Significance
Although plants and their herbivores account for most of macroscopic, terrestrial biodiversity, we do not fully understand the evolutionary origins of this high diversity. Coevolutionary theory proposes that adaptations between plants and their herbivores are reciprocal and that their interactions might have driven diversification and community composition. Contrary to this scenario of defense and counterdefense, we find an apparent asymmetry in the interactions between plants and herbivores. Specifically, despite the evolutionary constraints of long lifetimes for trees, plant–antiherbivore defenses may be more evolutionarily labile than herbivore adaptations to their hosts, allowing long-lived plant species to persist in the arms race with their insect herbivores. In contrast, herbivores may be evolutionarily “chasing” plants, feeding on species for which they have preadaptations.
Keywords: coevolution, defensive traits, herbivores, Inga, plant–herbivore interactions
Abstract
Coevolutionary models suggest that herbivores drive diversification and community composition in plants. For herbivores, many questions remain regarding how plant defenses shape host choice and community structure. We addressed these questions using the tree genus Inga and its lepidopteran herbivores in the Amazon. We constructed phylogenies for both plants and insects and quantified host associations and plant defenses. We found that similarity in herbivore assemblages between Inga species was correlated with similarity in defenses. There was no correlation with phylogeny, a result consistent with our observations that the expression of defenses in Inga is independent of phylogeny. Furthermore, host defensive traits explained 40% of herbivore community similarity. Analyses at finer taxonomic scales showed that different lepidopteran clades select hosts based on different defenses, suggesting taxon-specific histories of herbivore–host plant interactions. Finally, we compared the phylogeny and defenses of Inga to phylogenies for the major lepidopteran clades. We found that closely related herbivores fed on Inga with similar defenses rather than on closely related plants. Together, these results suggest that plant defenses might be more evolutionarily labile than the herbivore traits related to host association. Hence, there is an apparent asymmetry in the evolutionary interactions between Inga and its herbivores. Although plants may evolve under selection by herbivores, we hypothesize that herbivores may not show coevolutionary adaptations, but instead “chase” hosts based on the herbivore’s own traits at the time that they encounter a new host, a pattern more consistent with resource tracking than with the arms race model of coevolution.
Because plants and their insect enemies are strikingly species-rich groups, understanding their interactions is a foundational issue in ecology and evolution. Coevolutionary theory has long predicted that the arms race between plants and herbivores is the principal explanation for this great diversity (1). Coevolutionary and escape-and-radiate models suggest that herbivores might drive speciation in plants (1, 2). A number of recent, independent studies suggest that herbivore pressure contributes to the high local plant diversity, or coexistence, that is typical of plant communities in tropical rainforests (3–6). For herbivores, however, many questions remain with respect to factors shaping community structure, diversification, and coevolution. To begin to address these questions, we must understand the extent to which host choice is evolutionarily conserved. Although plant antiherbivore traits play a prominent role in determining host choice and need not track plant phylogeny, antiherbivore defenses are often not sufficiently considered. Here, we test hypotheses about herbivore host selection by extensively characterizing defenses of a speciose genus of trees co-occurring at one site, and by comparing phylogenies for both trophic groups.
The seminal work of Ehrlich and Raven (1) suggested that plants and insects reciprocally produce evolutionary change. This model predicts that evolutionary constraints (hereafter phylogenetic conservatism) will lead to phylogenetic signal for traits related to their interactions, for both hosts and herbivores. In other words, closely related plant species would have similar defenses and closely related herbivores would feed on closely related plants. Thus, the relationship between plants and herbivores, at both ecological and evolutionary levels, is expected to be strongly phylogenetically structured.
The Ehrlich and Raven model, and many subsequent studies, consider macroevolutionary processes across genera and families (7, 8). At these levels, phylogeny may be a good proxy for shared traits, and many resource acquisition traits show a phylogenetic signal. However, recent work at the species level suggests that herbivores have selected for divergence in defenses in closely related host species. Specifically, studies within several plant genera have found a poor pattern of congruence between their phylogenetic histories and the expression of defenses (3, 5, 6, 9, 10). Furthermore, within a community, neighboring plants are more likely to differ in defenses than expected by chance even if they are closely related (3, 5, 6). Following the notion that herbivores track or “chase” host defenses and not host species per se (11, 12), we would expect host choice at the level of plant species to mirror host defenses more than host phylogeny, a pattern that would diminish the role of plant phylogenetic relationships in the origin and structure of herbivore communities.
These predictions suggest that, in addition to phylogeny, focusing on ecologically relevant traits for host selection, such as plant defenses, is central to critical evaluation of the various hypotheses relating plant–insect interactions to community composition and diversity. These hypotheses must incorporate the multiple defenses used by plants (mechanical, developmental, phenological, biotic, chemical), and the diverse assemblages of herbivores that exert disparate selective pressures on their hosts. Moreover, we must consider that different defenses can evolve independently. This would provide a high-dimensional niche space for plants and herbivores, with substantial potential for adaptive radiation in both trophic groups. Therefore, understanding traits and their evolution at the level of species can deliver important insights into the processes structuring plant and herbivore communities.
Here, we test the role of plant–insect interactions in shaping herbivore host association and community structure. We focus our study on the species-rich neotropical tree genus Inga (Fabaceae, subfamily Mimosoideae) and its associated herbivores at Los Amigos Research Center, located in the lowland Amazon region of Madre de Dios, Peru. Inga includes ∼300 described species and occurs in moist and wet forests throughout the New World tropics. In Los Amigos and many neotropical forests, Inga constitutes one of the most diverse and abundant tree genera. For example, in 25 ha of forest in Amazonian Ecuador, there are >40 Inga species representing 6% of stems >1 cm (13).
We previously reported that defensive traits in Inga diverge among close relatives as well as among neighbors (3). Although these results suggest that herbivores may affect evolutionary change and local community assembly composition in Inga, much remains unknown regarding Inga’s natural enemies. To address this gap, we investigated the interactions between Inga and its herbivores by asking the following: (i) Are different antiherbivore traits of leaves evolving independently? (ii) Do Inga defensive traits and/or phylogenetic relationships predict host use by herbivores? (iii) Do the major lepidopteran clades that feed on Inga differ in their relationships to Inga traits and Inga phylogeny? (iv) Are closely related herbivores feeding on closely related plants?
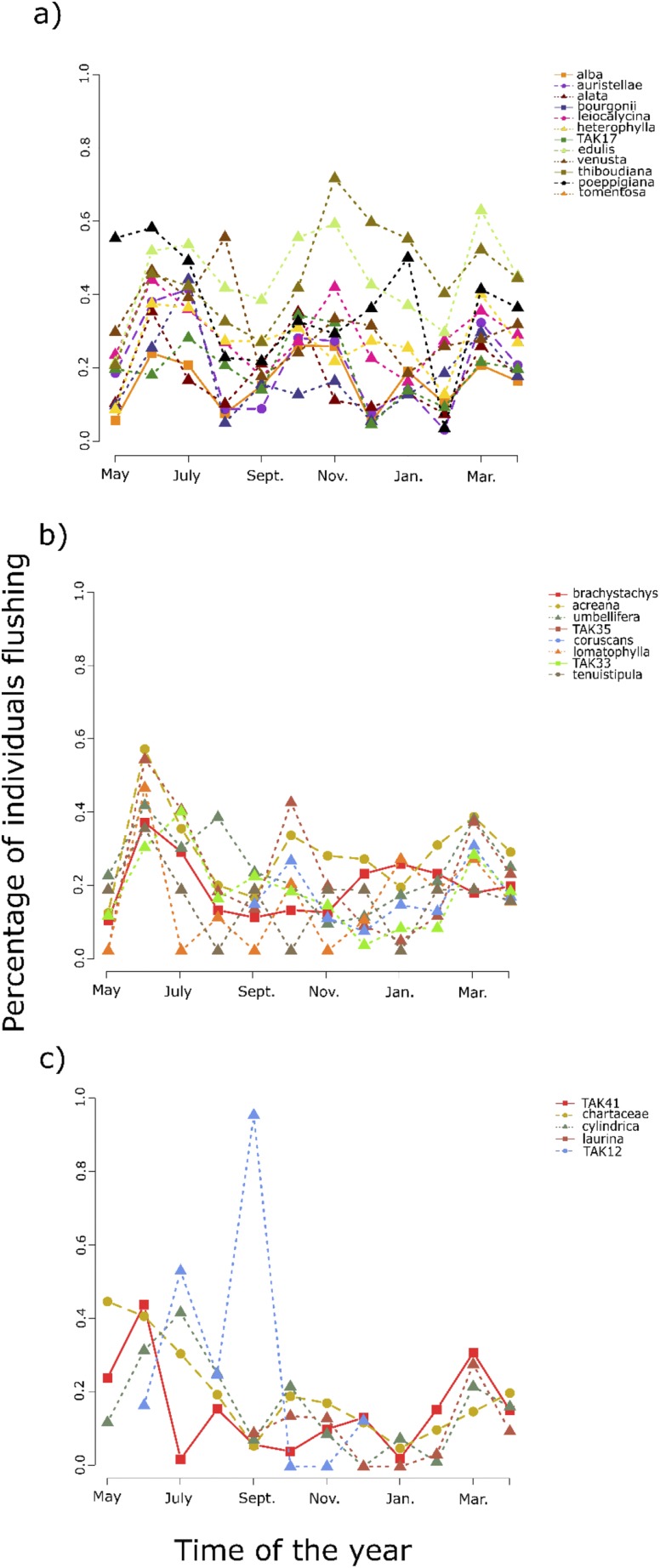
At Los Amigos, we characterized the defensive traits of expanding leaves for 33 species of Inga. We focused on expanding leaves as the majority of leaf damage occurs during this short window before leaves toughen (14). We included multiple classes of antiherbivore traits to capture as complete an understanding of the entire defensive profile as possible. We recorded the presence of defensive compounds, particularly several different classes of flavonoids, tannins, saponins, and metabolites containing amines. Total production of secondary metabolites in Inga comprises about 40–50% of leaf dry weight (15). Detrimental effects for lepidopteran herbivores have been observed in the laboratory at 0.5–2% of diet for whole-leaf extracts and specific fractions, suggesting that these metabolites are highly toxic (16–19). We also characterized the broader defense phenotype of each Inga species in terms of physical defenses (length and density of the nonglandular trichomes), biotic defenses (the number and identity of protective ants visiting the leaves), developmental defenses (leaf expansion rate and chloroplast development; ref. 20), and phenological defenses (timing and synchrony in leaf production; Fig. S1; refs. 21 and 22). Leaf expansion rate and chloroplast development (developmental defenses) have been recognized as adaptations that minimize vulnerability to herbivores (20, 22–24). More rapid expansion shortens the vulnerable period when leaves are tender and preferred by herbivores (22). Species with delayed chloroplast development have lower concentrations of energy and nitrogen and thus lose fewer resources per given amount of herbivory (22–24). Synchronization and timing of leaf production (phenological defenses) have been shown to be important defensive strategies (21, 22, 25). Species may synchronize leaf production at a population level within species to satiate herbivores (21). Meanwhile, temporal separation of leaf production among species may be favored as a strategy for partial escape from herbivory (20).
Fig. S1.
Leaf production of 25 Inga species in Los Amigos between 2007 and 2008 as the proportion of individuals flushing (A) continuously, (B) moderately continuously, and (C) episodic (highly synchronized).
We DNA barcoded and quantified the abundance of Lepidoptera associated with the expanding leaves of each Inga species, and developed multilocus phylogenies for the most abundant lepidopteran clades, the superfamily Gelechioidea, and the families Erebidae and Riodinidae. We also developed a multilocus phylogeny for Inga. We use these data and phylogenetic hypotheses to address how, for plant species within a single genus and at a single site, antiherbivore traits influence the assembly of the herbivore community.
Results and Discussion
Are Different Antiherbivore Traits of Leaves Evolving Independently?
Most of the antiherbivore traits we measured show weak and nonsignificant correlations across species, with a few key exceptions (Table S1). Species of Inga with a higher density of leaf trichomes also exhibit longer trichomes (physical defenses, r2 = 0.74, P < 0.001). Rapid leaf expansion correlates with lower chlorophyll content (developmental defenses, r2 = −0.53, P < 0.01) as has been found in other studies (3, 22). Species that were more similar in the mean number of ants visiting the extrafloral nectaries, were also visited by similar species of ants (biotic defenses, partial Mantel test controlling for phylogenetic relatedness, r = 0.28, P = 0.02).
Table S1.
Pairwise correlations between defense traits among Inga species
| Leaf defense traits | Trichome length | Leaf expansion rate | Leaf chlorophyll content | Mean number of ants per nectary | Ant visitor community to extrafloral nectaries | Chemistry | Timing of leaf production | Synchrony in leaf production |
| Trichome density, no. of hairs per 2 cm2 | 0.79†,* | 0.1† | −0.07† | −0.08† | 0.08 | 0.01 | −0.013 | 0.31† |
| Trichome length, mm | −0.06† | −0.15† | −0.05† | 0.22 | 0.07 | −0.04 | −0.2† | |
| Leaf expansion rate, % per d−1 | −0.55†,* | 0.09† | 0.08 | 0.20 | −0.012 | 0.15† | ||
| Leaf chlorophyll content, mg per m−2 | −0.24† | 0.04 | 0.07 | −0.04 | −0.22† | |||
| Mean number of ants per nectary | 0.28* | 0.08 | −0.05 | 0.14† | ||||
| Ant visitor community to extrafloral nectaries | 0.02 | 0.03 | 0.002 | |||||
| Chemistry (presence/absence of secondary compounds) | 0.06 | 0.03 | ||||||
| Timing of leaf production (mean angle) | 0.07 |
Correlation coefficients with † are based on the phylogenetic generalized linear model (PGLS); the rest are partial Mantel r. Significant values (P < 0.05) are marked with an asterisk.
We performed a phylogenetic principal-component analysis (PPCA) on traits represented by continuous data to test the hypothesis that different defense categories evolve independently (i.e., are orthogonal in trait space). Consistent with the trait correlation analyses, PPCA determined five significant axes of defense variation [eigenvalues >0.7; Jolliffe cutoff (26)], with each axis being highly correlated with a different defense mechanism. The first axis was highly correlated with trichome density and length (physical defenses, r = 0.94 for both traits), the second axis with leaf expansion rate and chlorophyll content (developmental defenses, r = 0.67 and r = −0.81, respectively), the third axis with timing of leaf production (phenological defense, r = 0.87), the fourth axis with the mean number of ants visiting extrafloral nectaries (biotic defenses, r = 0.73), and the last axis with synchrony in leaf production (r = −0.68).
Because none of the PPCA-derived axes was correlated with chemical defenses (Table S2), antiherbivore traits in Inga clearly fall into six independent axes of defense expression or categories: physical, developmental, biotic, timing, synchrony, and chemical. Given that each defense category varies largely independently of the others, plants may have many axes of trait divergence. Despite the possibility that some trait combinations may be missing due to trade-offs or physiological constraints, it seems very likely that the defensive phenotypes of plants can respond to selection in complex ways. This would support the hypothesis that antiherbivore defenses may provide a highly dimensional niche space in which many species of plants and herbivores, some of which are otherwise ecologically similar, are distinctive and can stably co-occur.
Table S2.
Correlations between chemical defenses and phylogenetically PCA-derived axes (PPCA) of defense variation
| PPCA axes | R | P (reps = 9,999) |
| PPCA 1 (physical defenses) | 0.06 | 0.20 |
| PPCA 2 (developmental defenses) | 0.09 | 0.10 |
| PPCA 3 (timing in leaf production) | 0.15 | 0.06 |
| PPCA 4 (biotic defenses) | 0.11 | 0.82 |
| PPCA 5 (synchrony in leaf production) | 0.03 | 0.51 |
Correlation coefficients are partial Mantel r.
Do Inga Defensive Traits and/or Phylogenetic Relationships Predict Host Use by Herbivores?
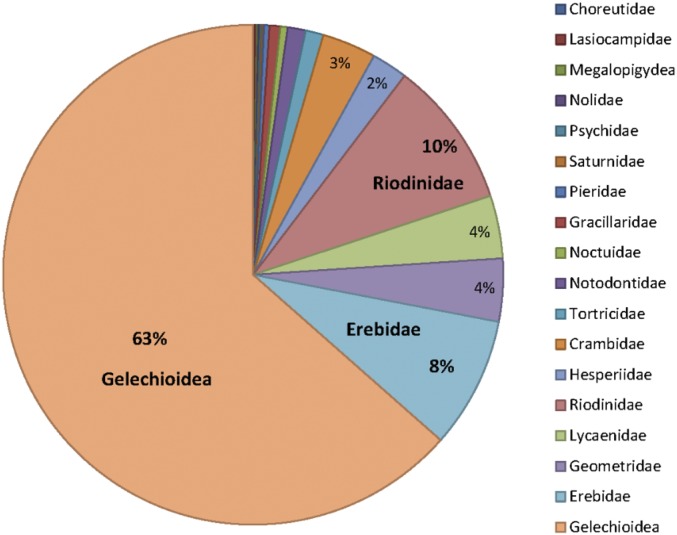
Although Inga species host a diversity of herbivores, we focus on the Lepidoptera because extensive field observations demonstrate that these are responsible for most of the damage to expanding leaves. The herbivore community was characterized with a sample of 1,576 individuals comprising 174 molecular operational taxonomic units (MOTUs) based on DNA sequences for the widely used cytochrome oxidase c subunit 1 barcode. These comprise representatives of 19 families of Lepidoptera, feeding on 33 Inga species (Fig. S2).
Fig. S2.
Lepidopteran herbivore families associated with Inga in Los Amigos. Percentages represent the relative abundances. All individual herbivores were barcoded with the mitochondrial locus COI. In addition, nuclear genes (elongation-factor 1α, EF-1α, and wingless Wg) were sequenced for the Gelechioidea, Riodinidae, and Erebidae.
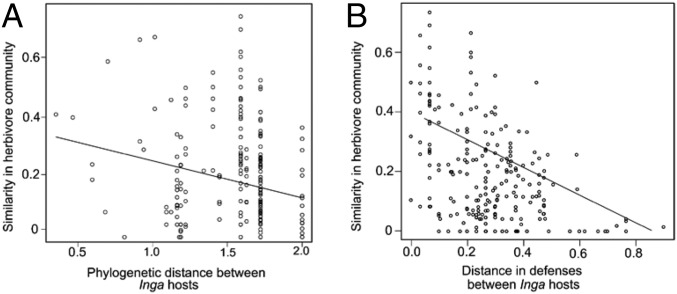
We determined whether differences in total herbivore assemblages for pairs of Inga hosts were related to Inga phylogeny and/or defensive traits. Matrix correlation analyses using the entire sample of Lepidoptera reveals a negative relationship of assemblage similarity with host phylogenetic distance, such that more closely related Inga species showed greater lepidopteran community similarity (partial Mantel r = −0.25, P = 0.02; Fig. 1A and Table 1). We also found a negative correlation between assemblage similarity and defense distance between Inga hosts, such that Inga species with similar defenses are attacked by similar herbivores (partial Mantel r = −0.50, P = 0.01; Fig. 1B and Table 1). The much higher correlation in the partial Mantel test for defenses indicates that similarity in defensive traits between Inga species predicts host associations for lepidopteran herbivores much more strongly than phylogenetic relatedness of Inga.
Fig. 1.
Relationship between the similarity of lepidopteran communities (1, Bray–Curtis index) on host plants vs. (A) phylogenetic distance between Inga hosts and (Mantel r = −0.25, P = 0.02), (B) distance in defenses between Inga hosts for all pairwise combinations of plants (partial Mantel r = −0.50, P = 0.001).
Table 1.
Summary statistics for the relationship between herbivore communities and host plant traits
| Host plant traits | All herbivores | Gelechioidea | Riodinidae | Erebidae |
| R | r | r | r | |
| Phylogeny | −0.25* | −0.24* | −0.2 | −0.04 |
| All defenses | −0.50* | −0.42* | −0.33* | −0.16 |
r represents the Mantel and partial Mantel correlations between the dissimilarity in host plant traits and their herbivore communities measured by the Bray–Curtis index. Significant values (P < 0.05) are marked with an asterisk.
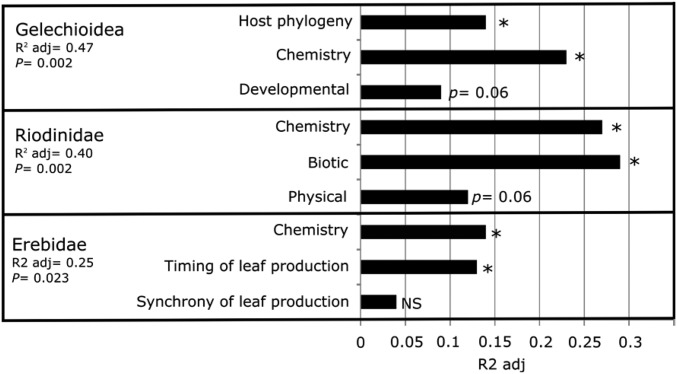
To quantify the extent to which host phylogeny and/or host defenses structure associated herbivore assemblages, and determine which host traits are the strongest predictors (i.e., most important), we also performed distance-based redundancy analyses (dbRDAs). The dbRDAs showed that plant defensive traits explained much of the variation in the lepidopteran assemblage (R2adj = 0.40, P = 0.001). Thirty percent of the total variation was explained solely by host chemistry (chemistry R2adj = 0.31, P = 0.001), with an additional 6% explained by physical defenses (trichome density and length R2adj = 0.06, P = 0.02). Neither host phylogeny nor the other four defenses were selected as significant variables.
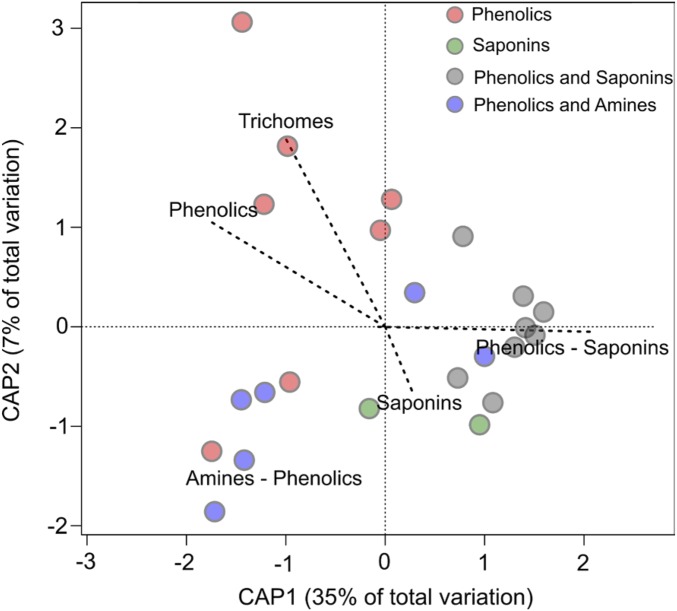
The ordination diagram of the herbivore assemblages associated with each Inga species (a grouping of Inga hosts in “herbivore space”; Fig. 2) supported these findings by clustering assemblages from Inga species that share similar secondary metabolite profiles. This result is of particular interest because, in contrast to previous studies including only a few compounds characteristic of particular species, genera, or families, our approach covered a range of chemical defenses, including saponins, flavonoids, tannins, and diverse amines. This suggests that studies with broader analyses of plant metabolites could be highly instructive.
Fig. 2.
Distance-based redundancy analysis plot of the most parsimonious model for the lepidopteran community similarity measured by the Bray–Curtis index (R2adj = 0.40, P = 0.001). Each point represents an Inga species and is color-coded by defense chemistry.
Do the Major Lepidopteran Clades That Feed on Inga Differ in Their Relationship to Inga Traits and Inga Phylogeny?
The Gelechioidea, Erebidae, and Riodinidae are the major lepidopteran clades feeding on young Inga leaves, comprising 52% of the species and 81% of the individuals found (Fig. S2). First, we examined whether these clades differed in their responses to Inga traits vs. Inga phylogeny. For all clades, matrix correlation analyses showed that the similarity in herbivore assemblage consistently decreases with increasing defense distance between Inga species (Table 1), and significantly so in two of three datasets. In contrast, plant relatedness had a significant effect on host choice only for the superfamily Gelechioidea, with similarity in herbivore assemblage decreasing with increasing phylogenetic distance between Inga species (Table 1). Host use by Riodinidae and Erebidae was not predicted by plant phylogeny (Table 1).
Variation partitioning analyses revealed that different groups of herbivores are associated with different host traits. For example, Gelechioidea are distinct from the other two clades in that, for most species, the larvae minimize predation by concealment, either by leaf-mining or by hiding between leaves bound together with silk. For this group, plant secondary metabolites (R2adj = 0.23, P = 0.02) were selected as the best predictor (Fig. 3), with higher abundance on Inga species that express saponins. To a lesser degree, phylogenetic relationships between Inga hosts (R2adj = 0.16, P = 0.005) also predicted host association. Because phylogeny is a synthetic measure for phylogenetically conserved traits, these results suggest that other conserved nutritive or defensive traits, not included in this study, are also important predictors of host association for this group of herbivores. Developmental defense was marginally significant, with Gelechioidea associated with species with a relatively slow rate of leaf expansion. Variation in leaf development could affect larvae survival, particularly for species that require longer periods of time for successful development and are confined to a single leaf during their entire larval stage.
Fig. 3.
Results of best-fit distance-based redundancy analyses (dbRDAs) models for the three most abundant lepidopteran families. Significant values (P < 0.05) are marked with an asterisk.
In contrast, for Riodinidae, phylogenetic relationships between Inga hosts were not a significant predictor. Instead, riodinids were more abundant on those Inga that receive greater ant visitation, with biotic defenses explaining as much as 30% of the total variation in community similarity (R2adj = 0.29, P = 0.014; Fig. 3). Given that ants commonly prey on caterpillars, this is unusual. However, the larvae of most Riodinidae minimize predation by recruiting ant bodyguards in exchange for honeydew secreted by the larvae; riodinid larvae are, in fact, myrmecophiles (27). Hence, a strong positive effect of ants on host selection and larval survival in Riodinidae is expected. Leaf chemistry also played a significant role in host associations; as for Gelechioidea, the preferred species were defended by saponins (R2adj = 0.27, P = 0.04; Fig. 3). Trichomes were marginally significant, with higher abundance on Inga species with more trichomes.
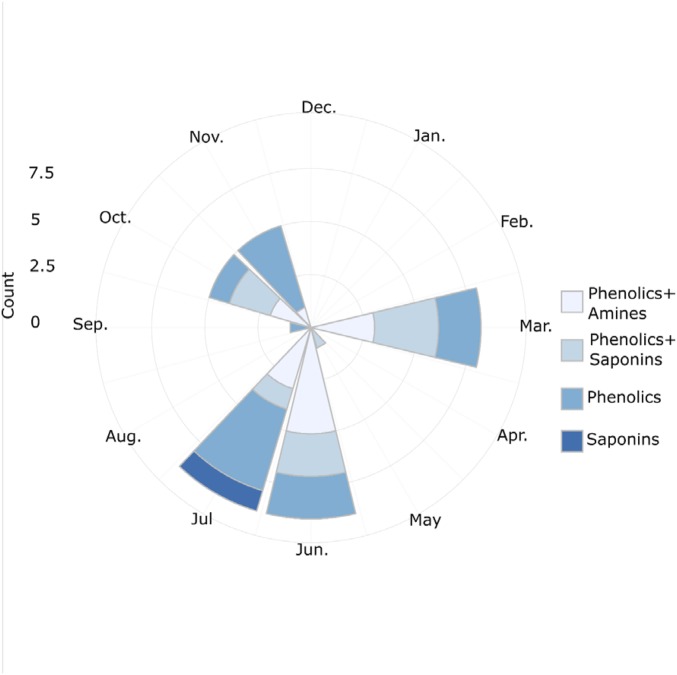
For Erebidae, phylogenetic relationships between Inga hosts were again not significant, but leaf chemistry, specifically amine-containing compounds (R2adj = 0.14, P = 0.01), and phenology were important predictors of abundance of Erebidae (Fig. 3). The phenology of young leaf production, including both the degree of synchrony and, for synchronous species, the date of their leaf flush, function as defenses (21). These plant traits restrict access of leaves to herbivores and are predicted to influence specialization of young-leaf feeders. Our analysis of Erebidae strongly supports this hypothesis, with a significant effect of peak month of leaf flush (R2adj = 0.13, P = 0.04). In particular, Erebidae preferred Inga species with flushing peaks in June–July and October–November, the beginning of the dry and wet seasons, respectively, over species that flushed at other times of the year. Moreover, only species of Inga that express amines had peaks in leaf production at around the same times of the year (partial Mantel r = 0.12, P = 0.02; Fig. S3), in episodes that are synchronous within species and staggered among species. These findings suggest that Erebidae closely track leaf production for their preferred hosts, and that flushing leaves simultaneously at a population level, is a strategy to satiate herbivores (21, 28). Our results reinforce long-standing observations that the key stages in the life cycles of herbivorous invertebrates, such as egg deposition, diapause, migration, and possibly mating, may be synchronized with the availability of their principal resource, expanding leaves (28–32) or developing inflorescences (33).
Fig. S3.
Stacked circular histogram of peak flushing events for Inga species at Los Amigos between 2007 and 2008. Flushing events are grouped by defense chemistry.
Although chemistry was important for all three clades, two clades preferred hosts that accumulate saponins, whereas Erebidae preferred amines. Clearly, chemistry alone actually is a complex of traits, many of which may evolve independently. Hence, the total number of orthogonal traits likely exceeds six.
We hypothesize that the differences among lepidopteran clades in which Inga defensive traits most influence host associations reflect differences in physiology, ecology, and natural history. These differences appear to be at the level of families; more closely related herbivores feed on suites of plants with similar defenses, whereas herbivore families diverge in terms of which defenses matter most for host choice. This result suggests that host choice may evolve slowly relative to plant defenses.
A final important point regards our result that plant defensive traits have a greater predictive power in explaining host associations than does plant phylogeny and that different herbivores respond to different plant defenses. These results highlight the limitations of using plant phylogeny alone to study the processes structuring herbivore communities. This is especially the case when variation in key defensive traits of local plant assemblages is not tightly correlated with their phylogenetic relationships, which seems to be an emerging pattern for plant communities (5, 6, 10, 34, 35). This underscores the importance of characterizing all antiherbivore traits for understanding the ecology and evolution of host range.
Are Closely Related Herbivores Feeding on Closely Related Plants?
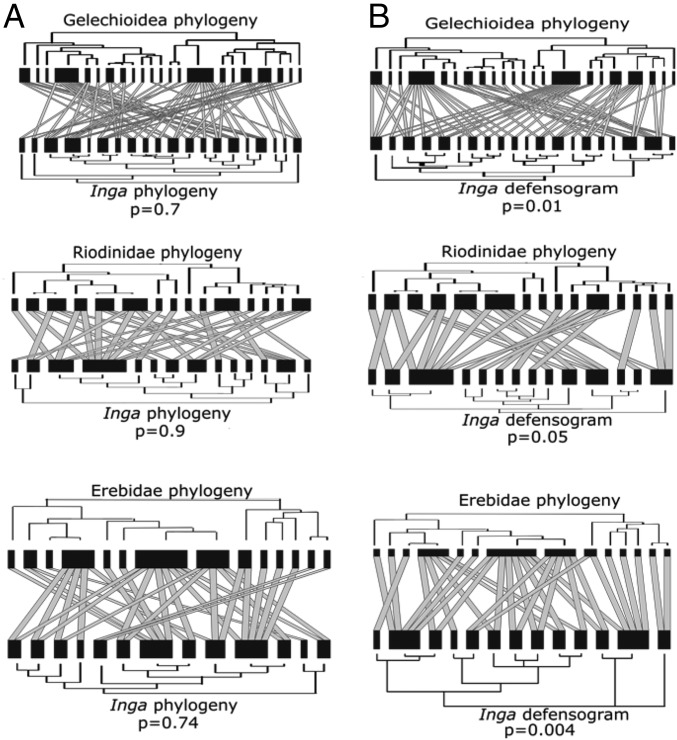
At an evolutionary level, our results are consistent with the idea that host defensive traits exert strong constraints on herbivore host choice, while herbivore traits that determine host choice may evolve relatively slowly. For example, the three lepidopteran families choose hosts based on distinctive sets of host traits and five out of six of the defense categories are important in constraining at least one host association. Nevertheless, we also hypothesize that the ensemble of herbivores attacking a given Inga species may change fairly readily as herbivores shift onto or add host species for which they have appropriate adaptations (36). Given low phylogenetic patterning of defensive traits in Inga, such a model predicts low topological congruence between Inga and herbivore phylogenies. For the three major herbivore clades, we found no indication of significant congruence between the Inga and herbivore phylogenies (Fig. 4A), a result further supported by the nonmonophyly of the specific lepidopteran groups associated with Inga. For Riodinidae and Erebidae, the species found feeding on Inga belong to several genera that are not closely related [e.g., Nymphidium, Sarota, Synargis for Riodinidae (37) and Coenipeta, Helia, Melese, Pelochyta, among others, for Erebidae (38)]. In addition, several of these species also occur on other genera of host plants. For example, the species of Nymphidium commonly found on Inga also occur on other legumes such as Zygia (Mimosoideae, very closely related to Inga), and the more distantly related Senna and Cassia (Caesalpinioideae; ref. 39, Janzen and Hallwachs, Caterpillars of ACG database: janzen.sas.upenn.edu/index.html). The fact that the sampling in the phylogenies of these two families is likely overdispersed across subclades also helps to interpret the effects of undersampling the herbivore phylogeny. A subsample that contains several lineages that are not closely related will err in the direction of phylogenetic divergence (40), because it would tend to inflate the average phylogenetic distance among herbivore species. Thus, trait conservation within the focal herbivore families that we report likely is a robust pattern.
Fig. 4.
Bipartite trophic network of Inga hosts and herbivores. (A) Phylogenies of Inga and Lepidoptera plotted in the margins. (B) Phylogenies of Lepidoptera and Inga defensogram plotted in the margins. For each network, lower bars represent host abundance and upper bars represent herbivore abundance.
For Gelechioidea, a poorly understood group with few larval feeding records in the tropics, we do not know whether one or more genera are associated with Inga. However, given the published phylogeny (41), the morphologies, and the feeding modes of the larvae that we observed (i.e., external feeders and leaf miners), it seems likely that the Gelechioidea that feed on Inga are not closely related.
Our analyses of both phylogenies and defensive traits do not support a model of reciprocal evolutionary change (Fig. 4A). Instead, they are consistent with macroevolutionary tracking of Inga defenses; that is, herbivore phylogenies are more significantly associated with a dendrogram of Inga defenses than expected by chance (Fig. 4B). More closely related herbivores preferred host species with similar defenses rather than closely related Inga. Our results are consistent with reports that the evolution of host use in herbivorous insects seems to be relatively more conserved with respect to host defenses rather than to host phylogeny, not only at the family level (42, 43) but also at finer taxonomic scales (10, 44).
Inga and Its Herbivores: Further Implications.
Do plant–herbivore interactions promote coexistence?
The idea that interactions between plants and herbivores may permit high local diversity by favoring coexistence has received considerable theoretical attention and some empirical study. One mechanism could be through increased niche differentiation for both plants and herbivores. Negative, density-dependent interactions with natural enemies could be a principal mechanism structuring plant community assembly because not sharing herbivores with neighbors gives the advantage of reduced damage or “enemy release” (45). Similarly, higher resource partitioning for insect herbivores may narrow niches, especially in tropical forests where herbivores are often highly specialized (46). Previously, we found that Inga species that are neighbors in Los Amigos and in Panama differ more in defense strategy than a random draw of the Inga community (3), and studies of other species-rich tropical genera, Bursera, Psychotria, and Piper, and at other sites, Mexico and Costa Rica, reveal the same pattern (4–6). Several results from the present study bolster this argument. First, we found that lepidopteran herbivores of Inga preferentially forage on subsets of species with similar defensive profiles, even though they are under the same selective pressures and community dynamics, and have the option to select any Inga from the community pool as host, suggesting that herbivore associations are constrained by differences in defensive traits. We also found that different groups of herbivores are associated with hosts based on different traits, in ways that make good sense given herbivore biology. Second, we also showed that antiherbivore defenses for Inga in Los Amigos fall into at least six independent axes of defense expression, providing a multidimensional niche space for coexistence within which a large number of co-occurring plant and herbivore species might sort in ecological time (3, 47). Last, as is noted below, plant–herbivore coevolution may be asymmetric, with more labile evolution of plant defenses. As has been suggested for mutualistic networks, the uneven dependency between partners in the interaction may promote stable coexistence (48). Thus, more attention to plant–herbivore interactions has the potential to reveal the mechanisms by which a considerable number of plant species coexist in tropical forests.
Asymmetry in plant vs. herbivore diversification.
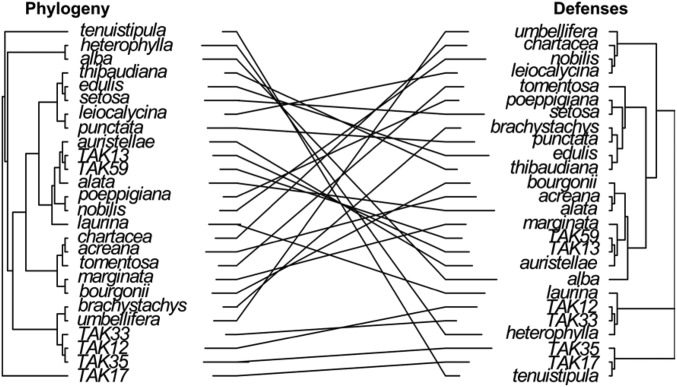
One long-standing prediction from coevolutionary theory is that defenses of plants and host specificity of insect herbivores should show phylogenetic signal, due to phylogenetic conservatism (1). However, our data do not support this prediction. We find that at least six different classes of defense adaptations can evolve independently. Only trichomes show significant (but not high) phylogenetic signal (Blomberg’s K̅ = 0.48, P = 0.05, Table S3) (3), while the other five axes of defense are independent of phylogeny and can be highly divergent among closely related Inga species (Fig. S4) (3). Furthermore, herbivore assemblages found on Inga species correlate better with host defenses than with host phylogeny (Fig. 1), and host associations for the three most abundant groups of herbivores show phylogenetic relationship with host defenses, but not host phylogeny (Fig. 4). These results strongly suggest that escape from herbivores, associated with rapid evolution of plant defenses (3), has been an important process in the diversification of Inga.
Table S3.
Measure of phylogenetic signal for each phylogenetically PCA-derived axis (PPCA) and the principal coordinates of the chemistry and ant species distance matrices (PCO) using Blomberg’s K
| Defensive traits | K statistic | P (reps = 9,999) |
| PPCA 1 (physical defenses) | 0.48 | 0.05 |
| PPCA 2 (developmental defenses) | 0.32 | 0.32 |
| PPCA 3 (timing in leaf production) | 0.24 | 0.81 |
| PPCA 4 (biotic defenses–ant number) | 0.23 | 0.81 |
| PPCA 5 (synchrony in leaf production) | 0.30 | 0.40 |
| Chemistry PCO1 (46% of variation) | 0.34 | 0.52 |
| Chemistry PCO2 (19% of variation) | 0.27 | 0.50 |
| Biotic PCO1 (ant species, 17% of variation) | 0.30 | 0.50 |
| Biotic PCO2 (ant species, 15% of variation) | 0.30 | 0.42 |
| Biotic PCO3 (ant species, 12% of variation) | 0.30 | 0.53 |
For PCO components, values in parentheses represent the percentage of variation explained by each component.
Fig. S4.
Comparison between the phylogenetic tree (Left) and the defensogram (defense dendrogram; Right) for Inga species.
In contrast, although adaptations in plant defenses should reduce herbivore fitness, leading to herbivore counteradaptations, the response of the herbivores to selection is less clear. The fact that closely related herbivores attack hosts with similar defense phenotypes rather than closely related ones, imply that herbivores are not tracking species per se but are tracking resources for which they have appropriate adaptations: seasonal activity of females that matches the timing of leaf flushing, host-finding capabilities, avoiding larval predators (particularly ants), a larval period that matches the rate of leaf development, and avoiding the toxic effects of plant chemicals (11). Switches to a novel host with divergent defenses would require that an herbivore rapidly evolve multiple adaptations. However, genetic variation for correlated innovations in a suite of traits is considered improbable (12). If closely related herbivores are similar in the complex set of adaptations to their hosts such that they are constrained to feed on hosts with similar defenses and if plant defenses evolve rapidly, then a pattern of reciprocal diversification seems less likely. Thus, in contrast to a model of a tight coevolutionary process, the interactions between Inga and its herbivores appear to be asymmetric. While plants may evolve under selection by herbivores, herbivores may not show coevolutionary adaptations but, instead, may “chase” or track hosts based on host defenses (refs. 49–52; see ref. 53 for an alternative hypothesis).
This framework suggests that antiherbivore defenses may evolve more rapidly than the herbivore traits that determine host choice and/or ability to feed and grow successfully, allowing plant species to outpace the relatively short generation times of herbivorous insects. We propose that, despite constraints on rates of adaptation imposed by their long lifetimes, the evolutionary lability of Inga defensive traits allows them to persist in the arms race.
Materials and Methods
Study Site.
This study was carried out at the Los Amigos Research Center (12°34 S, 70°05 W; elevation, ∼270 m) located in a continual expanse of forests between two national parks in the lowland Amazon region of Madre de Dios, Peru. Los Amigos is a conservation concession that comprises 453 ha of primary tropical rainforest on a mixture of upland terraces and floodplains. Annual rainfall at Los Amigos is between 2,700 and 3,000 mm, and the mean monthly temperature ranges from 21 to 26 °C (54).
Characterization of Herbivores and Defensive Traits of Inga.
Herbivores and defense trait data were collected on expanding leaves from understory saplings of Inga species. To record host associations of lepidopteran herbivores, we visually searched young leaf flushes and collected only those larvae that were found feeding. All larvae, that is, caterpillars, were assigned to morphospecies in the field and subsequently to MOTUs (for MOTU assignment, see SI Text) in the laboratory using sequences from the mitochondrial gene cytochrome oxidase I (COI). MOTUs were allocated to taxonomic families by searching each consensus sequence against the National Center for Biotechnology Information (NCBI) BLAST web interface, with a minimum accepted similarity for assignment of 90%.
We recorded the presence or absence of several classes of phenolic compounds (10 classes), saponins (1 class), and metabolites containing amines for expanding leaves (3 classes, Table S4). Details on chemical procedures are reported in ref. 3. We assessed the length and density of trichomes per area (number of hairs per 2 cm2 on the basal leaf surface). Leaf expansion rate was determined as the percent increase in area per day. Chloroplast development was measured as the chlorophyll content (in milligrams per square decimeter) of leaves between 30% and 80% of full expansion. To measure timing and synchrony in leaf production, we monitored between 30 and 70 individuals per tree species for monthly leaf production. To estimate timing in leaf production, we calculated the mean angle (using circular statistics), which indicates the average date of peak flushing activity across all individuals (55). To estimate synchrony in leaf production we calculated the coefficient of variation (CV) of the number of plant individuals per species flushing each month. We also determined the identity and the abundance of ants visiting these nectaries (number of ants per nectary). See SI Text for detailed methods.
Table S4.
Inga chemotypes
| Chemotypes | ||||||||||||||
| Inga species | QAG | DHM | C/E | C/EG | C/ERG | GC/GE | GC/GEG | GC/GEC | FG | TYRG | TYMG | C\E PYR GAL | TYR | SAP |
| acreana | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| alata | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| alba | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| auristellae | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| bourgonii | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| brachystachys | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| capitata | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| chartacea | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| edulis | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| heterophylla | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| laurina | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| leiocalycina | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| marginata | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| nobilis | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| poeppigiana | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| punctata | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| sapindoides | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| setosa | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TAK12 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| TAK13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| TAK17 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TAK35 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| TAK59 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| tenuistipula | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| thibaudiana | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| tomentosa | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| umbellifera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| venusta | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
C/E, catechin/epicatechin; C/EG, catechin/epicatechin gallate; C/ERG, catechin/epicatechin–rhamnose–gallate; C/E PYR GAL, catechin/epicatechin pyranose gallate; DHM, dihydromyricetin; FG, flavone glycoside; GC/GE, gallocatechin/galloepicatechin; GC/GEC, gallocatechin/epigallocatechin–coumarate; GC/GEG, gallocatechin/epigallocatechin gallate; QAG, quinic acid–gallate; SAP, saponins; TYMG, tyramine gallate; TYR, tyrosine; TYRG, tyrosine gallate.
Phylogenetic Reconstructions.
Phylogenetic analyses for MOTUs allocated to the most abundant lepidopteran clades, Gelechioidea, Riodinidae, and Erebidae, were conducted using one to three individuals per MOTU and three gene fragments: nuclear elongation factor (EF-1α) and wingless (Wg), and mitochondrial COI. Phylogenetic relationships were inferred using a multilocus coalescent-based Bayesian species tree approach in *BEAST 2.2.0 (56), with substitution models and codon partition for each marker according to the results of analyses using PartitionFinder 1.1.0 (57). Final phylogenies were derived from three independent runs of 100 million generations combined using LogCombiner 1.8 (58) with a burn-in of 10 million generations and sampling every 10,000 generations in each run. BEAST model convergence was confirmed by examination of parameter estimate distribution in Tracer 1.6. All primer sequences, PCR and sequencing protocols, and details of BEAST model assessment for each clade are in Tables S5–S7.
Table S5.
PCR protocol for lepidopteran herbivores
| Reagents | Volume in standard PCRs, µL |
| Multiplex Master Mix (Qiagen) | 10 |
| Water | 6.6 |
| Q-solution (Qiagen) | 2.0 |
| Forward 1 (20 µM) | 0.2 |
| Reverse 2 (20 µM) | 0.2 |
| DNA template | 1.0 |
| Total | 20 |
Table S7.
Species tree parameters for reconstruction of phylogenetic relationships for Gelechioidea, Riodinidae, and Erebidae
| Clades | Substitution models | Species tree priors | Tree rooting |
| Gelechioidea | COI (1): TN93 | Strict clock | MOTUs from family Tortricidae |
| COI (2): TN93 | Constant population size | ||
| COI (3): TN93 | Yule speciation model | ||
| EF1α (1, 2): HKY | |||
| EF1α (3): HKY | |||
| Wg (1, 2): HKY | |||
| Wg (3): HKY | |||
| Riodinidae | COI (1): TN93+I | Strict clock | MOTUs from families Lycaenidae and Pieridae |
| COI (2): HKY | Constant population size | ||
| COI (3): TN93+G | Yule speciation model | ||
| EF1α (1): HKY+I | |||
| EF1α (2): HKY | |||
| EF1α (3): HKY+G | |||
| Wg (1, 2): HKY+G | |||
| Wg (3): HKY | |||
| Erebidae | COI (1): HKY+G | Strict clock | MOTUs from families Noctuidae, Nolidae, and Notodontidae |
| COI (2): HKY+I | Linear population size | ||
| COI (3): HKY+G | Yule speciation model | ||
| EF1α (1, 2): HKY+I | |||
| EF1α (3): GTR+G | |||
| Wg (1, 2): GTR+G | |||
| Wg (3): GTR+G |
Numbers in parentheses represent codon positions.
Phylogenetic relationships among Inga host species were inferred using seven chloroplast regions (rpoCI, psbA-trnH, rps16, trnL-F, trnD-T, ndhF-rpl32, rpl32-trnL) and the nuclear ribosomal internal transcribed spacer regions (ITS). PCR and sequencing protocols for chloroplast regions are given by ref. 3 and for ITS by refs. 59 and 60. The phylogeny was estimated using a maximum-likelihood framework using RAxML, with separate models for ITS and cpDNA (61). The phylogeny was subsequently time-calibrated using penalized likelihood (62), where the crown age was constrained to 6 My (following refs. 59–63). Details about DNA extraction and sequencing are in SI Text.
From the resulting tree, we extracted pairwise distances between Inga species. This phylogenetic distance matrix was used in all of the subsequent ecological analyses that involved the phylogeny of Inga. It is important to mention that, although we studied a limited number of species, the Inga community in Los Amigos is composed of phylogenetically scattered species (64). Thus, our Inga community phylogeny represents a random sampling from the whole-genus phylogeny.
Statistical Analyses.
Relationship between plant traits and phylogenetic signal.
Associations between physical, developmental, biotic, and phenological defenses were investigated using phylogenetic generalized least-squares (PGLS) regression (65). To assess the correlations between chemistry, the community of ants visiting Inga, and the other defensive strategies, we used partial Mantel tests, conditioned on a matrix of phylogenetic distances between Inga species to control for phylogenetic effects. The distance matrix for biotic (number of ants), developmental, physical, and chemical defenses, as well as synchrony in leaf production were calculated using the Manhattan dissimilarity index. For the ant visitor community, the Bray–Curtis index was used. Chemical dissimilarity between species was based on the presence/absence of secondary compounds (0/1), classified according to their structure. Because the timing in leaf production is a circular variable (mean angle), we used the angular separation method from the package circular (66) to calculate the distance matrix for this trait.
We also performed a phylogenetic PCA on continuous trait data to derive independent axes of defense variation, and to test the hypothesis that different defense phenotypes are able to evolve independently [evolutionary orthogonal in trait space (67)]. Phylogenetic signal was evaluated on the significant axes of defense variation and on the principal coordinates of the chemistry and ant species distance matrices by using Blomberg’s K̅ (68).
Constraints on host plant selection.
Differences in herbivore community structure were related to differences in phylogenetic relationships and/or defensive traits between pairs of Inga hosts using partial Mantel tests. Overlap in feeding records among host species was estimated using the Bray–Curtis dissimilarity index with relative abundance data. To quantify the extent to which host phylogeny and/or host defenses structure herbivore community and to determine which defense trait is more important, we used dbRDA with the square-root transformed herbivore community dissimilarity matrix as a response variable together with each one of the measured defensive traits, including chemistry as a dummy variable, and the principal coordinates of the phylogenetic and ant species distance matrix as explanatory variables.
Phylogenetic patterns of host use.
To investigate whether host shifts have occurred more often on Inga that are more similar in defenses or on Inga that are more closely related, we examined the congruence of the herbivore phylogenies with Inga phylogeny and Inga defenses using ParaFit (69). This statistical tool tests the significance of a hypothesis of congruence between parasites and hosts using distance matrices of associated taxa and a set of host–parasite links. Distances matrices for herbivores and plants were derived from their phylogenetic trees and from a dendrogram (hierarchical clustering) obtained from the total plant defense distance matrix. Model selection for the cluster was based on the correlation between the original distance matrix and the binary matrix representing the partitions in the cluster. The clustering algorithm “UPGMA” showed the highest correlation and hence was selected as the best model for the defenses dendrogram. Significance of the ParaFit test was assessed by permutation. All of the statistical analyses were performed in R Statistical Environment (R Core Developmental Team 2016), and details can be found in SI Text.
SI Text
Detailed Methods.
Study species.
Inga is a genus of trees in the legume family (subfamily Mimosoideae) and is found in lowland moist forests through the New World. There are ∼300 described species (70). At Los Amigos and elsewhere, the genus Inga constitutes one of the most diverse and abundant genera in any western Amazonian forest. For example, in 25 ha of forest in Amazonian Ecuador, there are >40 Inga species representing 6% of stems (13). At Los Amigos, we collected data on 33 species of Inga. We focused our study on understory saplings, a key stage in the life cycle of a tree (71).
Inga is associated with multiple insect herbivore taxa, including Coleoptera, Orthoptera, phloem-feeding Coreidae (Heteroptera), Diptera, Tenthredinoidea (Hymenoptera), Phasmatodea, and Lepidoptera. Lepidoptera cause the most damage to Inga leaves (72), and we therefore focus on them here.
To record host associations of lepidopteran herbivores, we visually searched a minimum of 10 young leaf flushes per tree species and collected only those larvae that were found feeding. Insects were collected by hand from the leaves between 2010 and 2011 for a period of 10 mo. All caterpillars were assigned to morphospecies in the field. Because no identification keys to the caterpillars of this region exist, all herbivores were subsequently assigned to molecular operational taxonomic units (MOTUs) on the basis of DNA barcode sequences for the mitochondrial gene, cytochrome oxidase I (COI) (see below). We recorded a total of 1,567 individuals in 174 MOTUs from 19 families of Lepidoptera (Fig. S2).
Plant defensive traits.
We focused our study on the defenses of expanding leaves because during this ephemeral stage they receive more than 75% of the damage accrued during the lifetime of a leaf (14, 22, 73). Therefore, the defensive traits most relevant for insect herbivores when selecting hosts would be those of young leaves.
Leaf defensive traits were collected from young leaves on 0.5- to 4-m-tall saplings in the shaded understory between 2007 and 2011. We measured multiple traits that capture the entire plant’s defensive profile. We recorded the presence or absence of several classes of phenolic compounds, saponins, and metabolites containing primary or secondary amines that have been shown to decrease the growth and survival of herbivores (73, 74). Details on chemical procedures are reported in ref. 3.
We assessed the length and density of trichomes (number of hairs per 2 cm2) in a minimum of 10 individuals per species. Young leaves are also defended against herbivory by expanding leaves rapidly and delaying the development of the chloroplast (23). Leaf expansion rate was determined as the percent increase in area per day for ∼13 individuals per species. Chlorophyll content of leaves between 30% and 80% of full expansion was estimated using three values from a Minolta SPAD 502DL meter (Spectrum Technologies). For calibration between SPAD units and chlorophyll content, a portable Spectronic 20 (Milton Roy) was first calibrated in the laboratory using expanding leaves. These were extracted with 90% acetone/10% water (vol/vol) containing Na2CO3, and centrifuged at 10,000 × g at 5 °C. Absorbances were obtained using a narrow-bandpass spectrometer at 647 and 664 nm. Chlorophyll content was determined using the equations of ref. 75. The same leaves were extracted in 95% ethanol containing Na2CO3, centrifuged at 10,000 × g at 5 °C, and transmittance measured at 663 and 725 nm. Regression analysis gave the following equation for the portable spectrophotometer:
where Chla + Chlb is the total content of chlorophyll a and b, and A663 and A725 are the absorbance readings for wavelengths of 663 and 725 nm, respectively.
In the field, the SPAD meter was calibrated using expanding leaves. The SPAD meter was used according to the manufacturer’s directions. For determination with the Spectronic 20, chlorophyll was extracted in 95% ethanol containing a small amount of NaHCO3, Na2CO3, or Na2HPO4 and centrifuged at 25 °C in a minicentrifuge (SC1006-R) at 2,000 × g. The relationship was nonlinear and the equation to convert SPAD units to chlorophyll in milligrams of chlorophyll a and b per square meter is as follows:
where Chl is the total content of chlorophyll (a and b) of the sample i, SPAD is the unitless reading from the SPAD 502DL meter, and α (0.0417) and β (0.9524) are the fitted parameters.
We measured the timing and synchrony in leaf production. Because these are two measures of food availability for insect herbivores, they therefore could play important roles in structuring herbivore assemblages (21, 22). To measure these traits, we monitored between 30 and 70 individuals per species for monthly leaf production. To estimate the timing of leaf production, or mean angle, we converted months to angles, from 0° = January to 360° = December at intervals of 30°. The mean angle for a species indicates the average date of peak flushing activity among the individuals. We evaluated the significance of the mean angle using the Rayleigh test (56). We estimated synchrony in leaf production by calculating the coefficient of variation (CV) of plant individuals per species flushing each month.
Inga leaves have extrafloral nectaries that produce nectar and attract protective ants only during the short period of leaf expansion. We determined the identity and the abundance of ants visiting these nectaries (number of ants per nectary) in ∼30 individuals per species. Ants were identified to genus, and in some cases to species based on morphology.
Herbivore phylogenies.
Phylogenetic analyses for the most abundant lepidopteran clades: the superfamily Gelechioidea, and the families Riodinidae and Erebidae were inferred using one to three individuals per MOTU (for MOTU assignment, see below), two nuclear loci: elongation factor 1α (EF-1α), wingless (Wg), and one mitochondrial locus, COI. DNA was extracted from legs or, for very small larvae, larger body parts. The remaining parts were preserved as vouchers. We extracted total genomic DNA in 50 µL of extraction buffer containing 5% Chelex 100 resin (Bio-Rad) as described in ref. 76. For COI, PCR amplification and DNA sequencing for most of our samples were generated at the Canadian Center for Barcoding using standard barcoding protocols (77, 78). For the nuclear loci and the remaining samples for COI, we performed PCR amplification with 1 µL of DNA extract, 0.2 μM of each primer, and 10 µL of Multiplex PCR kit (Qiagen) in a 20-µL reaction volume (Table S5). We used the same pair of primers for both amplification and sequencing. Primer sequences and annealing temperatures are in Table S6. PCR products were purified using a shrimp alkaline phosphatase protocol. Sequencing was performed using ABI BigDye chemistry (Perkin-Elmer Biosystems) on ABI 3730xl capillary sequencer. We sequenced all products in both directions. The sequences were assembled into contigs and manually edited using the program Sequencher version 5.1 (Gene Codes). The resulting sequences were subsequently aligned using the program MUSCLE (79).
Table S6.
Taxon-specific primer pairs used in this study with target marker, optimized annealing temperature, and amplified product size
| Primer pairs | Sequence, 5′–3′ | Target group | Locus | Annealing temperature, °C | Expected length, bp | Source |
| LepF1 | ATT CAA CCA ATC ATA AAG ATA TTG G | Lepidoptera | COI | 49 | 658 | Ref. 90 |
| LepR1 | TAA ACT TCT GGA TGT CCA AAA AAT CA | |||||
| EF51.9 | CAR GAC GTA TAC AAA ATC GG | Gelechioidea | EF1α | 53 | 511 | Ref. 91 |
| EFrcM4 | ACA GCV ACK GTY TGY CTC ATR TC | Erebidae | 55 | |||
| Riodinidae | 55.5 | |||||
| LepWg1 | GAR TGY AAR TGY CAY GGY ATG TCT GG | Gelechioidea | Wg | 60 | 403 | Ref. 92 |
| LepWg2 | ACT ICG CAR CAC CAR TGG AAT GTR CA | Erebidae | 57 | |||
| Riodinidae | 54 | |||||
| LepWg1a_mod | GAA TGT AAR TGT CAY GGY ATG TCY GG | Erebidae | Wg | 53 | 403 | This study |
| Lepwg2a_mod | ACT GCG CAG CAC CAR TKG AAT GTG CA | Riodinidae | 54.3 | |||
| LepWg1 | GAR TGY AAR TGY CAY GGY ATG TCT GG | Erebidae | Wg | 54 | 348 | This study |
| Ere_Wg_mod | GAT ACC CTC KIC CRC ARC | Riodinidae | 51.5 |
MOTU assignment used COI sequence data and the software package jMOTU (80), with a similarity cutoff of 15 bp (∼2.3%). MOTUs identified using Automatic Barcode Gap Discovery (ABGD) (81) were identical. MOTUs were allocated to taxonomic families by BLASTing each consensus sequence against the NCBI BLAST web interface, with a minimum accepted similarity for family assignment of 90%.
To recognize misidentified taxa and/or confirm the correct placement of MOTUs into families by BLAST, we carried out a number of trials with varying taxon composition (including all of the MOTUs regardless of their taxonomic family) using maximum-likelihood (ML) methods. The analyses used a GTR + Γ model of substitution, and the data were partitioned by genes. ML analyses were implemented using RAxML (62) at the CIPRES Web portal (82), and support nodes were evaluated with 1,000 bootstrap replicates of the data. This preliminary phylogeny provided molecular support for the placement of MOTUs into the families Erebidae and Riodinidae and the superfamily Gelechioidea. Arctiinae and Erebinae were recovered as subfamilies of Erebidae, with Noctuidae as the sister group. These results are in agreement with the most recent published phylogeny for Erebidae (38). For Riodinidae, our preliminary phylogeny conforms to the most recent classification (37), with Riodinidae being monophyletic and with a sister group relationship between Riodinidae and Lycaenidae. The genera found on Inga include Melese, Hypocrita, Pelochyta, and Areva in the Arctiinae; Coenipeta, Helia, and Letis for Erebinae; Nymphidium, Sarota, and Synargis for Riodinidae; and Iaspis, Ostrinotes, Techlopsis, Theritas, Strephonota, and Symbiopsis for Lycaenidae. The superfamily Gelechioidea was recovered as monophyletic, which is in congruence with published phylogenies for this group (41). Nevertheless, several MOTUs allocated to this group by BLASTing were removed from the eventual analyses because their placement in this preliminary phylogeny was doubtful, being placed within other, distantly related (nongelechiid clades). These probably belong to the family Tortricidae and the superfamily Pyraloidea, groups that at a larval stage look identical to Gelechioidea. Because the Gelechioidea are poorly known, these MOTUs could not be placed to genus by BLAST.
Phylogenetic relationships for MOTUs correctly allocated to Gelechioidea, Riodinidae, and Erebidae were inferred using multilocus coalescent-based Bayesian species tree in *BEAST 2.2.0 (57), with substitution and codon partition models for each marker set according to the suggestions of PartitionFinder 1.1.0 (58) (Table S7). Tree species priors and sequences used for tree rooting for each clade are specified in Table S7. Alternative models were assessed using Bayes factors, following the guidelines from ref. 83. Parameters were estimated from three independent runs of 100 million generations combined using LogCombiner 1.8 (59) with a burn-in of 10 million generations and sampling every 10,000 generations in each run. BEAST model convergence was confirmed by examination of parameter estimate distributions in Tracer 1.6 (84).
Plant phylogeny.
Phylogenetic relationships between Inga host species were inferred using seven chloroplast regions (rpoCI, psbA-trnH, rps16, trnL-F, trnD-T, ndhF-rpl32, rpl32-trnL) and the nuclear ribosomal internal transcribed spacer regions (ITS). DNA extractions used a modified CTAB protocol (85) or DNAeasy plant mini kits (Qiagen). PCR and sequencing protocols for chloroplast regions are given by ref. 3 and for ITS by refs. 60 and 61. Sequences were assembled using Sequencher, version 4.5 (Gene Codes Corporation), and aligned manually, which was unproblematic given low sequence divergence. Sequences were aligned using MAFFT, version 7.0 (86), and phylogenies were estimated in an ML framework using RAxML, with separate models for ITS and cpDNA (62). Phylogenies were subsequently time-calibrated using penalized likelihood (63), where the crown age was constrained to 6 My (following refs. 60 and 64).
From the resulting tree, we extracted pairwise distances between Inga species using the function “cophenetic” in the APE package (87) from the statistical programming language R, version 3.2.5 (R Development Core Team 2016). This phylogenetic distance matrix was used in all of the subsequent ecological analyses that involved the phylogeny of Inga.
Statistical Analyses.
Relationship between plant traits and phylogenetic signal.
Associations between defense traits were investigated by using matrix correlation analyses and phylogenetic generalized linear models (65). To estimate the relationship between continuous and noncontinuous traits (e.g., of noncontinuous traits: presence or absence of chemical compounds and ant visitor community to extrafloral nectaries), we calculated the distances between pairs of Inga species for each trait and examined their correlations using partial Mantel tests, controlling for phylogenetic correlations. The distance matrices for biotic (number of ants), developmental, physical, and chemical defenses, as well as synchrony in leaf production were calculated using the Manhattan dissimilarity index. For the ant visitor community, the Bray–Curtis index was used. Because the timing in leaf production is a circular variable (mean angle), we used the angular separation method from the package circular (66) to calculate the distance matrix for this trait.
We also performed a phylogenetic PCA (PPCA) on continuous trait data to derive evolutionary independent axes of defense variation (67). Phylogenetic signal was evaluated on the significant axes of defense variation from the PPCA and on the principal coordinates of the chemistry and ant species distance matrices by using Blomberg’s K̅ (68). If there is no phylogenetic signal, K̅ would be close to zero, whereas values approaching 1 would indicate that the trait value matches expectations under a Brownian model of evolution.
In a previous study and in this study, we showed chemistry and ant visitation to leaves to be divergent among close relatives in Inga (3). In contrast, developmental defenses show phylogenetic signal in the previous study but not in this study. Two potential reasons for these differences are that (i) we include more species of Inga and (ii) the present phylogeny resolution (the ITS marker is new, and this resolves several clades).
Constraints on host plant selection.
To examine whether differences in total herbivore community structure were related to differences in phylogenetic relationships and/or defensive traits between pairs of Inga hosts, we used Mantel and partial Mantel tests with 9,999 permutations. All of the feeding records that were limited to a single individual in a particular host were not included in these analyses. Overlap in feeding records among hosts was estimated using the Bray–Curtis dissimilarity index with relative abundance raw data. The resulting matrix was then compared with a phylogenetic distance matrix for Inga hosts and with a defense distance matrix conditioned on the phylogenetic pairwise distances between species. The defense distance matrix between Inga species was generated by averaging the distance matrices for the different defense traits such that they were all weighted equally. These analyses were performed in the vegan package (88).
To quantify the extent to which host phylogeny and/or host defenses structure herbivore community and to determine which defense trait is more important, we used redundancy analyses (RDAs). The herbivore community similarity matrix was used as a response variable in a partial distance-based redundancy analysis (dbRDA) together with each one of the measured defensive traits and the principal coordinates of the phylogenetic distance matrix as explanatory variables. The distance matrix was square-root transformed to avoid negative eigenvalues in the analyses (89). This analysis was performed without timing and synchrony in leaf production because we lacked data on these traits for some species. The analyses were run using sampling effort as a covariate. First, we ran a global test using all of the explanatory variables, and, because this analysis was statistically significant (P ≤ 0.05, 9,999 permutations), we performed variable selection using the function ordistep. We did this to avoid overfitting and to select the most parsimonious model. Adjusted R2 values for the selected model were computed using the function varpart. We also ran a more restricted analysis that included only species for which we had data on phenology of leaf production. This analysis produced very similar results. We performed these analyses in the vegan package.
To investigate whether past host shifts have occurred more often on Inga that are more similar in defenses or on Inga that are more closely related, we examined the congruence of the herbivore phylogenies with Inga phylogeny and Inga defenses using ParaFit (69) from the APE package. This statistical tool tests the significance of a hypothesis of coevolution between parasites and hosts using phylogenetic distance matrices of associated taxa and a set of host–parasite associations. Distance matrices for herbivores and plants were derived from their phylogenetic trees and from a cluster dendrogram we obtained from the total defense distance matrix using the “cophenetic” function in the APE package. We ran this analysis with 9,999 permutations. We perform the above analyses for the entire Lepidoptera community and for the three major clades associated with Inga separately: Gelechioidea, Erebidae, and Riodinidae.
Acknowledgments
We thank the Ministry of Agriculture of Peru for granting the research and exportation permits. We gratefully acknowledge Los Amigos Biological Station for institutional and logistical support. Invaluable field assistance was provided by Wilder Hidalgo and Silvana Lozano. We thank Axel Haussman, Suzy Khachaturyan, and Eric Murakami for help in the barcode identification of the insect herbivores. Kyle Harms suggested the short title for the manuscript. This work was supported by the Secretaría Nacional de Educación Superior, Ciencia, Tecnología e Innovación del Ecuador (SENESCYT) and grants from Conservation, Research and Education Opportunities and from the University of Utah: The Global Change and Sustainability Center and the International Student Center from the University of Utah (to M.-J.E.) and National Science Foundation Grants DEB-0640630 and Dimensions of Biodiversity DEB-1135733 (to P.D.C. and T.A.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The novel DNA sequences generated for this paper have been deposited in International Barcode of Life [(iBOL) sample IDs: IngaHerbiv0281–IngaHerbiv0908 and RCMJE LA01–RCMJE LA285) and GenBank (accessions nos. MF577083–MF578220). Average values of nonchemical leaf defensive traits are available in Dataset S1, and details about the sequences used for the phylogenies and their associated plant hosts are available in Dataset S2.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1707727114/-/DCSupplemental.
References
- 1.Ehrlich PR, Raven PH. Butterflies and plants: A study in coevolution. Evolution. 1964;18:586–608. [Google Scholar]
- 2.Marquis RJ, et al. Ode to Ehrlich and Raven or how herbivorous insects might drive plant speciation. Ecology. 2016;97:2939–2951. doi: 10.1002/ecy.1534. [DOI] [PubMed] [Google Scholar]
- 3.Kursar TA, et al. The evolution of antiherbivore defenses and their contribution to species coexistence in the tropical tree genus Inga. Proc Natl Acad Sci USA. 2009;106:18073–18078. doi: 10.1073/pnas.0904786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becerra JX. The impact of herbivore-plant coevolution on plant community structure. Proc Natl Acad Sci USA. 2007;104:7483–7488. doi: 10.1073/pnas.0608253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sedio B. 2013. Trait evolution and species coexistence in the hyperdiverse tropical forest tree genus psychotria, PhD thesis (University of Michigan, Ann Arbor, MI)
- 6.Salazar D, Jaramillo MA, Marquis RJ. Chemical similarity and local community assembly in the species rich tropical genus Piper. Ecology. 2016;97:3176–3183. doi: 10.1002/ecy.1536. [DOI] [PubMed] [Google Scholar]
- 7.Futuyma DJ, Agrawal AA. Macroevolution and the biological diversity of plants and herbivores. Proc Natl Acad Sci USA. 2009;106:18054–18061. doi: 10.1073/pnas.0904106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janz N. Ehrlich and Raven revisited: Mechanisms underlying codiversifcation of plants and enemies. Annu Rev Ecol Evol Syst. 2011;42:71–89. [Google Scholar]
- 9.Agrawal AA, Fishbein M. Plant defense syndromes. Ecology. 2006;87(7) Suppl:S132–S149. doi: 10.1890/0012-9658(2006)87[132:pds]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Becerra JX. Insects on plants: Macroevolutionary chemical trends in host use. Science. 1997;276:253–256. doi: 10.1126/science.276.5310.253. [DOI] [PubMed] [Google Scholar]
- 11.Brooks DR, McLennan DA. The Nature of Diversity: An Evolutionary Voyage of Discovery. Univ of Chicago Press; Chicago: 2002. [Google Scholar]
- 12.Agosta SJ. On ecological fitting, plant-insect associations, herbivore host shifts, and host plant selection. Oikos. 2006;114:556–565. [Google Scholar]
- 13.Valencia R, et al. 2004. Tropical Forest Diversity and Dynamism: Findings from a Large-scale Plot Network, eds Losos EC, Leigh, Jr EG (Univ of Chicago Press, Chicago), pp 609–628.
- 14.Coley PD, Aide TM. In: Plant–Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions. Price PW, Lewinsohn TM, Fernandes WW, Benson WW, editors. Wiley; New York: 1991. pp. 25–49. [Google Scholar]
- 15.Endara MJ, et al. Divergent evolution in antiherbivore defenses within species complexes at a single Amazonian site. J Ecol. 2015;103:1107–1118. [Google Scholar]
- 16.Coley PD, et al. Divergent defensive strategies of young leaves in two Neotropical species of Inga. Ecology. 2005;86:2633–2643. [Google Scholar]
- 17.Lokvam J, Kursar TA. Divergence in structure and activity of phenolic defenses in young leaves of two co-occurring Inga species. J Chem Ecol. 2005;31:2563–2580. doi: 10.1007/s10886-005-7614-x. [DOI] [PubMed] [Google Scholar]
- 18.Lokvam J, Clausen TP, Grapov D, Coley PD, Kursar TA. Galloyl depsides of tyrosine from young leaves of Inga laurina. J Nat Prod. 2007;70:134–136. doi: 10.1021/np060491m. [DOI] [PubMed] [Google Scholar]
- 19.Bixenmann RJ, Coley PD, Weinhold A, Kursar TA. High herbivore pressure favors constitutive over induced defense. Ecol Evol. 2016;6:6037–6049. doi: 10.1002/ece3.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kursar TA, Coley PD. The consequences of delayed greening during leaf development for light absorption and light use efficiency. Plant Cell Environ. 1992;15:901–909. [Google Scholar]
- 21.Aide TM. Patterns of leaf development and herbivory in a tropical understory community. Ecology. 1993;74:455–466. [Google Scholar]
- 22.Kursar TA, Coley PD. Convergence in defense syndromes of young leaves in tropical rainforests. Biochem Syst Ecol. 2003;21:929–949. [Google Scholar]
- 23.Kursar TA, Coley PD. Delayed development of the photosynthetic apparatus in tropical rainforest species. Funct Ecol. 1992;6:411–422. [Google Scholar]
- 24.Kursar TA, Coley PD. Delayed greening in tropical leaves: An anti-herbivore defense? Biotropica. 1992;24:256–262. [Google Scholar]
- 25.Lamarre GPA, Mendoza I, Fine PVA, Baraloto C. Leaf synchrony and insect herbivory among tropical tree habitat specialists. Plant Ecol. 2014;215:209–220. [Google Scholar]
- 26.Jolliffe IT. Principal Components Analysis. Springer; New York: 1986. [Google Scholar]
- 27.Pierce NE, et al. The ecology and evolution of ant association in the Lycaenidae (Lepidoptera) Annu Rev Entomol. 2002;47:733–771. doi: 10.1146/annurev.ento.47.091201.145257. [DOI] [PubMed] [Google Scholar]
- 28.Aide TM. Herbivory as a selective agent on the timing of leaf production in a tropical understory community. Nature. 1998;336:574–575. [Google Scholar]
- 29.Chew FS, Courtney SP. Plant apparency and evolutionary escape from insect herbivory. Am Nat. 1991;138:729–750. [Google Scholar]
- 30.Wolda H. Insect seasonality: Why? Annu Rev Ecol Syst. 1998;19:1–18. [Google Scholar]
- 31.Grøtan V, Lande R, Engen S, Saether BE, DeVries PJ. Seasonal cycles of species diversity and similarity in a tropical butterfly community. J Anim Ecol. 2012;81:714–723. doi: 10.1111/j.1365-2656.2011.01950.x. [DOI] [PubMed] [Google Scholar]
- 32.Srygley RB, Dudley R, Oliveira EG, Riveros AJ. El Niño, host plant growth and migratory butterfly abundance in a changing climate. Biotropica. 2013;46:90–97. [Google Scholar]
- 33.Pratt GF. Evolution of Euphilotes (Lepidoptera: Lycaenidae) by seasonal and host shifts. Biol J Linn Soc Lond. 1994;51:387–416. [Google Scholar]
- 34.Pellissier L, et al. Turnover of plant lineages shapes herbivore phylogenetic beta diversity along ecological gradients. Ecol Lett. 2013;16:600–608. doi: 10.1111/ele.12083. [DOI] [PubMed] [Google Scholar]
- 35.Whitfeld TJS, et al. Predicting tropical insect herbivore abundance from host plant traits and phylogeny. Ecology. 2012;93:S211–S222. [Google Scholar]
- 36.Menken SBJ. Pattern and process in the evolution of insect-plant associations: Yponomeuta as an example. Entomol Exp Appl. 1996;80:297–305. [Google Scholar]
- 37.Espeland M, et al. Ancient Neotropical origin and recent recolonisation: Phylogeny, biogeography and diversification of the Riodinidae (Lepidoptera: Papilionoidea) Mol Phylogenet Evol. 2015;93:296–306. doi: 10.1016/j.ympev.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Zahiri R, et al. Molecular phylogenetics of Erebidae (Lepidoptera, Noctuidea) Syst Ent. 2012;37:102–124. [Google Scholar]
- 39.DeVries PJ, Chacon IA. Toward a better understanding of host use and biodiversity in riodinid butterflies (Lepidoptera) J Res Lepid. 1992;31:103–126. [Google Scholar]
- 40.Winkler IS, Mitter C. In: The Evolutionary Biology of Herbivorous Insects: Specialization, Speciation, and Radiation. Tilmon KJ, editor. Univ of California Press; Berkeley: 2008. pp. 240–263. [Google Scholar]
- 41.Sohn JC, et al. Phylogeny and feeding trait evolution of the mega-diverse Gelechioidea (Lepidoptera: Obtectomera): New insights from 19 nuclear genes. Syst Entomol. 2015;41:112–132. [Google Scholar]
- 42.Berenbaum MR. Chemical mediation of coevolution: Phylogenetic evidence for Apiaceae and associates. Ann Miss Bot Gard. 2001;88:45–59. [Google Scholar]
- 43.Wahlberg N. The phylogenetics and biochemistry of host-plant specialization in Melitaeine butterflies (Lepidoptera: Nymphalidae) Evolution. 2001;55:522–537. doi: 10.1554/0014-3820(2001)055[0522:tpaboh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 44.Becerra JX, Venable DL. Macroevolution of insect-plant associations: The relevance of host biogeography to host affiliation. Proc Natl Acad Sci USA. 1999;96:12626–12631. doi: 10.1073/pnas.96.22.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yguel B, et al. Phytophagy on phylogenetically isolated trees: Why hosts should escape their relatives. Ecol Lett. 2011;14:1117–1124. doi: 10.1111/j.1461-0248.2011.01680.x. [DOI] [PubMed] [Google Scholar]
- 46.Dyer LA, et al. Host specificity of Lepidoptera in tropical and temperate forests. Nature. 2007;448:696–699. doi: 10.1038/nature05884. [DOI] [PubMed] [Google Scholar]
- 47.Coley PD, Kursar TA. Ecology. On tropical forests and their pests. Science. 2014;343:35–36. doi: 10.1126/science.1248110. [DOI] [PubMed] [Google Scholar]
- 48.Bascompte J, Jordano P, Olesen JM. Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science. 2006;312:431–433. doi: 10.1126/science.1123412. [DOI] [PubMed] [Google Scholar]
- 49.Janzen DH. When is coevolution? Evolution. 1980;34:611–612. doi: 10.1111/j.1558-5646.1980.tb04849.x. [DOI] [PubMed] [Google Scholar]
- 50.Agosta SJ, Klemens JA. Ecological fitting by phenotypically flexible genotypes: Implications for species associations, community assembly and evolution. Ecol Lett. 2008;11:1123–1134. doi: 10.1111/j.1461-0248.2008.01237.x. [DOI] [PubMed] [Google Scholar]
- 51.Berenbaum MR. In: Insects Life Cycles: Genetics, Evolution and Co-ordination. Gilbert F, editor. Springer; London: 1990. pp. 87–99. [Google Scholar]
- 52.Bernays E, Graham M. On the evolution of host specificity in phytophagous arthropods. Ecology. 1998;69:886–892. [Google Scholar]
- 53.Jermy T. Evolution of insect/host plant relationships. Am Nat. 1984;124:609–630. [Google Scholar]
- 54.Malhi Y, et al. An international network to understand the biomass and dynamics of Amazonian forests (RAINFOR) J Veg Sci. 2002;13:439–450. [Google Scholar]
- 55.Zar JH. Biostatistical Analysis. Prentice-Hall; Upper Saddle River, NJ: 1999. [Google Scholar]
- 56.Heled J, Drummond AJ. Bayesian inference of species trees from multilocus data. Mol Biol Evol. 2010;27:570–580. doi: 10.1093/molbev/msp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lanfear R, Calcott B, Ho SYW, Guindon S. Partitionfinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol. 2012;29:1695–1701. doi: 10.1093/molbev/mss020. [DOI] [PubMed] [Google Scholar]
- 58.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richardson JE, Pennington RT, Pennington TD, Hollingsworth PM. Rapid diversification of a species-rich genus of neotropical rain forest trees. Science. 2001;293:2242–2245. doi: 10.1126/science.1061421. [DOI] [PubMed] [Google Scholar]
- 60.Dexter KG, Pennington TD, Cunningham CW. Using DNA to assess errors in tropical tree identifications: How often are ecologists wrong and when does it matter? Ecol Monogr. 2010;80:267–286. [Google Scholar]
- 61.Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 62.Sanderson MJ. Estimating absolute rates of molecular evolution and divergence times: A penalized likelihood approach. Mol Biol Evol. 2002;19:101–109. doi: 10.1093/oxfordjournals.molbev.a003974. [DOI] [PubMed] [Google Scholar]
- 63.Lavin M. In: Neotropical Savannas and Seasonally Dry Forests: Plant Diversity, Biogeography and Conservation. Pennington RT, Lewis GP, Ratter JA, editors. CRC; Boca Raton, FL: 2006. pp. 433–447. [Google Scholar]
- 64.Dexter KG, et al. Dispersal assembly of rain forest tree communities across the Amazon basin. Proc Natl Acad Sci USA. 2017;114:2645–2650. doi: 10.1073/pnas.1613655114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garland T, Midford PE, Ives AR. An introduction to phylogenetically based statistical methods, with a new method for confidence intervals on ancestral values. Am Zool. 1999;39:374–388. [Google Scholar]
- 66.Lund U, Agostinelli C. 2015 Circular: R Package for Circular Statistics. Available at cran.r-project.org/web/packages/circular/circular.pdf. Accessed October 10, 2016.
- 67.Revell LJ. Size-correction and principal components for interspecific comparative studies. Evolution. 2009;63:3258–3268. doi: 10.1111/j.1558-5646.2009.00804.x. [DOI] [PubMed] [Google Scholar]
- 68.Blomberg SP, Garland T, Jr, Ives AR. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution. 2003;57:717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 69.Legendre P, Desdevises Y, Bazin E. A statistical test for host-parasite coevolution. Syst Biol. 2002;51:217–234. doi: 10.1080/10635150252899734. [DOI] [PubMed] [Google Scholar]
- 70.Pennington TD. The Genus Inga: Botany. Univ of Chicago Press; Chicago: 1997. [Google Scholar]
- 71.Green PT, Harms KE, Connell JH. Nonrandom, diversifying processes are disproportionately strong in the smallest size classes of a tropical forest. Proc Natl Acad Sci USA. 2014;111:18649–18654. doi: 10.1073/pnas.1321892112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kursar TA, Wolfe BT, Epps MJ, Coley PD. Food quality, competition, and parasitism influence feeding preference in a neotropical lepidopteran. Ecology. 2006;87:3058–3069. doi: 10.1890/0012-9658(2006)87[3058:fqcapi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 73.Brenes-Arguedas T, Coley PD, Kursar TA. Divergence in the chemical ecology of Inga between two Neotropical sites. J Ecol. 2008;96:127–135. [Google Scholar]
- 74.Lokvam J, Brenes-Arguedas T, Lee JS, Coley PD, Kursar TA. Allelochemic function for a primary metabolite: The case of l-tyrosine hyper-production in Inga umbellifera (Fabaceae) Am J Bot. 2006;93:1109–1115. doi: 10.3732/ajb.93.8.1109. [DOI] [PubMed] [Google Scholar]
- 75.Jeffery SW, Humphrey GF. New spectrophotometric equations for determining chlorophylls a, b, c1, and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz. 1975;167:191–194. [Google Scholar]
- 76.West SA, Cook JM, Werren JH, Godfray HCJ. Wolbachia in two insect host-parasitoid communities. Mol Ecol. 1998;7:1457–1465. doi: 10.1046/j.1365-294x.1998.00467.x. [DOI] [PubMed] [Google Scholar]
- 77.Ivanova NV, deWaard JR, Hebert PDN. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Mol Ecol Notes. 2006;6:998–1002. [Google Scholar]
- 78.deWaard JR, Ivanova NV, Hajibabaei M, Hebert PDN. In: Molecular Biology: Environmental Genetics. Martin C, editor. Humana Press; Totowa, NJ: 2008. pp. 275–293. [Google Scholar]
- 79.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones M, Ghoorah A, Blaxter M. jMOTU and Taxonerator: Turning DNA Barcode sequences into annotated operational taxonomic units. PLoS One. 2011;6:e19259. doi: 10.1371/journal.pone.0019259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Puillandre N, Lambert A, Brouillet S, Achaz G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol. 2012;21:1864–1877. doi: 10.1111/j.1365-294X.2011.05239.x. [DOI] [PubMed] [Google Scholar]
- 82.Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA (IEEE, Piscataway, NJ), pp 1–8.
- 83.Kass R, Raftery A. Bayes factors. J Am Stat Assoc. 1995;90:773–795. [Google Scholar]
- 84.Rambaut A, Suchard MA, Xie D, Drummond AJ. 2014 Tracer, version 1.6. Available at beast.bio.ed.ac.uk/Tracer. Accessed May 20, 2015.
- 85.Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- 86.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paradis E, et al. 2015 APE: Analyses of phylogenetics and evolution. Available at cran.r-project.org/web/packages/ape/ape.pdf. Accessed February 15, 2016.
- 88.Oksanen J, et al. 2015 Vegan: Community ecology package. Available at cran.r-project.org/web/packages/vegan/vegan.pdf. Accessed February 15, 2016.
- 89.Legendre L, Legendre P. Numerical Ecology. Elsevier; Oxford: 2012. [Google Scholar]
- 90.Hebert PDN, Penton EH, Burns JM, Janzen DH, Hallwachs W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc Natl Acad Sci USA. 2004;101:14812–14817. doi: 10.1073/pnas.0406166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cho S, et al. A highly conserved nuclear gene for low-level phylogenetics: Elongation factor-1 alpha recovers morphology-based tree for heliothine moths. Mol Biol Evol. 1995;12:650–656. [Google Scholar]
- 92.Brower AVZ, DeSalle R. Patterns of mitochondrial versus nuclear DNA sequence divergence among nymphalid butterflies: The utility of wingless as a source of characters for phylogenetic inference. Insect Mol Biol. 1998;7:73–82. doi: 10.1046/j.1365-2583.1998.71052.x. [DOI] [PubMed] [Google Scholar]