by CD8+ T cells depends on NFAT1 transcription factor and regulates Th differentiation. J Immunol. 2005;175:5931–5939. doi: 10.4049/jimmunol.175.9.5931. [DOI] [PubMed] [Google Scholar]
- 12.Ullman KS, Northrop JP, Verweij CL, Crabtree GR. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: The missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- 13.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 14.Pei Y, et al. Nuclear export of NF90 to stabilize IL-2 mRNA is mediated by AKT-dependent phosphorylation at Ser647 in response to CD28 costimulation. J Immunol. 2008;180:222–229. doi: 10.4049/jimmunol.180.1.222. [DOI] [PubMed] [Google Scholar]
- 15.Zhu P, et al. IL-2 mRNA stabilization upon PMA stimulation is dependent on NF90-Ser647 phosphorylation by protein kinase CbetaI. J Immunol. 2010;185:5140–5149. doi: 10.4049/jimmunol.1000849. [DOI] [PubMed] [Google Scholar]
- 16.Salerno F, Wolkers MC. T-cells require post-transcriptional regulation for accurate immune responses. Biochem Soc Trans. 2015;43:1201–1207. doi: 10.1042/BST20150154. [DOI] [PubMed] [Google Scholar]
- 17.Salerno F, Guislain A, Cansever D, Wolkers MC. TLR-mediated innate production of IFN-γ by CD8+ T cells is independent of glycolysis. J Immunol. 2016;196:3695–3705. doi: 10.4049/jimmunol.1501997. [DOI] [PubMed] [Google Scholar]
- 18.Piccirillo CA, Bjur E, Topisirovic I, Sonenberg N, Larsson O. Translational control of immune responses: From transcripts to translatomes. Nat Immunol. 2014;15:503–511. doi: 10.1038/ni.2891. [DOI] [PubMed] [Google Scholar]
- 19.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almeida JR, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciuffreda D, et al. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur J Immunol. 2008;38:2665–2677. doi: 10.1002/eji.200838336. [DOI] [PubMed] [Google Scholar]
- 22.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naïve and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000;1:47–53. doi: 10.1038/76907. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 24.Mackay LK, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016;352:459–463. doi: 10.1126/science.aad2035. [DOI] [PubMed] [Google Scholar]
- 25.Gingras AC, et al. Regulation of 4E-BP1 phosphorylation: A novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell JD, Delgoffe GM. The mammalian target of rapamycin: Linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh-hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol. 2008;20:250–258. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navarro MN, Cantrell DA. Serine-threonine kinases in TCR signaling. Nat Immunol. 2014;15:808–814. doi: 10.1038/ni.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corse E, Gottschalk RA, Allison JP. Strength of TCR-peptide/MHC interactions and in vivo T cell responses. J Immunol. 2011;186:5039–5045. doi: 10.4049/jimmunol.1003650. [DOI] [PubMed] [Google Scholar]
- 31.Zehn D, King C, Bevan MJ, Palmer E. TCR signaling requirements for activating T cells and for generating memory. Cell Mol Life Sci. 2012;69:1565–1575. doi: 10.1007/s00018-012-0965-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivanov P, Anderson P. Post-transcriptional regulatory networks in immunity. Immunol Rev. 2013;253:253–272. doi: 10.1111/imr.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang JH, Toita R, Kim CW, Katayama Y. Protein kinase C (PKC) isozyme-specific substrates and their design. Biotechnol Adv. 2012;30:1662–1672. doi: 10.1016/j.biotechadv.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Kumar V, et al. Functional interaction between RAFT1/FRAP/mTOR and protein kinase cdelta in the regulation of cap-dependent initiation of translation. EMBO J. 2000;19:1087–1097. doi: 10.1093/emboj/19.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Zhu L, Kuokkanen S, Pollard JW. Activation of protein synthesis in mouse uterine epithelial cells by estradiol-17β is mediated by a PKC-ERK1/2-mTOR signaling pathway. Proc Natl Acad Sci USA. 2015;112:E1382–E1391. doi: 10.1073/pnas.1418973112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herbert TP, Tee AR, Proud CG. The extracellular signal-regulated kinase pathway regulates the phosphorylation of 4E-BP1 at multiple sites. J Biol Chem. 2002;277:11591–11596. doi: 10.1074/jbc.M110367200. [DOI] [PubMed] [Google Scholar]
- 37.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci USA. 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang JG, et al. LFA-1-dependent HuR nuclear export and cytokine mRNA stabilization in T cell activation. J Immunol. 2006;176:2105–2113. doi: 10.4049/jimmunol.176.4.2105. [DOI] [PubMed] [Google Scholar]
- 39.Doller A, et al. Protein kinase C alpha-dependent phosphorylation of the mRNA-stabilizing factor HuR: Implications for posttranscriptional regulation of cyclooxygenase-2. Mol Biol Cell. 2007;18:2137–2148. doi: 10.1091/mbc.E06-09-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caccamo N, et al. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol. 2010;40:2211–2220. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 41.Precopio ML, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pereboom TC, van Weele LJ, Bondt A, MacInnes AW. A zebrafish model of dyskeratosis congenita reveals hematopoietic stem cell formation failure resulting from ribosomal protein-mediated p53 stabilization. Blood. 2011;118:5458–5465. doi: 10.1182/blood-2011-04-351460. [DOI] [PubMed] [Google Scholar]
- 43.Blázquez-Domingo M, Grech G, von Lindern M. Translation initiation factor 4E inhibits differentiation of erythroid progenitors. Mol Cell Biol. 2005;25:8496–8506. doi: 10.1128/MCB.25.19.8496-8506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]