Significance
Microorganisms residing within animal tissues as symbionts can be critically important to many aspects of animal biology. For example, the microbiomes of many insects, such as aphids, honeybees, and termites, can provide nutrients, deter pathogens, and help digest food. We examined whether caterpillars also engage in intimate microbial partnerships. Across a broad diversity of caterpillar species, we found that microbes in the gut are extremely low-abundance and predominantly leaf-derived, suggesting their transient nature. Furthermore, suppressing bacteria in tobacco hornworms (Manduca sexta) had no detectable effect on caterpillar growth or survival. With caterpillars as a prominent—but possibly not unique—example of relative autonomy, the degree of reliance on microbes is an underappreciated yet likely important dimension of animal biodiversity.
Keywords: insects, herbivory, Lepidoptera, symbiosis, mutualism
Abstract
Many animals are inhabited by microbial symbionts that influence their hosts’ development, physiology, ecological interactions, and evolutionary diversification. However, firm evidence for the existence and functional importance of resident microbiomes in larval Lepidoptera (caterpillars) is lacking, despite the fact that these insects are enormously diverse, major agricultural pests, and dominant herbivores in many ecosystems. Using 16S rRNA gene sequencing and quantitative PCR, we characterized the gut microbiomes of wild leaf-feeding caterpillars in the United States and Costa Rica, representing 124 species from 15 families. Compared with other insects and vertebrates assayed using the same methods, the microbes that we detected in caterpillar guts were unusually low-density and variable among individuals. Furthermore, the abundance and composition of leaf-associated microbes were reflected in the feces of caterpillars consuming the same plants. Thus, microbes ingested with food are present (although possibly dead or dormant) in the caterpillar gut, but host-specific, resident symbionts are largely absent. To test whether transient microbes might still contribute to feeding and development, we conducted an experiment on field-collected caterpillars of the model species Manduca sexta. Antibiotic suppression of gut bacterial activity did not significantly affect caterpillar weight gain, development, or survival. The high pH, simple gut structure, and fast transit times that typify caterpillar digestive physiology may prevent microbial colonization. Moreover, host-encoded digestive and detoxification mechanisms likely render microbes unnecessary for caterpillar herbivory. Caterpillars illustrate the potential ecological and evolutionary benefits of independence from symbionts, a lifestyle that may be widespread among animals.
Many animals are colonized by microbial symbionts that have beneficial and fundamentally important impacts on host biology. Microbes can regulate animal development, immunity, and metabolism, mediate ecological interactions, and facilitate the evolutionary origin and diversification of animal clades (1–7). These integral host–microbe relationships have led to a conceptualization of animals as “holobionts” (8–10), superorganism-like entities composed of the host plus its microbiome—defined here as the entire assemblage of commensal, pathogenic, and mutualistic microorganisms (11). Furthermore, the recent proliferation of microbiome surveys supports a widely held assumption that microbial symbioses are universal across animals (12, 13).
The Lepidoptera (butterflies, moths, and their caterpillar larvae), despite being key components of most terrestrial foodwebs and extraordinarily diverse (14), are one group in which the role of microbes remains ambiguous. Here we focus on caterpillars, which are the main—and in some Lepidoptera, the exclusive—feeding stage, and which have long been intensively studied in many fields (15). The vast majority of caterpillars are herbivores, and some herbivores rely on microbes to supplement nutrients, neutralize toxins, or digest plant cell walls (16, 17). However, considering caterpillars’ simple gut morphology and rapid digestive throughput, it has been speculated that microbes cannot persist in the caterpillar gut and do not contribute to digestion (18, 19). Indeed, microscopy-based studies report no, or minimal, microbial growth in caterpillar guts (20–22).
DNA- and culture-based investigations of caterpillar gut microbiomes have produced mixed findings, with conflicting implications for microbial involvement in caterpillar biology. Some studies report a highly abundant and consistent bacterial community (23–25), characteristics that may indicate a functional association with the host. Others report high intraspecific variability in composition and similarity between diet- and gut-associated microbes (26–29). Inconsistencies could arise from methodological factors such as contamination of low-biomass samples (30), starvation before sampling, sequencing of extracellular DNA, and the use of laboratory-raised insects or artificial diets (27, 31, 32). As an additional complication, many microbiome surveys do not distinguish between dead or dormant passengers [“transients” (33)] and persistent, living populations [“residents” (33) or “symbionts” sensu (34)]. Furthermore, microbes in the latter category may be parasitic or pathogenic, as well as beneficial. While microbes were known to cause disease in caterpillars as early as Louis Pasteur’s experiments on silkworms (35), their potential importance as mutualists remains unclear.
Do caterpillars depend on gut microbes for feeding and development? To answer this question, we first characterized gut microbial abundance and composition across a taxonomically and geographically broad array of wild caterpillars (SI Appendix, Fig. S1). Our analyses are focused on the digestive tract, the most likely habitat for microbial colonization, as abundant microbes have not been observed elsewhere in the caterpillar body (31, 36). We applied the same methods to 24 additional insect, bird, and mammal species that we expected to have functional microbiomes to assess the reliability of our protocol and to contextualize our findings. We then conducted a field-based experiment testing whether gut bacteria impact larval growth and survival of the model species Manduca sexta (Sphingidae). Our findings question the generality of animal–microbe symbioses and may inform a multitude of research programs based on caterpillar herbivory in both natural and managed ecosystems (e.g., refs. 37–39).
Results
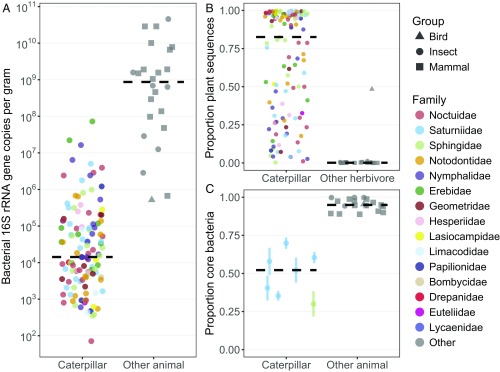
Using quantitative PCR and sequencing of the 16S rRNA gene, we found that wild caterpillars representing a broad diversity of Lepidoptera had gut bacterial densities multiple orders of magnitude lower than the microbiomes of other insects and vertebrate feces measured using identical methods (one-way ANOVA, F1,145 = 228.2, P < 0.0001, Fig. 1A) (SI Appendix, Table S1). Some animals host symbiotic fungi (40), but fungal biomass was also lower in caterpillar guts relative to other insects and vertebrates (median 6.1 × 102 vs. 9.5 × 104 rRNA gene copies per gram, respectively; one-way ANOVA, F1,145 = 36.03, P < 0.0001). While mitochondrial rRNA genes from fungi and other eukaryotes (as well as chloroplasts) are detectable using primers designed for bacteria and archaea (41), more targeted characterization of fungi in caterpillar guts is warranted. As another indicator of low microbial biomass, in most caterpillars over 80% of fecal 16S rRNA gene sequences were from plant chloroplasts or mitochondria versus ∼0.1% for other herbivores or omnivores with plant-rich diets (Wilcoxon rank-sum test, P < 0.0001, Fig. 1B). In a subset of caterpillars from which we sampled whole, homogenized midgut and hindgut tissue, plant DNA represented an even higher proportion of sequences in guts than in feces (SI Appendix, Fig. S2A). This pattern is more likely a function of plant DNA degradation during intestinal transit than of bacterial proliferation, as bacterial density remained similar or decreased slightly from midgut to feces, depending on the caterpillar species (SI Appendix, Fig. S2B).
Fig. 1.
Comparisons of bacterial density, relative abundance of plant DNA, and intraspecific variability between caterpillars and other animals expected to host functional microbiomes. Medians are indicated by black dashed lines, and points are horizontally jittered. Data for each species are listed in SI Appendix, Table S1. One caterpillar species yielding <100 total sequences was excluded. For species with multiple replicates, the median is plotted. (A) The density of bacterial 16S rRNA gene copies in caterpillar feces versus fecal (vertebrates) or whole-body homogenate (other insect) samples of other animals (n = 121 caterpillar species, 24 other species). Two caterpillar species with lower amplification than DNA extraction blanks are not shown. (B) The proportion of sequence libraries assigned to plant chloroplast or mitochondrial rRNA (n = 123 caterpillars, 21 other herbivores). (C) The proportion of bacterial sequences belonging to core phylotypes, defined for each species as those present in the majority of conspecific individuals analyzed. Included are species with at least three replicates with >100 bacterial sequences each (n = 7 caterpillars, 19 other animals). For species with more than three replicates, points show the median core size across all combinations of three individuals, and error bars show the interquartile range.
Animals with functionally important, resident microbiomes tend to host a high abundance of microbial taxa shared among conspecific individuals (e.g., refs. 42–44). Indeed, within species of the other insects and vertebrates analyzed here, microbiomes were largely made up of a common set of bacterial phylotypes (Fig. 1C). For example, >99% of sequences in any one honeybee belonged to phylotypes found in the majority of honeybees included in the analysis. In contrast, even when consuming the same species of food plant under similar conditions, caterpillars had a much lower proportion of their gut bacterial assemblage belonging to core phylotypes (one-way ANOVA, F1,24 = 165.3, P < 0.0001, Fig. 1C). In Schausiella santarosensis, which among the seven caterpillar species (mostly Saturniidae) examined had the highest median core size of ∼70%, four of its six core phylotypes were Methylobacterium, a typical inhabitant of leaf surfaces (45). This observation hints that many of the core taxa found in caterpillar guts may be transient, food-derived microbes.
Caterpillar gut microbiomes are dominated by leaf-associated bacteria, further suggesting that resident, host-specific symbionts are sparse or absent. The bacterial phylotypes present in the feces of at least half of the sampled individuals are Staphylococcus, Escherichia, Methylobacterium, Klebsiella, Enterococcus, and Sphingomonas (SI Appendix, Table S2). Of these, all but Staphylococcus—a potential caterpillar pathogen (46) or a transient from human skin (47)—are also among the 10 most common phylotypes found in paired leaf samples. Across caterpillars, a median 89.6% (interquartile range: 80.2–99.0%) of fecal bacterial sequences belonged to leaf-associated phylotypes. However, bacterial assemblages were not identical between leaves and caterpillar feces (PERMANOVA, pseudo-F1,196 = 12.54, R2 = 0.06, P = 0.001). Besides the potential growth of parasites and/or mutualists in the gut, this difference could arise from digestion filtering out subsets of the leaf microbiome.
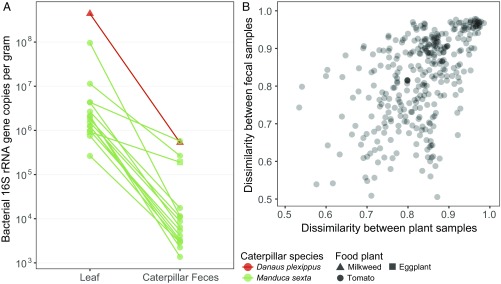
Low precision may partly explain the extensive variation in caterpillar gut bacterial loads (Fig. 1A) as these estimates are a product of bacterial sequence composition and total 16S rRNA gene counts (SI Appendix, SI Methods), both of which contain measurement error. However, transient inputs of leaf microbes also generate variation among caterpillar species and individuals. Leaf bacterial densities differed greatly within (tomato) and between (milkweed, eggplant, tomato) plant species, and these differences were reflected in the feces of monarch (Danaus plexippus) and M. sexta caterpillars feeding on them (linear regression, R2 = 0.24, P = 0.031; Fig. 2A). Furthermore, bacterial densities dropped by a median of 214-fold from leaves to feces (Fig. 2A), suggesting that any potential bacterial growth within the gut is relatively minor. The extent of this reduction varied widely (from 5- to 8,385-fold, Fig. 2A), possibly because of interindividual or interspecific differences in physiological traits that eliminate leaf microbes, such as gut pH. Variation in bacterial taxonomic composition among leaves and caterpillar feces was also correlated (Mantel test, r = 0.28, P = 0.001; Fig. 2B). In other words, caterpillars consuming leaves with more distinct bacterial assemblages had more distinct bacterial assemblages in their feces, as would be expected if gut microbes are diet-derived and only transiently present. Moreover, this process can explain a relationship between host relatedness and microbiome structure, a pattern sometimes termed “phylosymbiosis” (48). Specifically, although confamilial caterpillars in Costa Rica had marginally more similar gut bacterial assemblages than did caterpillars in different families (PERMANOVA, pseudo-F6,43 = 1.47, P = 0.053), they had also been feeding on plants with especially similar leaf microbiomes (PERMANOVA, pseudo-F6,42 = 1.73, P = 0.005).
Fig. 2.
The abundance and composition of caterpillar fecal bacteria compared with paired diet (leaf) samples. (A) The density of bacterial 16S rRNA gene copies in ground leaves versus feces for 16 caterpillars collected in Colorado. Parallel lines indicate an association between plant and fecal bacterial abundances across pairs. (B) The correlation between beta diversity (Bray–Curtis dissimilarity) across caterpillar fecal samples collected in Costa Rica and paired leaf-surface samples (n = 24 caterpillar species, 19 plant species; 26 individuals each). Here, only samples with >2,000 sequences are shown to facilitate visualization.
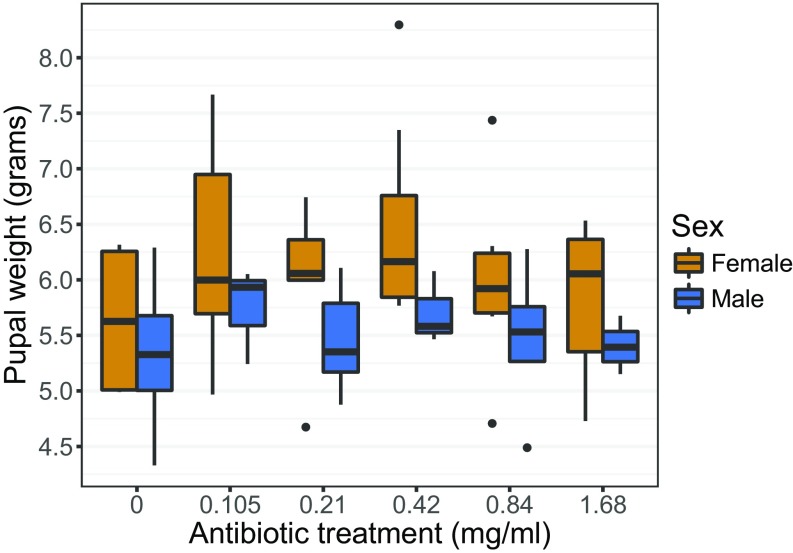
Supporting our claim that caterpillars lack resident gut microbiomes, we show experimentally that the growth and survival of field-collected M. sexta caterpillars are not dependent on gut bacterial activity. As measured by qPCR, wild M. sexta contain ∼61,000-fold lower bacterial loads than expected from allometric scaling relationships based on animals with resident microbiomes (ref. 49 and SI Appendix, Fig. S3). Feeding M. sexta antibiotics reduced this already low number of gut bacteria by 14- to 365-fold (range of medians across dosages), as measured using culture-dependent methods (linear regression, R2 = 0.13, P = 0.003; SI Appendix, Fig. S4A). Bacterial colony counts were correlated with the number of 16S rRNA gene copies (Pearson correlation, r = 0.38, P = 0.003; SI Appendix, Fig. S4B). Suppression of viable bacteria had no effect on pupal weight (linear regression, antibiotics: P = 0.45; sex: P = 0.014; interaction: P = 0.70; Fig. 3), which is predictive of fecundity in insects (50), nor on development time (linear regression, antibiotics: P = 0.19; sex: P = 0.023; interaction: P = 0.63; SI Appendix, Fig. S5A). Likewise, antibiotic treatment did not affect survival from larval hatching to adult emergence (logistic regression, 95% CI of odds ratio = 0.76–9.39, P = 0.19; SI Appendix, Fig. S5B), nor generally impact total feces production, a metric integrating leaf consumption and assimilation efficiency (linear regression, antibiotics: P = 0.07; sex: P = 0.002; interaction: P = 0.048). As expected with M. sexta (51), we found clear sexual size dimorphism, suggesting that our experimental design had sufficient power to detect biologically meaningful differences. Given that antibiotics reduced fecal bacteria to a variable extent within and among treatments (SI Appendix, Fig. S4A), we repeated the aforementioned analyses using gut bacterial abundance as the predictor variable. In all cases there was no significant relationship with host performance (P > 0.1), further indicating that reducing or eliminating gut bacteria from caterpillars does not reduce M. sexta fitness.
Fig. 3.
An increasing concentration of an antibiotic cocktail, applied to leaves before feeding, does not reduce M. sexta larval growth (n = 62). Males and females are plotted separately, as they were expected to differ in size. Fresh weight was measured 6 d after pupation. Pupal weight correlates with adult fecundity (50) and is often used as a proxy of insect fitness.
Discussion
Consistent with previous microscopy-based (20–22) and molecular studies (26–29), we found that microbial symbionts are generally absent or present only in low numbers in caterpillar guts. As expected for herbivores consuming microbe-rich leaf tissue, diet-derived microbes are transiently present in caterpillar guts, wherein they may be dead or inactive. That the microbial biomass in caterpillar guts is far lower than in the guts or whole bodies of many other animals (Fig. 1A), and also lower than in leaves (Fig. 2A), suggests a lack of persistent microbial growth. Moreover, any potential microbial metabolism might be too limited to substantially affect digestive processes, as illustrated by our observation that M. sexta caterpillars contain microbial loads orders of magnitude lower than comparably sized animals with resident microbiomes (SI Appendix, Fig. S3). Caterpillar gut microbiomes also exhibit high inter- and intraspecific variability in both abundance and composition (Figs. 1 and 2). Lacking resident populations, they may be easily influenced by the idiosyncrasies of which microbes are present on a given leaf and in what abundance, and which can survive transit through the digestive tract. Ingested microbes that die within the host could still be beneficial as food or by stimulating the immune system, but are not themselves symbionts [following the original definition of symbiosis as the “living together of different species” (referenced in ref. 34)].
In tandem with the transient nature of gut microbiomes across caterpillar species, the experiment on M. sexta suggests that microbes are unlikely to have cryptic, but essential, functions in caterpillar guts. Antibiotic suppression of viable gut bacteria had no apparent negative consequences for M. sexta, contrasting sharply with the many examples of major reductions in host growth or survival upon removal of beneficial symbionts (e.g., refs. 52–54). If anything, caterpillars treated with antibiotics showed slight (but not statistically significant) increases in performance (Fig. 3 and SI Appendix, Fig. S5B). Antibiotics increase weight gain of laboratory-bred caterpillars (55–57), and commercially made caterpillar diets often contain antibiotics. This effect might reflect microbial parasitism occurring in even apparently healthy caterpillars, or costly immune responses to the presence of pathogens (58). Aside from known leaf specialists, many of the most frequently detected bacterial genera in this study (SI Appendix, Table S2) have been reported to cause disease in caterpillars (36, 46, 59, 60). Additionally, even normally transient gut microbes can negatively affect caterpillars under certain circumstances, such as after ingestion of insecticidal toxins (61), and thus may be important to understanding caterpillar herbivory and pest management.
The lack of a resident gut microbiome in caterpillars may directly result from a digestive physiology that is unfavorable to microbial growth (18). The midgut, the largest section of the digestive tract in which caterpillars digest leaf material and absorb the resulting nutrients (62), is a particularly hostile environment for microbes (24). It is highly alkaline, with pH values often >10 (63) and as high as 12 (64), and contains host-encoded antimicrobial peptides (65). Additional attributes of the caterpillar gut that may hinder microbial colonization include a simple tube-like morphology without obvious microbe-housing structures (18), a continually replaced lining (the peritrophic matrix) covering the midgut epithelium (66), and short retention times [food transit takes ∼2 h in M. sexta (67)]. Although some insects harbor symbionts in specialized organs (68), to our knowledge, similar structures have not been reported in caterpillars. Buchner’s foundational survey of animal endosymbiosis describes Lepidoptera only as “a group in which no symbiont bearers have been discovered” (ref. 68, p. 817). Moreover, previous studies did not find microbes that were abundant outside of the gut (31, 36), although in infected populations the reproductive parasite Wolbachia may inhabit other larval tissues (69).
Without the aid of microbial symbionts, how are caterpillars able to overcome the dietary challenges posed by herbivory? Caterpillars use a combination of mechanical disruption, endogenously produced digestive enzymes, and high pH to extract easily solubilized nutrients, primarily from the contents of plant cells (18, 70, 71). Although this method of processing leaves is relatively inefficient, essential nutrients are not totally absent, so that caterpillars can compensate by simply eating more (18, 62). Some insects likely require microbes for detoxification (16), but many caterpillars possess host-encoded mechanisms for degrading or tolerating plant allelochemicals (72). However, there may be a vestigial role for microbes in these processes, as genomes of many Lepidoptera contain microbial genes encoding enzymes with related functions (73, 74). These gene acquisitions may have enabled a symbiont-free feeding strategy.
The caterpillars surveyed here are likely to be representative of most externally leaf-feeding Lepidoptera, as we included a range of diet breadths, from monophagous to highly generalist, and many of the most diverse families (SI Appendix, Fig. S1). However, a lack of resident gut microbiome in the caterpillar may not apply to the adult butterfly or moth. For example, adult honeybees have abundant gut microbes, while the larvae do not (75). Compared with caterpillars, adult butterflies host distinct bacterial communities (31) and high gut microbial loads (76). On the other hand, unlike honeybees or butterflies, many Lepidoptera do not feed as adults, and in these groups microbes may be altogether irrelevant to digestion or nutrition. However, we cannot exclude the possibility that microbial symbionts influence host fitness by their potential activities in eggs or pupae.
The extraordinary diversity and abundance of Lepidoptera (14) indicate that a symbiont-independent feeding strategy can be highly successful. Perhaps such success reflects a release from constraints imposed on other animals that do host and depend on symbionts. There are costs to engaging in mutualisms (e.g., refs. 77–79), and in a gut microbiome context one cost includes nutrient competition between host and microbes (80). A high availability of food allows caterpillars to “skim the cream” (62), assimilating simple nutrients that might otherwise be used by gut microbes and excreting recalcitrant material. In other words, “Why not do the digestion yourself rather than pay someone else to do it?” (ref. 81, p. 53). Another cost is the risk of gut microbes becoming pathogenic (61, 82) or of foodborne pathogens exploiting a gut environment that is hospitable to mutualists. The extreme conditions in the caterpillar midgut may lower these risks by limiting the growth of both pathogens and potential mutualists.
Dependence on microbes with different physiological tolerances than the host constrains overall niche breadth (7, 78). Compared with groups lacking functional microbiomes, animals whose biology is heavily influenced by microbial mutualists may be less able to switch to new food plants or new habitats over evolutionary time. Indeed, it has been argued that while microbial symbioses can provide novel ecological functions, they may also increase the extinction risk of host lineages (7, 83). As Lepidoptera represent one of the most species-rich animal radiations (84), a conspicuous question is whether independence from microbes may, in some cases, facilitate host diversification.
Caterpillars do not appear to be unique in lacking a resident microbiome that is important for feeding and development. Microbiomes of walking sticks (85), sawfly larvae (86, 87), a saprophagous fly (88), a parasitic horsehair worm (89), a leaf beetle (90, 91), and certain ants (92) display features similar to those that we observed in caterpillars. Our data suggest that some vertebrates also have minimal gut microbiomes and feed relatively autonomously. Feces of the herbivorous goose Branta bernicla had low bacterial loads and a high proportion of plant DNA, and the insectivorous bat Myotis lucifugus had similarly low fecal bacterial loads (SI Appendix, Table S1). These species exhibit caterpillar-like physiological traits such as a short gut and rapid digestive transit (93, 94). Additional examples in the literature might be obscured by contaminants masquerading as mutualists (95), a frequent absence of quantitative information (92) and experimental validation of microbial function in vivo, and publication bias against “negative results.”
While recent literature has documented extraordinary variation in the types of services provided by microbial symbionts, less explored is variation in the degree to which animals require any such services. Animals likely exist on a spectrum from tightly integrated host–microbe holobionts to simply animals, sensu stricto, in which a microbial presence is only relictual (i.e., mitochondria and horizontally transferred genes). Documenting the existence of microbially independent animals, as well as their ecological, physiological, and phylogenetic contexts, is a first step toward understanding the causes and consequences of evolutionary transitions along this continuum.
Methods
Sampling and Sequencing.
Caterpillar fecal samples (n = 185) were obtained from actively feeding, field-collected individuals in Arizona, Colorado, Massachusetts, and New Hampshire and in Área de Conservación Guanacaste, Costa Rica. To sample plant microbiomes, we collected leaves from the same branch used to feed caterpillars before fecal or gut sampling. All species were identified by morphology. Noncaterpillar animals were included if microbiome samples were available in our laboratory or were readily collectable during caterpillar sampling. We extracted DNA, PCR-amplified the 16S rRNA V4 gene region, and sequenced amplicons on an Illumina MiSeq in the same manner as previous insect microbiome studies (31, 91). These DNA extracts and primers were also used for quantitative PCR, which provides microbial biomass estimates concordant with those from microscopy (92) and culturing (SI Appendix, Fig. S4B). We did not find evidence that low amplification of caterpillar fecal bacteria is due to primer bias, PCR inhibitors, or storage methods (SI Appendix, SI Methods).
Antibiotic Experiment.
We collected M. sexta eggs from Datura wrightii plants near Portal, Arizona. Seventy-two newly hatched larvae were randomly and evenly divided among six treatments varying from 0 to 1.68 mg total antibiotics per milliliter of distilled water, and reared in separate unused plastic bags on D. wrightii foliage. Water with or without antibiotics was sprayed onto leaves, which were then briefly dried before feeding. The compounds used here (rifampicin, tetracycline, and streptomycin in a 1:2:4 ratio) have been shown to suppress bacterial symbionts in other insect herbivores (53, 96). More detail is provided in SI Appendix, SI Methods.
Data Analysis.
Statistical analyses were conducted in R. Differences in microbial loads, core sizes, and M. sexta performance were tested using linear models. M. sexta survival was analyzed using logistic regression. We used a Mantel test to estimate the rank correlation between leaf and fecal microbiome dissimilarities. A Wilcoxon rank-sum test was used for proportions of plant DNA. Differences in community composition were analyzed using PERMANOVA. DNA sequences, metadata, and R code are available at https://figshare.com/articles/Data_files_for_Hammer_et_al_Caterpillars_lack_a_resident_gut_microbiome_/4955648.
Supplementary Material
Acknowledgments
We thank the parataxonomists and staff of Área de Conservación Guanacaste, without whom this project would not have been possible. M. D. Bowers and H. A. Woods helped plan the study, and J. Dickerson, K. Vaccarello, and H. Layton provided invaluable assistance. We also thank M. Lyke, A. Beckley, and E. Connor for contributing samples, the reviewers and T. Sharpton for helpful comments, and the Southwestern Research Station and A. Dietz for administrative support. T.J.H. was supported by the American Philosophical Society’s Lewis and Clark Fund, the NSF Graduate Research Fellowship Program (Grant 1144083) and NSF Doctoral Dissertation Improvement Grant 1601787. D.H.J. and W.H. were supported by the University of Pennsylvania, Wege Foundation, Permian Global, the government of Costa Rica, and the Guanacaste Dry Forest Conservation Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Sequence data and additional files are publicly available from Figshare at https://figshare.com/articles/Data_files_for_Hammer_et_al_Caterpillars_lack_a_resident_gut_microbiome_/4955648.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707186114/-/DCSupplemental.
References
- 1.McFall-Ngai M, et al. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci USA. 2013;110:3229–3236. doi: 10.1073/pnas.1218525110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudakaran S, Kost C, Kaltenpoth M. Symbiont acquisition and replacement as a source of ecological innovation. Trends Microbiol. 2017;25:375–390. doi: 10.1016/j.tim.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Janson EM, Stireman JO, 3rd, Singer MS, Abbot P. Phytophagous insect-microbe mutualisms and adaptive evolutionary diversification. Evolution. 2008;62:997–1012. doi: 10.1111/j.1558-5646.2008.00348.x. [DOI] [PubMed] [Google Scholar]
- 4.Frago E, Dicke M, Godfray HCJ. Insect symbionts as hidden players in insect-plant interactions. Trends Ecol Evol. 2012;27:705–711. doi: 10.1016/j.tree.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Sommer F, Bäckhed F. The gut microbiota–Masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 6.Douglas AE. Symbiosis as a general principle in eukaryotic evolution. Cold Spring Harb Perspect Biol. 2014;6:1–14. doi: 10.1101/cshperspect.a016113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moran NA. The ubiquitous and varied role of infection in the lives of animals and plants. Am Nat. 2002;160(Suppl 4):S1–S8. doi: 10.1086/342113. [DOI] [PubMed] [Google Scholar]
- 8.Zilber-Rosenberg I, Rosenberg E. Role of microorganisms in the evolution of animals and plants: The hologenome theory of evolution. FEMS Microbiol Rev. 2008;32:723–735. doi: 10.1111/j.1574-6976.2008.00123.x. [DOI] [PubMed] [Google Scholar]
- 9.Bordenstein SR, Theis KR. Host biology in light of the microbiome: Ten principles of holobionts and hologenomes. PLoS Biol. 2015;13:e1002226. doi: 10.1371/journal.pbio.1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert SF, Sapp J, Tauber AI. A symbiotic view of life: We have never been individuals. Q Rev Biol. 2012;87:325–341. doi: 10.1086/668166. [DOI] [PubMed] [Google Scholar]
- 11.Lederberg J, McCray AT. ‘Ome Sweet ’Omics—A genealogical treasury of words. Scientist. 2001;15:8. [Google Scholar]
- 12.Russell JA, Dubilier N, Rudgers JA. Nature’s microbiome: Introduction. Mol Ecol. 2014;23:1225–1237. doi: 10.1111/mec.12676. [DOI] [PubMed] [Google Scholar]
- 13.Vavre F, Kremer N. Microbial impacts on insect evolutionary diversification: From patterns to mechanisms. Curr Opin Insect Sci. 2014;4:29–34. doi: 10.1016/j.cois.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Scoble MJ. The Lepidoptera. Oxford Univ Press; Oxford: 1992. [Google Scholar]
- 15.Stamp NE, Casey TM. Caterpillars: Ecological and Evolutionary Constraints on Foraging. Chapman & Hall Ltd.; New York: 1993. [Google Scholar]
- 16.Hammer TJ, Bowers MD. Gut microbes may facilitate insect herbivory of chemically defended plants. Oecologia. 2015;179:1–14. doi: 10.1007/s00442-015-3327-1. [DOI] [PubMed] [Google Scholar]
- 17.Douglas AE. The microbial dimension in insect nutritional ecology. Funct Ecol. 2009;23:38–47. [Google Scholar]
- 18.Appel H. The chewing herbivore gut lumen: Physicochemical conditions and their impact on plant nutrients, allelochemicals, and insect pathogens. In: Bernays EA, editor. Insect–Plant Interactions. CRC Press, Boca Raton; FL: 1994. pp. 209–223. [Google Scholar]
- 19.Bernays EA, Janzen DH. Saturniid and sphingid caterpillars: Two ways to eat leaves. Ecology. 1988;69:1153–1160. [Google Scholar]
- 20.Shannon AL, Attwood G, Hopcroft DH, Christeller JT. Characterization of lactic acid bacteria in the larval midgut of the keratinophagous lepidopteran, Hofmannophila pseudospretella. Lett Appl Microbiol. 2001;32:36–41. doi: 10.1046/j.1472-765x.2001.00854.x. [DOI] [PubMed] [Google Scholar]
- 21.Kukal O, Dawson TE. Temperature and food quality influences feeding behavior, assimilation efficiency and growth rate of arctic woolly-bear caterpillars. Oecologia. 1989;79:526–532. doi: 10.1007/BF00378671. [DOI] [PubMed] [Google Scholar]
- 22.Vilanova C, Baixeras J, Latorre A, Porcar M. The generalist inside the specialist: Gut bacterial communities of two insect species feeding on toxic plants are dominated by Enterococcus sp. Front Microbiol. 2016;7:1005. doi: 10.3389/fmicb.2016.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anand AAP, et al. Isolation and characterization of bacteria from the gut of Bombyx mori that degrade cellulose, xylan, pectin and starch and their impact on digestion. J Insect Sci. 2010;10:107. doi: 10.1673/031.010.10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broderick NA, Raffa KF, Goodman RM, Handelsman J. Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Appl Environ Microbiol. 2004;70:293–300. doi: 10.1128/AEM.70.1.293-300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kingsley VV. Persistence of intestinal bacteria in the developmental stages of the monarch butterfly (Danaus plexippus) J Invertebr Pathol. 1972;20:51–58. [Google Scholar]
- 26.Priya NG, Ojha A, Kajla MK, Raj A, Rajagopal R. Host plant induced variation in gut bacteria of Helicoverpa armigera. PLoS One. 2012;7:e30768. doi: 10.1371/journal.pone.0030768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staudacher H, et al. Variability of bacterial communities in the moth Heliothis virescens indicates transient association with the host. PLoS One. 2016;11:e0154514. doi: 10.1371/journal.pone.0154514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason CJ, Raffa KF. Acquisition and structuring of midgut bacterial communities in gypsy moth (Lepidoptera: Erebidae) larvae. Environ Entomol. 2014;43:595–604. doi: 10.1603/EN14031. [DOI] [PubMed] [Google Scholar]
- 29.Whitaker MR, Salzman S, Sanders J, Kaltenpoth M, Pierce NE. Microbial communities of lycaenid butterflies do not correlate with larval diet. Front Microbiol. 2016;7:1920. doi: 10.3389/fmicb.2016.01920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salter SJ, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014;12:87. doi: 10.1186/s12915-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammer TJ, McMillan WO, Fierer N. Metamorphosis of a butterfly-associated bacterial community. PLoS One. 2014;9:e86995. doi: 10.1371/journal.pone.0086995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lighthart B. Some changes in gut bacterial flora of field-grown Peridroma saucia (Lepidoptera: Noctuidae) when brought into the laboratory. Appl Environ Microbiol. 1988;54:1896–1898. doi: 10.1128/aem.54.7.1896-1898.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4:430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 34.Douglas AE. Symbiotic Interactions. Oxford Univ Press; Oxford: 1994. [Google Scholar]
- 35.Steinhaus EA. Microbial control—The emergence of an idea. Hilgardia. 1956;26:107–160. [Google Scholar]
- 36.Bucher GE. Pathogens of tobacco and tomato hornworms. J Invertebr Pathol. 1967;9:82–89. [Google Scholar]
- 37.Ehrlich P, Raven P. Butterflies and plants: A study in coevolution. Evolution. 1964;18:586–608. [Google Scholar]
- 38.L-M-Arnold A, et al. Forest defoliator pests alter carbon and nitrogen cycles. R Soc Open Sci. 2016;3:160361. doi: 10.1098/rsos.160361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearse IS, Altermatt F. Predicting novel trophic interactions in a non-native world. Ecol Lett. 2013;16:1088–1094. doi: 10.1111/ele.12143. [DOI] [PubMed] [Google Scholar]
- 40.Gibson CM, Hunter MS. Extraordinarily widespread and fantastically complex: Comparative biology of endosymbiotic bacterial and fungal mutualists of insects. Ecol Lett. 2010;13:223–234. doi: 10.1111/j.1461-0248.2009.01416.x. [DOI] [PubMed] [Google Scholar]
- 41.Rastogi G, Tech JJ, Coaker GL, Leveau JHJ. A PCR-based toolbox for the culture-independent quantification of total bacterial abundances in plant environments. J Microbiol Methods. 2010;83:127–132. doi: 10.1016/j.mimet.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Falony G, et al. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 43.Moran NA, Hansen AK, Powell JE, Sabree ZL. Distinctive gut microbiota of honey bees assessed using deep sampling from individual worker bees. PLoS One. 2012;7:e36393. doi: 10.1371/journal.pone.0036393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sudakaran S, Salem H, Kost C, Kaltenpoth M. Geographical and ecological stability of the symbiotic mid-gut microbiota in European firebugs, Pyrrhocoris apterus (Hemiptera, Pyrrhocoridae) Mol Ecol. 2012;21:6134–6151. doi: 10.1111/mec.12027. [DOI] [PubMed] [Google Scholar]
- 45.Vorholt JA. Microbial life in the phyllosphere. Nat Rev Microbiol. 2012;10:828–840. doi: 10.1038/nrmicro2910. [DOI] [PubMed] [Google Scholar]
- 46.Haloi K, Kalita MK, Nath R, Devi D. Characterization and pathogenicity assessment of gut-associated microbes of muga silkworm Antheraea assamensis Helfer (Lepidoptera: Saturniidae) J Invertebr Pathol. 2016;138:73–85. doi: 10.1016/j.jip.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Grice EA, et al. NISC Comparative Sequencing Program Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brooks AW, Kohl KD, Brucker RM, van Opstal EJ, Bordenstein SR. Phylosymbiosis: Relationships and functional effects of microbial communities across host evolutionary history. PLoS Biol. 2016;14:e2000225. doi: 10.1371/journal.pbio.2000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kieft TL, Simmons KA. Allometry of animal-microbe interactions and global census of animal-associated microbes. Proc Biol Sci. 2015;282:20150702. doi: 10.1098/rspb.2015.0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Honek A. Intraspecific variation in body size and fecundity in insects: A general relationship. Oikos. 1993;66:483–492. [Google Scholar]
- 51.Stillwell RC, Davidowitz G. A developmental perspective on the evolution of sexual size dimorphism of a moth. Proc Biol Sci. 2010;277:2069–2074. doi: 10.1098/rspb.2009.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salem H, Kreutzer E, Sudakaran S, Kaltenpoth M. Actinobacteria as essential symbionts in firebugs and cotton stainers (Hemiptera, Pyrrhocoridae) Environ Microbiol. 2013;15:1956–1968. doi: 10.1111/1462-2920.12001. [DOI] [PubMed] [Google Scholar]
- 53.Ceja-Navarro JA, et al. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat Commun. 2015;6:7618. doi: 10.1038/ncomms8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raymann K, Shaffer Z, Moran NA. Antibiotic exposure perturbs the gut microbiota and elevates mortality in honeybees. PLoS Biol. 2017;15:e2001861. doi: 10.1371/journal.pbio.2001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Hoeven R, Betrabet G, Forst S. Characterization of the gut bacterial community in Manduca sexta and effect of antibiotics on bacterial diversity and nematode reproduction. FEMS Microbiol Lett. 2008;286:249–256. doi: 10.1111/j.1574-6968.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 56.Murthy MR, Sreenivasaya M. Effect of antibiotics on the growth of the silkworm Bombyx mori L. Nature. 1953;172:684–685. doi: 10.1038/172684a0. [DOI] [PubMed] [Google Scholar]
- 57.Visôtto LE, Oliveira MGA, Guedes RNC, Ribon AOB, Good-God PIV. Contribution of gut bacteria to digestion and development of the velvetbean caterpillar, Anticarsia gemmatalis. J Insect Physiol. 2009;55:185–191. doi: 10.1016/j.jinsphys.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 58.Rolff J, Siva-Jothy MT. Invertebrate ecological immunology. Science. 2003;301:472–475. doi: 10.1126/science.1080623. [DOI] [PubMed] [Google Scholar]
- 59.Bulla LA, Rhodes RA, St. Julian G. Bacteria as insect pathogens. Annu Rev Microbiol. 1975;29:163–190. doi: 10.1146/annurev.mi.29.100175.001115. [DOI] [PubMed] [Google Scholar]
- 60.Peleg AY, et al. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob Agents Chemother. 2009;53:2605–2609. doi: 10.1128/AAC.01533-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broderick NA, et al. Contributions of gut bacteria to Bacillus thuringiensis-induced mortality vary across a range of Lepidoptera. BMC Biol. 2009;7:11. doi: 10.1186/1741-7007-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dow JAT. Insect midgut function. Adv Insect Phys. 1986;19:187–328. [Google Scholar]
- 63.Johnson K, Felton G. Potential influence of midgut pH and redox potential on protein utilization in insect herbivores. Arch Insect Biochem Physiol. 1996;32:85–105. [Google Scholar]
- 64.Dow JAT. Extremely high pH in biological systems: A model for carbonate transport. Am J Physiol. 1984;246:R633–R636. doi: 10.1152/ajpregu.1984.246.4.R633. [DOI] [PubMed] [Google Scholar]
- 65.Jiang H, Vilcinskas A, Kanost MR. Immunity in Lepidopteran insects. In: Söderhäll K, editor. Invertebrate Immunity. Springer; New York: 2010. pp. 181–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hegedus D, Erlandson M, Gillott C, Toprak U. New insights into peritrophic matrix synthesis, architecture, and function. Annu Rev Entomol. 2009;54:285–302. doi: 10.1146/annurev.ento.54.110807.090559. [DOI] [PubMed] [Google Scholar]
- 67.Brinkmann N, Tebbe CC. Leaf-feeding larvae of Manduca sexta (Insecta, Lepidoptera) drastically reduce copy numbers of aadA antibiotic resistance genes from transplastomic tobacco but maintain intact aadA genes in their feces. Environ Biosafety Res. 2007;6:121–133. doi: 10.1051/ebr:2007028. [DOI] [PubMed] [Google Scholar]
- 68.Buchner P. Endosymbiosis of Animals with Plant Microorganisms. John Wiley & Sons; New York: 1965. [Google Scholar]
- 69.Narita S, Nomura M, Kageyama D. Naturally occurring single and double infection with Wolbachia strains in the butterfly Eurema hecabe: Transmission efficiencies and population density dynamics of each Wolbachia strain. FEMS Microbiol Ecol. 2007;61:235–245. doi: 10.1111/j.1574-6941.2007.00333.x. [DOI] [PubMed] [Google Scholar]
- 70.Santos CD, Ferreira C, Terra WR. Consumption of food and spatial organization of digestion in the cassava hornworm, Erinnyis ello. J Insect Physiol. 1983;29:707–714. [Google Scholar]
- 71.Barbehenn RV. Digestion of uncrushed leaf tissues by leaf-snipping larval Lepidoptera. Oecologia. 1992;89:229–235. doi: 10.1007/BF00317222. [DOI] [PubMed] [Google Scholar]
- 72.Després L, David J-P, Gallet C. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol Evol. 2007;22:298–307. doi: 10.1016/j.tree.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 73.Sun BF, et al. Multiple ancient horizontal gene transfers and duplications in lepidopteran species. Insect Mol Biol. 2013;22:72–87. doi: 10.1111/imb.12004. [DOI] [PubMed] [Google Scholar]
- 74.Wybouw N, et al. A gene horizontally transferred from bacteria protects arthropods from host plant cyanide poisoning. eLife. 2014;3:e02365. doi: 10.7554/eLife.02365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martinson VG, Moy J, Moran NA. Establishment of characteristic gut bacteria during development of the honeybee worker. Appl Environ Microbiol. 2012;78:2830–2840. doi: 10.1128/AEM.07810-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ravenscraft A, Berry M, Hammer T, Peay K, Boggs C. Structure and function of the bacterial and fungal gut flora of Neotropical butterflies. bioRxiv. 2017 doi: 10.1101/128884. [DOI] [Google Scholar]
- 77.Frederickson ME, et al. The direct and ecological costs of an ant-plant symbiosis. Am Nat. 2012;179:768–778. doi: 10.1086/665654. [DOI] [PubMed] [Google Scholar]
- 78.Nougué O, Gallet R, Chevin L-M, Lenormand T. Niche limits of symbiotic gut microbiota constrain the salinity tolerance of brine shrimp. Am Nat. 2015;186:390–403. doi: 10.1086/682370. [DOI] [PubMed] [Google Scholar]
- 79.Simonsen AK, Dinnage R, Barrett LG, Prober SM, Thrall PH. Symbiosis limits establishment of legumes outside their native range at a global scale. Nat Commun. 2017;8:14790. doi: 10.1038/ncomms14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gaskins HR, Collier CT, Anderson DB. Antibiotics as growth promotants: Mode of action. Anim Biotechnol. 2002;13:29–42. doi: 10.1081/ABIO-120005768. [DOI] [PubMed] [Google Scholar]
- 81.Janzen DH. The natural history of mutualisms. In: Boucher DH, editor. The Biology of Mutualism. Oxford Univ Press; New York: 1985. pp. 40–99. [Google Scholar]
- 82.Young BC, et al. Severe infections emerge from the microbiome by adaptive evolution. bioRxiv. doi: 10.1101/116681. [DOI] [Google Scholar]
- 83.Bennett GM, Moran NA. Heritable symbiosis: The advantages and perils of an evolutionary rabbit hole. Proc Natl Acad Sci USA. 2015;112:10169–10176. doi: 10.1073/pnas.1421388112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wiens JJ, Lapoint RT, Whiteman NK. Herbivory increases diversification across insect clades. Nat Commun. 2015;6:8370. doi: 10.1038/ncomms9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shelomi M, Lo W-S, Kimsey LS, Kuo C-H. Analysis of the gut microbiota of walking sticks (Phasmatodea) BMC Res Notes. 2013;6:368. doi: 10.1186/1756-0500-6-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lucarotti CJ, Whittome-Waygood BH, Levin DB. Histology of the larval Neodiprion abietis (Hymenoptera: Diprionidae) digestive tract. Psyche. 2011;2011:1–10. [Google Scholar]
- 87.Whittome B, Graham RI, Levin DB. Preliminary examination of gut bacteria from Neodiprion abietis (Hymenoptera: Diprionidae) larvae. J Entomol Soc Ont. 2007;138:49–63. [Google Scholar]
- 88.Šustr V, Stingl U, Brune A. Microprofiles of oxygen, redox potential, and pH, and microbial fermentation products in the highly alkaline gut of the saprophagous larva of Penthetria holosericea (Diptera: Bibionidae) J Insect Physiol. 2014;67:64–69. doi: 10.1016/j.jinsphys.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 89.Hudson AJ, Floate KD. Further evidence for the absence of bacteria in horsehair worms (Nematomorpha: Gordiidae) J Parasitol. 2009;95:1545–1547. doi: 10.1645/GE-2145.1. [DOI] [PubMed] [Google Scholar]
- 90.Taylor EC. Cellulose digestion in a leaf eating insect, the Mexican bean beetle, Epilachna varivestis. Insect Biochem. 1985;15:315–320. [Google Scholar]
- 91.Hammer TJ, Dickerson JC, Fierer N. Evidence-based recommendations on storing and handling specimens for analyses of insect microbiota. PeerJ. 2015;3:e1190. doi: 10.7717/peerj.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanders J, et al. Dramatic differences in gut bacterial densities correlate with diet and habitat in rainforest ants. Integr Comp Biol. doi: 10.1101/114512. in press. [DOI] [PubMed] [Google Scholar]
- 93.Buchler ER. Food transit time in Myotis lucifugus Chiroptera: Vespertilionidae. J Mammal. 1975;56:252–255. [PubMed] [Google Scholar]
- 94.Buchsbaum R, Wilson J, Valiela I. Digestibility of plant constitutents by Canada Geese and Atlantic Brant. Ecology. 1986;67:386–393. [Google Scholar]
- 95.Lauder AP, et al. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome. 2016;4:29. doi: 10.1186/s40168-016-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilkinson TL. The elimination of intracellular microorganisms from insects: An analysis of antibiotic-treatment in the pea aphid (Acyrthosiphon pisum) Comp Biochem Physiol A Mol Integr Physiol. 1998;119:871–881. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.