Abstract
Background
Paroxysmal nocturnal hemoglobinuria (PNH), a rare clonal hematopoietic stem cell disorder, is characterized by chronic, uncontrolled complement activation leading to intravascular hemolysis and an inflammatory prothrombotic state. The EXPLORE study aimed to determine the prevalence of undiagnosed PNH in patients with aplastic anemia (AA), myelodysplastic syndrome (MDS), and/or other bone marrow failure (BMF) syndromes and the effect of PNH clone size on hemolysis.
Methods
Patients, selected from medical office chart reviews, had blood samples collected for hematologic panel testing and for flow cytometry detection of PNH clones.
Results
Granulocyte PNH clones ≥ 1% were detected in 199 of all 5,398 patients (3.7%), 93 of 503 AA patients (18.5%), 50 of 4,401 MDS patients (1.1%), and 3 of 130 other BMF patients (2.3%). Higher-sensitivity analyses detected PNH clones ≥ 0.01% in 167 of 1,746 patients from all groups (9.6%) and in 22 of 1,225 MDS patients (1.8%), 116 of 294 AA patients (39.5%), and four of 54 other BMF patients (7.8%). Among patients with PNH clones ≥ 1%, median clone size was smaller in patients with AA (5.1%) than in those with MDS (17.6%) or other BMF (24.4%), and the percentage of patients with lactate dehydrogenase levels (a marker for intravascular hemolysis) ≥ 1.5 × upper limit of normal was smaller in patients with AA (18.3%) than in those with MDS (42.0%).
Conclusions
These results confirm the presence of PNH clones in high-risk patient groups and suggest that screening of such patients may facilitate patient management and care.
Keywords: paroxysmal nocturnal hemoglobinuria, bone marrow dysfunction, clinical trial, flow cytometry, hematology
INTRODUCTION
Paroxysmal nocturnal hemoglobinuria (PNH) is characterized by chronic, uncontrolled complement activation leading to intravascular hemolysis and an inflammatory prothrombotic state (1,2). The etiology of this rare hematopoietic stem cell disorder is an acquired clonal genetic deficiency of glycosylphosphatidylinositol (GPI)-anchored and GPI-linked proteins on the surface of blood cells. Of particular importance in PNH are the GPI-anchored complement regulatory proteins CD55 (a decay accelerating factor) and CD59 (a membrane inhibitor of reactive lysis) (3). Deficiency of CD55 and CD59 on blood cells leads to increased cellular sensitivity to complement-mediated attack, resulting in hemolysis of red blood cells (RBCs) and activation of granulocytes, monocytes, and platelets (4).
Patients with PNH are at an elevated risk of life-threatening complications such as thrombosis, chronic kidney disease, and end-organ damage as well as disease-related morbidities including pulmonary hypertension, abdominal pain, dyspnea, and debilitating fatigue (5–10). PNH is associated with early mortality; up to 35% of PNH patients die within 5 years of diagnosis (10), and up to 50% of patients die within 10–15 years of diagnosis (11,12).
There is a well-established association between PNH and aplastic anemia (AA) (13); in fact, PNH often arises in patients with AA after effective immunosuppressive therapy (IST) (14). PNH cells have also been detected in some patients with other bone marrow failure (BMF) syndromes, such as refractory anemia (RA) myelodysplastic syndrome (MDS) (15,16). However, many of these studies had small sample sizes and employed variable diagnostic methods.
The association between PNH and BMF syndromes suggests that the selection pressure for PNH clonal expansion is mediated through the patient’s immune system (15). However, the mechanism by which PNH cells expand and obtain dominance over their non-PNH counterparts is not entirely understood (17). The presence of even minor populations of PNH cells in patients with AA or MDS may be an important prognostic indicator of a higher rate of response to IST (18,19).
Determining the association between BMF syndromes and PNH may represent an opportunity for early detection, diagnosis, and treatment of patients with unrecognized PNH within these populations. In fact, this association has prompted PNH testing guidelines to recommend testing for PNH in patients presenting with a wide range of clinical indications, including AA, MDSRA, unexplained cytopenia, Coombs-negative hemolytic anemia, thrombosis with unusual features, and intravascular hemolysis (20).
Here, we report the results of a large, multicenter, point-prevalence study (Examination of PNH, by Level of CD59 on Red and White Blood Cells [EXPLORE]) with the primary objective of determining the prevalence of PNH clones in patients diagnosed with AA, MDS, or other BMF syndromes.
METHODS
Study Design, Patients, and Assessments
EXPLORE is a multicenter prevalence study of PNH clone size, the percentage of cells with GPI anchor deficiency. Patients, of at least 10 years of age, were selected following review of patient medical charts by hematologists and oncologists from 1,294 centers in the United States and were included in the study if they had a diagnosis of AA (pancytopenia with low CD34+ cells, hypocellular marrow, and no evidence of leukemia), MDS (with normal or high CD34+ cells), and/or other BMF syndromes. (Details of the specific nature of other BMF as reported following medical office chart and author review are given in Supporting Information A.) Patients diagnosed with MDS were further classified according WHO-defined criteria for one of seven subtypes of MDS: RA, RA with ringed sideroblasts, refractory cytopenia with multilineage dysplasia, refractory cytopenia with multilineage dysplasia and ringed sideroblasts, RA with excess blasts (Type 1 and 2), 5q– syndrome, or unclassified. We specifically excluded from our analyses all patients with AA who also had a clinical diagnosis of PNH. The selection of patients for inclusion in the study was based on medical chart review and at the discretion of the reviewing physician; diagnoses were neither reviewed nor confirmed by the authors.
The primary endpoint was to identify the percentage of patients with AA, MDS, and/or other BMF syndromes who have an undiagnosed PNH white blood cell (WBC) clone ≥1%. Secondary endpoints in each subgroup of patients included the relationship between hemolysis, as assessed by lactate dehydrogenase (LDH) levels, and PNH clone size, as well as mean clone size in each subgroup. Additional analyses were performed on a subgroup of patients tested for PNH clone size after January 2008 when the use of fluorescent aerolysin (FLAER) became available in flow cytometry testing. FLAER has a strong binding affinity for GPI anchors and is more sensitive than CD59 in accurately detecting small populations of PNH cells, allowing identification of PNH clone sizes as small as 0.01%.
The study protocol was approved by each center’s institutional review board, and all patients provided informed consent before entering the study. Following selection, informed consent was obtained, inclusion and exclusion criteria were assessed, and baseline demographics, diagnosis, and medical history were recorded. All eligible patients, irrespective of any prior or current therapy, then had blood samples collected for chemistry and hematology panel testing and for flow cytometry detection of PNH clonal cells. Patients were excluded from the study if they had any condition that, in the opinion of the investigator, might interfere with the patient’s participation in the study, pose an added risk for the patient, or confound the assessment of the patient. The trial was registered at ClinicalTrials.gov as #NCT01192425.
Flow Cytometry
To eliminate interlaboratory variability in detection and reporting of PNH clones, a single reference flow cytometry laboratory (Dahl-Chase, Bangor, Maine) was used to determine the presence and size of PNH cell populations. RBCs were analyzed using a combination of the RBC-specific antibody CD235a-fluorescein isothiocyanate (clone KC16, Beckman Coulter, Miami, Florida) to delineate RBCs from other events and a R-phycoerythrin conjugate of the GPI-specific antibody CD59 (clone MEM43, Invitrogen, Carlsbad, California). Normal RBCs (type I cells) show bright expression of CD59, whereas PNH cells show either partial CD59 deficiency (type II cells) or complete CD59 deficiency (type III cells).
As the objective of this study was to identify the prevalence of PNH clones in WBCs, we used granulocytes as the primary population for the determination of PNH clone size. Although PNH clones can be identified in monocytes (serving as useful confirmation of the presence of a PNH clone in the WBCs), monocyte populations are typically smaller in peripheral blood, limiting the statistical confidence with which the smallest clone sizes can be identified. The complement-mediated lysis of PNH RBC clones and the potential dilution of clone sizes in patients administered RBC infusions renders this cell type suboptimal for the determination of true PNH clone size.
The initial WBC assay used CD66b (clone 80H3), CD24 (clone ALB9), and CD16 (clone 3G8) to assess GPI-deficient granulocytes gated using a combination of CD45 (clone J.33) and CD15 (clone 80H5) (all reagents from Beckman Coulter). GPI-deficient monocytes were identified with CD14 (clone RMO52, Beckman Coulter) and CD55 (clone 67, Invitrogen) gated using a combination of CD45, CD64 (clone 22), and CD33 (clone D3HL60.251, all from Beckman Coulter). The number of acquired cells varied depending on the cell count, with an initial assay sensitivity of 1%. Data generated with the initial assays were often difficult to interpret, as the expression of some of the individual GPI-linked structures is closely linked to the maturational status of the target cells. Thus, for sample types containing increased levels of immature cells (e.g., some MDS cases), a range of staining intensities was observed for some of the individual GPI-specific reagents that made the delineation of weakly stained immature cells from bona fide GPI-negative cells problematic.
The WBC assay was modified in January 2008 to take advantage of the newly available pan-GPI-reactive FLAER reagent (Cedar Lane Laboratories, Burlington, Ontario, Canada) to replace several of the antibody conjugates to individual GPI-molecules such as CD55, CD66b, and CD16. FLAER was used in combination with CD24 to detect FLAER-negative, CD24-negative, GPI-deficient granulocytes gated using a sequential combination of light scatter (to remove debris), CD45, and bright CD15 expression. The modified monocyte assay used a combination of FLAER and CD14 to detect FLAER-negative, CD14-negative GPI-deficient monocytes gated using a sequential combination of light scatter, CD45, and bright CD33/CD64 staining. The combination of optimized lineage-gating reagents with two GPI-specific reagents (for each lineage) and Boolean gating strategies allowed for a significantly increased level of assay sensitivity. Use of three high-sensitivity assays allowed for the reliable detection of PNH RBCs, granulocytes, and monocytes to the level of 0.01% without interference from other events, debris, and “false negatives.”
Variations of these assays have subsequently been included in a number of state-of-the-art protocols for detecting PNH cells by flow cytometry, such as the 2010 International Clinical cytometry Society (ICCS) Guidelines (20), the 2012 Practical Guidelines (21), and the Clinical and Laboratory Standards Institute guidelines for RBC diagnostic testing using flow cytometry (22).
Statistical Analyses
Descriptive statistics were calculated for continuous variables, with categorical variables being summarized using counts and percentage distribution. Assessments of differences between mean values were performed using two-sided t tests and differences in proportions using a Chi-squared test. All analyses were performed at the α = 0.05 level of significance. Only patients with a PNH clone size ≥ 1% were included in analyses of the whole patient population, whereas in the subset of patients assessed post January 2008 using the more sensitive FLAER reagent, patients with a minor PNH clone of size ≥0.01% to 1% were also included in the analyses.
RESULTS
Patient Characteristics
Of the 5,398 patients screened between July 2006 and July 2010, 4,401 (81.5%) had a diagnosis of MDS, 503 (9.3%) a diagnosis of AA, and 130 (2.4%) a diagnosis of other BMF. Of the 4,401 patients with MDS, 1,622 (36.9%) were reported as having a diagnosis of unclassified MDS, 1,288 (29.3%) had RA, 591 (13.4%) had RA with ringed sideroblasts, 381 (8.7%) had RA with excess blasts (types 1 and 2), 306 (7.0%) had refractory cytopenia with multilineage dysplasia, 138 (3.1%) had 5q– syndrome, and 75 (1.7%) had refractory cytopenia with multilineage dysplasia and ringed sideroblasts.
Baseline demographic and clinical characteristics of patients at the time of screening are presented in Table 1. There were approximately equal numbers of male and female patients, with most being of Caucasian origin. The median age of the participants was 75 years. There were considerable differences in mean age among the patient groups. The mean age was 74.2 years in patients diagnosed with MDS, 51.7 years in those diagnosed with AA, and 65.9 years in those diagnosed with other BMF. Only 19% of the patients with MDS were ≤65 years of age, compared with 72% of patients with AA and 44% of patients with other BMF. All 16 pediatric patients (age 18 years) had a diagnosis of AA.
Table 1.
Baseline Demographic and Clinical Characteristics of Patients at Screening
| Parameter | Overall patient population (N = 5398) |
|---|---|
| Male gender, n (%)a | 2845 (52.7) |
| Median (range) age at screening, years | 75 (11–103) |
| Race, n (%) | |
| Caucasian | 4516 (83.7) |
| Black, Hispanic, Asian | 818 (15.2) |
| Not reported | 64 (1.2) |
| History of transfusion, n (%)b | 3275 (60.7) |
| Mean (SD) hemoglobin (g/L)c | 108.6 (19.04) |
| Median (range) platelet count (× 109/L)d | 164.0 (2–2114) |
| Median (range) lactate dehydrogenase (U/L)e | 197 (63–2982) |
SD = standard deviation.
N = 5396.
N = 5382.
N = 5342.
N = 5213.
N = 5331.
Patients with Granulocyte PNH Clones ≥ 1%
Overall, 1.1% (50 of 4,401) of patients with MDS, 18.5% (93 of 503) of patients with AA, and 2.3% (3 of 130) of patients with other BMF had PNH clones ≥ 1% (Table 2). Although the range of PNH clone sizes was similar in each group, the median clone size was threefold greater in patients with MDS (17.6%) than in those with AA (5.1%) (Table 3). Among patients with PNH clones ≥ 1%, a larger percentage of patients had a clone size of ≥ 10% in the MDS group (27 of 50; 54.0%) than in the AA group (36 of 93; 38.7%).
Table 2.
Prevalence of Granulocyte PNH Clones by Diagnosis
| Diagnosis | Total population
|
FLAER assessmentsa
|
|||
|---|---|---|---|---|---|
| N | 1% or greater, n (%) | N | 0.01% or greater, n (%) | 1% or greater, n (%) | |
| All patientsb | 5398 | 199 (3.7) | 1746 | 167 (9.6) | 99 (5.7) |
| AA | 503 | 93 (18.5) | 294 | 116 (39.5) | 63 (21.4) |
| MDSc | 4401 | 50 (1.1)d | 1225 | 22 (1.8) | 12 (1.0) |
| RA | 1288 | 17 (1.3) | 334 | 7 (2.1) | 2 (0.6) |
| RCMDRS | 75 | 1 (1.3) | 30 | 2 (6.7) | 1 (3.3) |
| Unclassified | 1622 | 25 (1.5) | 425 | 8 (1.9) | 7 (1.6) |
| RARS | 591 | 3 (0.5) | 204 | 4 (2.0) | 2 (1.0) |
| RCMD | 306 | 3 (1.0) | 85 | 1 (1.2) | 0 |
| 5q– syndrome | 138 | 0 (0.0) | 47 | 0 | 0 |
| RAEB | 381 | 1 (0.3) | 100 | 0 | 0 |
| Other BMFc | 130 | 3 (2.3)d | 51 | 4 (7.8) | 1 (2.0) |
RCMDRS = refractory cytopenia with multilineage dysplasia and ringed sideroblasts; RARS = refractory anemia with ringed sideroblasts; RCMD = refractory cytopenia with multilineage dysplasia; RAEB = refractory anemia with excess blasts (types 1 and 2).
Only performed in patients assessed after January 2008.
All patients assessed regardless of diagnosis.
Includes 75 patients with MDS and other BMF. Other BMF included, but was not limited to, RA, pancytopenia, unexplained cytopenia, or myeloproliferative disorder (chronic myelomonocytic leukemia, polycythemia vera, essential thrombocythemia, or myelofibrosis).
P <.001 compared with patients with AA.
Table 3.
Clone Sizes and Elevated LDH Levels in Patients with Granulocyte PNH Clone Size of 1% or Greater
| Total population (N = 199)a | AA (N = 93) | MDS (N = 50) | Other BMF (N = 3) | |
|---|---|---|---|---|
| Clone size | ||||
| Median (%) | 13.2b | 5.1c | 17.6d | 24.4 |
| Range (%) | 1.0–99.7 | 1.0–96.7 | 1.0–99.7 | 1.2–97.8 |
| LDH > ULN, n (%) | 110 (55.3) | 43 (46.2) | 28 (56.0) | 1 (33.3) |
| LDH > 1.5 × ULN, n (%) | 65 (32.7) | 17 (18.3) | 21 (42.0) | 0 |
All patients assessed regardless of diagnosis.
N = 195.
N = 92.
N = 47.
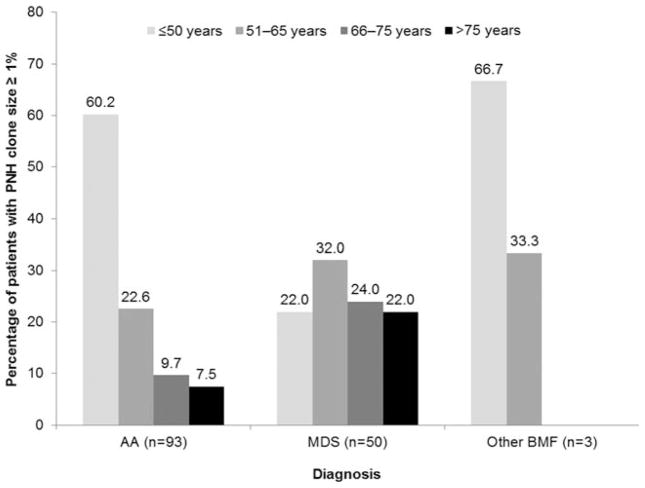
PNH clones ≥ 1% were detected in a greater percentage of patients < 65 years of age, and there was some evidence to suggest that the prevalence of PNH clones decreased with age (Fig. 1). PNH clones ≥ 1% were detected across MDS subtypes, with the exception of 5q– syndrome (Table 2). In the MDS group, PNH clones were more frequently reported in patients with diagnoses of RA, refractory cytopenia with multilineage dysplasia, or unclassified MDS.
FIG. 1.
Percentage of patients in each age category with a granulocyte PNH clone size of 1% or greater.
LDH released into the plasma from lysed RBCs is a validated measure of intravascular hemolysis (2), with LDH concentrations ≥ 1.5 × the upper limit of normal (ULN; 250 U/L) associated with significantly increased risks of thromboembolism (TE) and mortality (23). Among patients with PNH clones ≥ 1%, LDH levels > ULN were seen in 56.0% of patients with MDS, 46.2% of patients with AA, and 33.3% of patients with other BMF; LDH levels ≥ 1.5 × ULN were seen in 42.0% of patients with MDS, 18.3% of patients with AA, and none of the patients with other BMF (Table 3).
Patients with Granulocyte PNH Clones ≥ 1% and Cytopenias
Patients were assessed for various types of cytopenias (Table 4). Overall, cytopenia of any kind was reported in 2,302 patients (42.6%) in the total patient population. Of the patients with cytopenia, the majority had leukopenia and/or neutropenia, with just over a third of these patients having a diagnosis of thrombocytopenia. Approximately 18% of patients with cytopenia had all three conditions. The percentage of patients with a PNH clone ≥ 1% was similar in patients with any cytopenia, leukopenia, neutropenia, or bicytopenia (4.1–6.1%) but slightly higher in patients with thrombocytopenia (9.7%) and highest in patients with pancytopenia (12.6%).
Table 4.
Granulocyte PNH Clones in Patients with Cytopenia
| Diagnosis | All patients, n (N = 5398) | Cytopenic patients, % (N = 2302) | Patients with PNH clone size of 1% or greater, n (%)a |
|---|---|---|---|
| Any cytopenia | 2302 | – | 127 (5.5) |
| Leucopenia | 1869 | 81.2 | 105 (5.6) |
| Neutropenia | 1584 | 68.8 | 97 (6.1) |
| Thrombocytopenia | 781 | 33.9 | 76 (9.7) |
| Bicytopenia | 1088 | 47.3 | 45 (4.1) |
| Pancytopenia | 422 | 18.3 | 53 (12.6) |
Percentages based on number of patients with each diagnosis.
Thrombocytopenia was reported in 781 of the 2,302 patients with cytopenia (33.9%) with 76 of these 781 patients (9.7%) having PNH clones ≥ 1%. Of the patients with thrombocytopenia and PNH clones ≥ 1%, 46% had LDH levels above the upper limit of normal, and 21% had LDH ≥ 1.5 × ULN. Overall, younger patients (age ≤50 years) with thrombocytopenia were more likely to have a PNH clone size of ≥ 1% (42 of 145 patients; 29.0%) when compared with patients aged 51–65 years (19 of 172 patients; 11.0%) or >65 years (15 of 464 patients; 3.2%).
Patients Analyzed with FLAER after January 2008
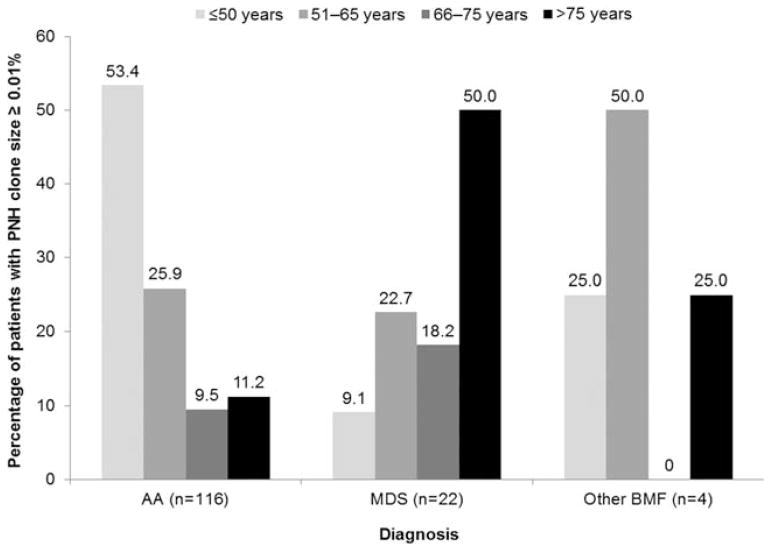
An additional analysis of PNH clones was conducted in a subgroup of 1,746 patients who were assessed for PNH clones using FLAER post January 2008. Overall, a PNH clone of ≥ 0.01% was observed in 167 (9.6%) of these patients. PNH clones of ≥ 0.01% were detected in 22 of 1,225 patients with MDS (1.8%), 116 of 294 patients with AA (39.5%), and 1 of 51 patients with other BMF (2.0%) (Table 2). Distribution of clone sizes was similar across all three patient groups: 40.0–45.7% of patients in each group had a clone size of 0.01 to 1%, and 54.3–60.0% in each group had a clone size of ≥ 1%. The distribution of PNH clones by age (Fig. 2) revealed that most patients (53.4%) with a diagnosis of AA and a PNH clone size ≥ 0.01% were aged ≤50 years. This was in contrast to patients with MDS, where 50.0% of patients with PNH clones ≥ 0.01% were >75 years of age. PNH clones ≥ 0.01% were most common in patients with refractory cytopenia with multilineage dysplasia and ringed sideroblasts and were not detected in patients with 5q– syndrome or RA with excess blasts (Table 2).
FIG. 2.
Percentage of patients in each age category with a granulocyte PNH clone size of 0.01% or greater.
DISCUSSION
It has been previously reported that bone marrow disorders are present in approximately 45% of patients with PNH (24), with studies suggesting that PNH cells are present in 57–70% of patients with AA (19), 20–50% of patients with MDS (25). and 50% of patients with other BMF (16). However, these findings were based primarily on retrospective analyses of data collected when Ham’s test, which only assesses the RBC population (26), was the standard technique employed in the detection of PNH cells. More recently, flow cytometry, and particularly FLAER, has been employed using monoclonal antibodies to cell-bound complement regulators that are absent from the surface of hematological cells, and the use of this technique to assess WBCs is now considered the standard for PNH diagnosis. In the multicenter EXPLORE study, we prospectively evaluated the presence of detectable PNH clones using flow cytometry in the largest population of patients to date with evidence of AA, MDS, and other BMF syndromes, as determined from review of medical office charts.
An unexpectedly high number of patients with MDS were included in the study, particularly patients with a diagnosis of unclassified MDS. As it was not possible to conduct in-depth diagnoses of each patient, we relied on the clinical judgment of the individual hematologists/oncologists to determine if the patient records they reviewed provided sufficient evidence for inclusion in one of the diagnostic categories.
Our analyses showed that PNH clone sizes ≥ 1% were prevalent in 18.5% of patients with AA, which is consistent with the 21% figure previously reported by Dunn et al. (15). PNH clones ≥ 1% were detected in a significantly smaller percentage of patients with MDS (1.1%) or other BMF (2.3%), although the median PNH clone sizes in these patient groups (17.6 and 24.4%, respectively) were approximately threefold to fivefold greater than the median clone size in patients with AA (5.1%). We are currently unable to explain why the relatively few patients with these disorders should have such a larger median clone size, although it should be noted that the range of clone sizes was similar in each of the diagnostic groups. The presence of even small PNH clones in patients with AA and MDS is of potential importance, as it has been shown that such patients have a higher response to IST (18,19).
The presence of a PNH clone ≥ 1% is clinically significant, as 56.0% of patients with MDS, 46.2% with AA, and 33.3% with other BMF showed evidence of intravascular hemolysis, as measured by LDH levels above the ULN. LDH levels ≥ 1.5 × ULN have been established as an important threshold associated with a significantly increased risk of TE and mortality in PNH patients (23), and our analysis showed that in patients with a PNH clone ≥ 1%, LDH concentrations at or above this threshold were seen in 42.0% of patients with MDS and 18.3% of patients with AA. These findings emphasize the importance of assessing and monitoring patients with these hematological disorders for the presence of PNH clones as well as clinical signs and symptoms associated with the disease.
This study demonstrated that approximately 10% of patients with thrombocytopenia had PNH clones ≥ 1%. PNH patients with thrombocytopenia have an increased incidence of TE, with evidence of increased platelet consumption, possibly as a result of the wide-spread formation of microthrombi (1,27,28). The lack of expression of CD55 and CD59 on blood cells and platelets in PNH increases the sensitivity of these cells to complement-mediated attack and may contribute to PNH-associated thrombotic tendencies (29). As 21% of thrombocytopenic patients with PNH clones ≥ 1% had LDH concentrations ≥ 1.5 × ULN, thrombocytopenic patients with elevated LDH may benefit from testing for PNH clones, as they may be at higher risk for developing TE.
The latest digital clinical flow cytometers are capable of rapidly analyzing samples and simultaneously collecting data on numerous parameters. This sophisticated and sensitive technique permits the accurate detection of PNH cells even when they are present in small numbers within a sample. This permits detection of PNH cells at an early stage of the disorder and prior to any clinical manifestations of the disease. Thus, high-sensitivity flow cytometry may facilitate early intervention with consequent improvement in patient management and potentially reduce morbidities associated with untreated PNH.
We were able to detect PNH clone cell sizes down to 0.01% in a subset of patients who were assessed after the introduction of FLAER to flow cytometry testing. Results from this subset of patients showed that 39.5% of patients with AA, 1.8% of patients with MDS, and 2.0% of patients with other BMF had a PNH clone. PNH clones ≥ 0.01% were also observed across most MDS subtypes, with the highest percentage of patients with PNH clones found in the subgroup with a diagnosis of refractory cytopenia with multilineage dysplasia and ringed sideroblasts. We observed that 53% of AA patients with a PNH clone size ≥ 0.01% were aged ≤50 years, whereas only 9% of MDS patients with a with a clone size ≥ 0.01% were in that age range. This suggests that younger patients with AA are more likely to develop PNH clones and further emphasizes the importance of screening for PNH in these patients.
The major limitation of this study is that it did not include a longitudinal assessment of clone sizes; measurements were made at entry into the study only. It is known that PNH clone size in individual patients is dynamic, with longitudinal studies reporting PNH clone size remaining stable in some patients but increasing or decreasing in others (19,30). There is considerable interest in determining the impact of changing clone sizes on risks of serious morbidity and mortality, the severity of PNH-related symptoms, and the effectiveness of therapies used to treat patients with PNH.
This study supports the PNH testing guidelines recently issued by the ICCS (20), which recommend high-sensitivity flow cytometry to detect PNH cells in patients at high risk for PNH. This includes patients with evidence of BMF syndromes, such as suspected or proven AA or hypoplastic anemia, RA-MDS, and cytopenias of unknown etiology. Although high-sensitivity flow cytometry assays are not essential for the diagnosis of hemolytic PNH, they are important in the diagnosis of PNH in patients with bone marrow disorders, where PNH cells may be present in much lower numbers, as such patients may be at a greater risk of progression to hemolytic PNH (20). The prognostic value of detecting PNH clones of small size using high-sensitivity assays is controversial. Longitudinal follow-up of these patients would be required to determine the significance of the presence of small PNH clones, particularly in patients with MDS or other BMF, as it is known that in patients with AA, PNH clones can change over time (16,31). ICCS guidelines recommend annual monitoring of patients with stable PNH clones and more frequent monitoring in patients exhibiting any change in clinical or hematologic parameters (20).
PNH is associated with significant morbidities and the life-threatening complications of TE and chronic kidney disease, the largest causes of death in PNH patients (23–25). Screening of high-risk patients and the detection of PNH clones may facilitate early intervention, improve patient management, and possibly prevent the morbidities and early mortality associated with untreated PNH.
In summary, we report on a large prospective multicenter study on the incidence of PNH clones in patients with AA, MDS, and other BMF syndromes with some interesting and unexpected findings. In the future, it will be important to demonstrate the long-term clinical significance of detecting a PNH clone and its effect on the natural history of the disease.
Acknowledgments
Grant sponsor: Alexion Pharmaceuticals.
The authors thank Mark Hughes, PhD, and Joshua Safran of Infusion Communications for analysis, writing, and editorial support. Dr. Weitz and Ms. Illingworth have received honoraria from Alexion Pharmaceuticals for speaking and consulting. Dr. Castro-Malaspina has received honoraria from Alexion Pharmaceuticals for an advisory board. All other authors declare no competing financial interests.
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- 1.Hill A, Richards SJ, Hillmen P. Recent developments in the understanding and management of paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2007;137:181–192. doi: 10.1111/j.1365-2141.2007.06554.x. [DOI] [PubMed] [Google Scholar]
- 2.Parker C, Omine M, Richards S, Nishimura J, Bessler M, Ware R, Hillmen P, Luzzatto L, Young N, Kinoshita T, Rosse W, Socié G International PNH Interest Group. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699–3709. doi: 10.1182/blood-2005-04-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dave SJ, Sodetz JM. Regulation of the membrane attack complex of complement. Evidence that C8 gamma is not the target of homologous restriction factors. J Immunol. 1990;144:3087–3090. [PubMed] [Google Scholar]
- 4.Cappellini MD. Coagulation in the pathophysiology of hemolytic anemias. Hematology Am Soc Hematol Educ Program. 2007:74–78. doi: 10.1182/asheducation-2007.1.74. [DOI] [PubMed] [Google Scholar]
- 5.Hillmen P, Richards SJ. Implications of recent insights into the pathophysiology of paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2000;108:470–479. doi: 10.1046/j.1365-2141.2000.01802.x. [DOI] [PubMed] [Google Scholar]
- 6.Kelly R, Richards S, Hillmen P, Hill A. The pathophysiology of paroxysmal nocturnal hemoglobinuria and treatment with eculizumab. Ther Clin Risk Manag. 2009;5:911–921. doi: 10.2147/tcrm.s3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodsky RA. Advances in the diagnosis and therapy of paroxysmal nocturnal hemoglobinuria. Blood Rev. 2008;22:65–74. doi: 10.1016/j.blre.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill A, Rother RP, Wang X, Morris SM, Jr, Quinn-Senger K, Kelly R, Richards SJ, Bessler M, Bell L, Hillmen P, Gladwin MT. Effect of eculizumab on haemolysis-associated nitric oxide depletion, dyspnoea, and measures of pulmonary hypertension in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2010;149:414–425. doi: 10.1111/j.1365-2141.2010.08096.x. [DOI] [PubMed] [Google Scholar]
- 9.Hillmen P, Elebute M, Kelly R, Urbano-Ispizua A, Hill A1, Rother RP, Khursigara G, Fu CL, Omine M, Browne P, Rosse W. Long-term effect of the complement inhibitor eculizumab on kidney function in patients with paroxysmal nocturnal hemoglobinuria. Am J Hematol. 2010;85:553–559. doi: 10.1002/ajh.21757. [DOI] [PubMed] [Google Scholar]
- 10.Kelly RJ, Hill A, Arnold LM, Brooksbank GL, Richards SJ, Cullen M, Mitchell LD, Cohen DR, Gregory WM, Hillmen P. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117:6786–6792. doi: 10.1182/blood-2011-02-333997. [DOI] [PubMed] [Google Scholar]
- 11.Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333:1253–1258. doi: 10.1056/NEJM199511093331904. [DOI] [PubMed] [Google Scholar]
- 12.Socié G, Mary JY, de Gramont A, Rio B, Leporrier M, Rose C, Heudier P, Rochant H, Cahn JY, Gluckman E. Paroxysmal nocturnal haemoglobinuria: long-term follow-up and prognostic factors. French Society of Haematology. Lancet. 1996;348:573–577. doi: 10.1016/s0140-6736(95)12360-1. [DOI] [PubMed] [Google Scholar]
- 13.Lewis SM, Dacie JV. The aplastic anaemia–paroxysmal nocturnal haemoglobinuria syndrome. Br J Haematol. 1967;13:236–251. doi: 10.1111/j.1365-2141.1967.tb08736.x. [DOI] [PubMed] [Google Scholar]
- 14.Schubert J, Vogt HG, Zielinska-Skowronek M, Freund M, Kaltwasser JP, Hoelzer D, Schmidt RE. Development of the glycosylphosphatitylinositol-anchoring defect characteristic for paroxysmal nocturnal hemoglobinuria in patients with aplastic anemia. Blood. 1994;83:2323–2328. [PubMed] [Google Scholar]
- 15.Dunn DE, Tanawattanacharoen P, Boccuni P, Nagakura S, Green SW, Kirby MR, Kumar MS, Rosenfeld S, Young NS. Paroxysmal nocturnal hemoglobinuria cells in patients with bone marrow failure syndromes. Ann Intern Med. 1999;131:401–408. doi: 10.7326/0003-4819-131-6-199909210-00002. [DOI] [PubMed] [Google Scholar]
- 16.Sugimori C, Mochizuki K, Qi Z, Sugimori N, Ishiyama K, Kondo Y, Yamazaki H, Takami A, Okumura H, Nakao S. Origin and fate of blood cells deficient in glycosylphosphatidylinositol-anchored protein among patients with bone marrow failure. Br J Haematol. 2009;147:102–112. doi: 10.1111/j.1365-2141.2009.07822.x. [DOI] [PubMed] [Google Scholar]
- 17.Brodsky RA. How do PIG-A mutant paroxysmal nocturnal hemoglobinuria stem cells achieve clonal dominance? Expert Rev Hematol. 2009;2:353–356. doi: 10.1586/ehm.09.35. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Chuhjo T, Yasue S, Omine M, Nakao S. Clinical significance of a minor population of paroxysmal nocturnal hemoglobinuria-type cells in bone marrow failure syndrome. Blood. 2002;100:3897–3902. doi: 10.1182/blood-2002-03-0799. [DOI] [PubMed] [Google Scholar]
- 19.Sugimori C, Chuhjo T, Feng X, Yamazaki H, Takami A, Teramura M, Mizoguchi H, Omine M, Nakao S. Minor population of CD55-CD59-blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308–1314. doi: 10.1182/blood-2005-06-2485. [DOI] [PubMed] [Google Scholar]
- 20.Borowitz MJ, Craig FE, Digiuseppe JA, Illingworth AJ, Rosse W, Sutherland DR, Wittwer CT, Richards SJ Clinical Cytometry Society. Guidelines for the diagnosis and monitoring of paroxysmal nocturnal hemoglobinuria and related disorders by flow cytometry. Cytometry B Clin Cytom. 2010;78B:211–230. doi: 10.1002/cyto.b.20525. [DOI] [PubMed] [Google Scholar]
- 21.Sutherland DR, Keeney M, Illingworth A. Practical guidelines for the high-sensitivity detection and monitoring of paroxysmal nocturnal hemoglobinuria clones by flow cytometry. Cytometry B Clin Cytom. 2012;82B:195–208. doi: 10.1002/cyto.b.21023. [DOI] [PubMed] [Google Scholar]
- 22.Red Blood Cell Diagnostic Testing Using Flow Cytometry: Approved Guidelines H52-A2. 2. Wayne, PA: Clinical and Laboratory Standards Institute (CLSI); 2013. [Google Scholar]
- 23.Lee JW, Jang JH, Kim JS, Yoon S-S, Lee J-H, Kim Y-K, Jo D-Y, Chung J, Sohn SK. Uncontrolled complement activation and the resulting chronic hemolysis as measured by LDH serum level at diagnosis as predictor of thrombotic complications and mortality in a large cohort of patients with paroxysmal nocturnal hemoglobinuria (PNH) Blood. 2011;18 Abstract 3166. [Google Scholar]
- 24.Muus P, Szer J, Schrezenmeier H, Brodsky RA, Bessler M, Socie G, Urbano-Ispizua A, Maciejewski JP, Rosse WF, Kanakura Y, Khursigara G, Karnell A, Bedrosian CL, Hillmen P. Evaluation of paroxysmal nocturnal hemoglobinuria disease burden: the patient’s perspective. A report from the International PNH Registry. Blood. 2010;116 Abstract 1525. [Google Scholar]
- 25.Galili N, Ravandi F, Palmero G, Bubis J, Illingworth A, Castro-Malaspina H, Raza A. Prevalence of paroxysmal nocturnal hemoglobinuria (PNH) cells in patients with myelodysplastic syndromes (MDS), aplastic anemia (AA), or other bone marrow failure (BMF) syndromes: interim results from the EXPLORE trial. J Clin Oncol. 2009;27(15 Suppl):7082. [Google Scholar]
- 26.Rosse WF. Paroxysmal nocturnal hemoglobinuria as a molecular disease. Medicine (Baltimore) 1997;76:63–93. doi: 10.1097/00005792-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Socié G, Muus P, Schrezenmeier H, Höchsmann B, Maciejewski JP, Weitz IC, Hill A, Bessler M, Risitano AM. Terminal complement inhibitor Eculizumab improves complement-mediated platelet consumption and thrombocytopenia in patients with paroxysmal nocturnal hemoglobinuria (PNH) Blood. 2009;114 Abstract 4030. [Google Scholar]
- 28.Weitz IC. Thrombosis in paroxysmal nocturnal hemoglobinuria—insights into the role of complement in thrombosis. Thromb Res. 2010;125(Suppl 2):S106–S107. doi: 10.1016/S0049-3848(10)70026-8. [DOI] [PubMed] [Google Scholar]
- 29.Helley D, de Latour RP, Porcher R, Rodrigues CA, Galy-Fauroux I, Matheron J, Duval A, Schved JF, Fischer AM, Socié G French Society of Hematology. Evaluation of hemostasis and endothelial function in patients with paroxysmal nocturnal hemoglobinuria receiving eculizumab. Haematologica. 2010;95:574–581. doi: 10.3324/haematol.2009.016121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pu JJ, Mukhina G, Wang H, Savage WJ, Brodsky RA. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol. 2011;87:37–45. doi: 10.1111/j.1600-0609.2011.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wanachiwanawin W, Siripanyaphinyo U, Piyawattanasakul N, Kinoshita T. A cohort study of the nature of paroxysmal nocturnal hemoglobinuria clones and PIG-A mutations in patients with aplastic anemia. Eur J Haematol. 2006;76:502–509. doi: 10.1111/j.0902-4441.2005.t01-1-EJH2467.x. [DOI] [PubMed] [Google Scholar]