Significance
A functional immune system requires a highly diverse repertoire of T cells to optimize protection against foreign pathogens while maintaining tolerance against self-antigens. Two critical pathways in the control of T cell tolerance are the cytokine TGF-β and Foxp3-expressing Treg cells. However, since TGF-β promotes Treg cell development, and Treg cells also produce the cytokine, whether TGF-β and Treg cells are part of the same regulatory module to repress self-reactive T cells or function as distinct pathways remains incompletely understood. Using a mouse model of autoimmune diabetes, this study elucidates a dominant role for a Foxp3-independent mechanism of TGF-β signaling in the regulation of T cell tolerance.
Keywords: autoimmunity, T cell, tolerance, TGF-β
Abstract
Peripheral T cell tolerance is promoted by the regulatory cytokine TGF-β and Foxp3-expressing Treg cells. However, whether TGF-β and Treg cells are part of the same regulatory module, or exist largely as distinct pathways to repress self-reactive T cells remains incompletely understood. Using a transgenic model of autoimmune diabetes, here we show that ablation of TGF-β receptor II (TβRII) in T cells, but not Foxp3 deficiency, resulted in early-onset diabetes with complete penetrance. The rampant autoimmune disease was associated with enhanced T cell priming and elevated T cell expression of the inflammatory cytokine GM-CSF, concomitant with pancreatic infiltration of inflammatory monocytes that triggered immunopathology. Ablation of the GM-CSF receptor alleviated the monocyte response and inhibited disease development. These findings reveal that TGF-β promotes T cell tolerance primarily via Foxp3-independent mechanisms and prevents autoimmunity in this model by repressing the cross talk between adaptive and innate immune systems.
A fundamental challenge faced by the immune system is the requirement for a highly diverse repertoire of T cells to optimize protection against foreign pathogens while maintaining tolerance against self-antigens. Thymic negative selection of high-affinity T cells is a major mechanism by which self-reactive T cells are eliminated from the repertoire (1), but this process is incomplete, and autoreactive T cells that reach the periphery must be regulated to prevent the development of autoimmunity. A multitude of mechanisms exists to maintain peripheral T cell tolerance, including T cell ignorance of self-antigens, CD4+CD25+ Foxp3-expressing Treg cells, and inhibitory signaling pathways (2). However, the relative contributions and interplay of these various tolerance mechanisms remain poorly understood.
The cytokine TGF-β is an important regulator of T cell tolerance. Mice with impaired or total loss of TGF-β signaling in T cells develop multifocal autoimmunity that is as severe as the disease observed in mice with global Tgfb1 deficiency (3, 4), demonstrating that T cells are critical targets of TGF-β regulation (5–7). However, mice with T cell-specific loss of TGF-β signaling also exhibit defects in the differentiation of thymic Treg (tTreg) cells (8), as TGF-β signaling has been shown to promote the survival of tTreg cell precursors (9). Furthermore, in addition to its role in supporting the tTreg cell lineage, TGF-β signaling induces Foxp3 expression and the differentiation of peripheral Treg (pTreg) cells (10–13), further linking TGF-β to this lineage of cells that is critical for the maintenance of immune tolerance.
The breach of tolerance that occurs in the absence of T cell-specific TGF-β signaling is not caused solely by altered differentiation and homeostasis of Treg cells (6, 7), suggesting that a major mechanism by which TGF-β maintains tolerance is through directly regulating autoreactive T cells. Additional support for the direct regulation of autoreactive T cells by TGF-β arises from a transgenic model of diabetes in which loss of TGF-β signaling among activated diabetogenic CD4+ T cells, but not Treg cells, induces disease (14). However, it remains possible that TGF-β inhibition of T cell activation and differentiation is dependent on transient expression of Foxp3 induced by TGF-β signaling (13, 15, 16). Indeed, Foxp3 induction in conventional human CD4+CD25− T cells has been demonstrated to inhibit T cell proliferation and affect gene expression (17, 18). Furthermore, Treg cells may engage the TGF-β pathway to promote T cell tolerance via TGF-β production and activation of the latent form of TGF-β (19–22). Thus, the intertwined relationship between the TGF-β–dependent and Treg cell-mediated immune suppressive pathways raises the question of whether these two key regulators exist as distinct tolerance modules or are part of the same module to control self-reactive T cells.
In this study, using models of T cell-specific TGF-β receptor II (TβRII) or Foxp3 deficiency in the context of the OT-II RIP-mOva transgenic system, we demonstrated a Foxp3-independent role for the TGF-β signaling pathway in the regulation of T cell tolerance. The loss of TGF-β signaling specifically in T cells resulted in the development of more rapid, fulminant diabetes than did the absence of Foxp3. The more severe disease that developed in OT-II RIP-mOva mice with T cell-specific deficiency of TβRII involved a heightened effector T cell phenotype and the recruitment of a pathogenic inflammatory monocyte response that was associated with enhanced T cell production of GM-CSF. These findings reveal an essential role for TGF-β in the direct, Foxp3-independent regulation of autoreactive T cells in the maintenance of peripheral T cell tolerance.
Results
OT-II T Cells from OT-II RIP-mOva Mice Are Not Ignorant of Their Cognate Antigen.
The use of transgenic mouse models has been instrumental in elucidating mechanisms of central and peripheral T cell tolerance. The study of mice coexpressing membrane ovalbumin (mOva) under the control of the rat insulin promoter (RIP) and transgenic OT-II T cells, which recognize the ovalbumin peptide in the context of MHC class II molecule I-Ab, demonstrated that OT-II T cells encounter their cognate antigen during thymic development and are subjected to negative selection (23). However, despite the process of negative selection, mature OT-II T cells exist in the periphery of double-transgenic OT-II RIP-mOva mice. Notably, however, OT-II RIP-mOva mice do not develop autoimmunity (9, 23), indicating that the peripheral OT-II T cells are regulated to prevent diabetes development.
To determine whether T cells from OT-II RIP-mOva mice are ignorant of their cognate antigen, we compared the activation profiles of T cells isolated from the nondraining and pancreas-draining lymph nodes of single-transgenic OT-II mice and double-transgenic OT-II RIP-mOva mice that had been crossed to a genetic background deficient in the recombinant activating gene 1 (Rag1). The majority of T cells from the nondraining and draining lymph nodes of both OT-II and OT-II RIP-mOva mice were naive, as defined by high CD62L expression and low CD44 expression (Fig. 1 A and B). However, examination of the early-activation marker CD69 revealed a distinct expression pattern in the draining lymph nodes of OT-II RIP-mOva mice. Only T cells from the draining lymph nodes of OT-II RIP-mOva mice exhibited up-regulation of CD69 expression, whereas T cells from the nondraining lymph nodes of OT-II RIP-mOva mice and T cells from both anatomic locations in single-transgenic OT-II mice expressed minimal levels of the marker (Fig. 1 C and D). These observations demonstrate that T cells from OT-II RIP-mOva mice are not ignorant of their cognate antigen, a potential mechanism of peripheral T cell tolerance (24), and suggest that active tolerance mechanisms are in place to prevent further activation of the T cells and the development of diabetes.
Fig. 1.
OT-II T cells encounter antigen in the pancreas-draining lymph nodes of OT-II RIP-mOva mice. (A) Flow cytometric analysis of CD62L and CD44 expression on T cells from the nondraining and pancreatic lymph nodes from single-transgenic OT-II Rag1-deficient and double-transgenic OT-II RIP-mOva Rag1-deficient mice. Numbers indicate the percentage of cells in the respective gates. (B) The graph shows percentages of naive CD62L+CD44lo T cells from nondraining and pancreatic lymph nodes from OT-II Rag1-deficient and OT-II RIP-mOva Rag1-deficient mice. Circles represent individual mice. Error bars indicate the mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test. ns, not significant. (C) Flow cytometric analysis of CD69 expression on T cells from the nondraining and pancreatic lymph nodes of OT-II Rag1-deficient and OT-II RIP-mOva Rag1-deficient mice. Numbers indicate the percentage of cells in the respective gates. (D) The graph shows the percentage of CD69-expressing T cells from the nondraining and pancreatic lymph nodes of OT-II Rag1-deficient and OT-II RIP-mOva Rag1-deficient mice. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test. *P < 0.05.
OT-II T Cell Tolerance Is Associated with Treg Cell Generation and TGF-β Signaling.
Treg cells and the TGF-β pathway are two critical regulators of T cell tolerance (25–27). To address the respective roles of these two tolerance mechanisms in our model system, we first determined the presence of Treg cells by analyzing the lymph nodes of single-transgenic OT-II and double-transgenic OT-II RIP-mOva mice for Foxp3-expressing CD4+CD25+ T cells. Indeed, OT-II RIP-mOva mice, but not OT-II mice that do not express the mOva as a self-antigen, contained Treg cells (Fig. 2 A and B), indicating that Treg cells may be a key mechanism by which diabetes development is prevented in OT-II RIP-mOva mice. Notably, the majority of Foxp3+ Treg cells did not express CD69 (Fig. S1A), revealing that the enhanced CD69 expression observed in T cells from the draining lymph nodes of OT-II RIP-mOva mice is due to the activation of conventional T cells (Fig. 1 C and D). To address the activity of the TGF-β signaling pathway, we performed flow cytometric analysis of phospho-Smad2/3 expression in T cells from the nondraining and draining lymph nodes of OT-II and OT-II RIP-mOva mice. T cells from the draining lymph nodes of OT-II RIP-mOva mice exhibited a consistent increase in levels of phospho-Smad2/3 expression compared with their nondraining lymph node counterparts (Fig. 2 C and D). This shift in phospho-Smad2/3 expression by draining lymph node T cells was more pronounced in OT-II RIP-mOva mice than in single-transgenic OT-II mice, suggesting that increased TGF-β signaling is in response to T cell interactions with their cognate antigen. These observations suggest that T cell tolerance in OT-II RIP-mOva mice may be promoted by Treg cells and/or TGF-β signaling.
Fig. 2.
TGF-β promotes T cell tolerance via Treg cell-independent mechanisms. (A) Flow cytometric analysis of Foxp3 and CD25 expression among lymph node T cells from OT-II Rag1-deficient and OT-II RIP-mOva Rag1-deficient mice. Numbers indicate the percentage of cells in the respective gates. (B) The graph shows the percentage of Foxp3+CD25+ cells among total lymph node T cells in OT-II Rag1-deficient and OT-II RIP-mOva Rag1-deficient mice. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test. **P ≤ 0.01. (C) Flow cytometric analysis of pSmad2/3 in T cells from the nondraining (dotted lines) and pancreatic (solid lines) lymph nodes of OT-II Rag1-deficient and OT-II RIP-mOva Rag1-deficient mice. The tinted gray histogram represents the fluorescence minus one (FMO) control. (D) The graph shows the mean fluorescence intensity (MFI) of pSmad2/3 staining in T cells from the nondraining and pancreatic lymph nodes of OT-II Rag1-deficient (white squares) and OT-II RIP-mOva Rag1-deficient (black circles) mice. Lines connect the pSmad2/3 MFI values from the nondraining and pancreatic lymph nodes of the same mouse. Statistical significance was calculated using the paired Student’s t test. (E) The graph shows the incidence of diabetes versus age for Tgfbr2−/− OT-II RIP-mOva Rag1-deficient (circles, n = 16), Foxp3sf OT-II RIP-mOva Rag1-deficient (squares, n = 11), and control OT-II RIP-mOva Rag1-deficient (triangles, n = 8) mice. (F) RIP-mOva Rag1-deficient mice were adoptively transferred with ER-Cre Tgfbr2fl/fl OT-II T cells (circles, n = 12), Foxp3sf OT-II T cells (squares, n = 7), or control OT-II T cells (triangles, n = 6) and were monitored for diabetes development. The graph shows the incidence of diabetes versus time after T cell transfer.
Fig. S1.
Treg cell phenotype and conditional ablation of TβRII in T cells. (A) Flow cytometric analysis of Foxp3 and CD25 expression among total (Left), CD69− (Center), and CD69+ (Right) CD4+TCRβ+ cells from the lymph nodes of control OT-II RIP-mOva Rag1-deficient mice. Numbers indicate the frequency of cells in the respective gates. (B) Flow cytometric analysis of Foxp3 and CD25 expression among CD4+TCRβ+ cells from the lymph nodes of control (Left) and Tgfbr2−/− (Center) OT-II RIP-mOva Rag1-deficient mice. (Right) The dot plot shows FMO control staining. The graph shows the frequency of FoxP3+CD25+ cells among total CD4+TCRβ+ cells from control and Tgfbr2−/− OT-II RIP-mOva Rag1-deficient mice. Circles represent individual mice. Error bars indicate mean ± SEM. (C) Representative flow cytometric analysis of TβRII expression on ER-Cre Tgfbr2fl/fl OT-II T cells transferred into RIP-mOva Rag1-deficient recipients that were untreated (solid line) or tamoxifen treated (dashed line) to induce Tgfbr2 deletion. The solid gray histogram represents the FMO control.
TGF-β Promotes T Cell Tolerance via Foxp3-Independent Mechanisms.
Our previous studies showed that T cell-specific deletion of TβRII, which is encoded by the Tgfbr2 gene, resulted in enhanced negative selection of OT-II T cells in double-transgenic OT-II RIP-mOva mice, whereas positive selection of OT-II T cells was not affected in the absence of mOva expression (9). Importantly, despite the exaggerated clonal deletion of OT-II T cells, loss of TGF-β signaling led to antigen-dependent T cell activation and the development of aggressive autoimmune diabetes (9). However, TGF-β signaling also promotes tTreg and pTreg cell development. Thus, the extent to which diabetes development in Tgfbr2−/− OT-II RIP-mOva mice is through TGF-β regulation of Treg cells versus direct regulation of autoreactive T cells was unclear.
To address this question in the model of diabetes, we crossed the Foxp3 scurfy (Foxp3sf) mice to the OT-II RIP-mOva background. We monitored control OT-II RIP-mOva mice, Foxp3sf OT-II RIP-mOva mice, and OT-II RIP-mOva mice with T cell-specific deletion of Tgfbr2 (Tgfbr2−/− OT-II RIP-mOva mice) for diabetes development. Whereas T cell-specific TβRII-deficient mice developed diabetes by 2 mo of age, Foxp3 deficiency resulted in much delayed disease with less than 50% disease penetrance at 5 mo of age (Fig. 2E). Consistent with our previous study (9), control OT-II RIP-mOva mice did not develop diabetes (Fig. 2E). Given the role of TGF-β signaling in the survival of tTreg cell precursors, we analyzed Tgfbr2−/− OT-II RIP-mOva mice for the presence of Foxp3+ Treg cells and found that Tgfbr2−/− OT-II RIP-mOva mice did contain Treg cells (Fig. S1B), indicating that the accelerated diabetes development in Tgfbr2−/− OT-II RIP-mOva mice is not attributable to an absence of Treg cells.
The distinct kinetics and penetrance of diabetes development in Tgfbr2−/− and Foxp3sf OT-II RIP-mOva mice indicate that, under certain circumstances, TGF-β promotes T cell tolerance primarily via Foxp3-independent mechanisms. However, a complication of the Tgfbr2−/− OT-II RIP-mOva system is the effect of TβRII deficiency on T cell negative selection (9). To circumvent the complication of altered thymic selection on T cell tolerance, we developed a transfer model of diabetes that allows the inducible termination of TGF-β signaling in mature T cells. OT-II T cells that coexpress floxed Tgfbr2 and the estrogen-receptor Cre (ERT2Cre Tgfbr2fl/fl OT-II) were adoptively transferred into RIP-mOva Rag1-deficient mice, which were treated with tamoxifen to induce Tgfbr2 deletion in T cells (Fig. 2F and Fig. S1C). As observed in the constitutive T cell-specific TβRII-deficient mice (Fig. 2E), acute Tgfbr2 deletion in T cells led to rampant diabetes (Fig. 2F). Importantly, the transfer of control or Foxp3sf OT-II T cells to RIP-mOva Rag1-deficient recipients did not cause disease in the time frame examined (Fig. 2F). These findings further reveal an important role for a Foxp3-independent mechanism of TGF-β control of peripheral T cell tolerance.
Enhanced Priming of Pancreas Draining Lymph Node T Cells in the Absence of TβRII.
To gain mechanistic insight into the Foxp3-independent functions of TGF-β in the control of T cell tolerance, we analyzed the phenotype of T cells in the pancreas-draining lymph nodes of control, Tgfbr2−/−, and Foxp3sf OT-II RIP-mOva mice. The majority of T cells from the draining lymph nodes of diabetic Tgfbr2−/− OT-II RIP-mOva mice exhibited an activated phenotype defined by high expression of CD44 and low expression of CD62L (Fig. 3 A and B). Diabetic Foxp3sf OT-II RIP-mOva mice also possessed a significantly higher frequency of activated T cells in the pancreas-draining lymph nodes compared with age-matched, nondiabetic control OT-II RIP-mOva mice (Fig. 3 A and B). However, the frequency of CD62LloCD44hi effector T cells in diabetic Foxp3sf OT-II RIP-mOva mice was significantly lower than that observed in diabetic Tgfbr2−/− OT-II RIP-mOva mice, indicating that the absence of TGF-β signaling has a greater impact on the regulation of T cell priming in the draining lymph node than does the absence of Treg cells. Consistent with the differences in CD62L and CD44 expression on T cells from the different strains of mice, T cells from the pancreas-draining lymph nodes of Tgfbr2−/− OT-II RIP-mOva mice produced the greatest amounts of IFN-γ (Fig. 3 C and D), a cytokine that is known to play an essential role in diabetes development (14, 28–30).
Fig. 3.
Enhanced T cell priming in the absence of TβRII in T cells. (A) Flow cytometric analysis of CD62L and CD44 expression on T cells from the pancreatic lymph nodes of nondiabetic control OT-II RIP-mOva Rag1-deficient, diabetic Tgfbr2−/− OT-II RIP-mOva Rag1-deficient, and diabetic Foxp3sf OT-II RIP-mOva Rag1-deficient mice. Numbers indicate the percentage of cells in the respective gates. (B) The graph shows the percentage of activated CD62L−CD44+ T cells in the pancreatic lymph nodes of nondiabetic control OT-II RIP-mOva Rag1-deficient, diabetic Tgfbr2−/− OT-II RIP-mOva Rag1-deficient, and diabetic Foxp3sf OT-II RIP-mOva Rag1-deficient mice. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test. *P < 0.05, ****P ≤ 0.0001. (C) Flow cytometric analysis of IFN-γ production by pancreatic lymph nodes of nondiabetic control OT-II RIP-mOva Rag1-deficient, diabetic Tgfbr2−/− OT-II RIP-mOva Rag1-deficient, and diabetic Foxp3sf OT-II RIP-mOva Rag1-deficient mice. Numbers indicate the percentage of cells in the respective gates. (D) The graph shows the percentage of IFN-γ–producing T cells in the pancreatic lymph nodes of nondiabetic control OT-II RIP-mOva Rag1-deficient, diabetic Tgfbr2−/− OT-II RIP-mOva Rag1-deficient, and diabetic Foxp3sf OT-II RIP-mOva Rag1-deficient mice. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test. **P ≤ 0.01.
Enhanced Effector Differentiation of Pancreas-Infiltrating T Cells in the Absence of TβRII.
In RIP-mOva mice, the highest concentration of the mOva antigen is found in the pancreas and the kidneys (31). Thus, in contrast to the discrepancy in T cell activation observed between T cells in the pancreas-draining lymph nodes of diabetic Tgfbr2−/− and Foxp3sf OT-II RIP-mOva mice (Fig. 3), where the T cells likely experience lower concentrations of the mOva antigen, pancreas-infiltrating T cells in both model systems might exhibit more similar differentiation phenotypes upon exposure to high amounts of their cognate antigen present in the target organ tissue. To determine whether the enhanced effector T cell phenotype in diabetic Tgfbr2−/− OT-II RIP-mOva mice was consistent in the draining lymph nodes and in the target tissue, we analyzed the phenotype of pancreas-infiltrating T cells in diabetic Tgfbr2−/− and Foxp3sf OT-II RIP-mOva mice. Diabetic Tgfbr2−/− and Foxp3sf OT-II RIP-mOva mice contained comparable frequencies of pancreas-infiltrating T cells, which were significantly higher than observed in nondiabetic OT-II RIP-mOva mice, which possessed very few pancreas-infiltrating T cells (Fig. 4A). Infiltration of the pancreas by OT-II T cells was also observed in the transfer model of diabetes. At the time points examined, a higher frequency of TβRII-deficient T cells was found in the pancreas compared with Foxp3sf T cells (Fig. S2A), which is consistent with the distinct disease manifestation profiles observed in the transfer setting (Fig. 2F).
Fig. 4.
Acute diabetes triggered by TβRII-deficient t cells is associated with an enhanced Th1 effector phenotype and the accumulation of pancreas-infiltrating inflammatory monocytes. (A) The graph shows the percentage of pancreas-infiltrating T cells in nondiabetic control OT-II RIP-mOva Rag1-deficient, diabetic Tgfbr2−/− OT-II RIP-mOva Rag1-deficient, and diabetic Foxp3sf OT-II RIP-mOva Rag1-deficient mice. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test. ***P ≤ 0.001; ns, not significant. (B) Flow cytometric analysis of IFN-γ production by pancreas-infiltrating T cells in diabetic Tgfbr2−/− OT-II RIP-mOva Rag1-deficient and Foxp3sf OT-II RIP-mOva Rag1-deficient mice. Numbers indicate the percentage of cells in the respective gates. (C) The graph shows the percentage of IFN-γ–producing T cells in the pancreas of diabetic Tgfbr2−/− OT-II RIP-mOva Rag1-deficient and Foxp3sf OT-II RIP-mOva Rag1-deficient mice. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test. *P < 0.05. (D) Flow cytometric analysis of Ly6C expression by pancreas-infiltrating T cells from diabetic Tgfbr2−/− OT-II RIP-mOva Rag1-deficient and Foxp3sf OT-II RIP-mOva Rag1-deficient mice. (E) The graph shows the percentage of Ly6C-expressing T cells in the pancreas of diabetic Tgfbr2−/− OT-II RIP-mOva Rag1-deficient and Foxp3sf OT-II RIP-mOva Rag1-deficient mice. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test. **P ≤ 0.01. (F) Flow cytometric analysis of Ly6C and Ly6G expression among CD45+CD11b+ cells in the pancreas of nondiabetic control OT-II RIP-mOva Rag1-deficient, diabetic Tgfbr2−/− OT-II RIP-mOva Rag1-deficient, and diabetic Foxp3sf OT-II RIP-mOva Rag1-deficient mice. Numbers indicate the percentage of cells in the respective gates. (G) The graph shows the percentage of Ly6C+ monocytes among CD11b+ cells in the pancreas of nondiabetic control OT-II RIP-mOva Rag1-deficient, diabetic Tgfbr2−/− OT-II RIP-mOva Rag1-deficient, and diabetic Foxp3sf OT-II RIP-mOva Rag1-deficient mice. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test. *P < 0.05. (H) Immunofluorescent staining of T cells and macrophages in the pancreata of Tgfbr2−/− OT-II RIP-mOva, Tgfbr2+/+ OT-II RIP-mOva, and C57BL/6 mice.
Fig. S2.
T cell and monocyte infiltration in the transfer model of diabetes. (A) The graph shows the frequency of TCRβ+ cells among total CD45+ cells in the pancreas of RIP-mOva Rag1-deficient mice that received ER-Cre Tgfbr2fl/fl OT-II cells + tamoxifen treatment to induce Tgfbr2 deletion (Tgfbr2−/− OT-II) or Foxp3sf OT-II cells. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test. **P ≤ 0.01. (B) Flow cytometric analysis of Ly6C expression on pancreas-infiltrating Tgfbr2−/− (Left) or Foxp3sf (Right) OT-II cells. Numbers indicate the percentage of cells in the respective gates. (C) The graph shows the frequency of Ly6C-expressing cells among total TCRβ+ cells in the pancreas of RIP-mOva Rag1-deficient mice that received Tgfbr2−/− or Foxp3sf OT-II cells. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test. **P ≤ 0.01. (D and E) Only RIP-mOva Rag1-deficient mice that received ER-Cre Tgfbr2fl/fl OT-II T cells were diabetic. Control and Foxp3sf OT-II T cell-recipient mice were nondiabetic at analysis. (D) Flow cytometric analysis of Ly6C and Ly6G expression among CD45+CD11b+ cells in the pancreas of RIP-mOva Rag1-deficient mice that received control, ER-Cre Tgfbr2fl/fl, or Foxp3sf OT-II T cells. Numbers indicate the percentage of cells in the respective gates. (E) The graph shows the percentage of Ly6C+ monocytes among CD11b+ cells in the pancreas of RIP-mOva Rag1-deficient mice that received control, ER-Cre Tgfbr2fl/fl, or Foxp3sf OT-II T cells. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test. *P < 0.05.
Pancreas-infiltrating T cells from both diabetic Tgfbr2−/− and Foxp3sf OT-II RIP-mOva mice produced high amounts of IFN-γ, although, as observed in the draining lymph nodes, a significantly higher frequency of T cells from Tgfbr2−/− OT-II RIP-mOva mice produced the cytokine than in Foxp3sf OT-II RIP-mOva mice (Fig. 4 B and C). The enhanced production of IFN-γ by T cells from both the draining lymph node and pancreas of Tgfbr2−/− OT-II RIP-mOva mice relative to Foxp3sf OT-II RIP-mOva mice suggested that TβRII-deficient T cells possessed a more differentiated effector phenotype than their TβRII-sufficient counterparts from diabetic Foxp3sf OT-II RIP-mOva mice. We further examined this by analyzing the expression of Ly6C, a marker that has been shown in viral infection models (32) to define more terminally differentiated, Th1 phenotype CD4+ T cells among pancreas-infiltrating T cells in diabetic Tgfbr2−/− and Foxp3sf OT-II RIP-mOva mice. Indeed, consistent with the phenotype of enhanced IFN-γ production, pancreas-infiltrating T cells from Tgfbr2−/− OT-II RIP-mOva mice expressed significantly higher amounts of the marker Ly6C than did T cells from Foxp3sf OT-II RIP-mOva mice (Fig. 4 D and E). The distinct patterns of Ly6C expression by TβRII-deficient versus Foxp3sf OT-II T cells was also observed in the transfer model of diabetes, in which an increased frequency of TβRII-deficient T cells expressed higher amounts of Ly6C than did Foxp3sf T cells (Fig. S2 B and C).
Acute Diabetes Triggered by TβRII-Deficient T Cells Is Associated with an Accumulation of Pancreas-Infiltrating Inflammatory Monocytes.
To gain mechanistic insights into the Foxp3-independent functions of TGF-β in control of T cell tolerance, we profiled the pancreatic immune cell infiltrates in diabetic Tgfbr2−/− OT-II RIP-mOva and diabetic Foxp3sf OT-II RIP-mOva mice. Compared with Tgfbr2+/+ OT-II RIP-mOva mice, a substantially increased population of CD11b+Ly6C+ inflammatory monocytes was present in TβRII-deficient but not in Treg cell-deficient mice (Fig. 4 F and G). This increase in CD11b+Ly6C+ inflammatory monocytes was recapitulated in the transfer model of diabetes in mice that received TβRII-deficient OT-II T cells but not Foxp3sf or control OT-II T cells (Fig. S2 D and E). In addition, immunofluorescent staining of the pancreata revealed T cell and macrophage infiltration in Tgfbr2−/− OT-II RIP-mOva mice but not in control Tgfbr2+/+ OT-II RIP-mOva or C57BL/6 mice (Fig. 4H). Intriguingly, high numbers of monocytes/macrophages are present in the pancreatic infiltrates of patients suffering from an aggressive subtype of type I diabetes termed “fulminant diabetes,” in which hyperglycemia occurs over the course of a few days (33, 34). Indeed the course of diabetes development is far more acute in Tgfbr2−/− than in Foxp3sf OT-II RIP-mOva mice (Fig. 2E), and the transfer of TβRII, but not Foxp3sf, OT-II T cells induces disease in a short time frame (Fig. 2F). These observations indicate that TGF-β signaling in T cells is specifically required to suppress inflammatory monocyte responses, the lack of which may account for the development of acute diabetes in mice and humans.
Pathogenic Role of Inflammatory Monocytes in Diabetes Triggered by TβRII-Deficient T Cells.
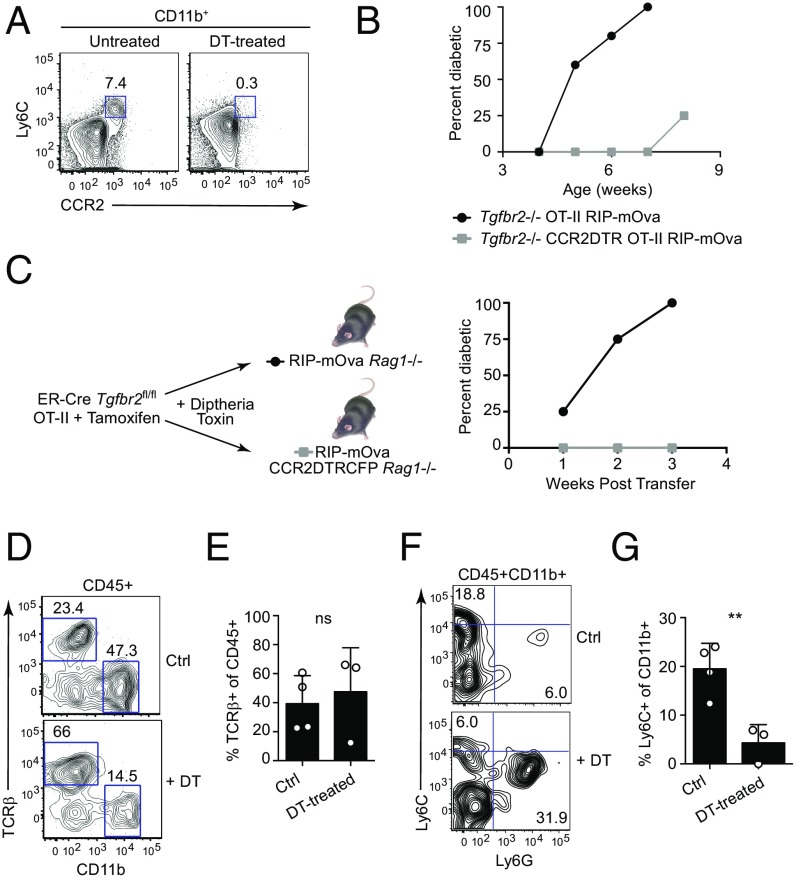
Whereas inflammatory monocytes are well-known innate immune cells mobilized during infection for host defense responses (35), recent studies have revealed that inflammatory monocytes are also essential for the induction of tissue damage in immunization models of autoimmune diseases such as experimental autoimmune encephalitis (EAE) (36, 37). Although T cells play an essential role in the immunization models of autoimmunity, the potential for directly activating monocytes by adjuvant-mediated triggering of pattern-recognition receptors remains. We wished to determine whether in a non–adjuvant-based model of sterile autoimmunity, inflammatory monocytes also played a pathogenic role in diabetes development. To address this question, we bred CCR2-DTR mice (38) to the Tgfbr2−/− OT-II RIP-mOva background to generate Tgfbr2−/− CCR2-DTR OT-II RIP-mOva mice, which allows for diphtheria toxin (DT) treatment and depletion of CCR2-expressing Ly6C monocytes. Compared with untreated mice, DT-treated CCR2-DTR OT-II RIP-mOva mice exhibited a marked depletion of Ly6C monocytes (Fig. 5A). To determine whether Ly6C monocytes played a pathogenic role in diabetes development, Tgfbr2−/− CCR2-DTR OT-II RIP-mOva mice were subjected to DT treatments starting at weaning (3 wk of age) and were monitored for blood glucose levels to assess diabetes development. As a control for the DT treatment, Tgfbr2−/− OT-II RIP-mOva mice that did not express the CCR2-DTR transgene were subjected to the same DT-treatment regimen. Strikingly, depletion of CCR2-expressing Ly6C monocytes dramatically delayed the kinetics of onset and penetrance of diabetes development (Fig. 5B).
Fig. 5.
Pathogenic role of inflammatory monocytes in diabetes triggered by TβRII-deficient T cells. (A) Flow cytometric analysis of Ly6C+CCR2+ cells in the spleens of untreated and DT-treated Tgfbr2−/− CCR2DTR OT-II RIP-mOva Rag1-deficient mice. Plots are gated on CD11b+ cells, and numbers indicate the percentage of cells in the respective gates. (B) The graph shows the incidence of diabetes with age in DT-treated Tgfbr2−/− OT-II RIP-mOva Rag1-deficient mice with (squares, n = 4) or without (circles, n = 4) the CCR2DTR transgene. (C) RIP-mOva Rag1-deficient mice with (squares, n = 3) or without (circle, n = 4) the CCR2DTR transgene were treated with DT following the adoptive transfer of ER-Cre Tgfbr2fl/fl OT-II T cells and subsequent tamoxifen treatment to induce Tgfbr2 deletion. The graph shows the incidence of diabetes versus time after T cell transfer. (D–G) Control RIP-mOva and RIP-mOva CCR2DTRCFP Rag1-deficient mice received ER-Cre Tgfbr2fl/fl T cells and were treated with tamoxifen to induce deletion of the Tgfbr2 allele. Both sets of recipient mice were treated with DT to control for nonspecific effects of DT. For simplicity, DT-treated RIP-mOva Rag1-deficient mice (no depletion of CCR2-expressing cells) are referred to as “Ctrl,” and DT-treated RIP-mOva CCR2DTRCFP Rag1-deficient mice in which CCR2-expressing cells are depleted are designated as “DT-treated.” (D) Flow cytometric analysis of TCRβ+ and CD11b+ cells among total CD45+ cells in the pancreas of DT-treated control RIP-mOva (Ctrl) and RIP-mOva CCR2DTR (+DT) Rag1-deficient mice that received Tgfbr2−/− OT-II cells. Numbers indicate the percentage of cells in the respective gates. (E) The graph shows the frequency of TCRβ+ cells among CD45+ cells in the pancreas of DT-treated control RIP-mOva (Ctrl) and RIP-mOva CCR2DTR (+DT) Rag1-deficient mice that received Tgfbr2−/− OT-II cells. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test; ns, not significant. (F) Flow cytometric analysis of Ly6C and Ly6G expression among CD45+CD11b+ cells in the pancreas of DT-treated control RIP-mOva (Ctrl) and RIP-mOva CCR2DTR (+DT) Rag1-deficient mice that received Tgfbr2−/− OT-II cells. Numbers indicate the percentage of cells in the respective gates. (G) The graph shows the frequency of Ly6C+ cells among CD45+CD11b+ cells in the pancreas of DT-treated control RIP-mOva (Ctrl) and RIP-mOva CCR2DTR (DT-treated) Rag1-deficient mice that received Tgfbr2−/− OT-II cells. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test. **P ≤ 0.01.
It has been reported that effector CD4+ T cells may also express the chemokine receptor CCR2 (39). To address the caveat that diabetogenic T cells may also be depleted by DT treatment, we generated RIP-mOva CCR2-DTR Rag1-deficient mice to be used in the transfer model of diabetes. In this model, only CCR2-expressing cells in the recipient mice would be ablated by DT treatment, whereas the transferred diabetogenic T cells would remain untouched, since the T cells do not express the CCR2-DTR transgene. As observed in the nontransfer model of diabetes (Fig. 5B), depletion of Ly6C monocytes by DT treatment inhibited disease development in the transfer model of diabetes (Fig. 5C). Notably, whereas the frequency of T cell infiltrates into the pancreas was comparable in both sets of recipients (Fig. 5 D and E), the frequency of Ly6C cells in the pancreas of DT-treated RIP-mOva CCR2-DTR Rag1-deficient mice was decreased compared with DT-treated control mice (Fig. 5 F and G). Collectively, these data demonstrate that the inflammatory monocyte response plays a pathogenic role in the development of diabetes that is triggered by loss of TGF-β control of peripheral T cell tolerance.
Pancreas-Infiltrating TβRII-Deficient T Cells Produce Increased Amounts of GM-CSF, Which Promotes Diabetes Development.
Given the preferential accumulation of inflammatory monocytes observed in the TβRII-deficient model of diabetes relative to that observed in diabetic Foxp3sf OT-II RIP-mOva mice, we wished to better understand the nature of the autoimmune response that leads to such a difference. GM-CSF, encoded by the gene Csf2, is a proinflammatory cytokine with crucial roles in myeloid cell homeostasis and monocyte activation (40–43). The cytokine, which can be produced by a variety of cell types, including T cells (44), has been demonstrated to contribute to the disease pathogenesis in an adjuvant-based model of EAE (45, 46).
In both the TβRII-deficient and Foxp3sf OT-II RIP-mOva models of diabetes, dysregulation of the T cell response is the critical trigger for disease. We wished to address whether the GM-CSF pathway contributed to the distinct diabetes profiles in TβRII-deficient and Foxp3sf OT-II RIP-mOva mice and analyzed GM-CSF production by T cells in the pancreatic lymph nodes and pancreas of diabetic TβRII-deficient and Foxp3sf OT-II RIP-mOva mice. In both models of diabetes, a greater frequency of T cells in the pancreas than in the pancreatic lymph nodes produced GM-CSF, and the GM-CSF–producing T cells coexpressed high levels of IFN-γ (Fig. 6A). As observed for IFN-γ (Fig. 3), a greater frequency of T cells from the pancreatic lymph nodes of diabetic TβRII-deficient OT-II RIP-mOva mice produced IFN-γ and GM-CSF than did T cells from the pancreatic lymph nodes of diabetic Foxp3sf OT-II RIP-mOva mice, although the difference was not statistically significant (Fig. 6B). In the pancreas, however, a significantly greater frequency of T cells from diabetic TβRII-deficient OT-II RIP-mOva mice coproduced IFN-γ and GM-CSF than did T cells from diabetic Foxp3sf OT-II RIP-mOva mice (Fig. 6B). In addition, in the T cell transfer model of diabetes, only TβRII-deficient OT-II T cells, but not Foxp3sf OT-II T cells, gave rise to IFN-γ and GM-CSF coproducers (Fig. S3), suggesting that TGF-β regulates GM-CSF production by a Foxp3-independent mechanism.
Fig. 6.
Pancreas-infiltrating TβRII-deficient T cells produce increased amounts of GM-CSF, which promotes diabetes development. (A) Flow cytometric analysis of IFN-γ and GM-CSF expression by T cells in the pancreatic lymph nodes (Upper) and pancreas (Lower) of diabetic Tgfbr2−/− and Foxp3sf OT-II RIP-mOva Rag1-deficient mice. Numbers indicate the percentage of cells in the gates. (B) The graph shows the percentage of IFN-γ+GM-CSF+ T cells in the pancreatic lymph nodes (Left) and pancreas (Right) of diabetic Tgfbr2−/− and Foxp3sf OT-II RIP-mOva Rag1-deficient mice. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test. **P ≤ 0.01; ns, not significant. (C) The graph shows diabetes incidence versus age among Tgfbr2−/− OT-II RIP-mOva Rag1-deficient mice (circles, n = 16), Tgfbr2−/− Csf2r/− OT-II RIP-mOva Rag1-deficient mice (squares, n = 7), and control OT-II RIP-mOva Rag1-deficient mice (triangles, n = 8). (D) The graph shows the frequency of TCRβ+ cells among CD45+ cells in the pancreas of control (Tgfbr2+/+), Tgfbr2−/−, and Tgfbr2−/−Csf2r−/− OT-II RIP-mOva Rag1-deficient mice. (E) The graph shows the frequency of Ly6C-expressing cells among total TCRβ+ cells in the pancreas of control (Tgfbr2+/+), Tgfbr2−/−, and Tgfbr2−/−Csf2r−/− OT-II RIP-mOva Rag1-deficient mice. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test; ns, not significant. (F) The graph shows the percentage of Ly6C+ cells among CD45+CD11b+ cells in the pancreas of control (Tgfbr2+/+), Tgfbr2−/−, and Tgfbr2−/− Csf2r−/− OT-II RIP-mOva Rag1-deficient mice. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test. *P < 0.05.
Fig. S3.
GM-CSF expression is enhanced in TβRII-deficient T cells, but its signaling is nonessential for T cell infiltration or effector differentiation. RIP-mOva Rag1-deficient mice received ER-Cre Tgfbr2fl/fl OT-II cells + tamoxifen treatment to induce Tgfbr2 deletion (Tgfbr2−/− OT-II) or Foxp3sf OT-II cells. Mice that received Tgfbr2−/− OT-II, but not Foxp3sf OT-II, cells were diabetic at the time of analysis. (A) Flow cytometric analysis of IFN-γ and GM-CSF expression among pancreas-infiltrating T cells in RIP-mOva Rag1-deficient mice that received Tgfbr2−/− or Foxp3sf OT-II cells. Numbers indicate the percentage of cells in the respective gates. (B) The graph shows the frequency of IFN-γ+GM-CSF+ cells among TCRβ+ cells in the pancreas of RIP-mOva Rag1-deficient mice that received Tgfbr2−/− or Foxp3sf OT-II cells. Circles represent individual mice. Error bars indicate mean ± SEM. Statistical significance was calculated using a two-tailed unpaired Student’s t test. **P ≤ 0.01.
To determine whether GM-CSF plays a pathogenic role in diabetes development in TβRII-deficient OT-II RIP-mOva mice, we bred Tgfbr2−/− OT-II RIP-mOva mice to Csf2rb−/− mice, which lack the β chain of the GM-CSF receptor, to generate Csf2rb−/− Tgfbr2−/− OT-II RIP-mOva mice. Compared with TβRII-deficient OT-II RIP-mOva mice with an intact GM-CSF signaling pathway, TβRII-deficient OT-II RIP-mOva mice lacking the β chain of the GM-CSF receptor exhibited delayed kinetics of diabetes onset and reduced penetrance of disease (Fig. 6C), demonstrating that GM-CSF signaling contributes to the pathogenesis of diabetes. Despite the distinct manifestations of diabetes development, the frequency of pancreas-infiltrating T cells was not significantly reduced in Csf2rb−/− compared with control Tgfbr2−/− OT-II RIP-mOva mice (Fig. 6D). Furthermore, among the pancreas-infiltrating T cells, a comparable frequency expressed Ly6C (Fig. 6E), indicating similar levels of Th1 differentiation. In contrast, Csf2rb−/− Tgfbr2−/− OT-II RIP-mOva mice exhibited a significant reduction in the inflammatory monocyte population compared with Tgfbr2−/− OT-II RIP-mOva mice with an intact GM-CSF signaling pathway (Fig. 6F).
Discussion
Despite our understanding of the critical roles that TGF-β and Foxp3+ Treg cells play in the maintenance of immune tolerance, the inherent relationship between these two pathways of immune suppression poses the fundamental question of whether they are part of the same regulatory module or have distinct mechanisms of T cell regulation. To address this, we used the transgenic OT-II RIP-mOva mouse model to compare the relative effects of TGF-β versus the transcription factor Foxp3, considered the master regulator of the Treg cell lineage, in the regulation of peripheral T cell tolerance. We observed that the absence of T cell-specific TGF-β signaling led to the development of more rapid, fulminant diabetes in OT-II RIP-mOva mice than did Foxp3 deficiency. The more aggressive disease that developed in the absence of TGF-β signaling was associated with an enhanced T cell effector phenotype and the recruitment of a pathogenic myeloid cell population that was in part dependent on GM-CSF signaling. These observations reveal that a Foxp3-independent mechanism of TGF-β signaling can play a dominant role in the regulation of peripheral T cell tolerance.
Although central tolerance is a critical aspect in the establishment and maintenance of immune tolerance, the process of clonal deletion is incomplete. Indeed, self-reactive T cells exist in the peripheral T cell repertoire of healthy individuals (47), demonstrating the necessity for additional tolerance mechanisms to keep such autoreactive T cells in check. The maintenance of peripheral T cell tolerance involves multiple pathways, ranging from immunological ignorance of self-antigens to more active tolerance mechanisms, such as Treg cells and inhibitory signaling pathways. T cell ignorance of self-antigens may occur due to antigen sequestration in tissues or because the self-antigen is presented at concentrations that are too low to result in productive T cell activation. However, the metrics used to define T cell activation may result in an overestimation of the prevalence of immunological ignorance as a mechanism of self-tolerance. Indeed in the RIP-mOva model system, the lack of T cell proliferation (48) and the maintenance of a CD62LhiCD44lo naive T cell phenotype (Fig. 1 and ref. 9) could be interpreted as ovalbumin-specific T cells being ignorant of their cognate antigen. However, our findings show that, despite maintaining a CD62LhiCD44lo naive phenotype, OT-II T cells in the pancreatic lymph nodes of OT-II RIP-mOva mice (but not in single-transgenic mice that do not express RIP-mOva as a self-antigen) up-regulate the early activation marker CD69, demonstrating that the T cells are not ignorant of their cognate antigen and thus indicating that mechanisms of active suppression keep these autoreactive T cells in check. Indeed, compared with single-transgenic OT-II mice, double-transgenic OT-II RIP-mOva mice contain Foxp3+ Treg cells and exhibit enhanced TGF-β signaling in the pancreatic lymph nodes. Our comparison of the effects of T cell-specific loss of TGF-β signaling versus Foxp3 deficiency on the development of autoimmunity demonstrates that in the OT-II RIP-mOva system TGF-β signaling is the dominant pathway controlling T cell tolerance.
The condition of lymphopenia has been associated with multiple autoimmune diseases in human patients, such as rheumatoid arthritis, systemic lupus erythematosus, and Sjogren’s syndrome (49). However, lymphopenia alone does not trigger the development of autoimmune disease, as the activity of immune suppressive pathways can control autoreactive T cell responses, and a second “hit” in addition to lymphopenia is thought to be required to trigger autoimmunity. Indeed, our findings demonstrate that OT-II RIP-mOva Rag1-deficient mice (which are by definition lymphopenic) do not develop diabetes until either the TGF-β or Foxp3+ regulatory modules are perturbed. The TGF-β pathway, in particular, has been shown to be critical for the inhibition of T cell responses in lymphopenic settings (50, 51). Importantly, a notable difference between Tgfbr2−/− and Foxp3sf OT-II RIP-mOva mice is the enhanced lymphopenia observed in OT-II RIP-mOva mice with T cell-specific deficiency of TGF-β signaling. We have previously reported that the TGF-β pathway plays an important role in promoting the survival of low-affinity T cells and that the absence of T cell-specific TGF-β signaling results in a peripheral T cell homeostasis defect in Tgfbr2−/− OT-II RIP-mOva mice (52) that is not present in Foxp3sf OT-II RIP-mOva mice with an intact TGF-β signaling pathway. This discrepancy in the degree of lymphopenia may contribute to the enhanced effector differentiation observed in Tgfbr2−/− OT-II RIP-mOva mice. However, the results from our T cell transfer model of diabetes further demonstrate that, in comparable lymphopenic environments, direct regulation of T cells by TGF-β signaling is a critical factor in preventing autoimmunity, as wild-type OT-II T cells and Foxp3sf OT-II T cells (which have intact TGF-β signaling but cannot undergo conversion into Treg cells or up-regulate transient expression of Foxp3) did not induce disease in the examined time frame.
Our findings demonstrate an important role for Foxp3-independent TGF-β regulation of peripheral T cell tolerance in OT-II RIP-mOva mice. Ultimately, however, Foxp3 deficiency also leads to diabetes development in the OT-II RIP-mOva system, which raises the question of how these distinct immune suppression pathways control autoreactive T cell responses. A key regulatory checkpoint is likely during T cell priming in the lymph node, when T cells encounter their cognate antigen and undergo activation before trafficking to the appropriate nonlymphoid tissues. Notably, it has been demonstrated that removal of the pancreas-draining lymph nodes in young, prediabetic NOD mice is sufficient to prevent diabetes development, demonstrating the critical role of lymph node T cell priming in the establishment of an autoimmune response (53). Both Treg cells (54, 55) and TGF-β signaling (56) have been implicated in the regulation of T cell activation in tissue-draining lymph nodes. Indeed, our findings demonstrate that both the absence of T cell-specific TGF-β signaling and Foxp3 deficiency result in increased T cell activation in the pancreas-draining lymph nodes in comparison with control OT-II RIP-mOva mice. However, T cell activation is far more pronounced in the absence of T cell-specific TGF-β signaling than in the absence of Treg cells. Whereas T cells in the pancreatic lymph nodes of Foxp3sf OT-II RIP-mOva mice produce very little cytokine, pancreatic lymph node TβRII-deficient T cells already produce copious amounts of the Th1 cytokine IFN-γ, which is known to promote diabetes development (14, 28–30). The limited (although significantly increased relative to control OT-II RIP-mOva mice) activation of T cells in the draining lymph nodes of Foxp3sf OT-II RIP-mOva mice is consistent with observations in another transgenic model of diabetes, in which Treg cells had little effect on T cell activation in the draining lymph node but were nonetheless important for the prevention of disease development (57). However, even in the pancreas, TβRII-deficient T cells produce more IFN-γ than do T cells from Foxp3sf OT-II RIP-mOva mice, indicating a more differentiated Th1 phenotype. In addition, in comparison with pancreatic T cells from diabetic Foxp3sf OT-II RIP-mOva mice, pancreatic TβRII-deficient T cells express much higher levels of the surface marker Ly6C, the expression of which has been shown to define more terminally differentiated Th1 cells in viral infection models (32). Collectively, these observations indicate that direct regulation by TGF-β is important for restraining the degree of effector differentiation of autoreactive T cells. Whether the differences in effector T cell differentiation in the pancreas and the distinct manifestations of diabetes observed in Tgfbr2−/− versus Foxp3sf OT-II RIP-mOva mice are attributable to differences due to the absence of TGF-β signaling during the initial priming phase or whether TGF-β continues to regulate effector T cell differentiation in tissues remains to be determined.
We find that the acute, fulminant diabetes that develops in OT-II RIP-mOva mice with T cell-specific loss of TGF-β signaling engages a heightened Th1 effector program and a pathogenic inflammatory monocyte response. Indeed, there is evidence that the innate immune response, particularly macrophages, also plays a role in autoimmune diabetes (58). Intriguingly, high numbers of monocytes/macrophages are present in the pancreatic infiltrates of patients suffering from fulminant diabetes (33, 34), which is noted for its aggressive and rapid disease progression. The pathogenic monocyte response in Tgfbr2−/− OT-II RIP-mOva mice is in part dependent on GM-CSF, a cytokine with established roles in myeloid cell homeostasis and monocyte activation (40–43). GM-CSF also promotes the development of autoimmune diseases such as arthritis and EAE (45, 46, 59, 60), both of which are associated with pathogenic myeloid cell responses (36, 37, 61). Indeed, GM-CSF signaling has been demonstrated to trigger an inflammatory signature in monocytes that contributes to their pathogenic function in autoimmunity (62). However, the direct activation of innate immune cells through pattern-recognition receptors is another plausible pathogenic mechanism in the adjuvant-based models (45, 46, 59, 62). In contrast, our findings demonstrate that in a spontaneous, non–adjuvant-based model of autoimmunity a dysregulated T cell response in a setting of sterile inflammation is sufficient to induce a pathogenic myeloid cell population that critically contributes to disease development. We observed a significantly higher frequency of GM-CSF–producing T cells in the pancreas of diabetic Tgfbr2−/− than in diabetic Foxp3sf OT-II RIP-mOva mice. The TβRII-deficient T cells that produced GM-CSF also coexpressed high levels of IFN-γ, and these T cells are reminiscent of populations of IFN-γ+GM-CSF+ T cells found to be enriched in the cerebrospinal fluid of multiple sclerosis patients and the synovial fluid of juvenile idiopathic arthritis patients (63–65). That this population of IFN-γ+GM-CSF+ coproducers is preferentially increased in pancreas-infiltrating T cells from diabetic Tgfbr2−/− OT-II RIP-mOva mice suggests that direct TGF-β control of T cells is particularly important in the regulation of GM-CSF production. Interestingly, Smad3-deficient T cells have been reported to produce enhanced amounts of GM-CSF (66), and the addition of TGF-β to cultures of human T cells can inhibit the production of GM-CSF (64). Future studies will determine whether TGF-β suppression of GM-CSF occurs via indirect or direct mechanisms and whether the Smad proteins have redundant roles in the regulation of this proinflammatory cytokine.
In conclusion, in this study, using a transgenic model of autoimmune diabetes, we have demonstrated a direct, Foxp3-independent mechanism of autoreactive T cell regulation by the TGF-β signaling pathway. These observations refine our understanding of two key regulatory modules in the control of peripheral T cell tolerance.
Materials and Methods
Mice.
Mice with T cell-specific deficiency of TβRII (designated “Tgfbr2−/− mice”) were generated by crossing Tgfbr2-floxed mice with the CD4-Cre transgene. OT-II RIP-mOva Rag1-deficient mice were crossed with Tgfbr2−/− mice to generate Tgfbr2−/− OT-II RIP-mOva Rag1-deficient mice. Foxp3sf mice were crossed to OT-II RIP-mOva Rag1-deficient mice to generate Foxp3sf OT-II RIP-mOva Rag1-deficient mice. Tgfbr2−/− OT-II RIP-mOva Rag1-deficient mice were further crossed to CCR2DTR mice or Csf2rb−/− mice to generate Tgfbr2−/− CCR2DTR OT-II RIP-mOva Rag1-deficient mice or Tgfbr2−/− Csf2r−/− OT-II RIP-mOva Rag1-deficient mice. ERT2-Cre Tgfbr2fl/fl OT-II Rag1-deficient, Foxp3sf OT-II Rag1-deficient, RIP-mOva Rag1-deficient, and RIP-mOva CCR2DTR Rag1-deficient mice were used for transfer experiments. All mice are fully backcrossed to the C57BL/6 background. All mice were maintained under specific pathogen-free conditions, and animal experimentation was conducted in accordance with Institutional Animal Care and Use Committee (IACUC) guidelines. Additional information is provided in SI Materials and Methods.
T Cell Transfer Model of Diabetes.
OT-II, ERT2-Cre Tgfbr2fl/fl OT-II, or Foxp3sf OT-II lymph node and spleen cells (all 1–2 × 106) were adoptively transferred into recipient mice. The following day, mice were subjected to the first of five consecutive daily i.p. injections of tamoxifen (Sigma). Tamoxifen was dissolved in ethanol and corn oil and administered to each mouse at 1 mg/d.
Diabetes Monitoring.
Blood glucose concentrations in experimental mice were monitored one or two times per week using the Bayer Contour Meter and Bayer Contour Test Strips. Mice with two consecutive readings of blood glucose concentration >250 mg/dL were considered diabetic.
Monocyte Depletion.
For depletion of CCR2-expressing cells, mice were treated i.p. with DT (Sigma) at a dose of 10 ng/g body weight every 3 d for the duration of blood glucose monitoring. For Tgfbr2−/− CC2RDTR RIP-mOva Rag1-deficient mice, DT treatments were started at weaning. For the transfer model of disease, DT treatments were started following the final tamoxifen treatment.
Immune Cell Isolation from Pancreas.
The pancreas was digested by incubation with 1 mg/mL Collagenase P (Roche) dissolved in 10% FBS HBSS (Gibco) with at 37 °C for 12 min. Digested pancreas samples were washed twice with 10% FBS HBSS and then once in 10% FBS RPMI-1640 medium (Memorial Sloan Kettering Core Media Preparation Facility). Islets were hand-picked and further treated with an enzyme-free Cell Dissociation Buffer (Gibco) for 10 min at 37 °C.
Flow Cytometry.
All samples were collected using an LSRII flow cytometer (Becton Dickinson), and flow cytometric data were analyzed using FlowJo software (Tree Star, Inc.). Detailed information about all antibodies used and staining procedures is provided in SI Materials and Methods.
Immunofluorescent Staining of Pancreas.
Pancreas tissues were fixed in 2% paraformaldehyde (PFA) overnight, followed by embedding in optimum cutting temperature (O.C.T.; Tissue-Tek) compound, and were cut into 14-μm sections. The pancreas sections were blocked with 3% BSA and stained with fluorophore-conjugated CD3 (clone 17A2; BioLegend) and F4/80 (clone BM8; BioLegend) antibodies at room temperature for 2 h. Then the sections were counterstained with DAPI and mounted with Vectashield mounting medium (Vector Laboratories). The images were captured by a Leica SP-5 upright confocal microscope and subsequently processed in Adobe Photoshop and Illustrator.
Statistical Analysis.
The paired Student’s t test was used to calculate statistical significance between pSmad2/3 levels in T cells from nondraining versus draining lymph nodes. Unpaired Student’s t test was used to calculate statistical significance for differences among other measurements between groups. A P value of ≤ 0.05 was considered statistically significant.
SI Materials and Methods
Mice.
Mice containing the CD4-Cre, OT-II, RIP-mOva, and floxed Tgfbr2 alleles were previously described (6, 31). CCR2DTR mice were provided by Eric Pamer of the Memorial Sloan Kettering Cancer Center, New York. Csf2rb−/− mice were provided by Alexander Lesokhin of the Memorial Sloan Kettering Cancer Center, New York. RIP-mOva mice were provided by William Heath of The Walter and Eliza Hall Institute of Medical Research, Parkville, Australia. ERT2-Cre, Foxp3sf, and Rag1−/− mice were purchased from Jackson Laboratory. Mice with T cell-specific deficiency of the TβRII (Tgfbr2−/− mice) were generated by crossing Tgfbr2-floxed mice with the CD4-Cre transgene. OT-II and RIP-mOva mice on Rag1-deficient backgrounds were crossed to generate OT-II RIP-mOva Rag1-deficient mice. OT-II RIP-mOva Rag1-deficient mice were crossed with Tgfbr2−/− mice to generate Tgfbr2−/− OT-II RIP-mOva Rag1-deficient mice. Foxp3sf mice were crossed to OT-II RIP-mOva Rag1-deficient mice to generate Foxp3sf OT-II RIP-mOva Rag1-deficient mice. Tgfbr2−/− OT-II RIP-mOva Rag1-deficient mice were crossed to CCR2DTR mice to generate Tgfbr2−/− CCR2DTR OT-II RIP-mOva Rag1-deficient mice. Tgfbr2−/− OT-II RIP-mOva Rag1-deficient mice were crossed to Csf2rb−/− mice to generate Tgfbr2−/− Csf2r−/− OT-II RIP-mOva Rag1-deficient mice. For transfer experiments OT-II Rag1-deficient mice were crossed to ERT2-Cre Tgfbr2-floxed (Tgfbr2fl/fl) mice to generate ERT2-Cre Tgfbr2fl/fl OT-II Rag1-deficient mice. Foxp3sf mice were crossed to OT-II Rag1-deficient mice to generate Foxp3sf OT-II Rag1-deficient mice. RIP-mOva Rag1-deficient mice were generated as recipient mice. RIP-mOva Rag1-deficient mice were crossed to CCR2DTR mice to generate RIP-mOva CCR2DTR Rag1-deficient mice. All mice are fully backcrossed to the C57BL/6 background. All mice were maintained under specific pathogen-free conditions, and animal experimentation was conducted in accordance with institutional guidelines.
Flow Cytometry.
Fluorochrome-conjugated antibodies to CD4 (clone RM4-5; Tonbo), CD11b (clone M1/70; eBioscience), CD25 (PC61.5; eBioscience), CD44 (clone IM7; eBioscience), CD45 (clone 30-F11; Invitrogen), CD62L (clone MEL-14; eBioscience), CD69 (clone HI.2F3; BD Pharmingen), Foxp3 (clone FJK-16s; eBioscience), GM-CSF (clone MP1-22E9; eBioscience), IFN-γ (clone XMG1.2; eBioscience), Ly6C (clone AL-21; BD Pharmingen), Ly6G (clone 1A8; BD Pharmingen), and TCR-β (clone H57-597; BD Pharmingen) were purchased from the indicated sources. TβRII expression was analyzed using a biotinylated TβRII antibody (R&D Systems) followed by staining with a fluorochrome-conjugated streptavidin antibody. Phosphorylated Smad2/3 was detected using a pSmad2/3 antibody (clone D27F4; Cell Signaling) followed by staining with anti-rabbit IgG (eBioscience). Cell-surface staining was performed by incubating cells with specific antibodies for 30 min on ice in the presence of 2.4G2 mAb to block FcγR binding. For Foxp3 detection, eBioscience or Tonbo Foxp3 transcription factor-staining kits were used. To assess T cell cytokine production, cells were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate (Sigma), 1 mM ionomycin (Sigma), and Golgi-Stop (BD Biosciences) for 4 h at 37 °C. Following the incubation, stimulated cells were stained for surface markers before intracellular cytokine staining was conducted using the BD Cytokine Kit. For detection of phosphorylated Smad2/3, lymph node cells were directly processed in a 2% PFA solution. Single-cell suspensions in 2% PFA were incubated for 10 min at room temperature. Fixed cells were permeabilized using the Tonbo transcription factor staining kit. Surface and intracellular antibodies were resuspended in a 1× solution of the Tonbo transcription factor permeabilization buffer, and cells were stained at room temperature for 1 h. Cells were then stained with a secondary antibody for 30 min at room temperature to detect pSmad2/3 expression. All samples were collected using an LSRII flow cytometer (Becton Dickinson), and flow cytometric data were analyzed using FlowJo software (Tree Star, Inc.).
Acknowledgments
We thank E. Pamer for providing CCR2DTR mice, A. Leoskhin for providing Csf2rb−/− mice, and members of the M.O.L. laboratory for helpful discussions. This work was supported by National Institute of Allergy and Infectious Diseases Grant R01 AI122264 (to M.O.L.), NCI Grant T32-CA9149-35 (to S.A.O.), a Howard Hughes Medical Institute Faculty Scholar Award (to M.O.L.), and Memorial Sloan Kettering Cancer Center Support Grant/Core Grant P30 CA008748.
Footnotes
Conflict of interest statement: The editor, A.Y.R., notes that he shares an institutional affiliation with the authors and coauthored a review article with M.O.L. in 2016.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706356114/-/DCSupplemental.
References
- 1.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: Learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 2.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 3.Shull MM, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulkarni AB, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 6.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, et al. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32:642–653. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Y, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlenner SM, Weigmann B, Ruan Q, Chen Y, von Boehmer H. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. J Exp Med. 2012;209:1529–1535. doi: 10.1084/jem.20112646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tone Y, et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishigame H, et al. Excessive Th1 responses due to the absence of TGF-β signaling cause autoimmune diabetes and dysregulated Treg cell homeostasis. Proc Natl Acad Sci USA. 2013;110:6961–6966. doi: 10.1073/pnas.1304498110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fantini MC, et al. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 16.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMurchy AN, et al. A novel function for FOXP3 in humans: Intrinsic regulation of conventional T cells. Blood. 2013;121:1265–1275. doi: 10.1182/blood-2012-05-431023. [DOI] [PubMed] [Google Scholar]
- 18.Scottà C, Soligo M, Camperio C, Piccolella E. FOXP3 induced by CD28/B7 interaction regulates CD25 and anergic phenotype in human CD4+CD25- T lymphocytes. J Immunol. 2008;181:1025–1033. doi: 10.4049/jimmunol.181.2.1025. [DOI] [PubMed] [Google Scholar]
- 19.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Fahlén L, et al. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737–746. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Worthington JJ, et al. Integrin αvβ8-mediated TGF-β activation by effector regulatory T cells is essential for suppression of T-cell-mediated inflammation. Immunity. 2015;42:903–915. doi: 10.1016/j.immuni.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards JP, Thornton AM, Shevach EM. Release of active TGF-β1 from the latent TGF-β1/GARP complex on T regulatory cells is mediated by integrin β8. J Immunol. 2014;193:2843–2849. doi: 10.4049/jimmunol.1401102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson MS, et al. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Legoux FP, et al. CD4+ T cell tolerance to tissue-restricted self antigens is mediated by antigen-specific regulatory T cells rather than deletion. Immunity. 2015;43:896–908. doi: 10.1016/j.immuni.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: Mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh SA, Li MO. TGF-β: Guardian of T cell function. J Immunol. 2013;191:3973–3979. doi: 10.4049/jimmunol.1301843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li MO, Flavell RA. TGF-beta: A master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hultgren B, Huang X, Dybdal N, Stewart TA. Genetic absence of gamma-interferon delays but does not prevent diabetes in NOD mice. Diabetes. 1996;45:812–817. doi: 10.2337/diab.45.6.812. [DOI] [PubMed] [Google Scholar]
- 29.von Herrath MG, Oldstone MB. Interferon-gamma is essential for destruction of beta cells and development of insulin-dependent diabetes mellitus. J Exp Med. 1997;185:531–539. doi: 10.1084/jem.185.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esensten JH, Lee MR, Glimcher LH, Bluestone JA. T-bet-deficient NOD mice are protected from diabetes due to defects in both T cell and innate immune system function. J Immunol. 2009;183:75–82. doi: 10.4049/jimmunol.0804154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurts C, et al. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall HD, et al. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity. 2011;35:633–646. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanafusa T, Imagawa A. Fulminant type 1 diabetes: A novel clinical entity requiring special attention by all medical practitioners. Nat Clin Pract Endocrinol Metab. 2007;3:36–45. doi: 10.1038/ncpendmet0351. [DOI] [PubMed] [Google Scholar]
- 34.Shibasaki S, et al. Expression of toll-like receptors in the pancreas of recent-onset fulminant type 1 diabetes. Endocr J. 2010;57:211–219. doi: 10.1507/endocrj.k09e-291. [DOI] [PubMed] [Google Scholar]
- 35.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mildner A, et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. 2009;132:2487–2500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- 37.King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hohl TM, et al. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6:470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang HH, et al. CCR2 identifies a stable population of human effector memory CD4+ T cells equipped for rapid recall response. J Immunol. 2010;185:6646–6663. doi: 10.4049/jimmunol.0904156. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton JA, Achuthan A. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol. 2013;34:81–89. doi: 10.1016/j.it.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Zhan Y, Xu Y, Lew AM. The regulation of the development and function of dendritic cell subsets by GM-CSF: More than a hematopoietic growth factor. Mol Immunol. 2012;52:30–37. doi: 10.1016/j.molimm.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Inaba K, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters JH, et al. Signals required for differentiating dendritic cells from human monocytes in vitro. Adv Exp Med Biol. 1993;329:275–280. doi: 10.1007/978-1-4615-2930-9_46. [DOI] [PubMed] [Google Scholar]
- 44.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 45.Codarri L, et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 46.El-Behi M, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richards DM, Kyewski B, Feuerer M. Re-examining the nature and function of self-reactive T cells. Trends Immunol. 2016;37:114–125. doi: 10.1016/j.it.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurts C, et al. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J Exp Med. 1997;186:2057–2062. doi: 10.1084/jem.186.12.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khoruts A, Fraser JM. A causal link between lymphopenia and autoimmunity. Immunol Lett. 2005;98:23–31. doi: 10.1016/j.imlet.2004.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang N, Bevan MJ. TGF-β signaling to T cells inhibits autoimmunity during lymphopenia-driven proliferation. Nat Immunol. 2012;13:667–673. doi: 10.1038/ni.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sledzińska A, et al. TGF-β signalling is required for CD4+ T cell homeostasis but dispensable for regulatory T cell function. PLoS Biol. 2013;11:e1001674. doi: 10.1371/journal.pbio.1001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ouyang W, et al. TGF-beta cytokine signaling promotes CD8+ T cell development and low-affinity CD4+ T cell homeostasis by regulation of interleukin-7 receptor alpha expression. Immunity. 2013;39:335–346. doi: 10.1016/j.immuni.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gagnerault MC, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of beta cell reactive T cells in NOD mice. J Exp Med. 2002;196:369–377. doi: 10.1084/jem.20011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tadokoro CE, et al. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donkor MK, et al. T cell surveillance of oncogene-induced prostate cancer is impeded by T cell-derived TGF-β1 cytokine. Immunity. 2011;35:123–134. doi: 10.1016/j.immuni.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J Exp Med. 2005;202:1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10:501–513. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- 59.Campbell IK, et al. Differentiation of inflammatory dendritic cells is mediated by NF-κB1-dependent GM-CSF production in CD4 T cells. J Immunol. 2011;186:5468–5477. doi: 10.4049/jimmunol.1002923. [DOI] [PubMed] [Google Scholar]
- 60.Cook AD, Braine EL, Campbell IK, Rich MJ, Hamilton JA. Blockade of collagen-induced arthritis post-onset by antibody to granulocyte-macrophage colony-stimulating factor (GM-CSF): Requirement for GM-CSF in the effector phase of disease. Arthritis Res. 2001;3:293–298. doi: 10.1186/ar318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Udalova IA, Mantovani A, Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol. 2016;12:472–485. doi: 10.1038/nrrheum.2016.91. [DOI] [PubMed] [Google Scholar]
- 62.Croxford AL, et al. The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity. 2015;43:502–514. doi: 10.1016/j.immuni.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 63.Noster R, et al. IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells. Sci Transl Med. 2014;6:241ra80. doi: 10.1126/scitranslmed.3008706. [DOI] [PubMed] [Google Scholar]
- 64.Hartmann FJ, et al. Multiple sclerosis-associated IL2RA polymorphism controls GM-CSF production in human TH cells. Nat Commun. 2014;5:5056. doi: 10.1038/ncomms6056. [DOI] [PubMed] [Google Scholar]
- 65.Piper C, et al. T cell expression of granulocyte-macrophage colony-stimulating factor in juvenile arthritis is contingent upon Th17 plasticity. Arthritis Rheumatol. 2014;66:1955–1960. doi: 10.1002/art.38647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye P, et al. GM-CSF contributes to aortic aneurysms resulting from SMAD3 deficiency. J Clin Invest. 2013;123:2317–2331. doi: 10.1172/JCI67356. [DOI] [PMC free article] [PubMed] [Google Scholar]