Significance
Hearing and balance impairments are major concerns and a serious burden for public health, but still lack an effective curative therapy. We assessed inner ear functions in a mouse model of Usher syndrome type 1, a developmental disorder characterized by profound congenital deafness and balance deficit, after local gene therapy. Viral transfer of the wild-type cDNA to the inner ear of the mutant mice shortly after birth resulted in a partial restoration of hearing and a long-lasting, almost complete, removal of the balance defect. The present results provide a basis for future clinical trials in humans.
Keywords: gene, therapy, balance, mouse, Usher
Abstract
Our understanding of the mechanisms underlying inherited forms of inner ear deficits has considerably improved during the past 20 y, but we are still far from curative treatments. We investigated gene replacement as a strategy for restoring inner ear functions in a mouse model of Usher syndrome type 1G, characterized by congenital profound deafness and balance disorders. These mice lack the scaffold protein sans, which is involved both in the morphogenesis of the stereociliary bundle, the sensory antenna of inner ear hair cells, and in the mechanoelectrical transduction process. We show that a single delivery of the sans cDNA by the adenoassociated virus 8 to the inner ear of newborn mutant mice reestablishes the expression and targeting of the protein to the tips of stereocilia. The therapeutic gene restores the architecture and mechanosensitivity of stereociliary bundles, improves hearing thresholds, and durably rescues these mice from the balance defects. Our results open up new perspectives for efficient gene therapy of cochlear and vestibular disorders by showing that even severe dysmorphogenesis of stereociliary bundles can be corrected.
Patients affected by both sensorineuronal hearing impairment and balance disorders due to inner ear defects are currently fitted with auditory prostheses (hearing aids or cochlear implants) and can benefit from balance rehabilitation therapy, but the outcomes are different from one patient to another. Some individuals with congenital profound deafness are able to have phone conversations thanks to cochlear implants, whereas others obtain little or no benefit from these devices, beyond becoming aware of environmental sounds (1, 2). Such variability has been reported in patients with Usher syndrome type 1 (USH1) (Online Mendelian Inheritance in Man no. 276900) (3, 4). Patients with USH1 suffer from congenital profound deafness and balance defects, and they subsequently undergo sight loss leading to blindness. The loss of visual input impedes lip reading and greatly limits vestibular defect compensation.
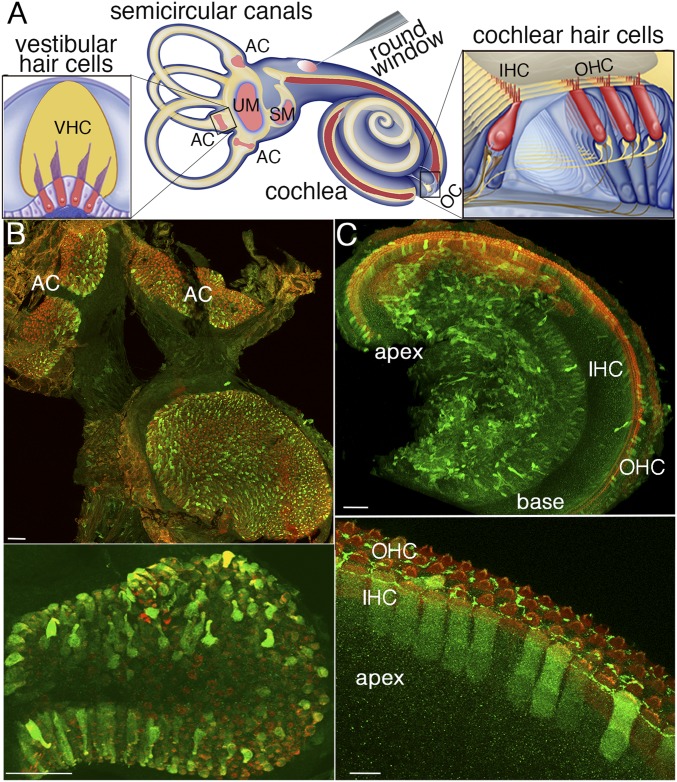
We tested local gene replacement as an alternative approach to cure deafness and balance disorders of USH1. The anatomy of the human inner ear is suitable for gene therapy. Its relatively isolated fluid-filled compartments allow one to deliver recombinant viral particles locally while minimizing the risk of systemic dissemination. The inner ear contains the hearing organ (the cochlea) and five vestibular end organs devoted to equilibration (the utricle and saccule, which detect linear acceleration, and three semicircular canals, each housing an ampulla, which detect angular accelerations of head rotation) (Fig. 1A). Sound and acceleration are detected by the mechanosensory receptor cells (hair cells) through deflection of their hair bundles, an array of modified microvilli known as stereocilia, organized into rows of graduated heights forming a staircase pattern. The tip link, a fibrous link connecting the tip of each transducing stereocilium to the side of its taller neighbor, gates the mechanoelectrical transduction channel(s) located at the tip of the shorter stereocilium (Fig. 2A). Mutations of USH1 genes affect hair bundle morphogenesis and tip-link maintenance in the inner ear hair cells (5).
Fig. 1.
AAV8 vector (Penn Vector Core) transduces vestibular and cochlear hair cells with different efficiencies. (A) Diagram of the mouse inner ear and viral injection through the round window of the cochlea. The vestibular sensory epithelia [AC, ampullar crista(e) of the three semicircular canals; SM, saccular macula; UM, utricular macula] and cochlear sensory epithelium (OC, organ of Corti) are drawn in pink and in red, respectively. Details of an AC and the OC are presented on the left side and right side of this diagram, respectively, with the hair cells (IHCs, OHCs, and VHCs) drawn in red. The AAV8-Sans-IRES-GFP (Penn Vector Core) recombinant virus injected through the cochlear round window of a mouse on P2.5 transduces the vast majority of VHCs (B, Upper) and transduces cochlear IHCs and OHCs more efficiently in the apical region than in the basal region of the cochlea (C, Upper), as shown by the GFP labeling (green) on P8.5. All hair cells are stained red by an anti-myosin VI antibody. Higher magnification views of the AC (B, Lower) and the OC (C, Lower) from the cochlear apical region are shown. (Scale bars: Upper, 50 μm; Lower, 10 μm.)
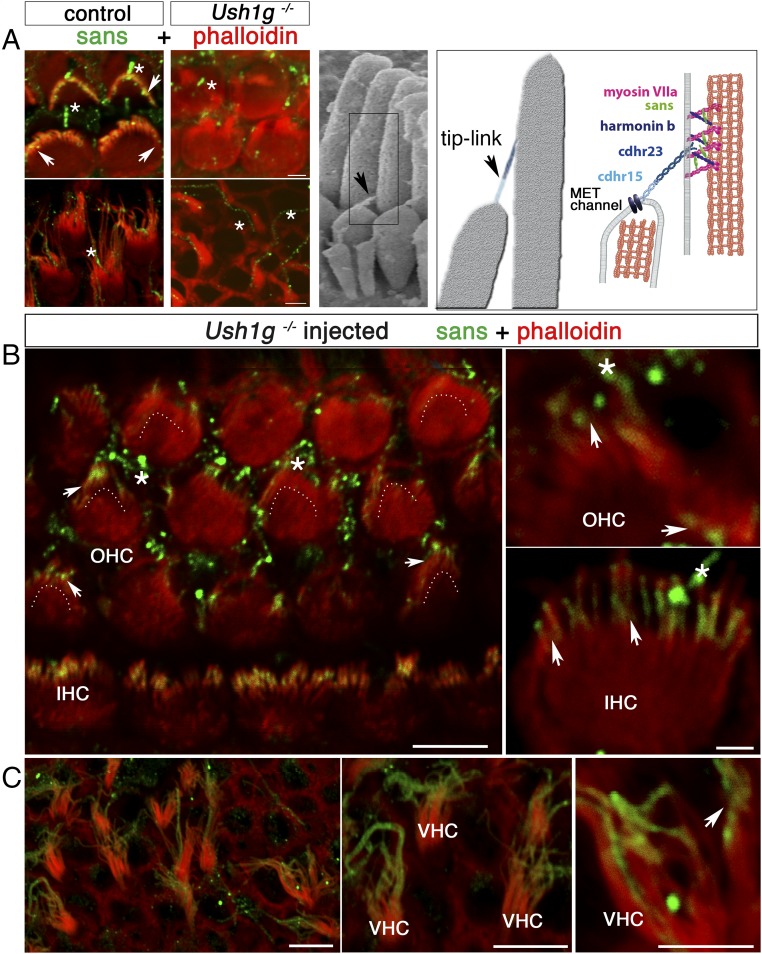
Fig. 2.
AAV8-Sans-IRES-GFP restores sans expression and targeting in the inner ear hair cells of Ush1g−/− mice. (A, Left) OHC (Upper) and VHC (Lower) hair bundles from P8.5 wild-type (control) and Ush1g−/− mice, immunostained for sans (green) and stained for F-actin with phalloidin (red). Sans is detected at the tips of the stereocilia in the wild-type mouse (white arrowheads), but not in the Ush1g−/− mouse. A nonspecific staining of the kinocilium is present both in the wild-type and Ush1g−/− mice (asterisks). (A, Center) Scanning electron micrograph of the IHC hair bundle, showing the tip links between adjacent stereocilia of different rows (black arrowhead). (A, Right) Diagram showing the tip-link lower and upper insertion points, the position of the mechanoelectrical transduction (MET) channel(s) at the tip of the shorter stereocilium, and the locations of the five USH1 proteins forming the tip link [cadherin-related proteins 15 (cdhr15) and 23 (cdhr23)] or presumably involved in its anchoring to the actin filaments of the taller stereocilium (harmonin b, sans, and myosin VIIa). The submembrane scaffold protein sans belongs to the tip-link upper insertion point molecular complex. Top views of the organ of Corti in the cochlear apical region (B) and of the utricular macula (C) of an injected Ush1g−/− mouse on P8.5 and high-magnification photographs of OHC, IHC, and VHC hair bundles are shown. Sans is targeted to the tips of the stereocilia in all hair cell types (arrowheads). The image in B is extracted from a larger tile scan and contains two tiles stitched together at the upper part of the image. Dashed lines in B indicate the position of the hair bundle base (V shape) in OHCs expressing the transgene. (Scale bars: 5 μm.)
Adenoassociated virus (AAV)-mediated gene transfer has emerged as a promising strategy for treating hereditary diseases (6, 7). AAV vectors have different cell tropisms, and can mediate high levels of transgene expression. These properties have led to their use in preclinical and clinical trials for several inherited disorders, including genetic forms of Parkinson’s disease, metabolic disorders, and retinal diseases, but they have not yet been used for inner ear diseases (6–10). Various viral vectors containing GFP as a reporter gene have been shown to transduce inner ear hair cells (11–15), and several studies have investigated the use of gene therapy to restore hearing and balance in animal models, with different degrees of success. However, to date, partial hearing improvement was only obtained for mouse models without severe dysmorphogenesis of the inner ear hair cells (16–22). Here, we focused on the genetic form of USH1 caused by mutations of USH1G, encoding the submembrane scaffold protein sans (23, 24). Ush1g−/− mutant mice display profound deafness and vestibular dysfunction, characterized by circling behavior and head tossing. The hair bundles of their cochlear hair cells and vestibular hair cells (VHCs) undergo abnormal morphogenesis and lack functional tip links (24, 25). We explored the feasibility, reliability, and long-term efficacy of local gene therapy in these mice.
Results
Viral cDNA Transfer Restores Sans Expression and Targeting in Cochlear and VHCs of Ush1g−/− Mice.
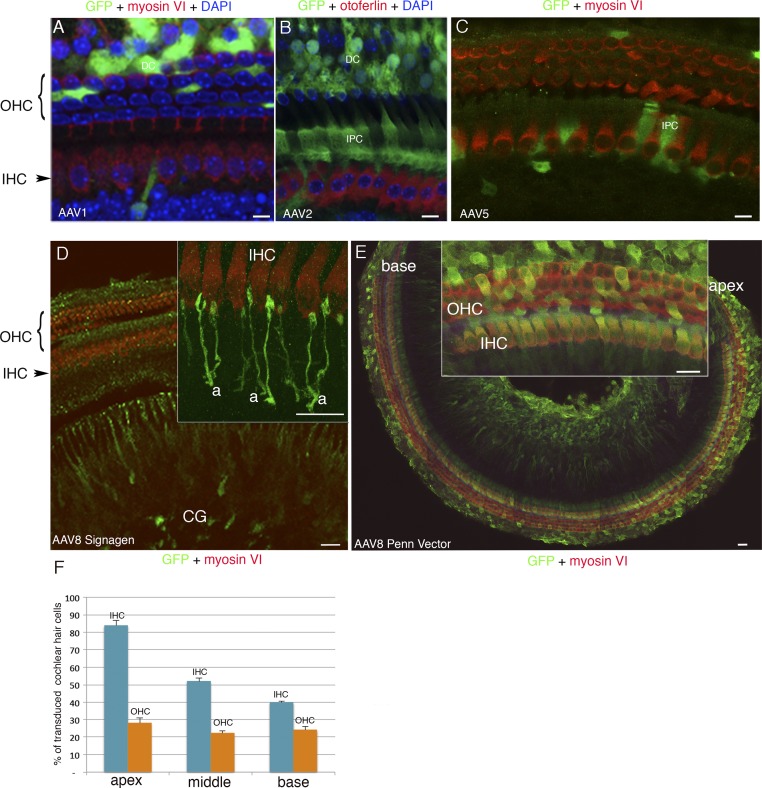
A number of AAV serotypes and over 100 naturally occurring AAV variants have been isolated from adenovirus stocks or from human/nonhuman primate tissues. They display different cell tropisms (14), but there are some discrepancies in their reported transduction efficiencies and cell tropisms in the inner ear (26–29). We thus investigated the ability of several AAV vectors to transduce the inner ear hair cells. We tested recombinant AAV1, AAV2, AAV5, and AAV8 vectors containing the green fluorescent protein (GFP) reporter gene driven by a hybrid promoter (CAG) consisting of the CMV enhancer fused to the chicken β-actin gene promoter. A single viral injection through the round window membrane separating the middle ear from the inner ear was performed in C57BL/6 wild-type mice on postnatal day 2.5 (P2.5) (Fig. 1A). The sensory epithelium of the cochlea (organ of Corti) and the vestibular end organs were microdissected on P8.5, and immunolabeled for otoferlin or myosin VI to visualize the inner ear hair cells, as well as for GFP. These recombinant AAV vectors transduced the inner ear cell types with different cell tropisms and transduction rates. In the cochlea, AAV1 (SignaGen) and AAV2 and AAV5 (Penn Vector Core) mostly transduced supporting cells of the organ of Corti, namely, Deiters’ cells and inner phalangeal cells (Fig. S1 A–C). AAV8 from SignaGen (with the CAG promoter) mainly transduced cochlear ganglion neurons (Fig. S1D), whereas AAV8 from Penn Vector Core (with the same promoter) efficiently transduced hair cells and supporting cells (Fig. S1E). There are two types of cochlear hair cells: the inner hair cells (IHCs), which are the genuine auditory sensory cells that transmit the encoded sensory signal to the central nervous system, and the outer hair cells (OHCs), which function as fine-tuned amplifiers of the sound stimulus (30) (Fig. 1A). After injection of AAV8 from Penn Vector Core, more IHCs expressed GFP at the apex of the cochlea (84 ± 3%) than at the base (40% ± 0.5%), whereas OHCs were transduced with roughly the same efficiency at the base (28 ± 3%) and at the apex (24 ± 2%) (n = 23 mice; Fig. S1 E and F). Surgery and intracochlear injection of the virus did not affect auditory brainstem responses (ABRs) tested from 2 to 12 wk after injection (Fig. 4A and Fig. S4; Mann–Whitney test, P = 0.7).
Fig. S1.
Cell tropism of AAV1, AAV2, AAV5, and AAV8 in the cochlea. The recombinant vectors AAV1 and AAV8 from SignaGen Laboratories or AAV2, AAV5, and AAV8 from Penn Vector Core, all containing the GFP reporter gene, were injected on P2.5 through the round window membrane into the left cochlea of 9, 6, 8, 8, and 31 mice, respectively. The sensory epithelium of the cochlea (organ of Corti) was microdissected on P8.5, and immunolabeled for myosin VI or for otoferlin, to visualize all hair cells or only IHCs, respectively, and for GFP. (A and B) Cell nuclei were stained blue with DAPI. (A–C) AAV1 (SignaGen Laboratories) and AAV2 (Penn Vector Core) mostly transduce supporting cells, namely, inner phalangeal cells (IPC) and/or Deiters’ cells (DC), and AAV5 transduces only a few supporting cells. AAV8-CAG from SignaGen Laboratories mostly transduces the cochlear ganglion (CG) neurons but not the hair cells (D) [Inset, detailed view of the IHCs and afferent nerve terminals (a) is shown], whereas AAV8-CAG from Penn Vector Core transduces both IHCs and OHCs (E), more efficiently in the apical region (E, Inset) than in the basal region of the cochlea as shown in a tile scan of images stitched into a large mosaic of nearly the entire organ of Corti (E). (F) Bar chart showing the proportions of IHCs and OHCs transduced with AAV8-CAG from Penn Vector Core in the apical, middle, and basal regions of the cochlea. (Scale bars: A–C, 5 μm; D and E, Inset, 10 μm; E, 50 μm).
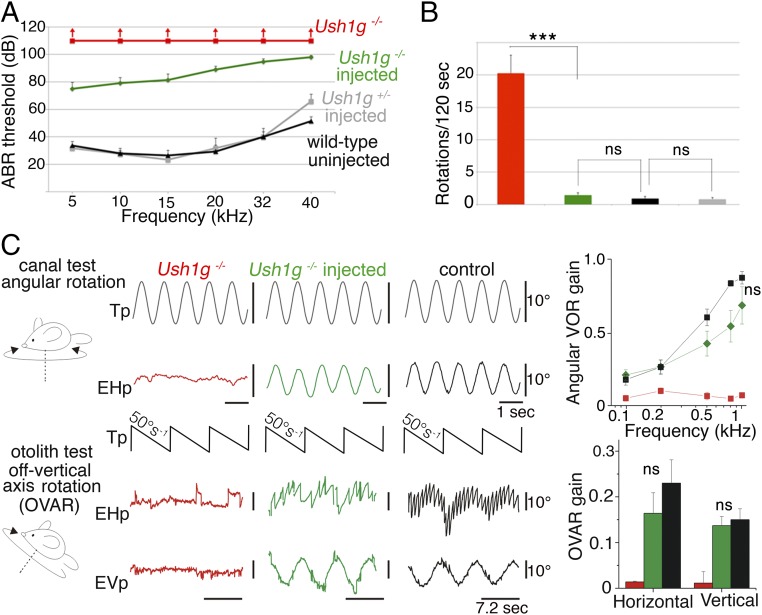
Fig. 4.
Sans cDNA transfer improves hearing thresholds of injected Ush1g−/− mice and almost completely restores their vestibular functions. (A) ABR thresholds in P30 wild-type mice (not injected) and P30 Ush1g+/− and Ush1g−/− mice injected or not injected with AAV8-Sans-IRES-GFP on P2.5. ABRs were recorded in response to 5- to 40-kHz tone bursts, and for sound intensities between 10 and 110 dB SPL. The ABR threshold elevation is about 50 dB for low-frequency sounds (5–15 kHz) in injected Ush1g−/− mice (n = 18 mice), instead of a complete hearing loss in the uninjected mutant mice (n = 3 mice). Note that the viral injection did not affect the ABR thresholds of Ush1g+/− heterozygous mice (n = 7), compared with the uninjected wild-type (control) mice (n = 6). (B) Bar chart showing the number of rotations in open-field recordings of mouse displacements over a period of 2 min (120 s) in 33-wk-old Ush1g+/− (control) and Ush1g−/− mice injected or not injected with the transgene on P2.5. ***P < 0.001. (C) Vestibulo-ocular recordings done between 10 and 12 mo after injection of the transgene. (Upper) Semicircular canal test. An angular VOR (aVOR) was recorded during horizontal sinusoidal rotations of the turntable (the representation of the table signal, Tp, is inverted for easy comparison). No aVOR is detected in the uninjected Ush1g−/− mouse (EHp, red trace), whereas the injected Ush1g−/− mouse (green trace) displays an aVOR response similar to that of the control mouse (black trace). The line graph shows the responses for stimuli of different speeds (from 0.1 to 1 Hz) (mean ± SEM, n = 5 mice in each group; ANOVA, P = 0.2). (Lower) Otolithic organ test using off-vertical axis rotation (OVAR). No eye response is detected in the Ush1g−/− mouse (EHp and EVp, red traces), whereas the injected Ush1g−/− mouse (green traces) and the control mouse (black traces) display compensatory eye movements. The bar chart shows the horizontal and vertical responses (mean ± SEM, n = 5 mice in each group; Mann–Whitney test, P = 0.3 and P = 0.5 for the comparison of the horizontal and vertical responses between the control and injected Ush1g−/− mice, respectively). EHp, eye horizontal position (eye movements to the right are represented upward); EVp, eye vertical position; ns, statistically not significant; Tp, table position.
Fig. S4.
Maintenance of the hearing improvement 12 wk after local gene therapy in Ush1g−/− mice. ABR thresholds in 12-wk-old wild-type mice (not injected, n = 5, black curve), Ush1g+/− mice injected with AAV8-GFP (n = 5, blue curve), and Ush1g−/− mice not injected (n = 5, red curve) or injected (n = 5, green curve) with AAV8-Sans-IRES-GFP on P2.5. ABRs were recorded in response to 5- to 40-kHz tone bursts, and for sound levels between 10 and 110 dB SPL. Despite the elevation of the ABR thresholds in the wild-type and injected Ush1g+/− control mice at this age (owing to the C57BL/6 genetic background of the mice) (34), a partial hearing improvement persists in injected Ush1g−/− mice compared with uninjected Ush1g−/− mice (Mann–Whitney test, P < 0.01 for 10 kHz and 15 kHz, P < 0.1 for 5 kHz and 20 kHz, and P > 0.1 for 32 kHz and 40 kHz).
We then investigated the efficiency with which the recombinant AAV8-Sans-internal ribosome entry site (IRES)-GFP virus (Penn Vector Core) containing the murine cDNA Sans, encoding the unique spliced transcript of Ush1g, transduced inner ear hair cells. Ush1g+/− mice were injected with the recombinant virus on P2.5, and 6 d later, the sensory epithelia of the cochlea and vestibular end organs were microdissected and immunolabeled either for GFP and myosin VI or for GFP and sans. The hair cell transduction efficacy of AAV8-Sans-IRES-GFP was similar to that of AAV8-GFP (Fig. 1 B and C). The rate of IHC transduction (i.e., GFP-expressing IHCs) was 87 ± 4% at the cochlear apex, gradually decreasing to 45 ± 6% at the cochlear base, and the rate of OHC transduction was 33 ± 6% at the apex, gradually decreasing to 25 ± 5% at the base (n = 24 mice; Fig. 1C). Surprisingly, in the vestibular end organs of the injected ears, the hair cells were transduced at a much higher rate, 91 ± 24% (n = 17 mice; Fig. 1B). Moreover, the injection of AAV8-Sans-IRES-GFP into the cochlea of Ush1g−/− mice restored a normal targeting of the sans protein to the tips of the stereocilia of transduced IHCs, OHCs, and VHCs on P8.5 (24, 25) (Fig. 2).
Viral Transfer of the Sans cDNA to the Hair Cells Rescues Ush1g−/− Mice from Hair Bundle Defects.
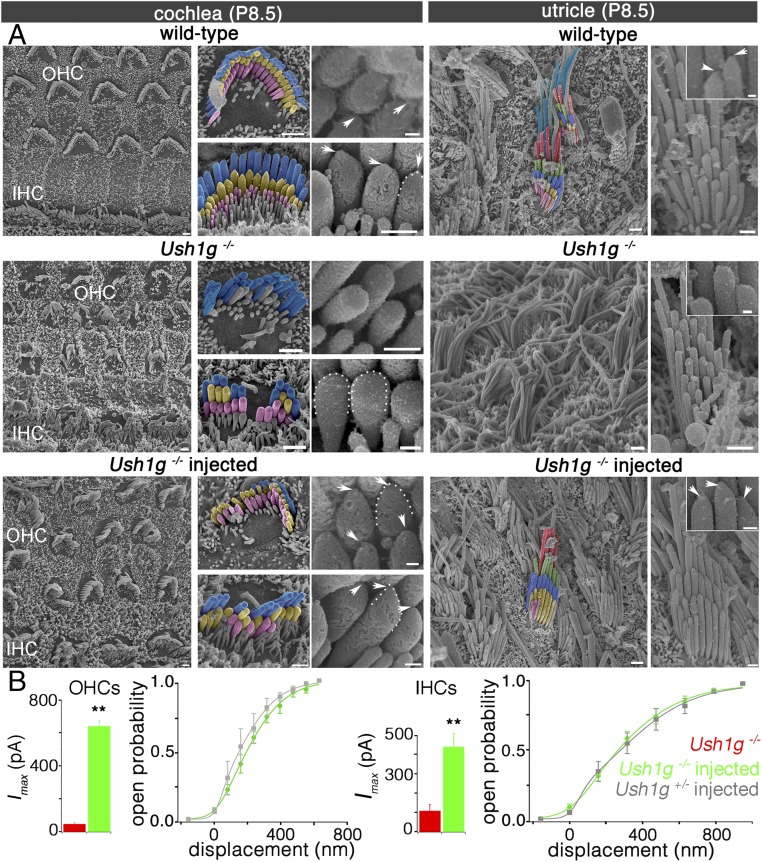
From embryonic day 17.5 (E17.5) onward, cochlear hair cells of Ush1g−/− mice display fragmented hair bundles with reduced numbers of stereocilia and mispositioned kinocilia (24, 25), and nearly all VHCs have collapsed hair bundles. The degeneration of the middle and short rows of stereocilia in IHCs and OHCs and the collapse of the VHC hair bundles are even more pronounced at P2.5 (Fig. 3A and Fig. S2). We investigated whether the viral expression of the Sans cDNA in the hair cells rescued the mice from these defects by scanning electron microscopy analysis. In the injected Ush1g−/− mice, the vast majority of hair bundles of IHCs, OHCs of the cochlear apex, and VHCs displayed a typical staircase pattern (81 ± 3% for cochlear hair cells, n = 72 cells and 70 ± 1% for VHCs, n = 304 cells from 10 mice). Notably, the stereocilia of the short and middle rows in most IHCs (93 ± 2%, n = 27 cells) and OHCs (68 ± 5%, n = 55 cells) in the apical region of the injected cochlea had prolate tips pointing toward their taller neighbors, just like in Ush1g+/− control mice. The stereocilia tips of most VHCs had their characteristic prolate shape too. Prolate tips are considered a hallmark of the presence of functional tip links resulting from the pulling force exerted by these links on the tips of the stereocilia (31). By contrast, the stereocilia tips had prolate shapes in only 4% of the VHCs (n = 204 cells) and none of the cochlear hair cells (n = 147 cells, from seven mice) of uninjected Ush1g−/− mice (Fig. 3A, Insets; Mann–Whitney test, P < 10−4 for all comparisons between injected and uninjected Ush1g−/− mice), which is consistent with the loss of tip links in these mice (24). Finally, we estimated the numbers of stereocilia per hair bundle, their projected heights, and their spacing, based on our scanning electron micrographs (Table S1). We found no significant differences in any of these parameters between the injected Ush1g−/− mice and Ush1g+/− mice (Table S1). Of note, the cochlear and vestibular hair bundles of injected and uninjected wild-type mice did not differ in appearance either, suggesting that the injection through the round window membrane did not impair the morphological development of the hair bundles.
Fig. 3.
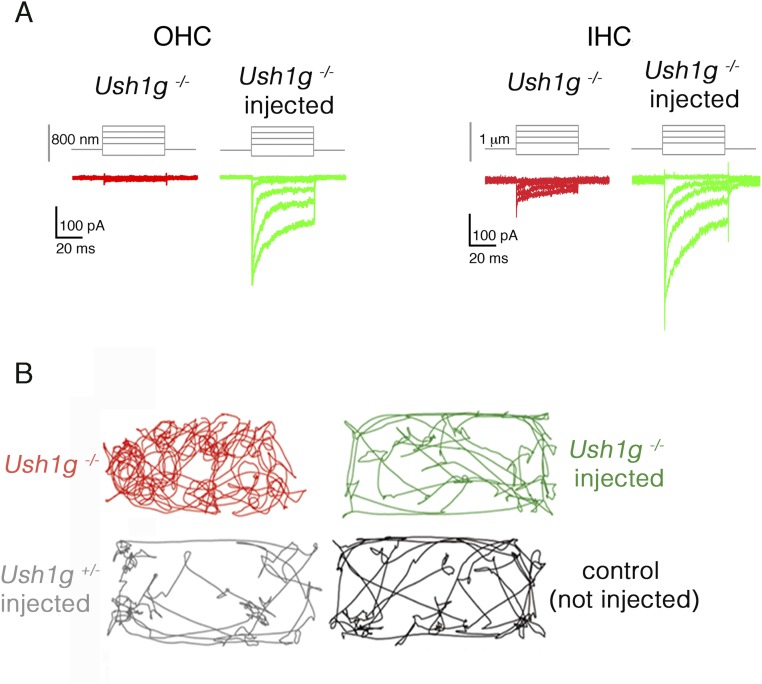
Sans cDNA transfer to inner ear hair cells rescues Ush1g−/− mice from hair bundle structural defects. (A) Low-, intermediate-, and high-magnification scanning electron micrographs showing the architecture of the hair bundles in cochlear OHCs and IHCs (Left) and in VHCs (utricle, Right) of a control wild-type mouse, an Ush1g−/− mouse, and an Ush1g−/− mouse injected with the Sans cDNA, on P8.5. In the wild-type mouse, the stereocilia tips have prolate shapes, which is the hallmark of functional tip links (arrowheads and dashed lines) (31), whereas they have rounded shapes in the Ush1g−/− mouse (dashed lines), in keeping with the loss of the tip links (24). Note the fragmentation of the hair bundles and the degeneration of some stereocilia in this mouse. In the injected Ush1g−/− mouse, the hair bundles have recovered their normal staircase architecture and the prolate shapes of stereocilia tips (arrowheads and dashed line). (Scale bars: 2 μm.) (B) Mechanoelectrical transduction (MET) currents recorded ex vivo in IHCs and OHCs of uninjected Ush1g−/− (red), injected Ush1g−/− (green), and injected Ush1g+/− (control, gray) P8.5 mice. Bar charts show the peak amplitudes of the MET currents (Imax) recorded in the hair cells of uninjected or injected Ush1g−/− mice (110.8 ± 30.8 pA in IHCs and 47.3 ± 5.7 pA in OHCs of uninjected Ush1g−/− mice vs. 424 ± 70 pA in IHCs and 641 ± 35 pA in OHCs of injected Ush1g−/− mice). Note the marked increase of Imax in the injected Ush1g−/− mice compared with the noninjected mice (Student’s t test, **P < 0.01 for both IHCs and OHCs). Line graphs show the MET channel opening probability plotted as a function of hair bundle displacement for IHCs and OHCs of injected Ush1g+/− (gray) and Ush1g−/− (green) mice. For both cell types, the two curves are superimposed.
Fig. S2.
Scanning electron micrographs of the hair bundles of cochlear hair cells and VHCs in wild-type and Ush1g−/− P2.5 mice. Low-, intermediate-, and high-magnification photographs show the hair bundles in cochlear hair cells and VHCs of a wild-type mouse and an Ush1g−/− mouse on P2.5. Note the fragmentation of the IHC and OHC hair bundles and the flaccid shape of the VHC hair bundles in the mutant mouse. The intermediate- and high-magnification photographs show the characteristic prolate shape of stereocilia tips (except in stereocilia of the tallest row), which is the hallmark of functional tip links, in the wild-type mouse but not in the mutant mouse, which lacks the tip links. (Scale bars: 2 μm.)
Table S1.
Comparative analysis of hair bundle geometric parameters in VHCs of Ush1g+/− and Ush1g−/− mice
| Ush1g+/− | Ush1g−/− | ||||
| Geometric parameter | Mean ± SD | n | Mean ± SEM | n | Statistical comparison |
| Projected height, tallest row | 5.8 ± 0.3 | 25 | 5.2 ± 0.2 | 58 | 0.12 |
| Projected length, tallest row | 9.6 ± 0.7 | 25 | 8.5 ± 0.4 | 58 | 0.19 |
| Interstereocilia distance, tallest row | 0.38 ± 0.04 | 25 | 0.33 ± 0.02 | 58 | 0.28 |
| No. of stereocilia, tallest row | 29.5 ± 2.7 | 25 | 32.2 ± 3.1 | 58 | 0.50 |
| No. of rows | 5.1 ± 0.2 | 24 | 4.7 ± 0.1 | 54 | 0.11 |
| Distance between rows | 0.79 ± 0.05 | 24 | 0.74 ± 0.04 | 54 | 0.48 |
| Apical cell surface area | 20.2 ± 3.2 | 25 | 24.6 ± 1.7 | 58 | 0.23 |
Measurements were based on scanning electron microscopy images of the utricle taken in Ush1g−/− mice (n = 10) and Ush1g+/− mice (n = 5) on P8. After selection of a representative set of hair bundles, various geometric parameters were extracted using a custom interface allowing a semiautomatized segmentation of stereocilia. Projected heights of the tallest stereocilia row were evaluated under similar angles of view for the two mutants. Statistical comparisons using the Student’s t test did not reveal any significant difference in the hair bundle parameters between the Ush1g−/− and Ush1g+/− mice. Values are given as mean ± SEM. All lengths and distances are given in micrometers, and areas are given in square micrometers; n is the number of cells analyzed.
In keeping with the morphological rescue of the hair bundles, the transduced IHCs and OHCs of injected Ush1g−/− P8.5 mice, identified on the basis of GFP fluorescence, displayed mechanoelectrical transduction currents of much higher peak amplitudes (424 ± 69.4 pA in IHCs, n = 11 and 641 ± 35 pA in OHCs, n = 12) than IHCs and OHCs of uninjected Ush1g−/− mice (110.8 ± 30.8 pA in IHCs, n = 5 and 47.3 ± 5.7 pA in OHCs, n = 4; Student’s t test, P < 0.01 for each comparison; Fig. 3B). In addition, the sensitivity of the transduction current response to hair bundle displacement was similar in injected Ush1g−/− mice and injected Ush1g+/− (control) mice (calculated mean sensitivity values: 1.12 ± 0.19 μm−1 and 1.30 ± 0.14 μm−1 for IHCs, and 1.73 ± 0.2 μm−1 and 2.24 ± 0.15 μm−1 for OHCs of injected Ush1g+/− and injected Ush1g−/− mice, respectively; unpaired t test, P > 0.1 for both comparisons) (24) (Fig. 3B and Fig. S3A).
Fig. S3.
Sans cDNA transfer to the inner ear of Ush1g−/− mice restores the mechanoelectrical transduction currents recorded in cochlear hair cells on P8.5, and the behavior of these mice in an open-field displacement test. (A) Examples of mechanoelectrical transduction currents recorded ex vivo in OHCs and IHCs of noninjected (red) and injected (green) P8.5 Ush1g−/− mice upon hair bundle displacements of different amplitudes (shown on top of the current traces). (B) Open-field recordings of mouse displacements over a period of 2 min (120 s) in 33-wk-old Ush1g+/− (control) and Ush1g−/− mice injected or not injected with the transgene on P2.5. The Ush1g−/− mouse explores the field by executing repetitive body turns, whereas the Ush1g−/− mouse injected on P2.5 does not display such a circling behavior, instead exploring the field just like the control mouse.
Partial Restoration of Hearing.
Ush1g−/− mice are profoundly deaf and show no identifiable ABRs, even in response to sounds of intensities up to 110 dB sound pressure level (SPL) (24) (Fig. 4A). After gene transfer, ABR waves could be recorded in 2- to 12-wk-old Ush1g−/− mice for sound intensities exceeding 75 dB SPL, indicating a substantial improvement in cochlear function. For sounds in the low-frequency range (5–15 kHz), the hearing threshold elevation was about 50 dB on P30, instead of a complete hearing loss in the uninjected mutant mice (n = 18 injected mice; one-way ANOVA test, P < 0.01; Fig. 4A). The improvement of hearing thresholds was less noticeable at higher frequencies (20–40 kHz), which is consistent with the decrease in cochlear hair cell transduction rates from the apical region to the basal region of the cochlea, where high-frequency tones are analyzed (32) (Fig. S1F). It is noteworthy that a partial hearing rescue was still present at the age of 12 wk, the oldest age at which the mice were tested (33) (Fig. S4). Thus, a single intracochlear injection of the transgene during the neonatal period was able to restore the hearing of loud sounds (≥75 dB) of low frequency in Ush1g−/− mice.
Complete Restoration of Vestibular Function.
Video-tracking in an open-field chamber showed that injected Ush1g−/− mice explored the field in a manner similar to control mice, without circling behavior (1.5 ± 0.4 turn in 2 min for injected Ush1g−/− mice, n = 10, vs. 20.3 ± 2.8 turns in 2 min for uninjected Ush1g−/− mice, n = 7; Student’s t test, P < 0.01; Fig. 4B, Fig. S3B, and Movies S1–S4). Additional behavioral tests were carried out to assess the vestibular functions in these mice, up to 53 wk after injection (31). In the balance platform test (SI Materials and Methods), the uninjected Ush1g−/− mice (n = 7) were unable to stay on the platform, whereas the injected Ush1g−/− mice (n = 10), like the Ush1g+/− control mice (n = 9), were able to spend about 1 min on the platform (58.4 ± 0.9 s vs. 27.6 ± 10.6 s for injected Ush1g−/− mice and uninjected mutant mice, respectively; Student’s t test, P < 0.01). Likewise, the injected Ush1g−/− mice responded normally in the trunk curl test, and were able to reach a horizontal landing surface rapidly when suspended by the tail, without curling the trunk toward their tail, whereas the uninjected mutant mice were unable to land on the horizontal surface, instead curling toward the base of the tail when suspended (Pearson’s χ2 test, P < 0.01). In a contact righting test, the injected Ush1g−/− mice could reorient their bodies rapidly upon inversion of the tube in which they were held, whereas the uninjected Ush1g−/− mice could not (Pearson’s χ2 test, P < 0.01). In the swim test described by Hardisty-Hughes et al. (34), the injected Ush1g−/− mice behaved just like the wild-type mice, whereas the uninjected Ush1g−/− mice displayed underwater tumbling, and had to be lifted out and rescued at once (Pearson’s χ2 test, P < 0.01). These results suggest that gene replacement therapy by early postnatal injection of AAV8-Sans into the inner ear restores the vestibular function in Ush1g−/− mice. Notably, this effect persisted in the long term, as no difference was found between the Ush1g+/− and injected Ush1g−/− mice when these tests were carried out 53 wk after treatment (platform test: Student’s t test, P > 0.1; swim test: Pearson’s χ2 test, P > 0.1; n = 5 mice for each genotype). We then evaluated the long-term contributions of the different vestibular end organs to the improvement in equilibration up to 67 wk. The functions of the semicircular canals and otolith organs (saccule and utricle) were assessed by recording vestibulo-ocular reflexes (VORs) and macula-ocular reflexes (MORs) in response to specific turntable movements, respectively (35). No compensatory eye movements in response to turntable rotation could be detected in Ush1g−/− mice, indicating a complete loss of function for both the semicircular canals and otolith organs, as expected. By contrast, all of the injected Ush1g−/− mice showed compensatory eye movements in the VOR and MOR tests (Fig. 4C). Vestibulo-ocular responses were indistinguishable from those of wild-type mice in two of the five injected mice. In the other three mice, semicircular canal organ responses were restored to a lesser degree than otolith organ responses, which were not significantly different from those of controls, demonstrating that a single intracochlear injection of the transgene results in a long-lasting complete restoration of the vestibular function in Ush1g−/− mice.
SI Materials and Methods
Viral Vector Constructs.
We used AAV1-CAG-GFP and AAV1-CMV-GFP, from SignaGen Laboratories, at a titer of 1.2 × 1012 genome copies (gc)/mL; AAV1-CAG-GFP, from Penn Vector Core, at a titer of 1.1 × 1013 gc/mL; AAV2-CAG-GFP, from Penn Vector Core, at a titer of 6.4 × 1013 gc/mL; AAV5-CMV-GFP, from Penn Vector Core, at a titer of 1.13 × 1013 gc/mL; and AAV8-CAG from SignaGen Laboratories and Penn Vector Core at titers of 1.6 × 1012 gc/mL and 1.4 × 1013 gc/mL, respectively.
The murine cDNA, Sans, corresponding to the unique spliced transcript of Ush1g reported (GenBank accession no. NM_176847), followed by an internal ribosome entry site (IRES) and the cDNA sequence of the GFP reporter gene, was inserted into the pENN.AAV.CB6.PI.rBG plasmid (Penn Vector Core), which was then packaged into the AAV8 capsid and produced by Penn Vector Core facility at a titer of 1.47 × 1013 gc/mL.
Intracochlear Injection.
Protocols were approved by the Animal Care and Use Committee of the Institut Pasteur. Intracochlear viral transduction was carried out as described by Akil et al. (16). Mice were anesthetized by hypothermia. A left postauricular incision was made and, using the cochlear basal turn and stapedial artery as landmarks, the otic bulla was exposed and opened. A glass micropipette with an outer tip diameter of 10 μm containing 2 μL of the viral vector preparation, with similar numbers of viral particles, was then inserted into the round window membrane, and the liquid was gently injected into the cochlea. The pipette was removed, the hole in the membrane was plugged with connective tissue, and the incision was sealed with biological glue (3M Vetbond).
Immunofluorescence.
After dissection, inner ears were perfused with 4% paraformaldehyde in PBS for 45 min at 4 °C. Cochleas and vestibular end organs were microdissected, rinsed three times for 10 min, and incubated for 1 h at room temperature in PBS supplemented with 20% normal horse serum and 0.3% Triton X-100. The samples were then incubated overnight with the primary antibodies: rabbit anti-sans (1/100), chicken anti-GFP (1:500; Abcam), antiotoferlin (1/250), and/or anti-myosin VI (1/200) (40, 41) in PBS supplemented with 1% horse serum. The samples were rinsed three times for 10 min in PBS, and then incubated for 1 h with ATTO-550–conjugated goat anti-rabbit IgG antibody (1:500 dilution; Sigma–Aldrich) and ATTO-488–conjugated goat anti-chicken IgG antibody (1:500 dilution; Sigma–Aldrich). Actin was labeled with ATTO-647N–conjugated phalloidin (1:200 dilution; Sigma–Aldrich). Samples were then mounted in Fluorsave (Calbiochem, USA). The z-stack images were captured with a Zeiss LSM-700 confocal microscope equipped with a Plan Apochromat 63×/1.4 N.A. oil immersion lens (Carl Zeiss).
Hair Cell Counting.
GFP reporter gene expression was used to evaluate the rate of transduction in injected ears: We divided the total number of IHCs or OHCs producing GFP by the total number of IHCs or OHCs immunolabeled for myosin VI. For mature stages, the rate of transduction was calculated in the middle and apical regions of each cochlea.
Electrophysiological Recordings.
Electrophysiological whole-cell patch-clamp recordings of hair cell mechanoelectrical transduction currents were performed in cochlear explants from P8 mice, as previously described (42). Cochleas were finely dissected, and the sensory epithelium (organ of Corti) was placed under nylon meshes and observed under a water immersion 40×/0.8 N.A. Olympus objective mounted on an Axioscope Zeiss microscope.
The extracellular and dissecting solutions both had the following compositions: 146 mM NaCl, 5.8 mM KCl, 1.5 mM CaCl2, 0.7 mM NaH2PO4, 2 mM sodium pyruvate, 10 mM glucose, and 10 mM Hepes (pH 7.4, 305 mosmol/kg). The intracellular solution contained 130 mM KCl, 10 mM NaCl, 3.5 mM MgCl2, 1 mM EGTA, 5 mM K2ATP, 0.5 mM GTP, and 5 mM Hepes (pH 7.3, 290 mosmol/kg).
Borosilicate patch pipettes (electrical resistance of 2–3 MΩ) were brought parallel to the hair cell rows through a hole in the reticular lamina. Hair cells were whole-cell voltage-clamped at room temperature (20–25 °C), at a holding potential of −80 mV, with an EPC-9 patch-clamp amplifier and Patchmaster software (HEKA). No correction was made for liquid junction potential. Series resistance was always below 10 MΩ and was compensated to 70%. Data were sampled at 100 kHz and filtered at 10 kHz with an eight-pole Bessel filter. Each hair bundle was mechanically stimulated by applying axial step displacements with a rigid glass rod that had been fire-polished before the experiment to yield a tip diameter of 2–3 μm. The probe was systematically positioned against the top of the hair bundle in the bundle’s plane of bilateral symmetry toward the tallest row of stereocilia, at an angle of ∼30° relative to the apical cell surface. The probe used for mechanical stimulation of the hair bundles was secured to a stack-type piezoelectric actuator (PA8/12; Piezo System Jena) driven by a low-voltage power supply (30V300; Piezo System Jena). Offline measurements were obtained with a displacement monitor containing photodiodes. The first 2 ms of the time course of probe motion were well described by an exponential increase, with a time constant of 100 ms. Data were analyzed in MATLAB, version 7.0 (MathWorks). Curves for mechanoelectrical transduction channel opening probability as a function of hair bundle displacement [Po(X)] were fitted with a three-state Boltzmann relationship. For sensitivity measurements, the mean value of the derivative of the three-state Boltzmann relationship was calculated for displacements corresponding to Po values between 0.2 and 0.8.
Audiological Tests.
ABRs to sound stimuli were recorded and analyzed as previously described (43). Briefly, mice were anesthetized with xylazine and ketamine, and placed in an attenuated-sound room. Three electrodes were placed at the vertex, the ipsilateral mastoid, and the lower back as the ground electrode. Pure-tone stimuli were used at frequencies of 5, 10, 15, 20, 32, and 40 kHz. Sound intensities of 10 to 110 dB, in 10-dB steps, were tested. Thresholds were determined as the lowest stimulus level resulting in recognizable ABR waves.
Behavioral Analysis.
We evaluated circling behavior with tracking software (Ethovision de Noldus Information Technology). The numbers of turns made in the clockwise and counterclockwise directions were counted in an open-field chamber (37 cm long and 18.5 cm wide) over a period of 120 s. We also did various tests to assess vestibular function, as previously described (34). The trunk curl test was carried out by holding the mouse by the tail and observing whether it managed to reach a horizontal landing surface (score of 1) or whether it curled its trunk toward its tail (score of 0). We also evaluated balance with the platform test: The mouse was positioned on a small platform (7 cm × 7 cm) at a height of 29 cm, and we observed how many times the mouse fell off the platform over a period of 1 min. The contact righting test was carried out by placing the mouse in a closed transparent tube and observing whether the mouse was able to right itself when the tube was rotated by 180° (score of 1 if the mouse can right itself and score of 0 if the mouse cannot). We also scored the swimming ability of each mouse in a container filled with water at 22–23 °C (score of 1 the mouse can swim and score of 0 if the mouse cannot swim).
Discussion
We demonstrate a long-term restoration of inner ear functions in a mouse model of USH1 by using a single AAV-mediated local gene therapy at an early postnatal stage. The recombinant AAV8, with the CAG promoter driving transgene expression, was found to be the most efficient of the AAV vectors tested for the transduction of inner ear hair cells. Virus injection through the round window membrane on P2.5 had no deleterious effect on inner ear development in wild-type mice. Furthermore, no adverse side effects were detected in the mice for up to 63 wk after virus injection. This approach therefore appears as a promising one to attempt in future extension to human patients.
Our results demonstrate that Sans cDNA delivery restores the production and localization of the sans protein in the IHCs, OHCs, and VHCs, suggesting that the exogenous protein was correctly targeted to the tip-link insertion points (24, 36) (Fig. 2). Moreover, the transduced cochlear hair cells of injected Ush1g−/− mice displayed markedly improved mechanoelectrical transduction currents in ex vivo experiments on P8.5 cochlear explants, which indicates that expression of the Sans cDNA in these cells effectively compensates for the absence of the native protein. From these results, we can infer that the exogenous protein most probably interacts with the other members of the USH1 protein complex (i.e., cadherin-related protein 23, cadherin-related protein 15, myosin VIIA, harmonin), as required for the correct functioning of the mechanoelectrical transduction channels (24) (Fig. 2A). Transduction currents can first be recorded on P0 and P2 in the hair cells of the cochlear basal and apical regions, respectively (37), whereas the transduction apparatus of VHCs is already functional on E17 (37, 38). In this study, the Sans cDNA was delivered by a viral vector on P2.5, and expression of the cDNA was first detected 48 h later (i.e., about 10 d after the normal onset of Ush1g expression on E14.5). Despite this delay, gene replacement therapy efficiently restored the structure and function of inner ear hair cells in Ush1g−/− mice and prevented the balance deficit while limiting the hearing impairment for low sound frequencies. The time window for treating deafness and balance disorders by gene transfer in patients with USH1 may therefore be larger than initially thought. In addition, the almost complete restoration of vestibular functions reported here reveals unanticipated temporal flexibility for the effective treatment of other early-onset balance disorders of genetic origin. Further studies are required to determine why the transgene injection did not fully restore cochlear function in Ush1g−/− mice. The higher rates of hair cell transduction in the vestibular end organs than in the cochlea raise the hope of a full restoration of hearing by increasing the transduction rates in the cochlear hair cells (22). It is worthy of note that hearing thresholds were significantly improved for sounds in the low-frequency range (5–15 kHz), which are analyzed in a cochlear apical region that, owing to its high curvature, cannot be reached by the electrode arrays of cochlear implants (39). Therefore, this gene therapy approach might be effective in association with current cochlear implants by partly restoring the tonotopic information that these implants cannot provide, and thus allowing for better sound perception.
This study constitutes a significant step toward the virus-mediated cure of a form of genetic deafness in humans. However, before considering translation to clinical trials, these results must be reproduced in larger animal models that are better predictors of responses in humans than rodents, based on the size and the kinetics of maturation of their cochlea.
Materials and Methods
Detailed information is provided in SI Materials and Methods. Animal experiments were carried out in accordance with INSERM and Institut Pasteur welfare guidelines. Animals were housed in the Institut Pasteur animal facilities accredited by the French Ministry of Agriculture for experiments on live mice. The intracochlear viral injection procedure, approved by the Animal Care and Use Committee of the Institut Pasteur, was carried out as described by Akil et al. (16). Immunofluorescence analyses were carried out as described elsewhere (40, 41). Scanning electron microscopy analysis was done as described elsewhere (24). Electrophysiological whole-cell patch-clamp recordings of hair cell mechanoelectrical transduction currents were carried out in cochlear explants from P8.5 mice, as described by Michalski et al. (42). ABRs to sound stimuli were recorded and analyzed as previously described (43). The various tests to assess vestibular function were carried out as previously reported (34, 35).
Supplementary Material
Acknowledgments
We thank Jean-Marc Panaud (Reprography Service, Institut Pasteur) for assistance with SEM image colorization. This work was supported by Fondation pour la Recherche Médicale (A.E.); the European Union Seventh Framework Programme under the grant agreement HEALTH-F2-2010-242013 (TREATRUSH); the European Commission (ERC-2011-ADG_294570); French state funds managed by Agence Nationale de la Recherche within the Investissements d’Avenir Programme (ANR-15-RHUS-0001); LabEx Lifesenses (ANR-10-LABX-65); and grants from the BNP Paribas Foundation, the FAUN-Stiftung, the LHW-Stiftung, and Errera Hoechstetter.
Footnotes
Conflict of interest statement: A patent involving A.E., C.P., and S.S. (PCT/EP2016/053613) has been deposited by the Institut Pasteur, INSERM, and CNRS.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1708894114/-/DCSupplemental.
References
- 1.Jeon EK, Turner CW, Karsten SA, Henry BA, Gantz BJ. Cochlear implant users’ spectral ripple resolution. J Acoust Soc Am. 2015;138:2350–2358. doi: 10.1121/1.4932020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivero RJ, Fan K, Angeli S, Balkany TJ, Liu XZ. Cochlear implantation in common forms of genetic deafness. Int J Pediatr Otorhinolaryngol. 2010;74:1107–1112. doi: 10.1016/j.ijporl.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman TB, Schultz JM, Ahmed ZM. Usher syndrome type 1: Genotype-phenotype relationships. Retina. 2005;25(Suppl):S40–S42. doi: 10.1097/00006982-200512001-00016. [DOI] [PubMed] [Google Scholar]
- 4.Liu XZ, et al. Cochlear implantation in individuals with Usher type 1 syndrome. Int J Pediatr Otorhinolaryngol. 2008;72:841–847. doi: 10.1016/j.ijporl.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Petit C, Richardson GP. Linking genes underlying deafness to hair-bundle development and function. Nat Neurosci. 2009;12:703–710. doi: 10.1038/nn.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins M, Thrasher A. Gene therapy: Progress and predictions. Proc Biol Sci. 2015;282:20143003. doi: 10.1098/rspb.2014.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonato M, et al. Progress in gene therapy for neurological disorders. Nat Rev Neurol. 2013;9:277–291. doi: 10.1038/nrneurol.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: Poised at the clinical crossroads. Mol Ther. 2012;20:699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotterman MA, Schaffer DV. Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genet. 2014;15:445–451. doi: 10.1038/nrg3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: Progress and challenges. Nat Rev Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 11.Iizuka T, et al. Noninvasive in vivo delivery of transgene via adeno-associated virus into supporting cells of the neonatal mouse cochlea. Hum Gene Ther. 2008;19:384–390. doi: 10.1089/hum.2007.167. [DOI] [PubMed] [Google Scholar]
- 12.Kilpatrick LA, et al. Adeno-associated virus-mediated gene delivery into the scala media of the normal and deafened adult mouse ear. Gene Ther. 2011;18:569–578. doi: 10.1038/gt.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konishi M, Kawamoto K, Izumikawa M, Kuriyama H, Yamashita T. Gene transfer into guinea pig cochlea using adeno-associated virus vectors. J Gene Med. 2008;10:610–618. doi: 10.1002/jgm.1189. [DOI] [PubMed] [Google Scholar]
- 14.Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: Vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Xia L, Yin S, Wang J. Inner ear gene transfection in neonatal mice using adeno-associated viral vector: A comparison of two approaches. PLoS One. 2012;7:e43218. doi: 10.1371/journal.pone.0043218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akil O, et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron. 2012;75:283–293. doi: 10.1016/j.neuron.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Askew C, et al. Tmc gene therapy restores auditory function in deaf mice. Sci Transl Med. 2015;7:295ra108. doi: 10.1126/scitranslmed.aab1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang Q, et al. Virally mediated Kcnq1 gene replacement therapy in the immature scala media restores hearing in a mouse model of human Jervell and Lange-Nielsen deafness syndrome. EMBO Mol Med. 2015;7:1077–1086. doi: 10.15252/emmm.201404929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chien WW, et al. Gene therapy restores hair cell stereocilia morphology in inner ears of deaf whirler mice. Mol Ther. 2016;24:17–25. doi: 10.1038/mt.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isgrig K, et al. Gene therapy restores balance and auditory functions in a mouse model of Usher syndrome. Mol Ther. 2017;25:780–791. doi: 10.1016/j.ymthe.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landegger LD, et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat Biotechnol. 2017;35:280–284. doi: 10.1038/nbt.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan B, et al. Gene therapy restores auditory and vestibular function in a mouse model of Usher syndrome type 1c. Nat Biotechnol. 2017;35:264–272. doi: 10.1038/nbt.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weil D, et al. Usher syndrome type I G (USH1G) is caused by mutations in the gene encoding SANS, a protein that associates with the USH1C protein, harmonin. Hum Mol Genet. 2003;12:463–471. doi: 10.1093/hmg/ddg051. [DOI] [PubMed] [Google Scholar]
- 24.Caberlotto E, et al. Usher type 1G protein sans is a critical component of the tip-link complex, a structure controlling actin polymerization in stereocilia. Proc Natl Acad Sci USA. 2011;108:5825–5830. doi: 10.1073/pnas.1017114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefèvre G, et al. A core cochlear phenotype in USH1 mouse mutants implicates fibrous links of the hair bundle in its cohesion, orientation and differential growth. Development. 2008;135:1427–1437. doi: 10.1242/dev.012922. [DOI] [PubMed] [Google Scholar]
- 26.Derby ML, Sena-Esteves M, Breakefield XO, Corey DP. Gene transfer into the mammalian inner ear using HSV-1 and vaccinia virus vectors. Hear Res. 1999;134:1–8. doi: 10.1016/s0378-5955(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 27.Raphael Y, Frisancho JC, Roessler BJ. Adenoviral-mediated gene transfer into guinea pig cochlear cells in vivo. Neurosci Lett. 1996;207:137–141. doi: 10.1016/0304-3940(96)12499-x. [DOI] [PubMed] [Google Scholar]
- 28.Sacheli R, Delacroix L, Vandenackerveken P, Nguyen L, Malgrange B. Gene transfer in inner ear cells: A challenging race. Gene Ther. 2013;20:237–247. doi: 10.1038/gt.2012.51. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, et al. Early postnatal virus inoculation into the scala media achieved extensive expression of exogenous green fluorescent protein in the inner ear and preserved auditory brainstem response thresholds. J Gene Med. 2013;15:123–133. doi: 10.1002/jgm.2701. [DOI] [PubMed] [Google Scholar]
- 30.Fettiplace R, Hackney CM. The sensory and motor roles of auditory hair cells. Nat Rev Neurosci. 2006;7:19–29. doi: 10.1038/nrn1828. [DOI] [PubMed] [Google Scholar]
- 31.Prost J, Barbetta C, Joanny JF. Dynamical control of the shape and size of stereocilia and microvilli. Biophys J. 2007;93:1124–1133. doi: 10.1529/biophysj.106.098038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat Neurosci. 2009;12:711–717. doi: 10.1038/nn.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- 34.Hardisty-Hughes RE, Parker A, Brown SD. A hearing and vestibular phenotyping pipeline to identify mouse mutants with hearing impairment. Nat Protoc. 2010;5:177–190. doi: 10.1038/nprot.2009.204. [DOI] [PubMed] [Google Scholar]
- 35.Romand R, et al. Retinoic acid deficiency impairs the vestibular function. J Neurosci. 2013;33:5856–5866. doi: 10.1523/JNEUROSCI.4618-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grati M, Kachar B. Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechanotransduction. Proc Natl Acad Sci USA. 2011;108:11476–11481. doi: 10.1073/pnas.1104161108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Géléoc GS, Holt JR. Sound strategies for hearing restoration. Science. 2014;344:1241062. doi: 10.1126/science.1241062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denman-Johnson K, Forge A. Establishment of hair bundle polarity and orientation in the developing vestibular system of the mouse. J Neurocytol. 1999;28:821–835. doi: 10.1023/a:1007061819934. [DOI] [PubMed] [Google Scholar]
- 39.Van Abel KM, et al. Hearing preservation among patients undergoing cochlear implantation. Otol Neurotol. 2015;36:416–421. doi: 10.1097/MAO.0000000000000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roux I, et al. Myosin VI is required for the proper maturation and function of inner hair cell ribbon synapses. Hum Mol Genet. 2009;18:4615–4628. doi: 10.1093/hmg/ddp429. [DOI] [PubMed] [Google Scholar]
- 41.Roux I, et al. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 42.Michalski N, et al. Harmonin-b, an actin-binding scaffold protein, is involved in the adaptation of mechanoelectrical transduction by sensory hair cells. Pflugers Arch. 2009;459:115–130. doi: 10.1007/s00424-009-0711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delmaghani S, et al. Hypervulnerability to sound exposure through impaired adaptive proliferation of peroxisomes. Cell. 2015;163:894–906. doi: 10.1016/j.cell.2015.10.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.