We humans are great apes, but share a surprisingly extensive suite of traits with social insects as well as with primates (1). These overlapping human–insectan phenotypes, which include divisions of labor, alloparental care, extensive food sharing, group–colony structures, collective decision making, and complex social cooperation, have indeed been considered responsible for the spectacular ecological and evolutionary successes of both social insects and humans, compared with other forms of animal life. Given the immense phylogenetic distance of humans from social insects, their common behavioral and life-history traits have thus far usually been ascribed to convergence, whereby shared selective pressures drive the evolution of social similarities despite highly divergent genetic and morphological substrates. In contrast, Shpigler et al. (2) demonstrate that a core, shared human–social insect phenotype, social responsiveness—as indicated by between-bee interactions and human diagnoses of autism—actually reflects shared genetic underpinnings. Shpigler et al.’s discovery provides novel insights into the genomic bases of both social adaptation and autism spectrum disorders, and implies a universality to social life with implications from philosophy to medicine. But how can taxa so otherwise different as honey bees and humans be similar with regard to genetic mechanisms of complex social behavior and its spectrum of expression?
The first comparative, evolutionary-genetic studies across highly diverse lineages focused on development and morphology, revealing that animal body plans for taxa as disparate as fruit flies and vertebrates were orchestrated by the same suite of “homeobox” genes (3). These findings were profoundly unexpected, but in retrospect make sense in terms of genetic “toolkits”: sets of master genes that build phenotypes, across lineages, as modular variations upon common themes (3, 4). More recent molecular genomic and bioinformatic methods, most notably RNA sequencing for quantification of gene expression and Gene Ontology analyses for classification of gene functions, have led to a burst of studies showing remarkable across-taxon similarities of gene-expression changes in response to the same environmental stimuli, such as territorial intrusion (e.g., ref. 5). These results extended deep homology to the brain and behavior, though only for relatively simple actions, such as aggressive responses to threat. What about the much more nuanced and complex social interactions that typify humans and highly social insects?
At its most basic, social behavior can be quantified in terms of social responsiveness, the degree to which an individual reacts to conspecifics. For honey bees, Shpigler et al. (2) evaluated social responsiveness of individual bees in two contexts: participation in the social opportunity of brood care of larvae, and engagement by social challenge of a potentially threatening noncolony mate. The common denominator of both is a willingness to take part in a socially salient interaction with conspecific individuals. Some bees responded consistently only to brood or only to the threat, but few responded to both, reflecting one facet of the strong divisions of labor that characterize honey bee social systems. Most importantly, a substantial proportion of bees (almost 15% of those tested), responded consistently to neither brood nor threat, and could be categorized as “socially unresponsive.”
The adaptive significance, if any, of such social indifference remains unclear, for honey bees as well as for the other social insect species with many inactive workers (6), and for humans (more on this point below). However, sequencing of RNA from brain tissue of the socially unresponsive bees, and comparison of their gene-expression profiles with those of the bees that were consistently socially interactive in brood care or defense tasks, revealed a set of genes that differed in expression levels: so-called differentially expressed genes. Gene Ontology analysis demonstrated that these differentially expressed genes were not a random subset of the genome, but were concentrated in specific gene-function categories including, for example, ion channels, chaperone proteins, and hormone signaling. As such, the gene set provides a functional-genomic “signature” for honey bee social responsiveness and division of social labor.
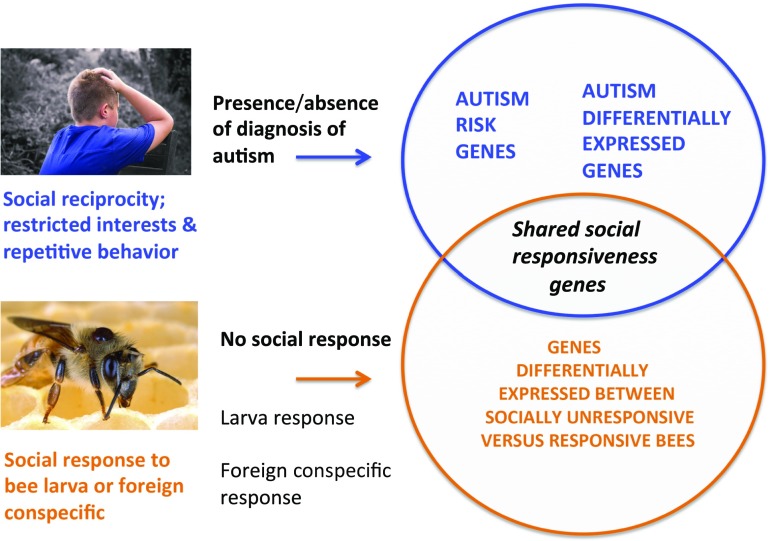
How does one measure social responsiveness among humans? One simple approach, and the one used here, is by its apparent extreme: reduced sociality among individuals with autism. Autism is formally defined in terms of: (i) deficits in social reciprocity, nonverbal communication behavior, and developing social relationships; and (ii) restricted interests and repetitive behavior. It exhibits high heritability (a strong genetic basis), and a genetic architecture involving both large single-gene or single-locus alterations and small, cumulative polygenic effects (7, 8). In addition to such germline differences, autism is also typified by differences in gene-expression profiles within the brain, such that individuals with autism diverge consistently from controls. The main upshot of Shpigler et al.’s study (2) is that they found statistically significantly high levels of overlap of the genes differentially expressed among honey bees varying in social responsiveness, with both human autism risk genes and human brain gene expression in autism versus controls (Fig. 1). These results are remarkable in indicating that a central aspect of sociality in humans and honey bees is underlain by an overlapping set of genes, with a strong implication of core, universal sociogenetic circuitry orchestrating social behavior in other animals as well. The gene sets involved, concentrating on GABA-ergic transmission and ion channel genes, also make functional sense in terms of what sorts of gene should most directly modulate social interaction and autism (9, 10).
Fig. 1.
Experimental and bioinformatic testing of the hypothesis that brain genes expressed in socially unresponsive honey bees overlap nonrandomly with autism risk genes, and with genes differentially expressed between individuals with autism and control individuals.
What are the implications of these results, for the study of social behavior, and for understanding autism? For analyzing sociality, the hypothesis is raised that social interactions and systems represent variations on surprisingly simple, shared genomic themes, that can be analyzed effectively even without detailed information on neural circuitry and brain structural–functional systems. Under this paradigm, brain circuits from bees to birds to humans are functionally, like brands and sizes of computers, quite similar (e.g., ref. 11). As such, among-taxon diversity is caused in large part by regulatory variation mediated by minor diversity in gene participation and functions within sociogenomic orchestras, subject to constraints on brainpower. Shorter causative paths from baseline genetic variation to emergent social behavioral variation bode well for the effectiveness of analyzing sociogenomics across additional diverse taxa, and finding “genes for altruism” (12), “genes for empathy,” and “genes for alloparental care,” among many others, playing the genetic equivalents of first violin or bass. In each case, variation in sequence and expression of the relevant genes can, moreover, also indicate genetically based trade-offs between, for example, different social functions, or social versus nonsocial functional domains.
What, in turn, can honey bee behavior teach us about autism? Most directly, it points us toward a key, adaptively varying phenotype at the center of autism-related cognition and behavior: social responsiveness itself. Social responsiveness can be as simple as replying to one’s name, forming an affective attachment, or engaging in a short conversation, the key point being that focus on such adaptive behaviors makes clear that to best study autism, we need to better understand typical social behaviors and how they can grade smoothly even into severe autism. Are there cognitive-genetic “switches” between social and nonsocial,
Shpigler et al.’s discovery provides novel insights into the genomic bases of both social adaptation and autism spectrum disorders, and implies a universality to social life with implications from philosophy to medicine.
or internally directed, attention that mediate responsiveness? If so, what genes control their thresholds and toggling? This logic of analyzing autism by studying typical sociality may seem counterintuitive, but it falls squarely into the standard medical paradigm of understanding disease as maladaptation and extremes of trade-offs, by determining what specific adaptive system or systems has become altered or disrupted, and how (13).
For honey bees, individually based divisions of labor allow escape from cognitive and behavioral trade-offs, and increased efficiencies. In contrast, humans switch more or less flexibly between different attentional and information processing modes, including the internally directed “default” mode versus “task-positive” modes that may be focused either socially empathically on people or on the worlds of concepts, systems, numbers, and things. Consider the “unresponsive honey bees” in this context: might they indeed be specialized for nonsocial or less-social tasks, such as building, foraging, hive-cooling, or spatial navigation? Did they just not receive the correct experimental marching orders for spurring their brain genes into more-specialized differential expression? Such nonsocial tasks may otherwise trade-off sharply with social ones, to the detriment of the collective. In autism, we have considerable evidence of trade-offs between social abilities and nonsocial ones, including visual-spatial skills, sensory-system acuity, aspects of abstract intelligence, systemizing and engineering abilities, and—at the extreme—the astounding abilities of savants (14). To a notable extent, then, autistic cognition and behavior appears to involve extremes of social–nonsocial trade-offs and benefits, as well as costs coming from bee-like specializations. Evidence of a genetic basis for such trade-offs comes, for example, from the NLGN3 gene R451C amino acid polymorphism in a mouse model of autism: mice with the low-sociality allele exhibit enhanced spatial learning (15). Dissecting the genetic bases of cognitive trade-offs among humans, as inspired by our social–insectan abilities in both social and nonsocial arenas, should be a top priority for future work.
The second domain of autism, restricted interests and repetitive behavior, has certainly served honey bees well in the coordination and effectiveness of their social world. Like social responsiveness, does it also share a functional-genomic basis with humans expressing autism, or perhaps obsessive-compulsive behavior (16)? Given the extensive and deep phenotypic and genetic similarities of humans with social insects, we clearly have much to learn from one another, to enhance understanding of both their behaviors and our own.
Acknowledgments
The author’s research is supported by Natural Sciences and Engineering Council of Canada Discovery Grant 2014-06505.
Footnotes
The author declares no conflict of interest.
See companion article on page 9653.
References
- 1.Crespi B. The insectan apes. Hum Nat. 2014;25:6–27. doi: 10.1007/s12110-013-9185-9. [DOI] [PubMed] [Google Scholar]
- 2.Shpigler HY, et al. Deep evolutionary conservation of autism-related genes. Proc Natl Acad Sci USA. 2017;114:9653–9658. doi: 10.1073/pnas.1708127114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pick L. Hox genes, evo-devo, and the case of the ftz gene. Chromosoma. 2016;125:535–551. doi: 10.1007/s00412-015-0553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubenstein DR, Hofmann HA. Proximate pathways underlying social behavior. Curr Opin Behav Sci. 2015;6:154–159. [Google Scholar]
- 5.Rittschof CC, et al. Neuromolecular responses to social challenge: Common mechanisms across mouse, stickleback fish, and honey bee. Proc Natl Acad Sci USA. 2014;111:17929–17934. doi: 10.1073/pnas.1420369111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charbonneau D, Dornhaus A. When doing nothing is something. How task allocation strategies compromise between flexibility, efficiency, and inactive agents. J Bioeconomics. 2015;17:217–242. [Google Scholar]
- 7.Tick B, Bolton P, Happé F, Rutter M, Rijsdijk F. Heritability of autism spectrum disorders: A meta-analysis of twin studies. J Child Psychol Psychiatry. 2016;57:585–595. doi: 10.1111/jcpp.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Torre-Ubieta L, Won H, Stein JL, Geschwind DH. Advancing the understanding of autism disease mechanisms through genetics. Nat Med. 2016;22:345–361. doi: 10.1038/nm.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coghlan S, et al. GABA system dysfunction in autism and related disorders: From synapse to symptoms. Neurosci Biobehav Rev. 2012;36:2044–2055. doi: 10.1016/j.neubiorev.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnashev N, Szepetowski P. NMDA receptor subunit mutations in neurodevelopmental disorders. Curr Opin Pharmacol. 2015;20:73–82. doi: 10.1016/j.coph.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Pfenning AR, et al. Convergent transcriptional specializations in the brains of humans and song-learning birds. Science. 2014;346:1256846. doi: 10.1126/science.1256846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson GJ, Hurd PL, Crespi BJ. Genes underlying altruism. Biol Lett. 2013;9:20130395. doi: 10.1098/rsbl.2013.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crespi BJ. The evolutionary etiologies of autism spectrum and psychotic affective spectrum disorders. In: Alvergne A, Jenkinson C, Faurie C, editors. Evolutionary Thinking in Medicine. Springer; Heidelberg: 2016. pp. 299–327. [Google Scholar]
- 14.Crespi BJ, Go MC. Diametrical diseases reflect evolutionary-genetic tradeoffs: Evidence from psychiatry, neurology, rheumatology, oncology and immunology. Evol Med Public Health. 2015;2015:216–253. doi: 10.1093/emph/eov021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaramillo TC, Liu S, Pettersen A, Birnbaum SG, Powell CM. Autism-related neuroligin-3 mutation alters social behavior and spatial learning. Autism Res. 2014;7:264–272. doi: 10.1002/aur.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo W, et al. Polygenic risk score and heritability estimates reveals a genetic relationship between ASD and OCD. Eur Neuropsychopharmacol. 2017;27:657–666. doi: 10.1016/j.euroneuro.2017.03.011. [DOI] [PubMed] [Google Scholar]