Significance
This report brings a new perspective on the decades-old question of how MHC and HLA complexes can potently protect against a variety of autoimmune diseases, including type 1 diabetes. We demonstrated that protection by the MHC-II Eα:Eβ complex operated via modulation of the composition of the intestinal microbiota during a critical early window of ontogeny, associated with modification of the local immune system. These findings prompt a model of HLA/MHC-mediated protection from autoimmunity, and raise the question of whether disease-protective alleles in other human autoimmune diseases or models thereof might operate by a similar mechanism. They also argue that treating infants and pregnant mothers with antibiotics should be minimized.
Keywords: microbiome, type 1 diabetes, neonatal, autoimmune disease, NOD mice
Abstract
Certain MHC-II or HLA-D alleles dominantly protect from particular autoimmune diseases. For example, expression of the MHC-II Eα:Eβ complex potently protects nonobese diabetic (NOD) mice, which normally lack this isotype, from spontaneous development of type 1 diabetes. However, the underlying mechanisms remain debated. We investigated MHC-II–mediated protection from type 1 diabetes using a previously reported NOD mouse line expressing an Eα transgene and, thereby, the Eα:Eβ complex. Eα16/NOD females vertically protected their NOD offspring from diabetes and insulitis, an effect that was dependent on the intestinal microbiota; moreover, they developed autoimmunity when treated with certain antibiotics or raised in a germ-free environment. Genomic and proteomic analyses revealed NOD and Eα16/NOD mice to host mild but significant differences in the intestinal microbiotas during a critical early window of ontogeny, and transfer of cecal contents from the latter to the former suppressed insulitis. Thus, protection from autoimmunity afforded by particular MHC/HLA alleles can operate via intestinal microbes, highlighting potentially important societal implications of treating infants, or even just their pregnant mothers, with antibiotics.
Type 1 diabetes (T1D) is an autoimmune disease characterized by T cell-provoked destruction of the insulin-producing β-cells of the pancreatic islets of Langerhans. Development of autoimmune diabetes is regulated by multiple genetic polymorphisms and largely unknown environmental factors. This and many other autoimmune diseases have their strongest genetic association with the HLA-D locus (1). Certain HLA-D haplotypes, such as DRB1*0401-DQB1*0302 and DRB1*0301-DQB1*0201, confer elevated risk for T1D; others, notably DRB1*1501-DQB1*0602, promote dominant protection (estimated to be as high as 97%) (2). Interestingly, an HLA allele that protects from one autoimmune disease can promote another.
The nonobese diabetic (NOD) mouse strain is the most widely studied animal model of autoimmune diabetes. All NOD mice develop a leukocytic infiltrate in their pancreatic islets, termed insulitis, around 3–4 wk of age, and a fraction of them progress to overt diabetes starting at about 12–14 wk, depending on the particular colony. Similar to the disease in humans, diabetes in NOD mice is a T cell-dependent, polygenic disorder that is modified by environmental factors. Parallel to the situation in humans, the MHC locus is by far the dominant genetic determinant in mice (1). The NOD strain expresses an unusual MHC-II A complex, termed Ag7, and does not express an E complex due to deletion of the Eα promoter (3). Remarkably, NOD mice genetically modified to express the Eα molecule in the appropriate cells, are completely protected from T1D and are either entirely or nearly devoid of insulitis (4–6).
Thus far, there is no clear consensus on the mechanism of E-mediated protection from T1D. An early model proposed that E complex expression leads to clonal deletion or anergizing of autoreactive T cells (7). But such a mechanism was rendered unlikely when clonal deletion in E-expressing NOD mice could be dissociated from protection from insulitis (5), and when E expression in the thymus was found to be neither necessary nor sufficient for protection (8). In addition, E+ NOD mice have islet cell-reactive T cells that can transfer disease to T cell-deficient NOD mice, arguing that they have a diabetes-competent T lymphocyte repertoire (9, 10). A second model argued for altered autoantigen presentation in E-expressing NOD mice: the E complex would outcompete Ag7 for limited pathogenic peptides. This mechanism also proved unlikely because E+ and E− antigen-presenting cells (APCs) from NOD mice similarly present peptide to and prime autoreactive T cells in vitro and in vivo (11, 12). A third proposed mechanism, that E complex expression alters the cytokine skewing of CD4+ T effector cells or promotes the generation of Foxp3+ T regulatory (Treg) cells, has been supported by data from some studies (5) but refuted by results from others (13).
There is a critical role for nongenetic (e.g., environmental) factors in the development of T1D in both humans and mice. The rapid rise of T1D incidence over the past few decades argues for an important nongenetic component to the pathogenesis of T1D (14), as do epidemiologic studies demonstrating that monozygotic twins have less than 50% concordance for T1D (15). Recent results have highlighted a role for the intestinal microbiota in promoting or protecting from several autoimmune diseases, including this one (16, 17). Since a few reports have suggested that MHC/HLA complexes can influence microbial colonization of the gut (18, 19), although others have appeared to disagree (20), we hypothesized that E-mediated protection from NOD autoimmunity might be an indirect effect, channeled through influences on the intestinal microbiota. Using multiple experimental approaches, we demonstrate this hypothesis to be true.
Results
Eα16/NOD Dams Transmitted Protection from Insulitis and T1D Vertically to Their NOD Progeny.
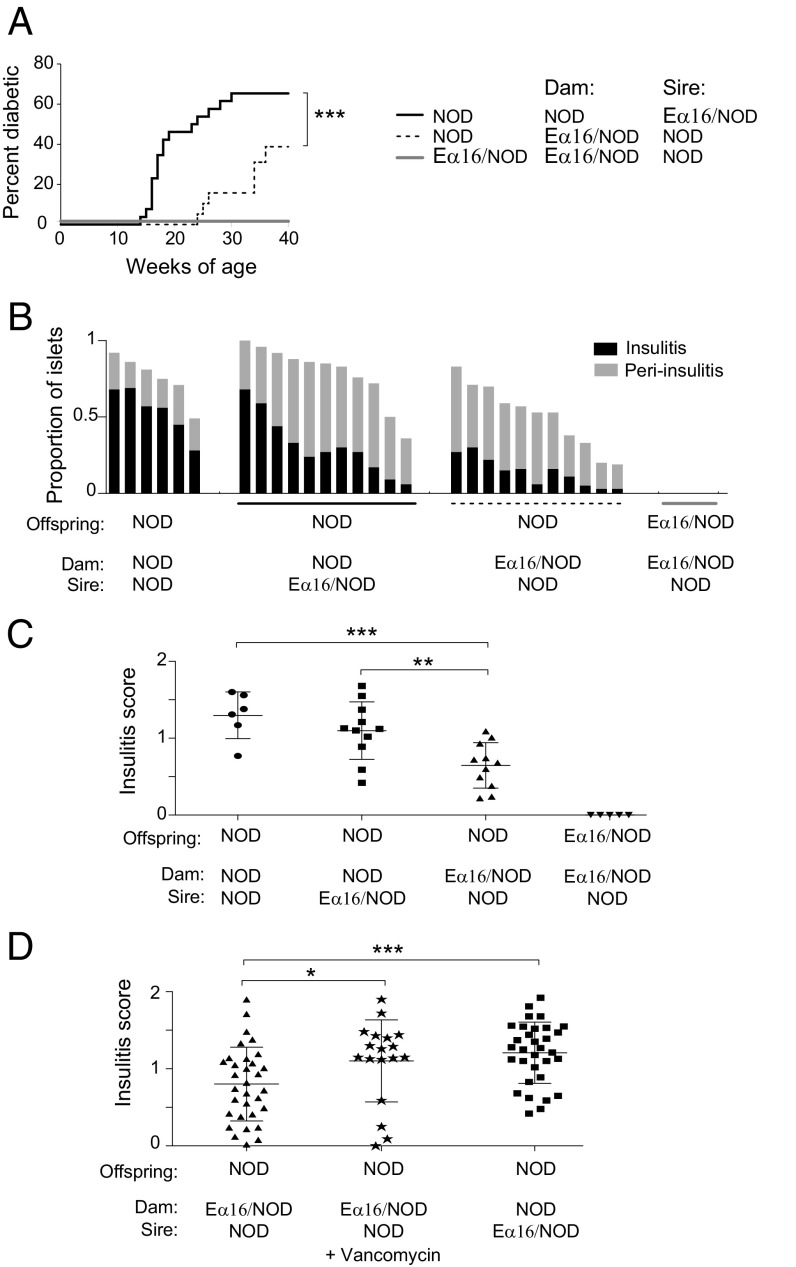
Since the maternal and neonatal environments can influence diabetes development in both NOD mice (21) and humans (22), we began by investigating whether protection from diabetes could be vertically transferred from an Eα16/NOD dam to her NOD progeny. Diabetes incidence was compared in cohorts of female NOD mice born to either NOD dams mated to Eα16/NOD sires or to the reciprocal combination. NOD progeny of Eα16/NOD dams had a significantly reduced incidence (39% vs. 65%) and delayed onset (21 vs. 14 wk of age) of diabetes vis á vis NOD progeny of NOD dams (Fig. 1A). Since multiple checkpoints are involved in the development of T1D, we investigated whether insulitis was also affected, and found that the NOD progeny of Eα16/NOD dams had significantly reduced insulitis as well (Fig. 1 B and C).
Fig. 1.
Eα16/NOD dams transmitted protection from insulitis and T1D vertically to their NOD progeny. (A) Diabetes incidence in a cohort of NOD mice born to NOD dams (n = 34), NOD mice born to Eα16/NOD dams (n = 24), and Eα16/NOD mice (n = 20). ***P = 0.0004 (Gehan–Breslow–Wilcoxon test). (B) Proportion of islets with insulitis or peri-insulitis at 10 wk of age. NOD mice born to NOD dams mated with NOD sires, NOD mice born to NOD dams mated with Eα16/NOD sires, NOD mice born to Eα16/NOD dams mated to NOD sires, and Eα16/NOD mice. (C) Composite insulitis score for each mouse in B. ***P = 0.001, **P = 0.005 (Mann–Whitney test). (D) Composite insulitis scores for vancomycin-treated Eα16/NOD dams that received oral vancomycin during the last 7–10 d of pregnancy. ***P = 0.0005, *P = 0.02 (Mann–Whitney test).
Genetic imprinting, maternal antibody transfer, and microbiome colonization are all possible mechanisms for such vertically transmitted protection. Since the microbiota can protect NOD mice from disease development in certain circumstances (17), we tested whether maternal microbes were responsible for E-mediated vertical suppression of insulitis by giving vancomycin in the drinking water of Eα16/NOD mothers during their last 7–10 d of pregnancy. This treatment resulted in an insulitis frequency in NOD progeny of Eα16/NOD dams that was significantly higher than that of offspring from their untreated counterparts, and was indistinguishable from that of progeny from standard NOD dams (Fig. 1D). Thus, E-mediated protection from autoimmunity was, at least to a degree, vertically transmitted to NOD progeny and was microbiota-dependent.
Treatment with Certain Antibiotics Induced Insulitis and Altered the Intestinal Microbiome in Eα16/NOD Mice.
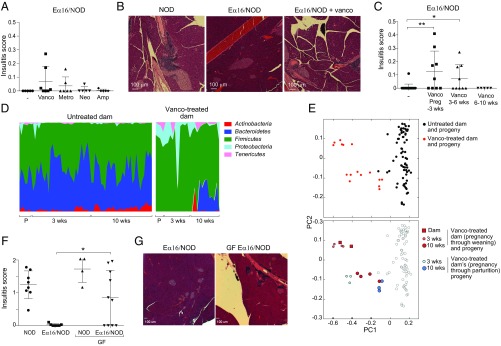
This conclusion implied that disruption or loss of the microbiota in Eα16/NOD mice might induce autoimmunity. As a first approach to evaluating this possibility, we gave female Eα16/NOD mice antibiotics in the drinking water, choosing vancomycin, metronidazole, neomycin, and ampicillin to survey a broad range of microbe sensitivities. Oral treatment from 3 to 6 wk of age with vancomycin or metronidazole, but not with neomycin or ampicillin, induced insulitis in a fraction of the Eα16/NOD mice (Fig. 2A); when it appeared, islet infiltration could be quite severe (Fig. 2B). While the fraction of Eα16/NOD mice showing insulitis under these conditions was quite low, this result contrasted with the extremely rare and mild insulitis observed with their untreated counterparts. Nonetheless, we sought to further optimize the treatment protocol. The effectiveness of vancomycin treatment showed a clear age-dependence. Giving this antibiotic either from the last 7–10 d of pregnancy until 3 wk after birth (optimally) or from 3 to 6 wk of age induced insulitis in Eα16/NOD mice, but administering it from 6 to 10 wk of age did not (Fig. 2C).
Fig. 2.
Antibiotic treatment induced insulitis and altered the intestinal microbiome in Eα16/NOD mice. (A) Insulitis composite score in Eα16/NOD mice treated with oral antibiotics provided in their drinking water from 3 to 6 wk of age. (B) Pancreas histology of 10-wk-old NOD mice (Left), Eα16/NOD mice (Center) or Eα16/NOD mice treated with vancomycin from 3 to 6 wk of age (Right). (C) Composite insulitis score of Eα16/NOD mice treated with vancomycin over different time periods, which include the last 7–10 d of pregnancy through weaning of the pups at 3 wk of age, 3–6 wk of age, or 6–10 wk of age. **P = 0.002, *P = 0.03 (Mann–Whitney test). (D) Phylum-level representation of 16S rRNA fecal microbiome from control or vancomycin-treated parents and their progeny at 3 and 10 wk of age. P, parents; 3 wk, 3-wk-old progeny; 10 wk, 10-wk-old progeny. (E) PCoA of unweighted UniFrac distances calculated from 16S rRNA gene sequencing of these fecal samples. (F) Insulitis scores from NOD, Eα16/NOD, and GF Eα16/NOD and NOD mice at 16–20 wk of age. *P = 0.02 (unpaired t test). (G) Pancreas histology from Eα16/NOD and GF Eα16/NOD mice.
Since oral vancomycin is not systemically absorbed, it appeared that disruption specifically of the intestinal microbiota might induce autoimmunity in a fraction of Eα16/NOD mice. We addressed this possibility by characterizing the fecal microbiome of vancomycin-treated pregnant dams, their progeny, and control mice unexposed to vancomycin by sequencing the V4 region of the 16S ribosomal RNA (rRNA) gene. As expected, oral administration of vancomycin changed the intestinal microbiome (reflected in feces) of the Eα16/NOD pregnant dams; the altered maternal microbiome was transmitted to their progeny, persisting through 10 wk of age (Fig. 2D). Principal coordinates analysis (PCoA) of unweighted UniFrac distances, with each dot representing the microbiome of an individual mouse, indicated that mice directly or indirectly exposed to vancomycin clustered separately from those that received no antibiotic (Fig. 2E, Upper). Most interesting, the altered microbiota was transmitted from vancomycin-treated Eα16/NOD dams to pups that had not been directly exposed to vancomycin. Consistent with this observation and the expected normalization of microbial flora over time, the maternal microbiomes clustered closer to those of their 3-wk-old than their 10-wk-old progeny (Fig. 2E, Lower).
Furthermore, we generated germ-free (GF) NOD and Eα16/NOD mice, and compared their degrees of insulitis. Sixty percent of GF Eα16/NOD mice showed substantial insulitis, with a severity similar to that of both their GF NOD littermates and standard NOD mice (Fig. 2 F and G). In short then, data from both the antibiotic-treatment and GF-housing experiments argued that microbes were critical elements of E-mediated protection from NOD diabetes. While the effects were not completely penetrant, it is important to keep in mind that only a few untreated Eα16/NOD individuals from our colony, of the hundreds examined over the past 25 y, exhibited any insulitis (5).
Eα16/NOD Mice Hosted a Distinct Intestinal Microbiome Early in Ontogeny.
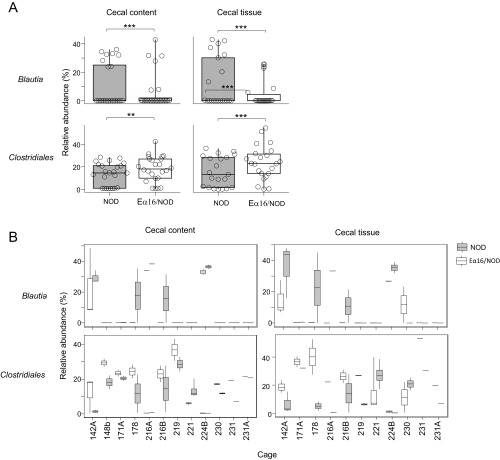
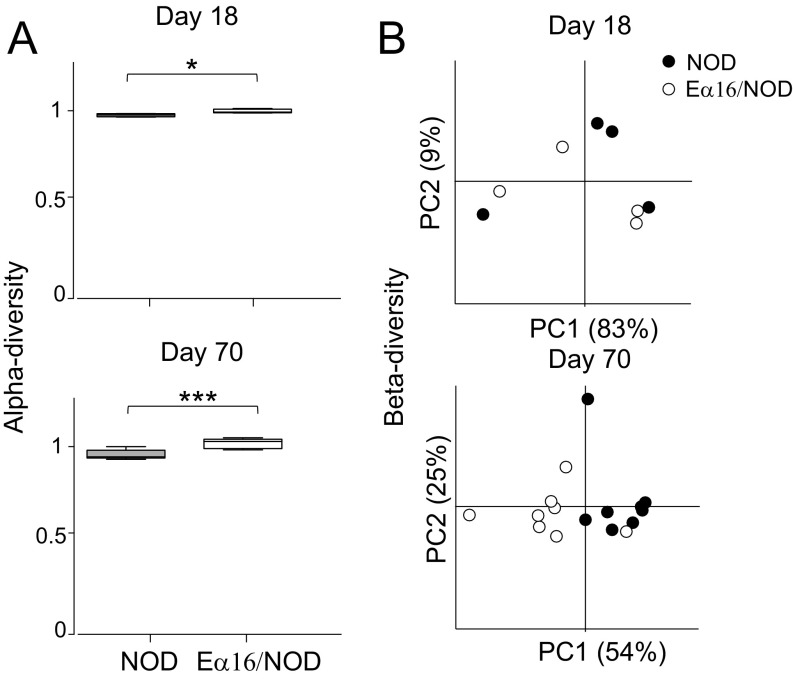
Islet autoimmunity begins when diabetogenic β-cell–derived self-antigens first appear in the pancreas-draining lymph nodes (PLNs) at days 15–18 in NOD mice (23), which also corresponds to the time when lymphatic connections from the gastrointestinal tract to the PLNs develop (24). Therefore, we tested whether expression of the E molecule impacted the microbiome of 18-d-old mice via 16S rRNA gene sequencing of cecal contents. To avoid detecting spurious associations between E-molecule expression and microbial taxa due to maternal or cage effects, we compared only cohoused NOD (n = 25) and Eα16/NOD (n = 25) littermates. The α-diversity of the cecal contents microbiome was significantly higher in Eα16/NOD than in NOD mice, and β-diversity was significantly lower, indicating that the microbiomes of the two types of mice were distinct (Fig. 3 A and B). Taxonomic comparisons by generalized linear mixed-effects modeling revealed a higher representation of microbes from the order Clostridiales [false-discovery rate (FDR) -corrected P = 1.2 × 10−3] and a lower proportion of the genus Blautia (FDR-corrected P = 7.2 × 10−6) in the microbiomes of Eα16/NOD mice (Fig. 3C, Fig. S1, and Table S1).
Fig. 3.
NOD and Eα16/NOD mice host distinctive intestinal microbiomes. (A) Boxplots of cecal microbiome α-diversity (PD whole tree) normalized as the ratio of each sample’s α-diversity to its cage mean. n = 25 Eα16/NOD and n = 25 NOD mice (**P = 0.004). (B) Boxplots of cecal microbiome β-diversity (weighted UniFrac distance) (**P = 0.0098). Unpaired t test with 10,000 Monte-Carlo simulations. (C) Boxplots of the relative abundance of the order Clostridiales in cecal contents and tissue. **P < 0.01, ***P < 0.001. (D) Cecal contents from NOD or Eα16/NOD donors were gavaged to NOD pups twice weekly from 2 to 5 wk of age. Insulitis was assessed at 10 wk of age. *P = 0.04 (unpaired t test).
Fig. S1.
Boxplots of the relative abundance of the order Clostridiales and the genus Blautia in cecal contents and tissue of 18-d-old NOD and Eα16/NOD mice showing distribution by individual mice (A) and individual cages (B). n = 25 Eα16/NOD and n = 25 NOD mice for cecal contents, and n = 21 Eα16/NOD and n = 21 NOD mice for cecal tissue. **P < 0.01, ***P < 0.001.
Table S1.
Differentially abundant taxa between Eα16/NOD and NOD cecal microbiomes
| Taxa | Tissue | Estimate | SE | P value | FDR | Higher representation | Statistically significant | Referencing | Age, d |
| (o) Clostridiales | Cecal contents | −0.39 | 0.083 | 2.5E-06 | 2.0E-05 | Eα16/NOD | * | Open | 18 |
| (o) Clostridiales | Cecal tissue | −0.47 | 0.081 | 5.5E-09 | 4.4E-08 | Eα16/NOD | * | Open | 18 |
| (o) Clostridiales | Cecal contents | −0.36 | 0.097 | 1.8E-04 | 1.2E-03 | Eα16/NOD | * | Closed | 18 |
| (o) Clostridiales | Cecal tissue | −0.50 | 0.089 | 2.0E-08 | 1.4E-07 | Eα16/NOD | * | Closed | 18 |
| (g) Blautia | Cecal contents | 0.66 | 0.120 | 3.6E-08 | 5.7E-07 | NOD | * | Open | 18 |
| (g) Blautia | Cecal tissue | 1.03 | 0.125 | 2.1E-16 | 3.5E-15 | NOD | * | Open | 18 |
| (g) Blautia | Cecal contents | 0.62 | 0.123 | 5.1E-07 | 7.2E-06 | NOD | * | Closed | 18 |
| (g) Blautia | Cecal tissue | 1.02 | 0.129 | 1.9E-15 | 2.7E-14 | NOD | * | Closed | 18 |
| (f) Erysipelotrichaceae | Cecal tissue | −0.54 | 0.234 | 2.1E-02 | 1.1E-01 | Eα16/NOD | Open | 18 | |
| (f) Erysipelotrichaceae | Cecal tissue | −0.72 | 0.280 | 1.0E-02 | 4.7E-02 | Eα16/NOD | * | Closed | 18 |
| (f) Ruminococcaceae | Cecal tissue | −0.43 | 0.178 | 1.5E-02 | 5.1E-02 | Eα16/NOD | Closed | 18 |
Generalized linear mixed-effect modeling on OTUs picked by closed referencing using the Greengenes database or OTUs generated using open reference de novo clustering. f, family; g, genus; o, order.
P < 0.05.
Since certain tissue-associated microbes may engender specific immune responses, we next investigated whether cecal tissue-associated microbes were differently represented between Eα16/NOD and littermate NOD mice. Consistent with the findings in cecal-content microbiomes, a higher representation of microbes from the order Clostridiales (FDR-corrected P = 1.4 × 10−7) and a lower proportion of the genus Blautia (FDR-corrected P = 2.7 × 10−14) were present in the cecal tissue microbiomes of Eα16/NOD mice (Fig. 3C, Fig. S1, and Table S1).
The microbiota differences were not uniform in all mice from different cages (Fig. S1), suggesting redundancy in the range of microbes affected by the E molecule. Therefore, we sought to identify combinations of operational taxonomic units (OTUs) affected by E (and interfering with diabetes) by constructing Random Forest classifiers of E or N genotypes based on the microbiome profiles. While success rates of 80–87% could be obtained, these proved not significantly different from chance by permutation analysis.
We also exploited a metaproteomic approach to investigate microbial protein expression in feces from littermate Eα16/NOD vs. NOD mice at day 18 and day 70 (n = 24 mice in total). Microbial proteomic data were aggregated according to both functional and taxonomic annotations (1,078 different functional-taxonomic features identified). α-Diversity was again significantly higher in Eα16/NOD individuals at day 18 and day 70. β-Diversity analysis revealed a strong cage/littermate effect at day 18, and clustering by mouse genotype at day 70 (Fig. S2).
Fig. S2.
(A) Boxplots of NOD mouse fecal metaproteomic α-diversity (Shannon index) normalized as the ratio of each sample’s α-diversity to its cage mean. n = 4 and 8 Eα16/NOD mice, and n = 4 and 8 NOD mice at day 18 and day 70, respectively (*P = 0.026 and ***P = 0.0002). (B) PCoA plot of NOD mice fecal metaproteomic β-diversity at day 18 and day 70.
Both the genomic and proteomic data argued that the Eα16/NOD and NOD microbiota were distinct during early life. The intestinal microbiomes of Eα16/NOD mice showed increased α-diversity, which has been associated with protection from T1D in genetically susceptible mice and humans (25, 26). To directly confirm this conclusion, we compared the ability of intestinal microbiota from Eα16/NOD vs. NOD donors to protect NOD recipients from insulitis. NOD mice gavaged twice weekly from 2 to 5 wk of age with Eα16/NOD cecal contents had a significantly reduced insulitis severity (median insulitis score of 0.55 vs. 1.23) compared with that of controls gavaged in parallel with NOD cecal contents (Fig. 3D).
Comparing the IgA-Bound Repertoire of Cecal Bacteria in NOD and Eα16/NOD Mice.
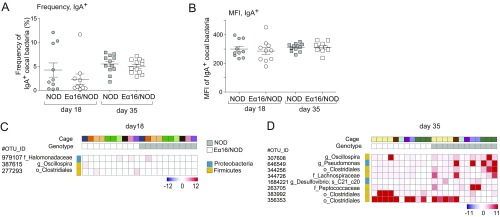
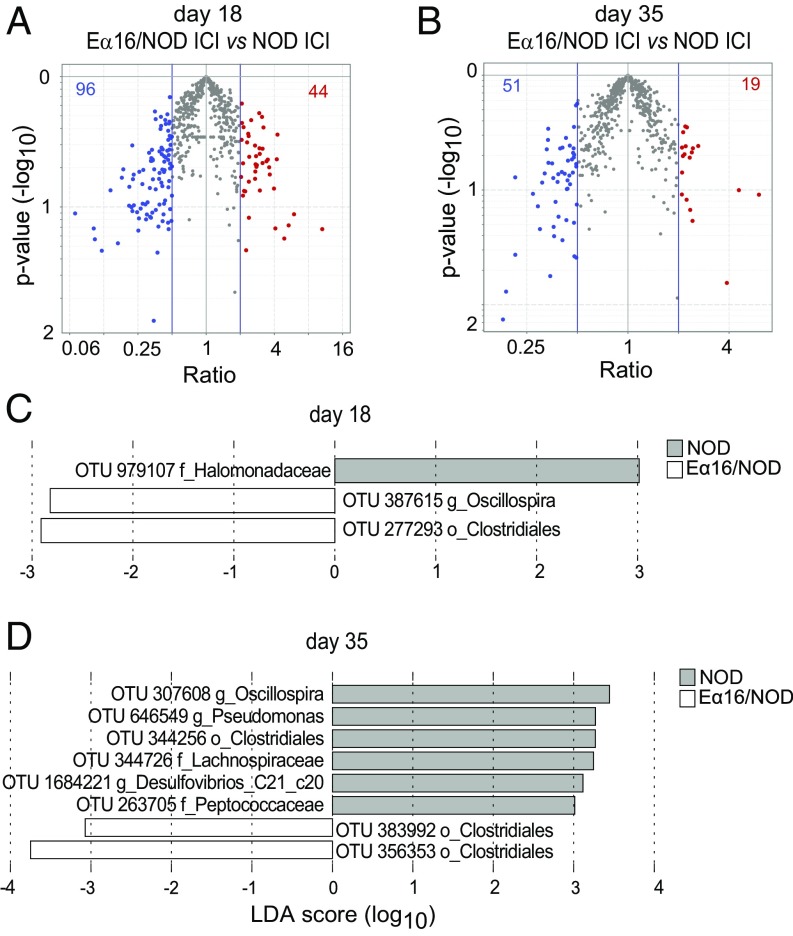
To explore one possible mechanism for E-mediated, microbiota-dependent protection in the NOD model of T1D, we investigated the IgA response to gut bacteria in NOD and Eα16/NOD littermates born to NOD mothers, using the recently developed IgA-seq method (27), to interrogate the composition of microbes that are bound, or not, to IgA molecules in the intestinal contents. Eα16/NOD and NOD littermates did not differ in the flow cytometric frequency or mean fluorescence intensity of IgA-coated cecal bacteria (Fig. S3), contrary to previous data demonstrating fluctuations in the proportion of IgA+ stool bacteria depending on the host MHC allele (19). In our IgA-sequencing data, direct comparisons between the genotypes revealed no OTUs that had significantly different IgA-coating index (ICI) values for either age group (Fig. 4 A and B). However, the LEfSe biomarker discovery tool (28) flagged a handful of OTUs that were overall differentially IgA-coated between NOD and their Eα16/NOD counterparts (Fig. 4 C and D), albeit with rather low scores. All four of the OTUs with higher representation in the Eα16/NOD IgA-coated microbiomes were from the order Clostridiales, while those with higher representations in NOD microbiomes were from the orders Clostridiales, Oceanospirillales, and Pseudomonales. When displayed on a per mouse basis and accounting for cage of origin, it appeared that the differential signals were scattered, with little uniformity, and were mostly due to a few mice (Fig. S3). We again applied a Random Forest approach to identify an effect of E on IgA coating of combinations of microbes, but no groups of OTUs were identified whose ICI significantly distinguished (by permutation testing) the two genotypes.
Fig. S3.
(A and B) Frequency and mean fluorescence intensity of IgA-coated cecal bacteria in 18- and 35-d-old NOD and Eα16/NOD mice. (C and D) Heatmaps depicting specific OTUs whose IgA-coated microbes may differ in 18- and 35-d-old NOD and Eα16/NOD mice.
Fig. 4.
IgA-bound repertoire of cecal bacteria is similar in NOD and Eα16/NOD mice. (A and B) Volcano plot comparing the ICI of the cecal microbiotas from NOD and Eα16/NOD mice at 18 and 35 d of age. (C and D) Comparison of the representation of specific IgA-coated taxa between the cecal microbiomes of 18- and 35-d-old NOD and Eα16/NOD mice.
Investigating the Intestinal Immune System in Eα16/NOD Mice.
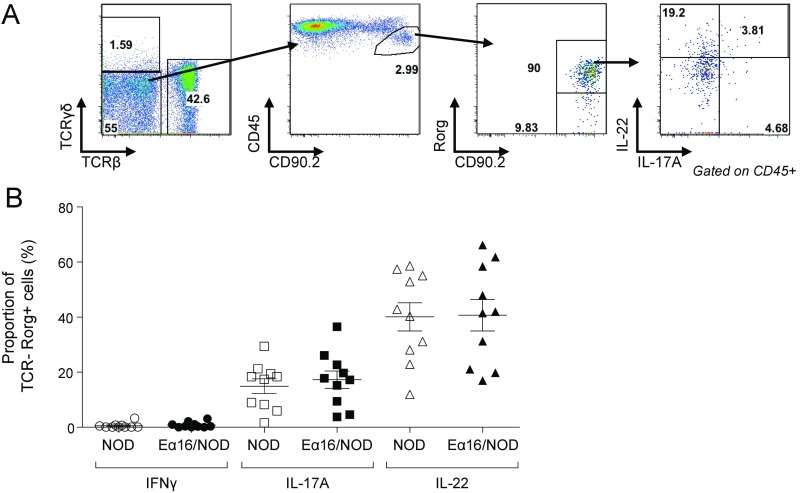
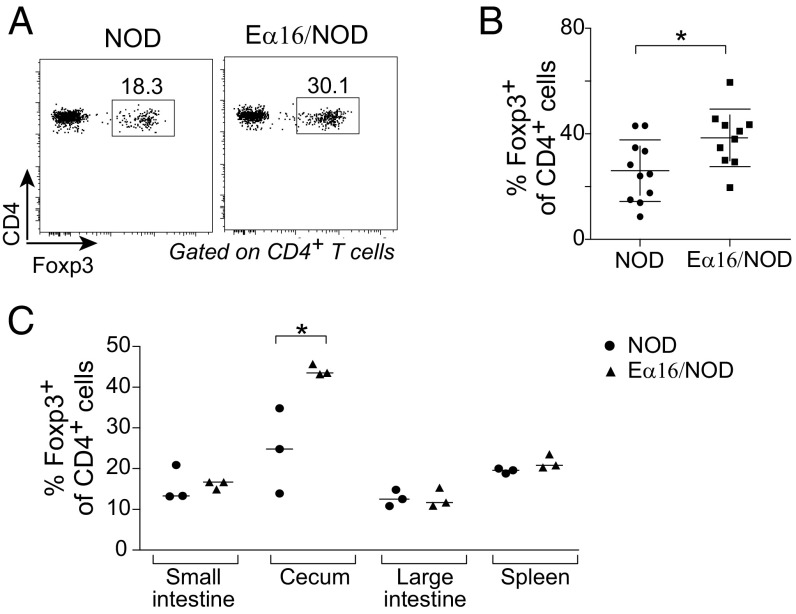
Finally, we explored whether the early-life microbiome alterations found in Eα16/NOD mice were associated with changes in the intestinal immune system. The innate and adaptive immune-system cell populations of the lamina propria of the large and small intestines, PLNs, Peyer’s patches, cecal patch, and spleen showed no robust differences in Eα16/NOD vs. NOD littermates, according to fraction and number of CD4+ T-helper cells expressing IFN-γ, IL-17, IL-10, or IL-22, CD8+ T cells, and myeloid cell populations, such as CD103-expressing cells. Since MHC-II expression on innate lymphoid cells (ILCs) is necessary to maintain homeostasis with commensal microbiota (29), we also compared the number and frequency of ILCs expressing the transcription factors Gata-3 or Rorγ, along with the number and frequency of ILC3 expressing IFN-γ, IL-17, or IL-22, and found no consistent differences (Fig. S4). However, we did observe an increased regulatory Treg cell frequency in the cecal lamina propria of Eα16/NOD mice at day 18 (Fig. 5 A and B). In contrast, there were similar populations of Treg in the small and large intestines, Peyer’s patches, cecal patch, and the PLNs (Fig. 5C).
Fig. S4.
Flow cytometric comparison of the ILC3 cell population cytokine production in littermate NOD and Eα16/NOD mice. (A) ILC3 gating strategy and representative plot. (B) Cytokine production by colonic ILC3 cell populations in NOD and Eα16/NOD mice.
Fig. 5.
Eα16/NOD mice had an enlarged representation of cecal Treg. (A) Cells were isolated from littermates at day 18 and analyzed by flow cytometry. Representative plot of Foxp3+CD4+ cell population. Cells were gated as TCRβ+CD19−CD45+. (B) Percentage of cecal lamina propria Foxp3+CD4+ T-cell from three independent experiments. (C) Percentage of Foxp3+CD4+ T cells. *P < 0.05.
Discussion
An association between certain MHC and HLA class II alleles and protection from particular autoimmune diseases, notably T1D, was discovered decades ago; yet the mechanisms underlying this dominant protection have remained mysterious. Here we showed that commensal microbes drove E-mediated protection from autoimmune diabetes, and that expression of the E complex shaped the intestinal microbiome during a critical early window of ontogeny.
How might E complex expression shape the intestinal microbiota in young NOD mice? One possibility is that an additional restriction element, the E complex, could allow immune responses against additional microbial antigens, thereby shaping the developing microbiota. In particular, there could be an effect on the nascent IgA repertoire via presentation of additional microbial antigens, and IgAs are known, in turn, to shape the intestinal microbiota (19, 30). We tested the hypothesis that the type of MHC class II allele present would influence the affinity and specificity of the IgA produced in response to the intestinal microbiota of young Eα16/NOD vs. NOD mice. However, we saw no differences in the composition of IgA-bound bacteria. Nonetheless, IgA binding may alter microbial localization and function without detectible impacts on microbiota composition, as has been reported in some contexts (31).
Second, E expression might influence antimicrobial peptide (AMP) secretion from intestinal epithelial cells. Expression of the E complex on APCs, leading to more cognate interactions with CD4+ T cells, could enhance APC activation, which in turn could trigger intestinal epithelial cells to secrete AMPs, known to impact intestinal microbiota localization and composition (32, 33). Third, E complex expression might promote host production of a microbe-specific metabolic substrate, conferring a selective advantage to specific microbes that can use this resource (34).
How does the Eα16/NOD intestinal microbiota prevent autoimmunity? A likely possibility is that the microbiome influences the development of the local intestinal immune system that, in turn, somehow prevents insulitis. Indeed, the intestinal microbiome changes in Eα16/NOD mice occurred at the time insulitis typically initiates (∼3 wk of age) (23). This timing corresponds to a wave of islet cell apoptosis (35) and establishment of a lymphatic connection between the intestinal immune system (24) and the PLNs, which is critical for the development of insulitis and T1D (36). The notion of an intestinal impact on the development of T1D is supported by studies in humans and mice, and is often referred to as the “leaky gut hypothesis” (37). In support of a role for the E complex working via such mechanisms, cell transfer experiments indicated that protection is mediated by the E-expressing macrophage or dendritic cell lineage (13), which is supported by genetic ablation studies showing a requirement for E-expression on the CD11c+ but not CD19+ cell lineage (12). Our observation of increased Treg cell proportions in the cecum of Eα16/NOD mice could represent an effect of early-life microbial stimulation on CD11c+ tolerogenic dendritic cells. Interestingly, Ooi et al. (38) have recently reported that the dominant protective effect of HLA-DR1 allele on development of Goodpasture’s disease can be attributed to shaping of the self-epitope–specific Treg repertoire.
Perhaps our study’s most important message is a societal one, assuming a translation of our findings on the NOD model to human T1D patients. Antibiotic treatment of infants, or just their pregnant mothers, can potentially subvert ordinarily potent diabetes-protective genetic elements.
Materials and Methods
Mice.
The generation of Eα16/NOD mice has previously been described (5). All mouse experiments were approved by the Institutional Animal Care and Use Committee of Harvard Medical School.
Diabetes and Insulitis Assessments.
Diabetes and insulitis were assessed as previously described (39). Insulitis was scored by two independent readers who were blinded to the identity of the slides.
Gavage.
NOD mice were gavaged biweekly starting at 2 wk of age using a 22-gauge straight oral gavage needle (VWR 20068-608) with either NOD or Eα16/NOD cecal contents.
Antibiotic Treatment.
For antibiotic treatment, 1 g/L of ampicillin sodium salt (Sigma), 1 g/L of metronidazole (Acros Organics) plus the sweetener Equal 2.5 g/L, 0.5 g/L vancomycin hydrochloride (Acros Organics), or 1 g/L of neomycin (Fisher BioReagents) were used. Pregnant dams were provided with 0.5 g/L of vancomycin hydrochloride in their drinking water during the last 7–10 d pregnancy.
Sample Collection and DNA Isolation.
Fresh fecal pellets, cecal contents, and cecal tissues were collected into sterile Eppendorf tubes under a laminar flow hood and stored at −80 °C until processing. Genomic DNA was isolated as previously described (39).
16S rRNA Gene Sequencing and Analysis.
The 16S rRNA gene sequencing of the V4 variable region was performed at the Broad Institute or Biopolymers Facility at Harvard Medical School on the Illumina MiSeq platform using the protocol previously described (40). Sequences were processed and curated using QIIME v1.9.0, as pick_closed reference otus.spy (41). To control for maternal and cage effects, each sample’s α-diversity value was normalized by dividing it against the mean α-diversity value calculated from all mice from its cage. This cage-normalized α-diversity value was then used for comparisons between NOD and Eα16/NOD mice. To compare β-diversity, weighted and unweighted UniFrac distances were calculated between each pair of NOD mice and each pair of Eα16/NOD mice. To control for maternal and cage effects, UniFrac distances were calculated between cohoused littermate pairs.
Linear Mixed Effect Modeling.
Data files from QIIME were analyzed in the R environment. Taxon differential abundance was calculated for the taxa that have greater than 1% relative abundance across all tested samples using generalized linear mixed-effects models with genotype and sample type as fixed effects and cage number as random effects (42). Multiple tests were corrected for FDR.
IgA-Seq Analyses.
Cecal content was collected from NOD and Eα16/NOD littermates, and frozen at −80 °C until further use. Sorting of IgA-bound cecal bacteria was carried out as previously described (27).
Preparation of Intestinal Cells for Immunologic Analysis and Flow Cytometry.
Small intestine, cecum, and colon lamina propria cell suspensions and flow cytometry were prepared as previously described (39). The cecal patch was removed from the cecum before this tissue was used for the preparation of the lamina propria cell suspensions.
SI Materials and Methods
Mice.
Eα16/NOD mice were maintained at the Jackson Laboratory, from where they were periodically imported into our specific pathogen-free facility at the New Research Building at Harvard Medical School. Mice were then bred at the New Research Building and their progeny used for experiments. Mice were maintained free of segmented filamentous bacteria, which were confirmed by routine testing of mouse feces for the presence of segmented filamentous bacteria by PCR, as previously described (39). Litters were weaned at 18–21 d of age. NOD and Eα16/NOD littermates remained cohoused. GF Eα16/NOD mice were generated by Caesarian-section delivery of Eα16/NOD and NOD pups under aseptic conditions and fostering to GF Swiss-Webster dams. GF NOD mice were also kindly provided by A. Chervonsky, Department of Pathology, Committee on Immunology, The University of Chicago, Chicago. Mice were confirmed to be GF by aerobic/anaerobic culture and 16S rRNA gene PCR. All mouse experiments were approved by the Institutional Animal Care and Use Committee of Harvard Medical School.
Diabetes and Insulitis Assessments.
Starting at 10 wk of age, mice were tested weekly for the onset of diabetes by screening their urine glucose levels (Diastix). After two consecutive positive urine glucose levels, the onset of diabetes was then confirmed by blood glucose measurement >300 mg/dL. Mice were screened until 40 wk of age. For the evaluation of insulitis, pancreata were dissected and immediately fixed in 10% buffered formalin (Sigma-Aldrich), then embedded in paraffin, sectioned, and stained with H&E. Each islet was given a score with 0 = no insulitis, 1 = peri-insulitis, and 2 = insulitis. At least 50 islets were scored for most samples. The composite insulitis score for each mouse is calculated as follows: two times the number of islets with insulitis plus the number of islets with peri-insulitis divided by the total number of islets per pancreas.
Cecal Contents and Tissue Collection.
For cecal luminal content sample collection, the cecum was opened longitudinally and gently vortexed in 50-mL Falcon tubes with 5 mL of sterile PBS to release the cecal contents. Cecal contents were transferred into three sterile 1.5-mL Eppendorf tubes and centrifuged at 0.8 × g for 5 min. Cecal tissues were washed three times in sterile PBS. The samples were immediately placed on dry ice and stored at −80 °C.
16S rRNA Gene Sequencing and Analysis.
Sequencing was performed according to manufacturer’s specification with the addition 5–30% PhiX and generation of paired-end 250 base pair in length reads. The overlapping region were stitched together and further processed in a data curation pipeline implemented in QIIME v1.9.0 as pick_closed reference otus.spy (41). OTU tables were then filtered to remove very low abundance OTUs (<0.00005%). Samples with fewer than 10,000 sequences were excluded from analysis. The α- and β-diversity metrics were calculated using OTU tables rarified to 10,000 sequences. The Greengenes database, release May 2013, was used for analysis requiring a phylogenetic tree. P values were calculated using unpaired t test with 10,000 Monte-Carlo simulations.
Metaproteomic Analysis.
Proteins were extracted from stool samples as described previously, without any differential centrifugation step as pretreatment (43). Peptide mixtures were generated according to the filter-aided sample preparation with minor modifications detailed elsewhere (44, 45). Peptide mixtures were subjected to LC-MS/MS analysis using an LTQ-Orbitrap Velos mass spectrometer (Thermo Scientific) interfaced with an UltiMate 3000 RSLCnano LC system (Thermo Scientific), according to established procedures and parameters (46). Peptide identification was carried out using Sequest-HT as search engine, Percolator as peptide validator tool (both embedded in Proteome Discoverer v1.4), and an in-house mouse gut metagenomes assembly as sequence database (to which mouse and soybean sequences from UniProt, release 2015_03, were appended), as detailed in an earlier study (46). Functional and taxonomic annotation of database sequences were carried out by extracting protein family information upon blastp search (e-value threshold 10−5) against the SwissProt database (release 2015_03), and performing lowest common ancestor-based classification with MEGAN5 (47) upon blastp search (e-value threshold 10−5) against the NCBI-nr database, respectively. Principal component analysis of metaproteomic data were carried out using Perseus (coxdocs.org/doku.php?id=perseus:start).
Preparation of Cecal Contents for Gavage.
Ceca from three to five 6- to 10-wk-old NOD or Eα16/NOD mice were dissected, opened longitudinally in a laminar flow hood, and contents were separated from tissue by manually shaking for 10 s in 5 mL/cecum of cold, sterile PBS. Cecal tissue was removed and the large aggregates were allowed to settle. The cecal liquid was decanted off into sterile Eppendorf tubes and stored at −80 °C.
IgA-Seq Analyses.
Cecal content was collected from NOD and Eα16/NOD littermates, and frozen at −80 °C until further use. Sorting of IgA-bound cecal bacteria was carried out as previously described (27). Briefly, cecal contents were homogenized by bead-beating in Lysing Matrix D tubes (MP Biomedicals). The cecal slurry was filtered through 40 μM cell strainers and centrifuged to obtain a bacterial pellet. Bacteria were blocked with 10% rat serum (Jackson ImmunoResearch) in PBS containing 1% BSA, and then labeled with a PE-conjugated anti-IgA antibody (Clone mA-6E1; eBioscience). Samples were separated into IgA+ and IgA− fractions by FACS on a FACSAria. Bacteria were pelleted and stored at −80 °C until the DNA extraction step. DNA was isolated from the bacterial pellets by phenol-chloroform extraction and subjected to 16S rRNA gene sequencing as described above. The extent of IgA-coating for any given OTU, also known as the ICI, was calculated as the ratio of the OTU relative abundance in the IgA+ fraction to its relative abundance in the IgA− fraction.
Acknowledgments
We thank Dirk Gevers for helpful advice on microbiome sequencing experiments; S. Edwards, A. T. Sherpa, and K. Hattori for assistance with mice; and H. Paik and L. Yang for help with informatics. This work was supported by the JPB Foundation and a gift from the Howalt family (to C.B. and D.M.); by a Pediatric Infectious Disease Society Fellowship Award, JDRF advanced Post-Doctoral Fellowship 10-2013-105, a Child Health Research Center K12 Award, and NIH Grant K08AI114970 (all to M.S.); and by National Science Foundation Fellowship DGE1144152 (to L.K.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1712280114/-/DCSupplemental.
References
- 1.Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Curr Diab Rep. 2011;11:533–542. doi: 10.1007/s11892-011-0223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todd JA, Wicker LS. Genetic protection from the inflammatory disease type 1 diabetes in humans and animal models. Immunity. 2001;15:387–395. doi: 10.1016/s1074-7613(01)00202-3. [DOI] [PubMed] [Google Scholar]
- 3.Mathis DJ, Benoist C, Williams VE, 2nd, Kanter M, McDevitt HO. Several mechanisms can account for defective E alpha gene expression in different mouse haplotypes. Proc Natl Acad Sci USA. 1983;80:273–277. doi: 10.1073/pnas.80.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimoto H, Kikutani H, Yamamura K, Kishimoto T. Prevention of autoimmune insulitis by expression of I-E molecules in NOD mice. Nature. 1987;328:432–434. doi: 10.1038/328432a0. [DOI] [PubMed] [Google Scholar]
- 5.Böhme J, Schuhbaur B, Kanagawa O, Benoist C, Mathis D. MHC-linked protection from diabetes dissociated from clonal deletion of T cells. Science. 1990;249:293–295. doi: 10.1126/science.2115690. [DOI] [PubMed] [Google Scholar]
- 6.Lund T, et al. Prevention of insulin-dependent diabetes mellitus in non-obese diabetic mice by transgenes encoding modified I-A β-chain or normal I-E α-chain. Nature. 1990;345:727–729. doi: 10.1038/345727a0. [DOI] [PubMed] [Google Scholar]
- 7.Reich EP, Sherwin RS, Kanagawa O, Janeway CA., Jr An explanation for the protective effect of the MHC class II I-E molecule in murine diabetes. Nature. 1989;341:326–328. doi: 10.1038/341326a0. [DOI] [PubMed] [Google Scholar]
- 8.Parish NM, Chandler P, Quartey-Papafio R, Simpson E, Cooke A. The effect of bone marrow and thymus chimerism between non-obese diabetic (NOD) and NOD-E transgenic mice, on the expression and prevention of diabetes. Eur J Immunol. 1993;23:2667–2675. doi: 10.1002/eji.1830231042. [DOI] [PubMed] [Google Scholar]
- 9.Mellanby RJ, Phillips JM, Parish NM, Cooke A. Both central and peripheral tolerance mechanisms play roles in diabetes prevention in NOD-E transgenic mice. Autoimmunity. 2008;41:383–394. doi: 10.1080/08916930801991021. [DOI] [PubMed] [Google Scholar]
- 10.Trembleau S, Gregori S, Penna G, Gorny I, Adorini L. IL-12 administration reveals diabetogenic T cells in genetically resistant I-Ealpha-transgenic nonobese diabetic mice: Resistance to autoimmune diabetes is associated with binding of Ealpha-derived peptides to the I-A(g7) molecule. J Immunol. 2001;167:4104–4114. doi: 10.4049/jimmunol.167.7.4104. [DOI] [PubMed] [Google Scholar]
- 11.Nakano N, Kikutani H, Nishimoto H, Kishimoto T. T cell receptor V gene usage of islet β cell-reactive T cells is not restricted in non-obese diabetic mice. J Exp Med. 1991;173:1091–1097. doi: 10.1084/jem.173.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai S, Serra P, Clemente-Casares X, Slattery RM, Santamaria P. Dendritic cell-dependent in vivo generation of autoregulatory T cells by antidiabetogenic MHC class II. J Immunol. 2013;191:70–82. doi: 10.4049/jimmunol.1300168. [DOI] [PubMed] [Google Scholar]
- 13.Johnson EA, Silveira P, Chapman HD, Leiter EH, Serreze DV. Inhibition of autoimmune diabetes in nonobese diabetic mice by transgenic restoration of H2-E MHC class II expression: Additive, but unequal, involvement of multiple APC subtypes. J Immunol. 2001;167:2404–2410. doi: 10.4049/jimmunol.167.4.2404. [DOI] [PubMed] [Google Scholar]
- 14.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 15.Redondo MJ, et al. Genetic determination of islet cell autoimmunity in monozygotic twin, dizygotic twin, and non-twin siblings of patients with type 1 diabetes: Prospective twin study. BMJ. 1999;318:698–702. doi: 10.1136/bmj.318.7185.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chervonsky AV. Influence of microbial environment on autoimmunity. Nat Immunol. 2010;11:28–35. doi: 10.1038/ni.1801. [DOI] [PubMed] [Google Scholar]
- 17.Mathis D, Benoist C. The influence of the microbiota on type-1 diabetes: On the threshold of a leap forward in our understanding. Immunol Rev. 2012;245:239–249. doi: 10.1111/j.1600-065X.2011.01084.x. [DOI] [PubMed] [Google Scholar]
- 18.Gomez A, et al. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PLoS One. 2012;7:e36095. doi: 10.1371/journal.pone.0036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubinak JL, et al. MHC variation sculpts individualized microbial communities that control susceptibility to enteric infection. Nat Commun. 2015;6:8642. doi: 10.1038/ncomms9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hov JR, et al. The influence of the autoimmunity-associated ancestral HLA haplotype AH8.1 on the human gut microbiota: A cross-sectional study. PLoS One. 2015;10:e0133804. doi: 10.1371/journal.pone.0133804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greeley SA, et al. Elimination of maternally transmitted autoantibodies prevents diabetes in nonobese diabetic mice. Nat Med. 2002;8:399–402. doi: 10.1038/nm0402-399. [DOI] [PubMed] [Google Scholar]
- 22.Warram JH, Krolewski AS, Gottlieb MS, Kahn CR. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med. 1984;311:149–152. doi: 10.1056/NEJM198407193110304. [DOI] [PubMed] [Google Scholar]
- 23.Höglund P, et al. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turley SJ, Lee JW, Dutton-Swain N, Mathis D, Benoist C. Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc Natl Acad Sci USA. 2005;102:17729–17733. doi: 10.1073/pnas.0509006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krych Ł, Nielsen DS, Hansen AK, Hansen CH. Gut microbial markers are associated with diabetes onset, regulatory imbalance, and IFN-γ level in NOD mice. Gut Microbes. 2015;6:101–109. doi: 10.1080/19490976.2015.1011876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kostic AD, et al. DIABIMMUNE Study Group The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17:260–273. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palm NW, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–1010. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segata N, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hepworth MR, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macpherson AJ, Köller Y, McCoy KD. The bilateral responsiveness between intestinal microbes and IgA. Trends Immunol. 2015;36:460–470. doi: 10.1016/j.it.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Kinnebrew MA, et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee S, Hooper LV. Antimicrobial defense of the intestine. Immunity. 2015;42:28–39. doi: 10.1016/j.immuni.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 34.Pickard JM, et al. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature. 2014;514:638–641. doi: 10.1038/nature13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med. 2003;198:1527–1537. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gagnerault MC, Luan JJ, Lotton C, Lepault F. Pancreatic lymph nodes are required for priming of beta cell reactive T cells in NOD mice. J Exp Med. 2002;196:369–377. doi: 10.1084/jem.20011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaarala O, Atkinson MA, Neu J. The “perfect storm” for type 1 diabetes: The complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555–2562. doi: 10.2337/db08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ooi JD, et al. Dominant protection from HLA-linked autoimmunity by antigen-specific regulatory T cells. Nature. 2017;545:243–247. doi: 10.1038/nature22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kriegel MA, et al. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci USA. 2011;108:11548–11553. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gevers D, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolker BM, et al. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol Evol. 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Tanca A, Palomba A, Pisanu S, Addis MF, Uzzau S. Enrichment or depletion? The impact of stool pretreatment on metaproteomic characterization of the human gut microbiota. Proteomics. 2015;15:3474–3485. doi: 10.1002/pmic.201400573. [DOI] [PubMed] [Google Scholar]
- 44.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 45.Tanca A, Biosa G, Pagnozzi D, Addis MF, Uzzau S. Comparison of detergent-based sample preparation workflows for LTQ-Orbitrap analysis of the Escherichia coli proteome. Proteomics. 2013;13:2597–2607. doi: 10.1002/pmic.201200478. [DOI] [PubMed] [Google Scholar]
- 46.Tanca A, et al. A straightforward and efficient analytical pipeline for metaproteome characterization. Microbiome. 2014;2:49. doi: 10.1186/s40168-014-0049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huson DH, Weber N. Microbial community analysis using MEGAN. Method Enzymol. 2013;531:465–485. doi: 10.1016/B978-0-12-407863-5.00021-6. [DOI] [PubMed] [Google Scholar]