Significance
The accumulation of toxic tau protein, as in Alzheimer’s disease, is regulated by the 90-kDa heat shock protein (Hsp90) chaperone system. Inhibition of Hsp90 has been shown to reduce tau levels. However, Hsp90 inhibition can be problematic due to a lack of blood–brain barrier permeability and established toxicities. Here, we demonstrate that the Hsp90 cochaperone, ATPase homolog 1 (Aha1), dramatically increases the production of aggregated tau in vitro and in a mouse model of neurodegenerative disease. Moreover, inhibition of Aha1 reduced tau accumulation in cultured cells. These data identify Aha1 as a target for the treatment of tauopathies.
Keywords: tau oligomers, Aha1, Alzheimer’s disease, chaperones, Hsp90
Abstract
The microtubule-associated protein tau (MAPT, tau) forms neurotoxic aggregates that promote cognitive deficits in tauopathies, the most common of which is Alzheimer’s disease (AD). The 90-kDa heat shock protein (Hsp90) chaperone system affects the accumulation of these toxic tau species, which can be modulated with Hsp90 inhibitors. However, many Hsp90 inhibitors are not blood–brain barrier-permeable, and several present associated toxicities. Here, we find that the cochaperone, activator of Hsp90 ATPase homolog 1 (Aha1), dramatically increased the production of aggregated tau. Treatment with an Aha1 inhibitor, KU-177, dramatically reduced the accumulation of insoluble tau. Aha1 colocalized with tau pathology in human brain tissue, and this association positively correlated with AD progression. Aha1 overexpression in the rTg4510 tau transgenic mouse model promoted insoluble and oligomeric tau accumulation leading to a physiological deficit in cognitive function. Overall, these data demonstrate that Aha1 contributes to tau fibril formation and neurotoxicity through Hsp90. This suggests that therapeutics targeting Aha1 may reduce toxic tau oligomers and slow or prevent neurodegenerative disease progression.
The microtubule-associated protein tau (MAPT, tau) accumulates and aggregates in a family of neurodegenerative diseases called tauopathies (1), with the most common being Alzheimer’s disease (AD) (2). In particular, the pathogenic formation of oligomeric tau species is thought to be a major contributor to disease progression (3). Therefore, strategies aimed at reducing oligomeric tau accumulation could hold therapeutic promise for these diseases (4).
Molecular chaperones, including the 90-kDa heat shock protein (Hsp90), regulate protein folding, degradation, and accumulation (5). Of the proteins regulated by Hsp90, often referred to as “clients,” tau is one of the most thoroughly characterized (6). In the past decade, Hsp90 emerged as one of the next breakthrough drug targets for diseases of aging, particularly for neurodegenerative diseases like tauopathies (7). Small molecules inhibiting the ATPase activity of Hsp90 showed great promise in preclinical models, prompting the development of a host of clinical leads (8), but the translation of this preclinical success into patients has been disappointing. Not only have many leads suffered from poor blood–brain barrier permeability (9), but toxicity has also dampened enthusiasm (10, 11). This has led to the pursuit of Hsp90 cochaperones as distinct drug targets offering an alternative to Hsp90 (5, 12).
Activator of Hsp90 ATPase homolog 1 (Aha1) is the only one of these cochaperones known to stimulate Hsp90 ATPase activity (13). This small 38-kDa cochaperone binds to the N-terminal and middle domains of Hsp90, inducing a partially closed conformation that accelerates the progression of the ATPase cycle dramatically (13, 14). Therefore, small molecules targeting the interaction of Hsp90 with Aha1 could be beneficial in disease by reducing ATPase activity (15, 16). Here, we sought to determine if Aha1 could facilitate the pathogenesis of tau by stimulating Hsp90 activity. We determined that Aha1 stimulation of Hsp90 activity can drive tau fibril and oligomer formation, in vitro. Overexpressing Aha1 in a transgenic model of tauopathy increased neurotoxic oligomeric and insoluble tau. This tau accumulation enhanced both neuron loss and behavioral deficits. Moreover, inhibiting the interaction between Aha1 and Hsp90, using a small molecule, reduced insoluble tau accumulation in cultured cells. Our findings suggest that targeting Hsp90 cochaperones may enable inhibition of tau aggregation, which could reenergize the translational appeal of the Hsp90 chaperone network as a drug target.
Results
Aha1 Enhances Hsp90-Dependent Tau Aggregation.
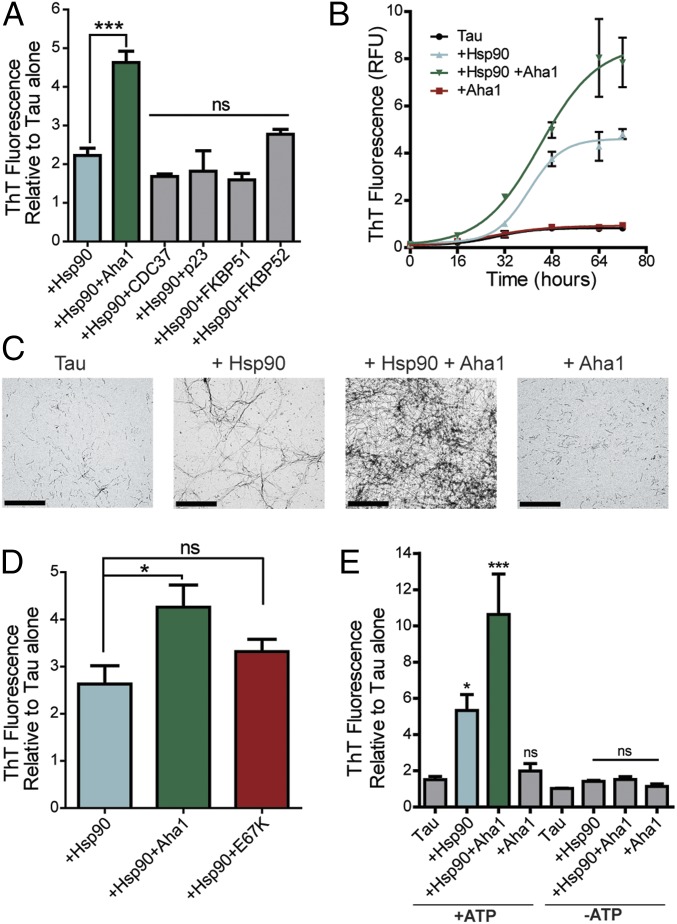
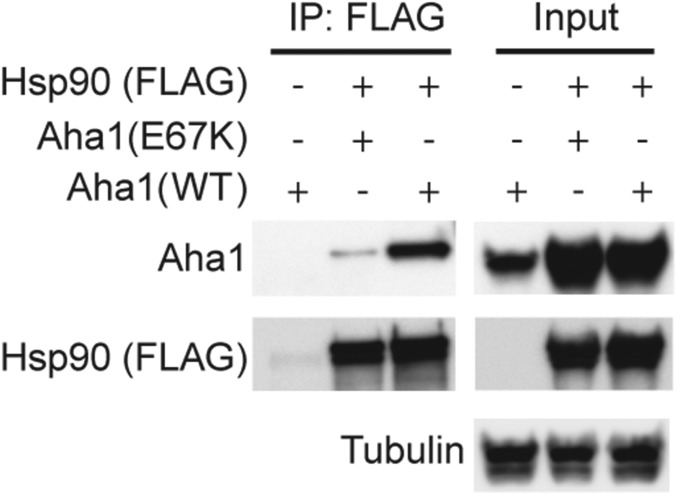
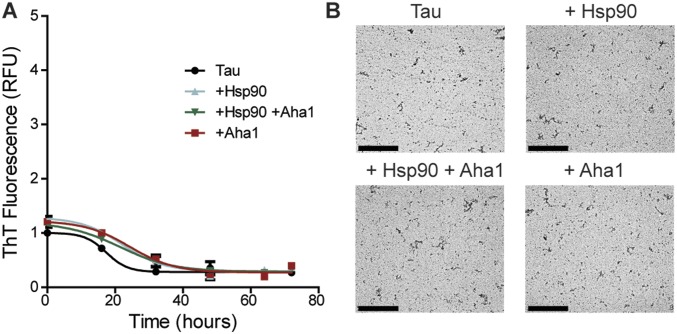
Since Hsp90 has been shown to exacerbate tau fibril formation (17), we screened five established Hsp90 cochaperones to determine whether they had an inhibitory or stimulatory effect on this process. Recombinant P301L tau was incubated with Hsp90 in the presence of ATP with or without cochaperone proteins, as indicated (Fig. 1A). Aha1 was the only cochaperone to show a significant enhancement of tau fibril formation, while CDC37, p23, FKBP51, and FKBP52 were not significantly different from Hsp90 alone. We then examined the effects of Hsp90 and Aha1 on tau fibril formation over time. We found the most potent inducer of tau fibril formation was Hsp90 and Aha1 combined (Fig. 1B). Moreover, Aha1 alone did not affect tau aggregation. These results were also confirmed using transmission electron microscopy (TEM), which shows an increase in tau fibrils in the presence of Hsp90 and an exacerbation of fibrils when both Hsp90 and Aha1 are present (Fig. 1C), suggesting that Aha1 could be responsible for the formation of toxic tau oligomers and larger aggregates. Additionally, a mutant, Aha1-E67K, which does not bind to Hsp90 (Fig. S1), did not enhance tau fibril formation (Fig. 1D). Since heparin is a known tau aggregation inducer, and tau aggregation can be modulated by DTT, we conducted control experiments to check if the aggregation behavior of tau can be affected by Hsp90, Aha1, or their combination in the absence of heparin or DTT. Tau did not fibrillate under these conditions within the time frame examined (Fig. S2). Moreover, since Aha1 is a known stimulator of Hsp90 ATPase activity (13, 14), we next investigated the effects of these proteins on tau aggregation in the absence of ATP. We found that ATP was essential for Aha1/Hsp90-mediated tau aggregation (Fig. 1E). Together, these data indicate that Aha1 uses ATP to enhance Hsp90-mediated tau aggregation.
Fig. 1.
Hsp90 and Aha1 synergize to form tau aggregates. (A) Recombinant P301L tau fibril formation measured by thioflavin T (ThT) fluorescence, comparing the effect of five different recombinant cochaperone proteins with Hsp90 and ATP (results represent the mean ± SEM, n = 3; ***P < 0.001). ns, not significant. (B) Recombinant P301L tau fibril formation measured by ThT fluorescence over a period of 72 h with or without the addition of Hsp90 and Aha1 (results represent the mean ± SEM, n = 3). (C) Representative 20,000× TEM images of recombinant P301L tau fibrils formed in the presence of indicated chaperone proteins with ATP. (Scale bars: 2 μm.) (D) Recombinant P301L tau fibril formation was measured by ThT fluorescence in the presence ATP and chaperones as indicated (results represent the mean ± SEM, n = 3; *P < 0.05). (E) Recombinant P301L tau fibril formation measured by ThT fluorescence with varying mixtures of Hsp90, Aha1, and ATP as indicated (results represent the mean ± SEM, n = 3; ***P < 0.001, *P < 0.05).
Fig. S1.
E67K-Aha1 mutation reduces tau aggregation in vitro. Western blot of immunoprecipitated (IP) Hsp90 (FLAG) from iHEK cells transfected with either Aha1-WT or Aha1-E67K.
Fig. S2.
Tau fibril formation without heparin and DTT. (A) Recombinant P301L tau fibril formation measured by thioflavin T (ThT) fluorescence over a period of 72 h with or without the addition of Hsp90 and Aha1 (results represent the mean ± SEM, n = 3). RFU, relative fluorescence units. (B) Representative 20,000× TEM images. (Scale bars: 2 μm.)
KU-177 Inhibits Interaction Between Hsp90 and Aha1.
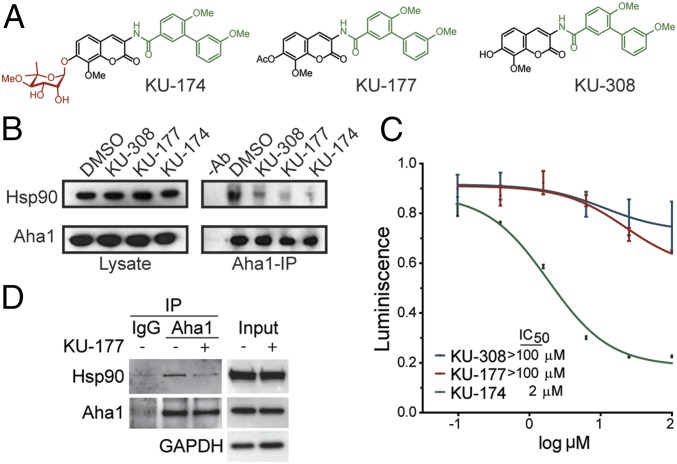
There are no commercially available Aha1-specific inhibitors. We generated novobiocin analogs designed to bind to both Hsp90 and Aha1 (KU-174) or to only Aha1 (KU-177 and KU-308) (Fig. 2A). Immunoprecipitation of Aha1 from PC3-MM2 cells revealed that Aha1 and Hsp90 complexes were inhibited by KU-308, KU-177, and KU-174 (Fig. 2B). Hsp90-mediated refolding of denatured luciferase was inhibited with KU-174 (Fig. 2C), indicating that this compound directly inhibits Hsp90, consistent with a previous report (18). However, both KU-308 and KU-177, which lack the noviose sugar required for Hsp90 binding (Fig. 2A, red), did not inhibit luciferase refolding (Fig. 2C). This suggests that these compounds do not directly inhibit Hsp90, as they were engineered to specifically bind to Aha1. Because of these characteristics, we chose to use KU-177 as our lead compound. We further tested the ability of KU-177 to inhibit the interaction between Hsp90 and Aha1 in HEK cells. Consistent with the PC3-MM2 cells, immunoprecipitation of Aha1 revealed that KU-177 inhibited the binding of Aha1 to Hsp90 (Fig. 2D).
Fig. 2.
KU-177 inhibits interaction between Hsp90 and Aha1. (A) Chemical structure of the novobiocin analogs KU-174, KU-177, and KU-308. The noviose sugar moiety (red) is required for Hsp90 binding of novobiocin analogs and is absent in KU-177 and KU-308. The biaryl amide moiety (green) has been shown to interact with Aha1 (18). (B) Immunoprecipitated Aha1 from PC3-MM2 cells treated with ±10 μM KU-308, KU-177, or KU-174 for 24 h was analyzed by Western blot. Without antibody (−Ab) indicates a mock immunoprecipitation. (C) Comparison of Hsp90-mediated luciferase refolding activity in PC3-MM2 cell treated with DMSO or 100, 25, 6.25, 1.56, 0.39, and 0.097 μM KU-308, KU-177, or KU-174 for 2 h. The IC50 value for KU-177 is shown (R2 = 0.98). Dose–response curves for KU-308 and KU-177 suggest the IC50 values would be higher than the range of concentrations examined here (KU-308, KU-174: n = 3; KU-177: n = 2). (D) Immunoprecipitated Aha1 from iHEK cells treated ±10 μM KU-177 for 24 h was analyzed by Western blot.
KU-177 Inhibits Tau Aggregation in Vitro.
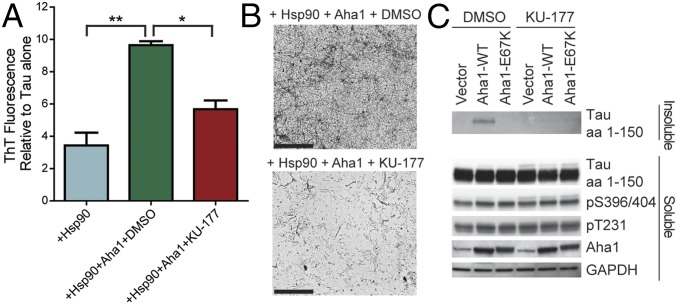
We investigated the ability of KU-177 to inhibit Aha1-mediated tau aggregation. Recombinant P301L tau was incubated with Hsp90 alone or with Hsp90 and Aha1, and then treated with KU-177 or DMSO as a control. KU-177 was able to significantly reduce tau fibril formation compared with the DMSO control (Fig. 3A). KU-177 showed a robust reduction in tau fibril formation, as observed by TEM (Fig. 3B). Inducible HEK (iHEK)-P301L cells transfected with Aha1-WT or Aha1-E67K were treated with KU-177 and harvested to examine soluble and sarkosyl-insoluble tau. We see that both the mutant Aha1-E67K and the Aha1 inhibitor KU-177 were able to reduce insoluble tau (Fig. 3C). We also noted that KU-177 increased soluble, phosphorylated tau.
Fig. 3.
KU-177 inhibits Aha1 enhancement of Hsp90-mediated tau aggregation. (A) Recombinant P301L tau fibril formation measured by thioflavin T (ThT) fluorescence, comparing the effect of 10 μM KU-177 or DMSO on tau fibril formation (results represent the mean ± SEM, n = 3; **P < 0.01, *P < 0.05). (B) Representative 20,000× TEM images of recombinant P301L tau fibrils formed with KU-177 or DMSO control. (Scale bars: 2 μm.) (C) iHEK-P301L cells transfected with Aha1-WT, Aha1-E67K, or empty vector were treated with 10 μM KU-177 or DMSO and harvested, and soluble and sarkosyl-insoluble fractions were then prepared. Blots were probed by antibodies as indicated.
Aha1 Colocalization with Tau Tangles Correlates with Disease Progression in Human AD Brain.
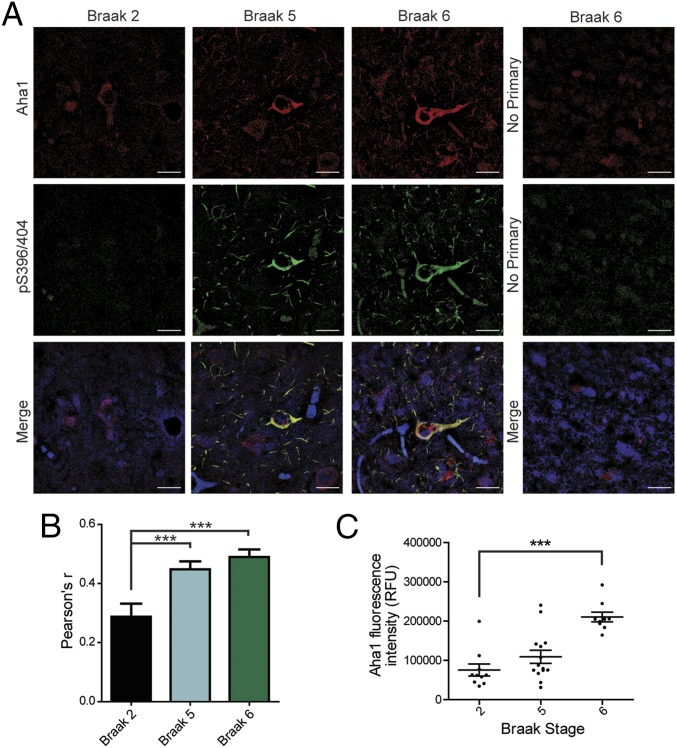
We evaluated postmortem human brain samples from patients with AD or healthy age-matched controls for Aha1 localization in relation to tau tangles (Fig. 4A). We found a significant increase in the amount of colocalization between Aha1 and tau tangles as shown by immunofluorescence (pS396/404, PHF1) in AD samples compared with controls (Fig. 4B). There was a positive correlation between Aha1 immunofluorescence intensity and tau Braak staging (Fig. 4C). This suggests a role for Aha1 in pathological tau progression.
Fig. 4.
Human AD samples show colocalization between Aha1 and tau tangles. (A) Tissue samples from the medial temporal gyrus of patients at Braak stage 2, 5, or 6 were stained for Aha1 (red), pS396/404 tau tangles (green), and neuronal Nissl (Neurotrace, blue), and then imaged using confocal microscopy; images were taken at a magnification of 60×. (Scale bars: 20 μm.) Representative, control sections lacking primary antibody are shown on the Far Right. (B) Quantification of colocalization between Aha1 and phosphorylated tau tangles (pS396/404) (results represent the mean Pearson’s correlation coefficient ± SEM, n = 10 images; ***P < 0.001). (C) Scatter plot of the intensity of Aha1 fluorescence and Braak staging (results represent the mean fluorescence intensity ± SEM; Braak stage 2: n = 10 images, Braak stage 5: n = 14 images, Braak stage 6: n = 9 images; ***P < 0.001). RFU, relative fluorescence units.
Aha1 Overexpression in rTg4510 Mice Increased Oligomeric and Insoluble Tau Species.
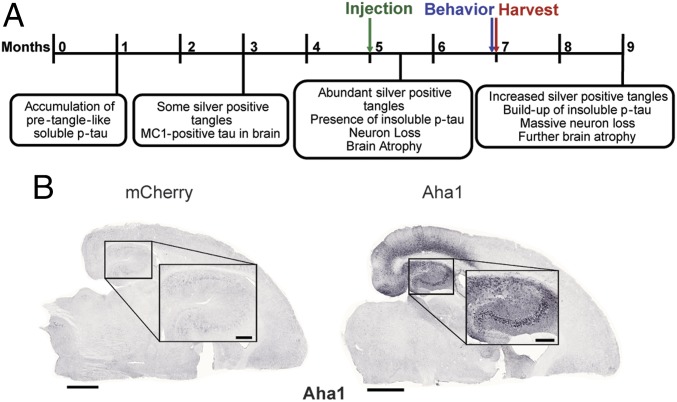
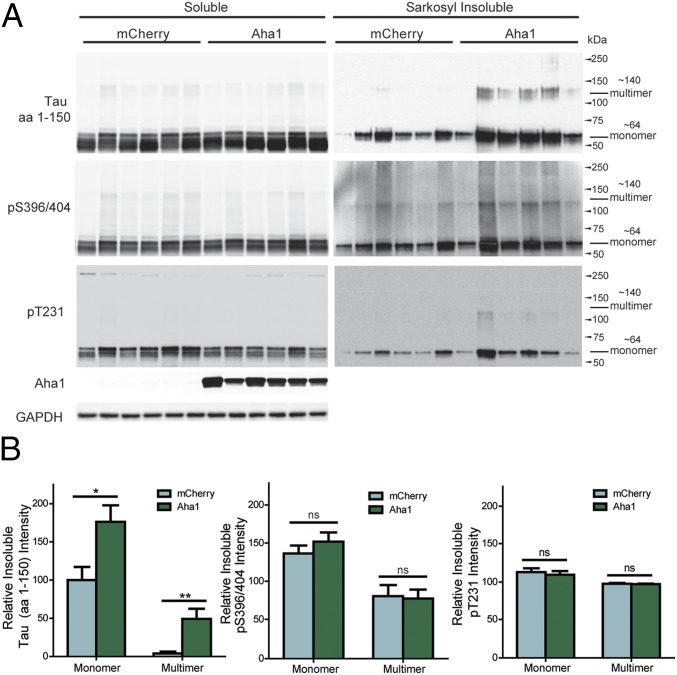
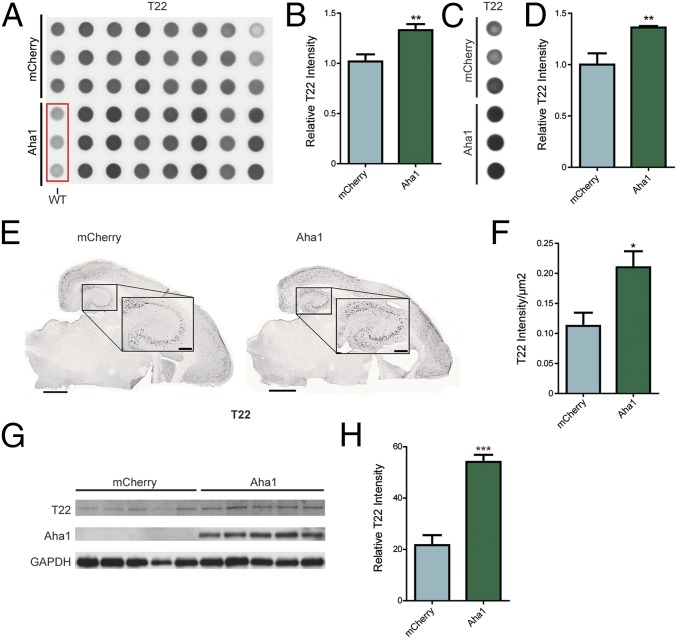
Five-month-old rTg4510 mice received bilateral hippocampal injections of adenoassociated virus serotype 9 (AAV9)-Aha1 (n = 9) or AAV9-mCherry (n = 8) (Fig. 5A). Immunohistochemical staining revealed that Aha1 was overexpressed throughout the hippocampus (Fig. 5B). Aha1 overexpression significantly increased monomeric and multimeric sarkosyl-insoluble tau in the hippocampus (Fig. 6); sarkosyl-insoluble tau was not detected in wild-type mice (Fig. S3). Insoluble phosphorylated tau was not significantly increased. Aha1 overexpression also increased toxic T22-tau oligomer levels (17) both in individual mouse samples (Fig. 7 A and B) and in pooled samples from each treatment group (Fig. 7 C and D). This increase of T22-tau oligomers in Aha1-overexpressing mice was further confirmed using immunohistochemistry (Fig. 7 E and F) and semidenaturing Western blotting (Fig. 7 G and H).
Fig. 5.
Viral transduction leads to sustained overexpression of Aha1 in the hippocampus of rTg4510 mice. (A) Characteristic phenotype of rTg4510 tau transgenic mouse model along with experimental design time points. (B) Representative images of brain sections showing viral expression of Aha1 protein in AAV9-injected Aha1 and mCherry control littermates. (Scale bars: whole slice, 1,000 μm; Inset, 250 μm.)
Fig. 6.
Aha1 overexpression in rTg4510 mice leads to increases in insoluble tau species. (A) Western blot analysis of soluble and sarkosyl-insoluble fractions from hippocampal tissue of rTg4510 mice expressing either AAV9-Aha1 or AAV9-mCherry. Six representative samples from AAV9-Aha1– and AAV9-mCherry–injected mice are shown. (B) Quantification of Western blots of sarkosyl-insoluble total (amino acids 1–150), pS396/404, and pT231 tau (results represent the mean ± SEM relative to the level of monomeric tau in AAV9-mCherry–injected mice (mCherry, n = 8; Aha1, n = 9; *P < 0.05, **P < 0.01). ns, not significant.
Fig. S3.
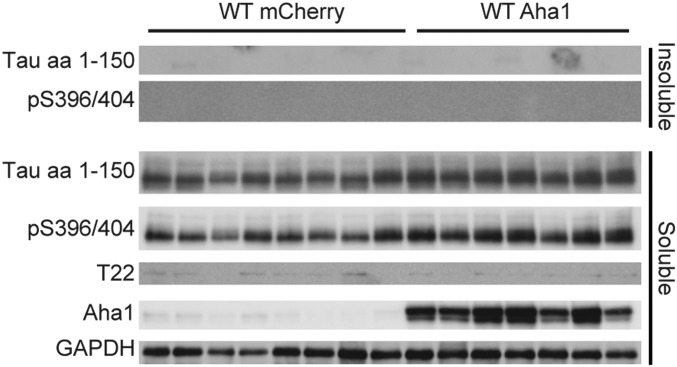
Tau solubility in WT mice. Western blot analysis of soluble and sarkosyl-insoluble fractions from hippocampal tissue of WT mice expressing either AAV9-Aha1 (n = 7) or AAV9-mCherry (n = 8). One rTg4510 mouse sample was included as a comparison.
Fig. 7.
Aha1 overexpression in rTg4510 mice leads to increases in pathological tau species. (A) Dot blot of hippocampal tissue of individual mice shown in triplicate probed by T22. (B) Quantification of dot blot (results represent the mean ± SEM; mCherry, n = 8; Aha1, n = 8; **P < 0.01). (C) Dot blot of pooled hippocampal tissue shown in triplicate probed by T22. (D) Quantification of dot blot (results represent the mean ± SEM of triplicate samples taken from the pooled fractions; n = 3; **P < 0.05). (E) Representative images of brain tissue slices stained with T22 from AAV9-mCherry– and AAV9-Aha1–injected mice. (Scale bars: whole slice, 1,000 μm; Inset, 250 μm.) (F) Quantification of the T22-positive area in the hippocampal field of view (Inset from E) (results represent the mean ± SEM; mCherry, n = 8; Aha1, n = 9; *P < 0.05). (G) Samples from AAV9-Aha1 and AAV9-mCherry mice were run on a semidenaturing gel and probed by T22 (1:500, approximately 75 kDa) along with other antibodies as indicated. (H) Quantification of T22 Western blot (results represent the mean ± SEM; mCherry, n = 6; Aha1, n = 7; ***P < 0.001).
Aha1 Overexpression in rTg4510 Mice Leads to Neuronal Loss and Cognitive Impairments.
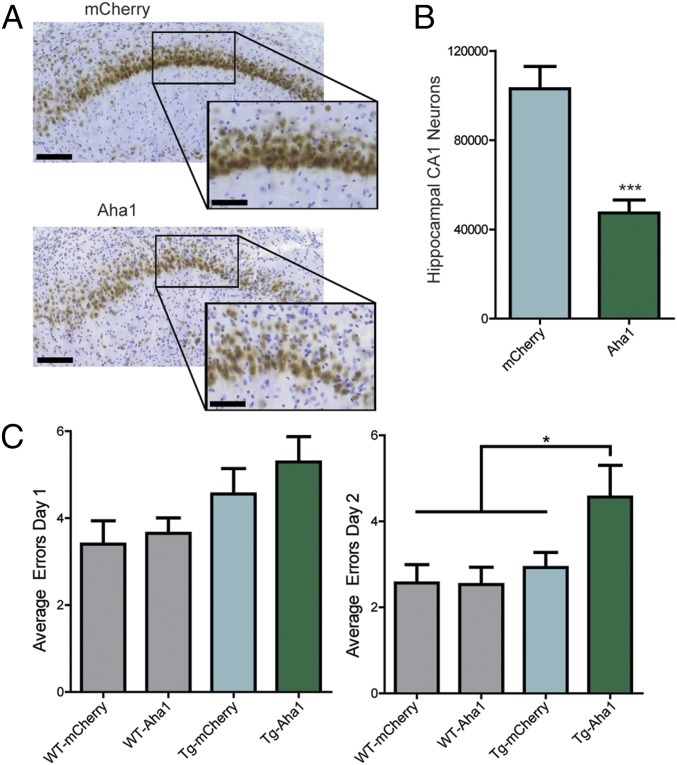
Using unbiased stereology, rTg4510 mice overexpressing Aha1 showed a significant reduction in hippocampal CA1 neurons compared with mCherry controls (Fig. 8 A and B). Learning and memory were evaluated in mice injected with AAV9-Aha1 (n = 9) and AAV9-mCherry (n = 8) using the 2-d radial arm water maze (RAWM). Animals overexpressing Aha1 made significantly more errors in locating the submerged escape platform compared with mCherry-overexpressing littermates, demonstrating a memory recall deficit (Fig. 8C). Overall, these data demonstrate that Aha1 enhances Hsp90-mediated tau aggregation. This interaction results in increased oligomeric and insoluble tau concomitant with neuronal loss and memory deficits.
Fig. 8.
Aha1 overexpression in rTg4510 mice leads to cognitive impairments. (A) Representative images of NeuN-stained neurons in the CA1 region of the hippocampus (brown) counterstained with Cresyl violet (purple) from AAV9-mCherry– and AAV9-Aha1–injected mice (Insets). (Scale bars: 100 μm.) (B) Quantification of unbiased stereology (results represent the mean ± SEM; mCherry, n = 7; Aha1, n = 8; ***P = 0.0003). (C) RAWM was performed on AAV9-Aha1 and AAV9-mCherry rTg4510 (Tg) and WT littermates as indicated. Average errors from day 1 (training) and day 2 (memory) are shown (results represent the mean ± SEM; n ≥ 9; *P < 0.05).
Discussion
In this study, we identified the Hsp90 cochaperone Aha1 as a potential therapeutic target for the treatment of tauopathies. Our data suggest that Aha1 increased tau fibril formation, resulting in insoluble tau accumulation by stimulating Hsp90 ATPase activity. Expression of Aha1 not only increased insoluble tau levels but also significantly increased T22 immunoreactive tau oligomers. This increase in pathological tau levels manifested in neuronal loss and cognitive deficits. Furthermore, we demonstrated that the Aha1 inhibitor KU-177 reduced the accumulation of insoluble P301L tau in cultured cells. This suggests that Aha1 may be a promising target for the development of therapeutics directed toward reducing tau aggregation.
Previous work has focused on Hsp90 as a therapeutic target to reduce the toxic load of amyloidogenic proteins in cells (19). However, this endeavor has been challenging as Hsp90 has many client proteins within the cell and inhibiting this chaperone can lead to many pleiotropic effects (10, 20). Compounds that target specific Hsp90 cochaperones (12) are being investigated for their potential to be less toxic as well as more specific (5). Targeting the Hsp90/p23 and Hsp90/CDC37 complexes with celastrol analogs (21–24) or withanolides (25–27) has been investigated. However, these compounds still bind Hsp90 and have effects similar to Hsp90 inhibitors (27, 28). Alternatively, small- molecule inhibitors of Hsp90/HOP complexes disrupt this complex by binding directly to HOP (29). One of these compounds, C9, was shown to have anticancer effects similar to direct Hsp90 inhibition, without inducing heat shock response (30). Until recently, there were no known small-molecule inhibitors of Aha1. Ghosh et al. (18) identified compounds that bind to either Hsp90 or Aha1 based on the novobiocin scaffold. More recently, two additional Aha1/Hsp90 inhibitors were identified (31). These compounds demonstrated protection against pathologies related to cystic fibrosis, but it is still unclear if these inhibitors bind directly to Hsp90 or Aha1.
Here, we demonstrated that the Aha1-binding inhibitor KU-177 reduced Hsp90/Aha1-mediated toxic tau accumulation. Further studies will be required to determine the pharmacokinetics, brain distribution, and efficacy of KU-177 and future classes of Aha1 inhibitors. Collectively, this study identified a role for Aha1 in the progression of tauopathies. This suggests inhibition of Aha1 may prevent or reverse the accumulation of pathogenic tau.
Materials and Methods
Antibodies.
The following antibodies were used: anti-Aha1 antibodies (SMC-172D, StressMarq; ab83036 for immunoprecipitation, Abcam), anti-Hsp90α (SMC-149B; StressMarq), anti-GAPDH (60004-1-Ig; Proteintech), anti-NeuN (MAB377B; Millipore), H-150 anti-tau (sc-5587; Santa Cruz Biotechnology), and anti-tau pT231 (55313-025; Anaspec). PHF1 anti-tau (pS396/404) was a kind gift from Peter Davies, Feinstein Institute for Medical Research, Manhasset, NY. T22 anti-tau oligomer was a kind gift from Rakez Kayed, University of Texas Medical Branch, Galveston, TX.
Plasmids and Viral Vectors.
Aha1 WT and Aha1 E67K expression plasmids were generated in our laboratory using the pCMV6 backbone. AAV9-Aha1 and AAV9-mCherry were generated in our laboratory for murine gene therapy studies.
Protein Expression.
The details of the expression and purification of recombinant human P301L tau, Aha1, Aha1 E67K, p23, FKBP51, FKBP52, and CDC37 are described in SI Materials and Methods. Hsp90α protein was a kind gift from Johannes Buchner, Technical University of Munich, Munich.
TEM.
Ten microliters of protein samples was adsorbed onto square mesh copper grids (EMS300-Cu) for 60 s and washed twice with 10 μL of deionized water, and excess water was removed by wicking with filter paper. Samples were negatively stained with 1% uranyl acetate for 30 s and dried overnight. Grids were viewed using a JEOL 1400 Digital Transmission Electron Microscope, and images were captured with a Gatan Orius wide-field camera. Fields shown are representative.
Thioflavin T Fluorescence Assay.
Ten micromolar P301L tau was incubated with 400 nM indicated chaperone in 100 μM sodium acetate (pH 7.0) buffer with 2 mM DTT, 2.5 μM heparin (3,000 Da), and 10 μM thioflavin T in 100-μL volumes in a 96-well, black, clear-bottom plate (07-200-525; Fisher) for 3 d at 37 °C. Fluorescence was read at 440 nm excitation and 482 nm emission in a BioTek Synergy H1 plate reader at indicated time points. All conditions were performed at least in duplicate.
Cell Culture and Transfection.
The iHEK-P301L cells (32) and luciferase-expressing PC3-MM2 cells (32) were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin (Invitrogen). Inducible cells were incubated with 3 μg of tetracycline for 72 h. Forty-eight hours before harvest, transfections were performed with 2.5 μL of Lipofectamine 2000 (Invitrogen) per 1 μg of DNA, which was incubated in serum-free Opti-MEM for 5 min before adding the mixture drop-wise to the cells. KU-177 was added 24 h before harvest at indicated concentrations. Cells were harvested in Tsaio TBS buffer [50 mM Tris base, 274 mM NaCl, 5 mM KCl (pH 8.0)] containing protease inhibitors. Samples were prepared as previously described (33) to obtain soluble (S1) and sarkosyl-insoluble (P3) fractions.
Coimmunoprecipitation.
Coimmunoprecipitation of Hsp90α with Aha1 from PC3-MM2 and iHEK-P301L cells incubated with the indicated compounds for 24 h was performed as previously described (18).
Luciferase refolding assay.
Compound dissolved in DMSO at the indicated concentrations or a DMSO control was evaluated in a luciferase refolding assay in PC3-MM2 cells as previously described (34), and dose–response curves of the luminescence signal relative to DMSO control were generated using GraphPad Prism 5.0.
Human Tissue Processing.
Brain tissue samples from the medial temporal gyrus of patients with Braak stage 2, 5, or 6 were provided by the University of California Alzheimer’s Disease Research Center and the Institute for Memory Impairments and Neurological Disorders. Samples were fixed in 4% paraformaldehyde overnight; sucrose gradients up to 30% were then used, and tissue was sectioned on a sliding microtome at 25-μm-thick sections. Sections were stored at 4 °C in Dulbecco’s PBS supplemented with 0.065% sodium azide until they were used for immunohistochemistry.
Animal Studies and Tissue Processing.
The rTg4510 (Jackson Laboratories) and nontransgenic control mice received bilateral stereotaxic hippocampal (X = ±3.6, Y = −3.5, Z = +2.68) injections of AAV9 vector (miniature CMV + chicken β-actin; 1012) at 5 mo of age [n = 20 (10 transgenic [seven male, three female] and 10 nontransgenic [seven male, three female]); n = 19 (nine transgenic [six male, three female] for Aha1, 10 nontransgenic [six male, four female] for mCherry)]. Each injection delivered 2 μL of AAV9 particles. At 7 mo of age, the mice were used for behavioral testing using the RAWM task. Upon completion of the RAWM task, the brains were harvested after cardiac perfusion with 0.9% saline. The right hemisphere from each mouse was dissected, and the hippocampus was then snap-frozen and stored at −80 °C until processed as previously described (33) to obtain S1 and P3 fractions. The left hemisphere from each mouse was fixed in 4% paraformaldehyde overnight, and sucrose gradients up to 30% were then used, with 25-μm-thick tissue sections were generated using a sliding microtome for general histochemical staining and 50-μm sections generated for stereology studies. Sections were stored at 4 °C in Dulbecco’s PBS supplemented with 0.065% sodium azide until they were used for immunohistochemistry.
Western Blot and Dot Blot Analysis.
Cell and mouse brain tissues samples were analyzed by Western blot using 4–15% SDS gradient gels (BioRad). Antibody dilutions were 1:1,000 unless otherwise stated, and all secondary antibodies were used at 1:1,000 (Southern Biotech). Blots were developed using ECL (Pierce) on a LAS-4000 mini imager (GE Healthcare). For dot blots, proteins were applied onto a wet nitrocellulose membrane and dried by vacuum. Dried membranes were blocked and developed as described above.
Semidenaturing Western Blot.
Tissue was homogenized using sonication, and the low-speed spin fraction was collected after centrifugation at 13,000 × g for 15 min. Samples were then mixed with 2× Laemmli sample buffer (BioRad) containing 2.1% SDS, and run on a blot using 4–15% SDS gradient gels (BioRad) without boiling the samples or adding β-mercaptoethanol.
RAWM.
The RAWM task was performed as previously described (35). Briefly, a circular black tank with a six-arm metal insert was filled with water, and a platform was submerged 1 cm below the surface of the water at the end of a designated goal arm. Animals were permitted 60 s to locate the platform, during which time an observer blinded to treatment manually scored the number of errors. An error was defined as an entry into an incorrect arm or the absence of an arm choice within 15 s. Mice were trained over 2 d with 12 trials per day, which were divided into four blocks of three trials each. Average errors were calculated for each mouse on day 1 and day 2. Groups were evaluated separately each day with one-way ANOVA, using a least significant difference test to compare groups.
Immunohistochemistry.
All immunohistochemistry was done using free-floating sections as previously described (36). Human tissue was stained as previously described using immunofluorescent secondary antibodies (17, 37). A detailed description of the immunohistochemistry methods can be found in SI Materials and Methods. Sections stained for stereology were blocked and permeabilized as described above and incubated overnight at room temperature with biotinylated anti-NeuN (1:3,000). Following washes, avidin–biotin complex (ABC) conjugation, and peroxidase development, tissue was mounted on charged glass slides and allowed to dry overnight. A 0.05% Cresyl violet counterstain was applied to slides and then briefly and quickly destained with 0.3% acetic acid in water before dehydration.
Microscopy.
Bright-field–stained tissue was imaged using a Plan-Apochromat (PLAN-APO) 20×/0.88 objective on a Zeiss Axioscan.Z1 slide scanner. Brain tissue immunofluorescently stained was imaged using a Leica TCS SP2 for image analysis. A Zeiss LSM 880 AxioObserver laser scanning confocal microscope was used for representative images. Fields of view were selected in the cortex based on tau-positive staining. A 63×/1.40 PLAN APO oil objective was used to take a minimum of ten 1-μm Z-stacked images with Argon (for tau-positive signal in green), and Red HeNe (for Aha1-positive and Neurotrace signal in red).
Imaging Analysis.
Bright-field image analysis was performed using NearCYTE software (www.nearcyte.org) as previously described (17). This program was used to outline regions of interest, and thresholds were then set manually until all of the user-determined positive cells were selected with as little nonspecific area selected as possible. Using the batch process option, the area positive ratio was automatically calculated for each slide.
Fluorescent image analysis was performed using ImageJ (NIH). Background was subtracted from the red channel using the Gaussian Blur tool (radius = 50 μm), and the new blurred image was then subtracted from the original image. The red channel was also despeckled before image analysis. Both channels were set to a consistent threshold, and colocalization between the red and green channels was then quantified with a Pearson’s coefficient. The intensity of red fluorescence was also measured to make a scatter plot showing levels of Aha1 in relation to Braak staging.
Stereology.
Neurons were stained with anti-NeuN and Cresyl violet, and those positive for both were counted in the CA1 of the hippocampus. A computerized stereological system, connected to a Leica DM4000B microscope with a Prior motorized stage, was used to outline the area using distinct landmarks in the brain at a magnification of 4× (37, 38). Every eighth section was sliced at 50 μm to be used for stereology, and only sections containing hippocampi (as determined by analyzer) were counted (mCherry: n = 7 animals, approximately nine sections per animal, approximately five reference points per section; Aha1: n = 8 animals, approximately nine sections per animal, approximately five reference points per section). After the initial analysis, the mCherry control group was reanalyzed at a higher stringency level. Neurons were counted in this region by using randomly designated areas in the computer-generated grid using a 100× oil immersion lens. Neurons were counted when they were located within the 3D dissectors or touching the inclusion lines, and the top 1 μm and bottom 1 μm of tissue were excluded. After analysis of all tissue, the number of neurons per animal was multiplied by 4.5 to reflect the total number of neurons throughout the hippocampus.
Statistical Analysis.
To compare two groups, a t test was used. Groups larger than two were evaluated using one-way ANOVA with Dunnett’s multiple comparison test. P values below 0.05 were considered significant.
Study Approval.
All studies were carried out following the guidelines set by the University of South Florida’s Institutional Animal Care and Use Committee in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International regulations. All human tissue was acquired under approved Institutional Review Board protocols for the University of California, Irvine. Patient samples were deidentified and approved for studies of this nature, with written informed consent provided to use the tissue for research purposes.
SI Materials and Methods
Protein Expression and Purification.
Recombinant human P301L tau, Aha1, Aha1 E67K, p23, FKBP51, FKBP52, and CDC37 were cloned into bacterial expression vector pet28a with a His tag, followed by a tobacco etch virus (TEV) sequence. Plasmids were transformed into Escherichia coli (BL21) one-shot star cells and plated onto kanamycin-agar plates. Plates were grown at 37 °C for ∼16 h. Ten milliliters of LB with kanamycin starter cultures was then inoculated with a colony, and the starter culture was grown for 8 h. One-liter cultures were then inoculated at a 1:100 dilution and grown to an OD600 of 0.8. Cultures were induced with the addition of 1 mM isopropyl-β-d-thiogalactopyranoside, and the incubator temperature was reduced to 16 °C. Cultures were then grown for 14 h. Cells were then pelleted at 3,500 × g for 30 min, and supernatant was discarded. Pellets were resuspended in lysis buffer [20 mM Tris⋅HCl (pH 8.0), 500 mM NaCl, 10 mM imidazole with protease inhibitors] and frozen at −80 °C. Bacterial pellets were then thawed and lysed by sonication. Lysates were then spun at 50,000 × g for 1 h. Next, the supernatant was purified by nickel affinity chromatography (Nickel Resin, PI88222; Fisher). Protein purity and expression were then checked by Coomassie-stained SDS/PAGE. Next, the protein was digested with TEV protease, removing the His tag. Finally, proteins were purified by a size exclusion column (200 pg, HiLoad 16/600; Superdex). Proteins were then stored at −80 °C.
Immunohistochemistry.
Briefly, sections were incubated in PBS with 10% MeOH and 3% H2O2 to block endogenous peroxidases. After PBS washes, tissue was permeabilized for 30 min by 0.2% Triton X-100 with 1.83% lysine and 4% serum in PBS. Following permeabilization, tissue was incubated overnight at room temperature with either anti-Aha1 (rat, 1:7,000) or anti-T22 (rabbit, 1:700). Following PBS washes, biotinylated goat anti-rat (1:1,000) or goat anti-rabbit (1:3,000) secondary antibody was added for 2 h. An ABC kit (Vectastain) was used to increase visibility. Following three PBS washes, tissue was incubated with 0.05% diaminobenzidine plus 0.5% nickel and developed with 0.03% H2O2. Sections were then mounted on charged slides, allowed to dry overnight, and dehydrated in alcohol gradients. Slides were coverslipped with distyrene, plasticizer, and xylene (DPX) mountant following clearing with Histoclear (National Diagnostics).
The tissue was permeabilized as described above and incubated at room temperature overnight with rat anti-Aha1 (1:100), and mouse anti-PHF1 (1:100). Following washes, sections were incubated for 2 h with Alexa Fluor-488–labeled goat anti-rat (1:1,000) or Alexa Fluor 594-labeled goat anti-mouse (1:1,000) secondary antibodies. Following secondary incubation, sections were stained with Neurotrace (1:25; Invitrogen) for 20 min. After fluorescent secondary, human tissue was incubated in 0.1% Sudan Black B in 70% ethanol (Sigma) for 20 min to reduce autofluorescence, and then washed three times with 0.2% Tween in PBS. Tissue was mounted after three washes and coverslipped with ProLong Gold antifade (Invitrogen) reagent.
Imaging Analysis.
Bright-field image analysis was performed using NearCYTE software (www.nearcyte.org). This program was used to outline regions of interest, and thresholds were then set manually until all of the user-determined positive cells were selected with as little nonspecific area selected as possible. Using the batch process option, the area positive ratio was automatically calculated for each slide.
Acknowledgments
We thank Dr. Peter Davies for the PHF1 antiphosphorylated tau (pS396/404) antibody. We also thank Dr. Rakez Kayed for providing the T22 anti-tau oligomer antibody. We acknowledge Dr. Peter Mouton for his stereology expertise, Dr. Vladamir Uversky for insightful edits, and Dr. Andrew Lesniak for providing the NearCYTE software. We also thank Drs. Nicole Berchtold and Carl Cotman for access to human tissue samples from the University of California, Irvine, Alzheimer’s Disease Research Center, which is funded by NIH/National Institute on Aging Grant P50 AG16573. This work was supported by NIH Grants NS073899 and MH103848 and by Veteran’s Health Administration Grants BX001637 and BX002475. This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital. The contents of this publication do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Conflict of interest statement: C.A.D., L.B.S., B.S.J.B., J.K., and L.J.B. are the coinventors for the following provisional patent application: “The Hsp90 Activator Aha1 Drives Production of Pathological Tau Aggregates.”
This article is a PNAS Direct Submission. M.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707039114/-/DCSupplemental.
References
- 1.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 2.Ballatore C, Lee VM-Y, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 3.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: Separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 4.Sahara N, et al. Molecular chaperone-mediated tau protein metabolism counteracts the formation of granular tau oligomers in human brain. J Neurosci Res. 2007;85:3098–3108. doi: 10.1002/jnr.21417. [DOI] [PubMed] [Google Scholar]
- 5.Blair LJ, Sabbagh JJ, Dickey CA. Targeting Hsp90 and its co-chaperones to treat Alzheimer’s disease. Expert Opin Ther Targets. 2014;18:1219–1232. doi: 10.1517/14728222.2014.943185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karagöz GE, et al. Hsp90-Tau complex reveals molecular basis for specificity in chaperone action. Cell. 2014;156:963–974. doi: 10.1016/j.cell.2014.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pratt WB, Gestwicki JE, Osawa Y, Lieberman AP. Targeting Hsp90/Hsp70-based protein quality control for treatment of adult onset neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 2015;55:353–371. doi: 10.1146/annurev-pharmtox-010814-124332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: Are we there yet? Clin Cancer Res. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inda C, Bolaender A, Wang T, Gandu SR, Koren J., 3rd Stressing out Hsp90 in neurotoxic proteinopathies. Curr Top Med Chem. 2016;16:2829–2838. doi: 10.2174/1568026616666160413141350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler LM, Ferraldeschi R, Armstrong HK, Centenera MM, Workman P. Maximizing the therapeutic potential of HSP90 inhibitors. Mol Cancer Res. 2015;13:1445–1451. doi: 10.1158/1541-7786.MCR-15-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong DS, et al. Targeting the molecular chaperone heat shock protein 90 (HSP90): Lessons learned and future directions. Cancer Treat Rev. 2013;39:375–387. doi: 10.1016/j.ctrv.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Gaali S, et al. Selective inhibitors of the FK506-binding protein 51 by induced fit. Nat Chem Biol. 2015;11:33–37. doi: 10.1038/nchembio.1699. [DOI] [PubMed] [Google Scholar]
- 13.Wolmarans A, Lee B, Spyracopoulos L, LaPointe P. The mechanism of Hsp90 ATPase stimulation by Aha1. Sci Rep. 2016;6:33179. doi: 10.1038/srep33179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Richter K, Reinstein J, Buchner J. Integration of the accelerator Aha1 in the Hsp90 co-chaperone cycle. Nat Struct Mol Biol. 2013;20:326–331. doi: 10.1038/nsmb.2502. [DOI] [PubMed] [Google Scholar]
- 15.Okayama S, et al. p53 protein regulates Hsp90 ATPase activity and thereby Wnt signaling by modulating Aha1 expression. J Biol Chem. 2014;289:6513–6525. doi: 10.1074/jbc.M113.532523. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Wang X, et al. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127:803–815. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 17.Blair LJ, et al. Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J Clin Invest. 2013;123:4158–4169. doi: 10.1172/JCI69003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh S, et al. Hsp90 C-terminal inhibitors exhibit antimigratory activity by disrupting the Hsp90α/Aha1 complex in PC3-MM2 cells. ACS Chem Biol. 2015;10:577–590. doi: 10.1021/cb5008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao H, Michaelis ML, Blagg BSJ. Hsp90 modulation for the treatment of Alzheimer’s disease. Adv Pharmacol. 2012;64:1–25. doi: 10.1016/B978-0-12-394816-8.00001-5. [DOI] [PubMed] [Google Scholar]
- 20.Schulz R, Dobbelstein M, Moll UM. HSP90 inhibitor antagonizing MIF: The specifics of pleiotropic cancer drug candidates. Oncoimmunology. 2012;1:1425–1426. doi: 10.4161/onci.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang F, et al. Optimization and biological evaluation of celastrol derivatives as Hsp90-Cdc37 interaction disruptors with improved druglike properties. Bioorg Med Chem. 2016;24:5431–5439. doi: 10.1016/j.bmc.2016.08.070. [DOI] [PubMed] [Google Scholar]
- 22.Zhang T, et al. A novel Hsp90 inhibitor to disrupt Hsp90/Cdc37 complex against pancreatic cancer cells. Mol Cancer Ther. 2008;7:162–170. doi: 10.1158/1535-7163.MCT-07-0484. [DOI] [PubMed] [Google Scholar]
- 23.Patwardhan CA, et al. Gedunin inactivates the co-chaperone p23 protein causing cancer cell death by apoptosis. J Biol Chem. 2013;288:7313–7325. doi: 10.1074/jbc.M112.427328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chadli A, et al. Celastrol inhibits Hsp90 chaperoning of steroid receptors by inducing fibrillization of the Co-chaperone p23. J Biol Chem. 2010;285:4224–4231. doi: 10.1074/jbc.M109.081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu M, et al. Structure-activity relationship (SAR) of withanolides to inhibit Hsp90 for its activity in pancreatic cancer cells. Invest New Drugs. 2014;32:68–74. doi: 10.1007/s10637-013-9987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Y, et al. Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem Pharmacol. 2010;79:542–551. doi: 10.1016/j.bcp.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinadinos C, et al. Low endogenous and chemical induced heat shock protein induction in a 0N3Rtau-expressing Drosophila larval model of Alzheimer’s disease. J Alzheimers Dis. 2013;33:1117–1133. doi: 10.3233/JAD-2012-121534. [DOI] [PubMed] [Google Scholar]
- 28.Westerheide SD, et al. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- 29.Yi F, Regan L. A novel class of small molecule inhibitors of Hsp90. ACS Chem Biol. 2008;3:645–654. doi: 10.1021/cb800162x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pimienta G, Herbert KM, Regan L. A compound that inhibits the HOP-Hsp90 complex formation and has unique killing effects in breast cancer cell lines. Mol Pharm. 2011;8:2252–2261. doi: 10.1021/mp200346y. [DOI] [PubMed] [Google Scholar]
- 31.Ihrig V, Obermann WMJ. Identifying inhibitors of the Hsp90-Aha1 protein complex, a potential target to drug cystic fibrosis, by alpha technology. SLAS Discov. 2017;22:923–928. doi: 10.1177/2472555216688312. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh S, et al. Diverging novobiocin anti-cancer activity from neuroprotective activity through modification of the amide tail. ACS Med Chem Lett. 2016;7:813–818. doi: 10.1021/acsmedchemlett.6b00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramsden M, et al. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L) J Neurosci. 2005;25:10637–10647. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall JA, et al. Novobiocin analogues that inhibit the MAPK pathway. J Med Chem. 2016;59:925–933. doi: 10.1021/acs.jmedchem.5b01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat Protoc. 2006;1:1671–1679. doi: 10.1038/nprot.2006.275. [DOI] [PubMed] [Google Scholar]
- 36.Dickey C, et al. Aging analysis reveals slowed tau turnover and enhanced stress response in a mouse model of tauopathy. Am J Pathol. 2009;174:228–238. doi: 10.2353/ajpath.2009.080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abisambra JF, et al. Tau accumulation activates the unfolded protein response by impairing endoplasmic reticulum-associated degradation. J Neurosci. 2013;33:9498–9507. doi: 10.1523/JNEUROSCI.5397-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mouton PR, Pakkenberg B, Gundersen HJ, Price DL. Absolute number and size of pigmented locus coeruleus neurons in young and aged individuals. J Chem Neuroanat. 1994;7:185–190. doi: 10.1016/0891-0618(94)90028-0. [DOI] [PubMed] [Google Scholar]