Significance
Most species extinctions after habitat loss are delayed. Thus, there are important, yet insufficiently appreciated, opportunities to conserve species through habitat restoration. Here, we assess the impact of targeted habitat restoration on how long tropical bird species might persist in two tropical biodiversity hotspots—the Eastern Arc Mountains of Tanzania and the Atlantic Forest of Brazil. Persistence times could be increased up to 56-fold by regenerating forest among the largest and closest forest fragments at these two localities. Given the unusually large numbers of threatened and endemic species that occur in other biodiversity hotspots, opportunities to enhance species persistence through habitat restoration should be explored elsewhere.
Keywords: species credit, tropical biodiversity hotspots, understory birds, persistence time, relaxation half-life–area relationship
Abstract
The Eastern Arc Mountains of Tanzania and the Atlantic Forest of Brazil are two of the most fragmented biodiversity hotspots. Species–area relationships predict that their habitat fragments will experience a substantial loss of species. Most of these extinctions will occur over an extended time, and therefore, reconnecting fragments could prevent species losses and allow locally extinct species to recolonize former habitats. An empirical relaxation half-life vs. area relationship for tropical bird communities estimates the time that it takes to lose one-half of all species that will be eventually lost. We use it to estimate the increase in species persistence by regenerating a forest connection 1 km in width among the largest and closest fragments at 11 locations. In the Eastern Arc Mountains, regenerating 8,134 ha of forest would create >316,000 ha in total of restored contiguous forest. More importantly, it would increase the persistence time for species by a factor of 6.8 per location or ∼2,272 years, on average, relative to individual fragments. In the Atlantic Forest, regenerating 6,452 ha of forest would create >251,000 ha in total of restored contiguous forest and enhance species persistence by a factor of 13.0 per location or ∼5,102 years, on average, relative to individual fragments. Rapidly regenerating forest among fragments is important, because mean time to the first determined extinction across all fragments is 7 years. We estimate the cost of forest regeneration at $21–$49 million dollars. It could provide one of the highest returns on investment for biodiversity conservation worldwide.
Habitat loss is the most important cause of species extinction (1). Extinctions from habitat loss are often delayed rather than immediate, because many species that tend to linger in the habitat fragments do not have viable populations and are doomed to eventual local extinction (2–4). Consequently, studies that characterize species richness in fragmented landscapes often underestimated how many species will go extinct (5–7). This delayed loss of species in habitat remnants is called faunal collapse or relaxation (2). Extinction debt (8) is the number or proportion of species that will eventually become extinct as a community reaches a new equilibrium (2, 9).
This time lag provides an important but insufficiently appreciated opportunity for conservation through habitat restoration (10). The increase in relaxation time resulting from habitat restoration has been termed species credit (11). Habitat restoration results in an increase in population size—and therefore, viability—because of an expansion in available habitat. Importantly, connecting fragments allows immigration from source populations that rescue floundering populations (12–15).
Estimating the rate at which species are lost in habitat remnants is a challenge. Occasionally, we have explicit data on the numbers of species initially within an area (S0) and subsequent data on numbers of species in habitat remnants over time. Examples include studies of species loss in Amazonian forest fragments (16), manmade islands in Chiew Larn Reservoir in Thailand (17), and North American and Tanzanian national parks (18–20). More often, we lack data on the numbers of species before habitat loss and only know the number of species at some time later (21–25).
In a large meta-analysis of studies, Halley et al. (26) used various approaches to infer S0 and therefore, to estimate the relaxation half-life or the time that it takes to lose one-half of all species that will be eventually lost for mammals, birds, reptiles, invertebrates, and plants. One method for inferring initial species richness is through the continental species–area relation: S0 = bAz (26). This relates species richness to area for regions contained within a large “continental” area. The exponent z tends to be quite small (usually between 0.1 and 0.2), reflecting the fact that the continental sample will have most of the species within the larger area. It contrasts to the island species–area relation, of the same form but where z is much larger (often larger than 0.25), reflecting the fact that small, isolated areas have many fewer species than larger ones.
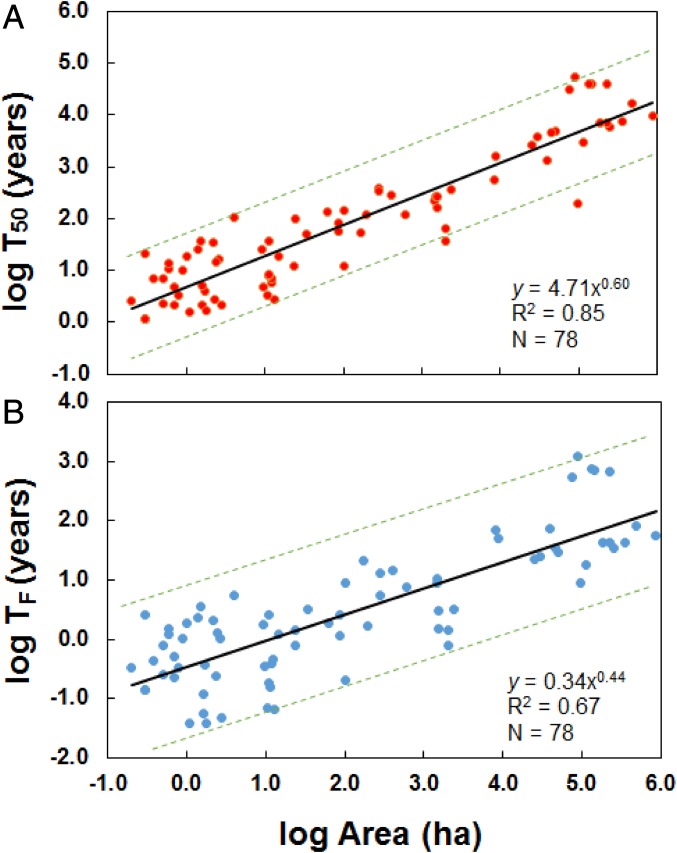
Here, using an empirical relaxation half-life vs. area relation for tropical bird communities (Fig. 1A), we assessed the increase in relaxation time, the delayed time to first extinction, and change in species number over time in bird communities that would be achieved by restoring contiguous forest among forest fragments. We used the continental species–area relation (SAR) to estimate the initial species richness in these fragments. These data were a subset of the bird community data presented in the work by Halley et al. (26). Two highly fragmented global tropical biodiversity hotspots, the Eastern Arc Mountains of Tanzania and the Atlantic Forest of Brazil, provided our examples. We simulated explicit spatial restorations of forest 1 km in width between the largest and closest forest fragments at 11 locations in these two biodiversity hotspots.
Fig. 1.
Relation between (A) log relaxation half-life (T50) and log area and (B) log time to first determined extinction (TF) and log area for tropical bird communities in habitat remnants. The regression line is shown in black, and dashed green lines are the 95% prediction intervals. Data are from 14 tropical bird community studies, a subset of the bird community data in the work by Halley et al. (26). The 95% prediction intervals were calculated in R (70).
We restrict the analysis to forest-dependent understory bird species. Such species forage or move through understory forest habitat and can be most reliably surveyed using mist nets, the predominant long-term survey technique used in our study locations (27–29). These species and especially, insectivores and forest interior species are also particularly at risk from habitat loss and fragmentation (27, 30–33).
Results
Relaxation Half-Life and Time to First Determined Extinction.
Assuming a hyperbolic decay over time in species number (6, 26, 34), we estimate the observed relaxation half-life, T50, vs. area relation to be
| [1] |
where A is area (in hectares). If log area is regressed on log half-life using least squares regression, log area explains 85% of the variation in log half-life for tropical bird communities in habitat remnants that spanned six orders of magnitude in size (Fig. 1A).
The relation of observed time for the first bird extinction caused by habitat loss vs. area is
| [2] |
where TF is the time to first extinction, and A is area (in hectares). If log area is regressed on log time to first extinction, log area explains 67% of the variation (Fig. 1B). For fragments <49 ha in size, time to first extinction is <1 y—a result in accord with studies of birds in Amazon fragments that were surveyed before and immediately after deforestation (16).
Restoration of Contiguous Forest.
In the Eastern Arc Mountains of Tanzania, regenerating 8,134 ha of forest among the largest and closest forest fragments (n = 42) at nine locations (Fig. 2 and SI Appendix, Figs. S1–S8) would create 316,663 ha in total of restored contiguous forest (Table 1). Of the 8,134 ha of regenerated forest, 1,592 ha occur within existing protected areas at locations that are predominantly secondary regenerating forest largely because of past fire, and 6,542 ha occur in nonprotected matrix habitats.
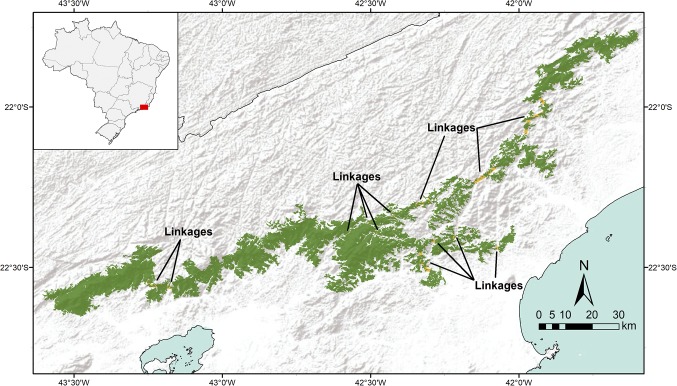
Fig. 2.
Map of the East Usambara Mountains, one of nine study locations in the Eastern Arc Mountains of Tanzania. The proposed linkage of the largest and closest forest fragments in the East Usambara Mountains would create 28,788 ha of contiguous forest. Forest fragments, totaling 27,702 ha, are shown in green, and linkages identified for regeneration, totaling 1,086 ha, are shown in yellow.
Table 1.
Summary by study location of number and area of fragments, regenerated forest area, and restored contiguous forest area in the Eastern Arc Mountains of Tanzania and the Atlantic Forest of Brazil
| Biodiversity hotspot and study location | Fragments | Regenerated forest (ha) | Restored contiguous forest (ha) | |||
| N | Minimum (ha) | Maximum (ha) | Total (ha) | |||
| Eastern Arc Mountains of Tanzania | ||||||
| East Usambara | 9 | 227 | 7,658 | 27,702 | 1,086 | 28,788 |
| West Usambara (east) | 2 | 1,366 | 3,598 | 4,934 | 142 | 5,076 |
| West Usambara (west) | 3 | 417 | 4,898 | 5,830 | 151 | 5,981 |
| Nguu | 5 | 377 | 9,068 | 14,739 | 546 | 15,285 |
| Nguru | 4 | 198 | 19,620 | 28,237 | 1,673 | 29,910 |
| Uluguru | 2 | 8,000 | 15,025 | 23,025 | 180 | 23,205 |
| Rubeho | 4 | 92 | 31,093 | 35,712 | 1,013 | 36,725 |
| Udzungwa (east) | 11 | 204 | 80,975 | 161,955 | 3,306 | 165,261 |
| Udzungwa (west) | 2 | 841 | 5,553 | 6,394 | 38 | 6,432 |
| Overall | 42 | 92 | 80,975 | 308,528 | 8,134 | 316,663 |
| Atlantic Forest of Brazil | ||||||
| Rio de Janeiro | 20 | 479 | 52,883 | 202,790 | 2,605 | 205,395 |
| Pontal do Paranapanema, São Paulo | 9 | 404 | 35,138 | 42,710 | 3,847 | 46,557 |
| Overall | 29 | 404 | 52,883 | 245,500 | 6,452 | 251,952 |
Regenerated forest area is the total area of native forest required to be regenerated and/or replanted to reconnect the largest and closest forest fragments at a study location. Restored contiguous forest area is the combined area of all fragments and regenerated forest at a study location.
In the Atlantic Forest of Brazil, regenerating 6,489 ha of forest among the largest and closest forest fragments (n = 29) at two locations (Fig. 3 and SI Appendix, Fig. S9) would create 251,952 ha in total of restored contiguous forest (Table 1).
Fig. 3.
Map of the Rio de Janeiro study location, one of two study locations in the Atlantic Forest of Brazil. The proposed linkage of the largest and closest forest fragments here would create 205,395 ha of contiguous forest. Forest fragments, totaling 202,790 ha, are shown in green, and linkages identified for regeneration, totaling 2,605 ha, are shown in yellow.
Impact of Forest Regeneration on Species Credit.
Across nine locations in the Eastern Arc Mountains of Tanzania, overall mean (±SE) half-life in restored contiguous forest is 2,168 (±571) y (range, 787–6,365 y) compared with 754 (±125) y (range, 71–4,149 y) in individual fragments. Forest regeneration would increase half-life for bird communities here by an average factor of 6.8 per location (range, 1.6–20.3) or ∼2,272 y relative to individual fragments.
In the Atlantic Forest of Brazil, overall mean (±SE) half-life for bird communities in restored contiguous forest is 5,115 (±2,137) y (range, 2,978–7,252 y) compared with 824 (±162) y (range, 173–3,213 y) in individual fragments. Forest regeneration increases half-life here by an average factor of 13.0 per location (range, 10.9–15.1) or ∼5,102 y relative to individual fragments.
Extinction Forecasts.
Rapidly regenerating forest among the largest and closest forest fragments at study localities in these two biodiversity hotspots is important to minimize species losses. In the Eastern Arc Mountains of Tanzania, mean (±SE) time to first determined extinction in forest fragments across nine locations is 7 (±1) y (range, 1–26 y) compared with 15 (±3) y (range, 8–36 y) in contiguous forest. In the Atlantic Forest of Brazil, mean (±SE) time to first determined extinction in forest fragments is 7 (±1) y (range, 3–22) compared with 30 (±9) y (range, 20–39 y) in contiguous forest.
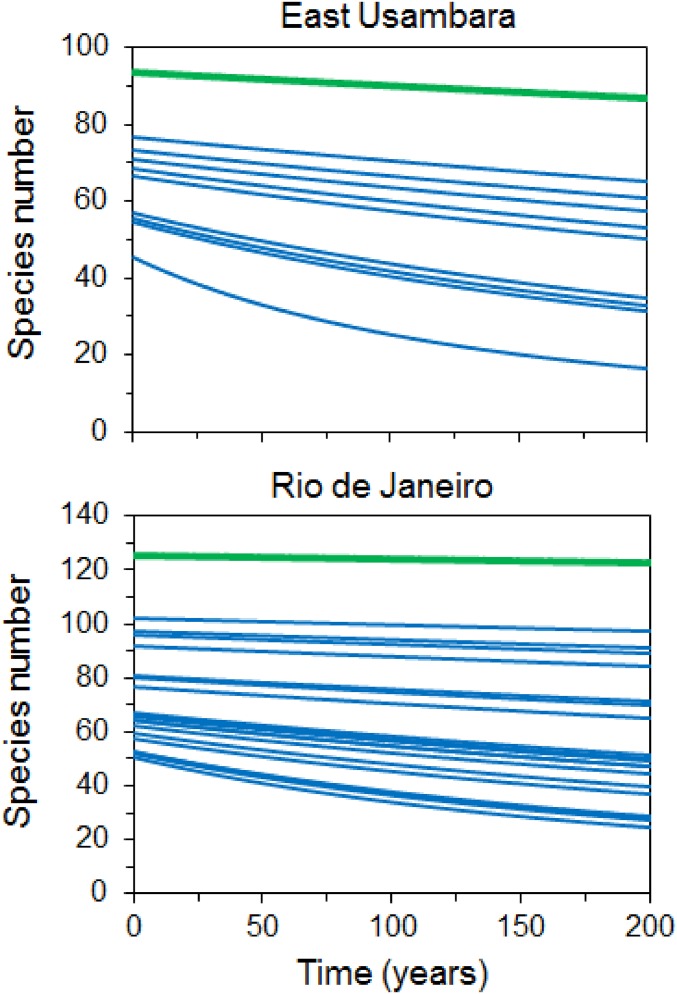
A comparison of projected bird species numbers over 200 y in restored contiguous forest vs. forest fragments further highlights the conservation value of forest restoration among the largest forest fragments in these two biodiversity hotspots (Fig. 4 and SI Appendix, Figs. S9 and S10). Error estimates are presented in SI Appendix, Table S2.
Fig. 4.
Comparison of projected change in understory bird species number over time in restored contiguous forest, shown in green, vs. individual forest fragments, shown in blue, in the East Usambara Mountains study location in the Eastern Arc Mountains of Tanzania and in the Rio de Janeiro study location in the Atlantic Forest of Brazil.
Discussion
The nature of Eqs. 3–7 is that all fragments lose species eventually. Thus, the aim of conservation is to delay that process as much as possible, certainly postponing extinctions that might happen in a few years or decades. Regenerating forest among the largest and closest forest fragments at 11 locations at biodiversity hotspots in Tanzania and Brazil could dramatically postpone and likely prevent many forest bird extinctions in these two highly fragmented tropical biodiversity hotspots. Regenerating ≈8,100 ha of forest in the Eastern Arc Mountains and 6,500 ha in the Atlantic Forest would reconnect ≈77% of all remaining forest in the Eastern Arc (35), 23% in the Brazilian state of Rio de Janeiro, and 33% in the Pontal do Paranapanema region in the state of São Paulo (36).
Rapid regeneration of forest among forest fragments is important to minimize species loss over time, because the mean time to first habitat-determined extinction across all forest fragments in these two biodiversity hotspots is 7 y. Most forest fragmentation and loss in these two regions has occurred over the last 300 y (35, 37), although the westernmost portion of the Pontal do Paranapanema region in São Paulo was 78% contiguous forest until as recently as 1956 (38). It is likely that many of the smallest forest fragments (≲100 ha) in our study localities have already lost a large fraction of their original species. The most important current conservation value of these smallest fragments is in the context of species credit. They can facilitate the restoration of linkages among the largest forest fragments.
Given current patterns of land use in matrix habitats and average distance among fragments in the study locations, it is highly feasible to regenerate forest in these two biodiversity hotspots. In the Eastern Arc Mountains of Tanzania, secondary regenerating forest comprises nearly two-thirds (62%) of current land use in proposed forest regeneration sites (SI Appendix, Table S1), and human population density and use are low. Furthermore, the average distance among fragments across the nine study locations is <2.0 km, while at three of nine locations, the mean distance is <850 m (SI Appendix, Table S1). In the Atlantic Forest of Brazil, most potential forest regeneration sites in Rio de Janeiro state are low-productivity cattle pasture or other degraded land. In the Pontal de Paranapanema, São Paulo study location, the predominant land uses are sugar cane fields and improved cattle pastures and thus, have higher value than in Rio de Janeiro. The average distance among fragments across these two study locations is <5.3 km (SI Appendix, Table S1). In the Atlantic Forest, private landowners are required under the Forest Code to maintain forested riparian buffers or Area de Preservação Permanente (APPs) of a minimum 30 m in width and for larger landowners, 20% of the overall property holdings (i.e., Legal Reserve) as forest [although there are recent debates about the revised forest code (39, 40)]. Consequently, there is a significant backlog of legally required restoration in the region.
Regenerating forest in these two biodiversity hotspots would require the development of a broad package of incentives focused on individuals and communities using matrix habitats to establish and conserve forest cover between existing protected fragments. Based on the per hectare cost of establishing the Derema corridor (41, 42), an important linkage between the two largest nature reserves in the East Usambara Mountains in the Eastern Arc Mountains of Tanzania (35), we estimate that the cost of regenerating forest cover at the nine locations there would be less than $21 million dollars. In the Atlantic Forest of Brazil, we estimate that the cost of regenerating forest at the two locations would be approximately $49 million. This assumes an average cost of $7,500 per hectare, which comes from recent establishment of the Fazenda Dourada and Rosanela linkages in the Rio de Janeiro and Pontal de Paranapanema, São Paulo regions, respectively.
These cost estimates directly depend on linkage length and width. In this analysis, we assumed a width of 1 km (Methods) as the minimum width necessary to permit all understory bird species in our study locations to disperse between fragments. This assumption, we believe, errs on the side of caution. Long-term (30-y) mark recapture data for understory bird species in the East and West Usambara Mountains in the Eastern Arc Mountains (27) indicate that many understory bird species here could almost certainly disperse between fragments through narrower linkages. To estimate the absolute minimum width of a linkage that would permit all bird species to move between fragments would require extensive species-specific data on bird dispersal and movement through linkages varying in width. Unfortunately, such data are not available but should be an area for future research.
Additionally, the likelihood that understory bird species could successfully disperse between fragments through reforested linkages is almost certainly dependent also on the age and habitat structure of the linkage. Given that mean time to first extinction across all forest fragments in our study regions is less than a decade, might reforestation proceed too slowly to permit particularly some of the most extinction-prone species to be rescued by immigrants (43)? Recent reviews of reforestation projects in the Atlantic Forest suggest that the time required to regenerate biologically diverse and structurally complex forest is 10–40 y (44, 45).
On the other hand, monitoring of animal movement through recently regenerated forested linkages in the Atlantic Forest also indicates that many extinction-prone species, including golden lion tamarins (Leontopithecus rosalia) and panthers (Puma concolor), can disperse through linkages that are <10 y in age. To estimate whether the most extinction-prone understory bird species in our study regions, particularly insectivores and forest interior species (27, 30–33), would be the last species to be rescued by immigrants would also require species-specific data on bird movement through regenerated forest varying in age and habitat structure.
There is an additional benefit of regenerating forest among the largest and closest forest fragments in these two regions. Climate change models suggest that tropical lowland species and especially those with narrow geographic ranges are particularly extinction-prone (46). Forest regeneration among the largest and closest fragments would permit plant and animal species to move upslope between 535 and 1,210 m in elevation, on average, relative to individual fragments in the Eastern Arc Mountains of Tanzania (SI Appendix, Table S1) and between 965 and 1,982 m in elevation in the Rio de Janeiro study location (SI Appendix, Table S1). In the Pontal do Paranapanema, São Paulo study location, elevation varies relatively little among fragments (SI Appendix, Table S1).
Given the unusually large number of endemic and highly threatened plant and animal species in these two biodiversity hotspots, forest regeneration between the largest and closest forest fragments could provide one of the highest returns on investment worldwide for biodiversity conservation. Given the highly fragmented nature of most of the other tropical biodiversity hotspots (47, 48), we believe that important opportunities almost certainly exist in these other tropical biodiversity hotspots to enhance species credit through forest regeneration. Given the rapid rate of species loss in small habitat remnants after habitat destruction (16, 17, 26), rapid action is essential to obtain maximum benefit.
Methods
Study Sites.
Myers et al. (47) identified 25 global biodiversity hotspots, which are defined as sites that contain unusually high numbers of endemic plant and animal species and have lost greater than 70% of their original habitat. The Eastern Arc Mountains of Tanzania and Kenya and the Atlantic Forest of Brazil, Argentina, and Paraguay are two of the most fragmented of these biodiversity hotspots (35, 37, 38, 47–49).
We identified 11 study locations, nine in the Eastern Arc Mountains of Tanzania and two in the Atlantic Forest of Brazil. For these, it is highly feasible to regenerate forest among the largest and closest forest fragments because of current land use in matrix habitats and the size and distance among fragments (35–37).
Fragment and Linkage Selection Criteria.
In the Eastern Arc Mountains of Tanzania, nearly all large forest fragments have some form of protected area status as national park, nature reserve, or forest reserve (central, regional, or local government, respectively). On the other hand, very little of the intervening matrix between the largest forest blocks is protected apart from that located within several of the largest protected areas. To minimize the financial and social costs that would be associated with regenerating forest between the largest and closest forest fragments in the Eastern Arc Mountains, we restricted fragment selection to fragments (i) >1,000 ha and <7.0 km apart or (ii) >90 ha and <2.5 km apart, which could link to or serve as a stepping stone to create a contiguous forest block >5,000 ha, and (iii) bordered >1 km in width by secondary regenerating forest or low-density agriculture. Within two of the largest protected areas (Udzungwa National Park and Shume Magamba Forest Reserve), we adopted less restrictive fragment selection criteria. Here, we restricted fragment selection to fragments (i) >450 ha and <8.0 km apart or (ii) >90 ha and <6.0 km apart, which could link to or serve as a stepping stone to create a contiguous forest block >5,000 ha. Estimation of forest cover is presented in SI Appendix, SI Text.
In many regions, riparian habitats along streams and rivers are natural linkages among habitat remnants. In the Eastern Arc Mountains, regenerating forest between fragments along streams and rivers in matrix habitats is impractical because of intensive small-scale subsistence agriculture which occurs here. Consequently, we identified reforestation sites for linkages at localities where small-scale subsistence agriculture and human population density were low, which provided the shortest and most direct link between fragments.
In the Atlantic Forest of Brazil, much of the native forest at the two study locations is within state or federal protected areas. Technically, all remaining Atlantic Forest of Brazil has some legal protection. In practice, deforestation and degradation continue, and there are legal means to circumvent the biome-wide protection laws. Nearly all of the intervening matrix between forest fragments is privately owned. We restricted fragment selection to those (i) >400 ha and <16.5 km apart that could link to or serve as a stepping stone to create a contiguous forest block >45,000 ha and (ii) bordered >1 km in width by pasture or sugar cane, small-scale, or commercial agriculture.
Regenerating riparian habitats along streams and rivers in the Atlantic Forest of Brazil is more feasible than in the Eastern Arc Mountains, largely because the Forest Code requires private landowners to maintain forested riparian buffers. We identified reforestation sites for linkages along streams and rivers in localities where there were ongoing restoration programs of riparian habitats. In other localities, we identified reforestation sites for linkages that provided the shortest and most direct link between fragments.
Estimation of Regenerated Forest Width.
Because of the very limited gap crossing ability of many of the forest-dependent understory bird species (27, 35, 50–53) (SI Appendix, SI Text), regenerating forest among the largest and closest forest fragments is critical to conserving intact forest bird communities. Forest regeneration among forest fragments would also obviously greatly enhance species credit for many other taxa having limited gap-crossing ability, including many arboreal primates (54, 55), sloths (56), scarab beetles (57), Euglossine bees (58), forest chameleons (59), and animal-dispersed plants (60, 61).
Long-term survey data for understory bird species in the East and West Usambara Mountains in the Eastern Arc Mountains of Tanzania suggest that the minimum linkage width among fragments to conserve intact understory bird communities is ∼1 km (35, 50). We adopted this criterion. This estimate is based on doubling the median distance of encounter of the understory bird species captured, on average, the farthest from the forest edge over 30 y (red-capped forest warbler Orthotomus metopias, 279 m) and providing a 200-m buffer around a ∼600-m core (35, 50). Similar patterns of distribution of bird species from the forest edge have been reported in the Atlantic Forest of Brazil, Paraguay, and Argentina (53).
Estimation of the Half-Life of a Fragment.
If we assume that speciation and immigration through nonforested habitats are negligible, the decay of species richness in an isolated fragment given by Halley et al. (ref. 26, equation A12) is
| [3] |
Here, S0 is original species number, S(t) is current species number, and t is length of time in years between S0 and S(t). This decay is also a function of two parameters: the half-life time t50 for each fragment and the parameter α that is the same for all fragments; these can be estimated from the plot of S(t) as a function of t using nonlinear regression. However, Halley et al. (26) showed that, with minimal error, we can estimate t50 according to the simpler hyperbolic model used by Terborgh (3) and Halley and Iwasa (34):
| [4] |
where T50 is empirical half-life or time (in years) to lose one-half of those which will eventually be lost species. The parameter α can then be estimated from the slope of the graph of T50 (on a log–log scale) against area.
Empirical Time to First Determined Extinction, TF.
The time to the first extinction is given by equation 3 in the work by Halley et al. (26). It is derived in supplementary note 2 of the paper in ref. 26. The formula is
| [5] |
Noting that we have found from empirical data that α = 0.6 and also using, for t50, its empirically derived value T50, we estimate an empirical time to the first extinction to be
| [6] |
Thus, we can estimate the empirical time to first extinction to be T50/S0 for each point on the graph and then fit a power law model.
Species Number as a Function of Time.
Thus, using the parameter α = 0.6 in Eq. 3 above, we can show that the time decay of species richness in an isolated fragment is approximately
| [7] |
The value of S0 was estimated using the continental SAR:
| [8] |
The exponent z tends to range between 0.1 and 0.2 for various taxa (62–64). For our work, we assumed an exponent of z = 0.15 (6) and a constant b = 20 species per 1 ha based on long-term survey data for understory bird species in the East and West Usambara Mountains (27) in the Eastern Arc Mountains of Tanzania. The latter value is also consistent with observations from the Atlantic Forest of Brazil (65). Eqs. 3–7 are reliant on the assumption that there is negligible dispersal between forest fragments. This assumption breaks down if there is an extensive habitable matrix (66), if fragments are not well-isolated or there are strong dispersal capacities for many species (67), and if there is extensive regeneration (68). Some progress toward dealing with incomplete isolation has been made (69). However, we regard this assumption as tenable because of the very limited gap-crossing ability of many understory bird species in our study regions (SI Appendix, SI Text), and natural regeneration is retarded or suppressed.
Supplementary Material
Acknowledgments
We thank the Tanzania Wildlife Research Institute and the Tanzania Commission for Science and Technology for permission to conduct this study. We also thank the Danish International Development Agency, the Field Museum of Natural History, the Chicago Zoological Society, the Sophie Danforth Conservation Fund, National Geographic Society Grants 524-94 and 977815, the Earthwatch Institute, the John D. and Catherine T. MacArthur Foundation, the Critical Ecosystem Partnership Fund, the World Wide Fund for Nature, and the Earth Point Corporation for support. C.N.J. received support from the Ciência Sem Fronteiras Program of Brazil Grant A025_2013 during part of this study.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.F.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705834114/-/DCSupplemental.
References
- 1.IUCN 2016 The IUCN red list of threatened species, version 2016-3. Available at www.iucnredlist.org. Accessed December 22, 2016.
- 2.Diamond JM. Biogeographic kinetics: Estimation of relaxation times for avifaunas of southwest pacific islands. Proc Natl Acad Sci USA. 1972;69:3199–3203. doi: 10.1073/pnas.69.11.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terborgh J. Preservation of natural diversity: The problem of extinction prone species. Bioscience. 1974;24:715–722. [Google Scholar]
- 4.Lovejoy TE, et al. Edge and other effects of isolation on Amazon forest fragments. In: Soulé ME, editor. Conservation Biology: The Science of Scarcity and Diversity. Sinauer; Sunderland, MA: 1986. pp. 257–285. [Google Scholar]
- 5.Pimm SL, Raven P. Biodiversity. Extinction by numbers. Nature. 2000;403:843–845. doi: 10.1038/35002708. [DOI] [PubMed] [Google Scholar]
- 6.Halley JM, Sgardeli V, Triantis KA. Extinction debt and the species-area relationship: A neutral perspective. Glob Ecol Biogeogr. 2014;23:113–123. [Google Scholar]
- 7.Pimm SL, Brooks T. Conservation: Forest fragments, facts, and fallacies. Curr Biol. 2013;23:R1098–R1101. doi: 10.1016/j.cub.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Tilman D, May RM, Lehman CL, Nowak MA. Habitat destruction and the extinction debt. Nature. 1994;371:65–66. [Google Scholar]
- 9.Kuussaari M, et al. Extinction debt: A challenge for biodiversity conservation. Trends Ecol Evol. 2009;24:564–571. doi: 10.1016/j.tree.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Wearn OR, Reuman DC, Ewers RM. Extinction debt and windows of conservation opportunity in the Brazilian Amazon. Science. 2012;337:228–232. doi: 10.1126/science.1219013. [DOI] [PubMed] [Google Scholar]
- 11.Hanski I. Extinction debt and species credit in boreal forests: Modeling the consequences of different approaches to biodiversity conservation. Ann Zool Fennici. 2000;37:271–280. [Google Scholar]
- 12.MacArthur RA, Wilson EO. The Theory of Island Biogeography. Princeton Univ Press; Princeton: 1967. [Google Scholar]
- 13.Diamond JW. The island dilemma: Lessons of modern biographic studies for the design of nature reserve. Biol Conserv. 1975;7:129–146. [Google Scholar]
- 14.Brown JH, Kodric-Brown A. Turnover rates in insular biogeography: Effect of immigration on extinction. Ecology. 1977;58:445–449. [Google Scholar]
- 15.Hylander K, Ehrlén J. The mechanisms causing extinction debts. Trends Ecol Evol. 2013;28:341–346. doi: 10.1016/j.tree.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Ferraz G, et al. Rates of species loss from Amazonian forest fragments. Proc Natl Acad Sci USA. 2003;100:14069–14073. doi: 10.1073/pnas.2336195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson L, et al. Near-complete extinction of native small mammal fauna 25 years after forest fragmentation. Science. 2013;341:1508–1510. doi: 10.1126/science.1240495. [DOI] [PubMed] [Google Scholar]
- 18.Newmark WD. A land-bridge island perspective on mammalian extinctions in western North American parks. Nature. 1987;325:430–432. doi: 10.1038/325430a0. [DOI] [PubMed] [Google Scholar]
- 19.Newmark WD. Extinction of mammal populations in western North American national parks. Conserv Biol. 1995;9:512–526. [Google Scholar]
- 20.Newmark WD. Insularization of Tanzanian parks and the local extinction of large mammals. Conserv Biol. 1996;10:1549–1556. [Google Scholar]
- 21.Wilson EO. In: Biodiversity. Wilson EO, Peter EM, editors. National Academy Press; Washington, DC: 1988. pp. 3–18. [Google Scholar]
- 22.Pimm SL, Russell GJ, Gittleman JL, Brooks TM. The future of biodiversity. Science. 1995;269:347–350. doi: 10.1126/science.269.5222.347. [DOI] [PubMed] [Google Scholar]
- 23.Pimm SL, Askins RA. Forest losses predict bird extinctions in eastern North America. Proc Natl Acad Sci USA. 1995;92:9343–9347. doi: 10.1073/pnas.92.20.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.May RM, Lawton JH, Stork NE. In: Extinction Rates. Lawton JH, May RM, editors. Oxford Univ Press; Oxford: 1995. pp. 1–24. [Google Scholar]
- 25.Brooks TM, Pimm SL, Oyugi JO. Time lag between deforestation and bird extinction in tropical forest fragments. Conserv Biol. 1999;13:1140–1150. [Google Scholar]
- 26.Halley JM, Monokrousos N, Mazaris AD, Newmark WD, Vokou D. Dynamics of extinction debt across five taxonomic groups. Nat Commun. 2016;7:12283. doi: 10.1038/ncomms12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korfanta NM, Newmark WD, Kauffman MJ. Long-term demographic consequences of habitat fragmentation to a tropical understory bird community. Ecology. 2012;93:2548–2559. doi: 10.1890/11-1345.1. [DOI] [PubMed] [Google Scholar]
- 28.Callens T, et al. Genetic signature of population fragmentation varies with mobility in seven bird species of a fragmented Kenyan cloud forest. Mol Ecol. 2011;20:1829–1844. doi: 10.1111/j.1365-294X.2011.05028.x. [DOI] [PubMed] [Google Scholar]
- 29.Banks-Leite C, Ewers RM, Metzger JP. Unraveling the drivers of community dissimilarity and species extinction in fragmented landscapes. Ecology. 2012;93:2560–2569. doi: 10.1890/11-2054.1. [DOI] [PubMed] [Google Scholar]
- 30.Newmark WD. Tropical forest fragmentation and the local extinction of understory birds in the Eastern Usambara Mountains, Tanzania. Conserv Biol. 1991;5:67–78. [Google Scholar]
- 31.Stratford JA, Stouffer PC. Local extinctions of terrestrial insectivorous birds in a fragmented landscape near Manaus, Brazil. Conserv Biol. 1999;13:1416–1423. [Google Scholar]
- 32.Lees AC, Peres CA. Rapid avifaunal collapse along the Amazonian deforestation frontier. Biol Conserv. 2006;133:198–221. [Google Scholar]
- 33.Powell LL, Cordeiro NJ, Stratford JA. Ecology and conservation of avian insectivores of the rainforest understory: A pantropical perspective. Biol Conserv. 2015;188:1–10. [Google Scholar]
- 34.Halley JM, Iwasa Y. Neutral theory as a predictor of avifaunal extinctions after habitat loss. Proc Natl Acad Sci USA. 2011;108:2316–2321. doi: 10.1073/pnas.1011217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newmark WD. 2002. Conserving Biodiversity in East African Forests: A Study of the Eastern Arc Mountains, Ecological Studies No. 155 (Springer, New York)
- 36.Mata Atlântica SOS; Instituto Nacional de Pesquisas Espaciais 2013 Atlas dos remanescentes florestais da Mata Atlântica, período de 2011 a 2012. Available at https://www.sosma.org.br. Accessed March 27, 2017.
- 37.Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM. The Brazilian Atlantic Forest: How much is left and how is the remaining forest distributed? Implications for conservation. Biol Conserv. 2009;142:1141–1153. [Google Scholar]
- 38.Uezu A, Metzger JP. Forest fragmentation: Restoration opportunity and urgency. PLoS One. 2016;11:e0147909. doi: 10.1371/journal.pone.0147909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soares-Filho B, et al. Cracking Brazil’s forest code. Science. 2014;344:363–364. doi: 10.1126/science.1246663. [DOI] [PubMed] [Google Scholar]
- 40.Calmon M, et al. Emerging threats and opportunities for large-scale ecological restoration in the Atlantic Forest of Brazil. Restor Ecol. 2011;19:154–158. [Google Scholar]
- 41.Tanzania Forest Conservation and Management Project 2006 Resettlement action plan for farm plots displaced for biodiversity conservation in the Derema Forest Corridor. Available at documents.worldbank.org/curated/en/733321468117290219/Tanzania-Forest-Conservation-and-Management-Project-resettlement-action-plan. Accessed January 11, 2016.
- 42.Sumbi P. 2010 Facilitating the compensation payments for the Derema Forest Reserve, East Usambara Mountains. CEPF final project completion report. Available at www.cepf.net/Documents/Final_WWFTanzania_Derema_compensation_payments.pdf. Accessed January 11, 2016.
- 43.Laurance WF. Ecological correlates of extinction proneness in Australian tropical rain forest mammals. Conserv Biol. 1991;5:79–89. [Google Scholar]
- 44.Rodrigues RR, Lima RAF, Gandolfi S, Nave AG. On the restoration of high diversity forests: 30 Years of experience in the Brazilian Atlantic Forest. Biol Conserv. 2009;142:1242–1251. [Google Scholar]
- 45.Rodrigues RR, et al. Large-scale ecological restoration of high-diversity tropical forests in SE Brazil. For Ecol Manage. 2011;261:1605–1613. [Google Scholar]
- 46.Colwell RK, Brehm G, Cardelús CL, Gilman AC, Longino JT. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science. 2008;322:258–261. doi: 10.1126/science.1162547. [DOI] [PubMed] [Google Scholar]
- 47.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 48.Sloan S, Jenkins CN, Joppa LN, Gaveau DLA, Laurance WF. Remaining natural vegetation in the global biodiversity hotspots. Biol Conserv. 2014;177:12–24. [Google Scholar]
- 49.Newmark WD. Forest area, fragmentation, and loss in the Eastern Arc Mountains: Implications for the conservation of biological diversity. J East Afr Nat Hist. 1998;87:29–36. [Google Scholar]
- 50.Newmark WD. The role and design of wildlife corridors with examples from Tanzania. Ambio. 1993;12:500–504. [Google Scholar]
- 51.Newmark WD, Mkongewa VJ, Sobek AD. Ranging behavior and habitat selection of terrestrial insectivorous birds in northeast Tanzania: Implications for corridor design in the Eastern Arc Mountains. Anim Conserv. 2010;13:474–482. [Google Scholar]
- 52.Awade M, Metzger JP. Using gap-crossing capacity to evaluate functional connectivity of two Atlantic rainforest birds and their response to fragmentation. Austral Ecol. 2008;33:863–871. [Google Scholar]
- 53.Zurita G, Pe’er G, Bellocq MI, Hansbauer MM. Edge effects and their influence on habitat suitability calculations: A continuous approach applied to birds of the Atlantic forest. J Appl Ecol. 2012;49:503–512. [Google Scholar]
- 54.Schwarzkopf L, Rylands AB. Primate species richness in relation to habitat structure in Amazonian rainforest fragments. Biol Conserv. 1989;48:1–12. [Google Scholar]
- 55.Dale VD, Pearson SM, Offerman HL, O’Neill RV. Relating patterns of land-use change to faunal biodiversity in the Central Amazon. Conserv Biol. 1994;8:1027–1036. [Google Scholar]
- 56.Montgomery FF, Sunquist ME. In: The Ecology of Arboreal Folivores. Montgomery GG, editor. Smithsonian Institution; Washington, DC: 1978. pp. 329–359. [Google Scholar]
- 57.Klein BC. Effects of forest fragmentation on dung and carrion beetle communities in central Amazonia. Ecology. 1989;70:1715–1725. [Google Scholar]
- 58.Powell AH, Powell GVN. Population dynamics of male euglossine bees in Amazonian forest fragments. Biotropica. 1987;19:176–179. [Google Scholar]
- 59.Shirk PL, et al. Impact of habitat alternation on endemic Afromontane chameleons: Evidence for historical population declines using hierarchical spatial modelling. Divers Distrib. 2014;20:1186–1199. [Google Scholar]
- 60.Corderio NJ, Howe HF. Low recruitment of trees dispersed by animals in African forest fragments. Conserv Biol. 2001;15:1733–1741. [Google Scholar]
- 61.Cordeiro NJ, Howe HF. Forest fragmentation severs mutualism between seed dispersers and an endemic African tree. Proc Natl Acad Sci USA. 2003;100:14052–14056. doi: 10.1073/pnas.2331023100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson MP, Mason LG, Raven PH. Ecological parameters and plant species diversity. Am Nat. 1968;102:297–306. [Google Scholar]
- 63.Rahbek C. The relationship among area, elevation, and regional species richness in neotropical birds. Am Nat. 1997;149:875–902. doi: 10.1086/286028. [DOI] [PubMed] [Google Scholar]
- 64.Brook BW, Sodhi NS, Ng PK. Catastrophic extinctions follow deforestation in Singapore. Nature. 2003;424:420–426. doi: 10.1038/nature01795. [DOI] [PubMed] [Google Scholar]
- 65.Ulrich W, et al. Environmentally and behaviourally mediated co-occurrence of functional traits in bird communities of tropical forest fragments. Oikos. 2017 doi: 10.1111/oik.04561. [DOI] [Google Scholar]
- 66.Koh LP, Ghazoul J. A matrix-calibrated species-area model for predicting biodiversity losses due to land-use change. Conserv Biol. 2010;24:994–1001. doi: 10.1111/j.1523-1739.2010.01464.x. [DOI] [PubMed] [Google Scholar]
- 67.Van Houtan KS, Pimm SL, Halley JM, Bierregaard RO, Jr, Lovejoy TE. Dispersal of Amazonian birds in continuous and fragmented forest. Ecol Lett. 2007;10:219–229. doi: 10.1111/j.1461-0248.2007.01004.x. [DOI] [PubMed] [Google Scholar]
- 68.Stouffer PC, Strong C, Naka LN. Twenty years of understorey bird extinctions from Amazonian rain forest fragments: Consistent trends and landscape-mediated dynamics. Divers Distrib. 2009;15:88–97. [Google Scholar]
- 69.Sgardeli V, Iwasa Y, Varvoglis H, Halley JM. A forecast for extinction debt in the presence of speciation. J Theor Biol. 2017;415:48–52. doi: 10.1016/j.jtbi.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 70.R Core Team 2016. The R Stats Package (R Foundation for Statistical Computing, Vienna), Version 3.3.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.