Abstract
Pharmacoperones correct the folding of otherwise misfolded protein mutants, restoring function (i.e. providing “rescue”) by correcting their trafficking. Currently most pharmacoperones possess intrinsic antagonist activity because they were identified using methods initially aimed at discovering such functions. Here, we describe an ultra-high throughput homogeneous cell-based assay with a cAMP detection system, a method specifically designed to identify pharmacoperones of the vasopressin type 2 (V2) receptor (V2R); a GPCR that, when mutated, is associated with nephrogenic diabetes insipidus. Previously developed methods to identify compounds capable of altering cellular trafficking of V2R were modified and used to screen a 645K compound collection by measuring the ability of library compounds to rescue a mutant hV2R [L83Q], using a cell-based luminescent detection system. The campaign initially identified 3,734 positive modulators of cAMP. The confirmation and counterscreen identified only 147 of the active compounds with an EC50 ≤ 5 μM. Of these, 83 were reconfirmed as active through independently-obtained pure samples and were also inactive in a relevant counterscreen. Active and tractable compounds within this set can be categorized into three predominant structural clusters, described here, in the first report detailing the results of a large scale pharmacoperone HTS campaign.
Keywords: cAMP, Pharmacoperone, GPCR, protein folding, protein trafficking
Introduction
Vasopressin type 2 receptor (V2R), is a G-protein coupled receptor (GPCR), a 7-transmembrane protein, found in the distal convoluted tubule and the collecting ducts of the kidney. V2R normally responds to vasopressin and stimulates mechanisms that concentrate urine and maintain water homeostasis. Mutation of the V2R results in mistrafficking of the receptor which in turn results in vasopressin-unresponsive cells. This deficit leads to nephrogenic diabetes insipidus (NDI).1, 2 To date there are no known drugs capable of reversing receptor mediate misfolding associated NDI and current treatment options are limited to alleviating symptoms.3 Of the 188 reported allelic variants or mutations found in the V2R, at least 70 have been reporter to result in traffic defective receptors.2 The cell based assay described here and elsewhere incorporates the use of the L83Q mutation which, results in the misfolded V2R and subsequently loss of receptor function. While other mutants are predicted to work, the choice to use the L83Q mutant for HTS was biased on its ability to lead to NDI and also because the level of receptor function, when in the presence of vasopressin, goes from basal (unrescued) and reverts to wild type (rescued) activity when previously treated with the pharmacoperone.
Pharmacoperones are small molecules that enter cells and serve as a “molecular scaffold” to correct the folding of otherwise misfolded proteins. SR121463, a pharmacoperone of V2R, has been demonstrated by many labs to restore a mutated V2R’s response to vasopressin.4, 5 Notably the binding affinity of this molecule to V2R demonstrates incredible selectivity and relatively constant low nanomolar Ki profiles, regardless of species tested. SR121463 was also previously reported to be active on at least 8 NDI associated V2R mutants.6 Unfortunately, this compound has the limitation of also being a V2R antagonist, which limits its potential for therapeutic translation. Thus we need to find V2R pharmacoperones lacking antagonist activity. We have previously reported the development of a 1536-well HTS assay to identify V2R pharmacoperones.5 We now report the further optimization of this homogeneous no-wash assay, its adaptation to a fully-automated robotics platform, and the completion of a large-scale HTS campaign of the Scripps drug diversity library (SDDL) to identify V2R pharmacoperones. Multiple counterscreens and confirmation assays were applied to confirm selective dose-dependent activity. To assure accuracy, de novo powder samples of the most active and selective compounds were obtained and analyzed to verify activity. The most potent, selective, and chemically tractable V2R pharmacoperones were clustered by structure class and are identified in this report.
Materials and Methods
Cell Culture and cAMP Assay
SR121463, a V2R peptidomimetic antagonist was used in the current study as a known pharmacoperone drug, was generously provided by Dr. Claudine Serradeil at Sanofi-Aventis and used as received. The V2R pharmacoperone assay was modified compared to our previous report5 with the following changes: Cells were cryogenically frozen in a 9:1 serum to DMSO ratio in order to maintain a large batch of uniform cells to be used in different time periods in the screening effort. On the day of screening, cells were thawed and added to 1,536 well white, solid bottom, tissue culture treated plates (part 789173-F, Greiner Bio-one, Monroe, NC) (3 μL/well, 2000 cells/well) in growth media. This was followed by a 25 nL pin-tool addition of test compounds and controls (positive control is 200 nM SR121463; negative control is DMSO only). The plates were incubated for 17 h at 37 °C, 5% CO2 and humidified conditions prior to the addition of 5 μM vasopressin (final) in 1 μL stimulation buffer (growth media without FBS, plus 2.5 mM IBMX). After stimulation, plates were re-incubated for 2 h prior to their removal to equilibrate at room temperature (10 min) followed by addition of 1 μL of 5X cAMP-Glo detection reagent (Promega Corp., Madison, WI). After incubation at room temperature for 19 min, 5 μL of 1X Kinase-Glo (Promega) was added to each well to quantitate residual ATP levels through the production of a luminescent endpoint. Plates were incubated for 10 min at room temperature and then luminescence was measured using a PerkinElmer ViewLux plate imager (PerkinElmer Lifesciences, Waltham, MA). The optimized counterscreen was identical to the primary screen described, except that cells were cultured in the presence of 1 μg/mL doxycycline for 36 h prior to plating and during all phases of the experiment. Doxycycline treatment turns off the expression of the mutant transcript of V2R and leaves the cells devoid of the receptor.
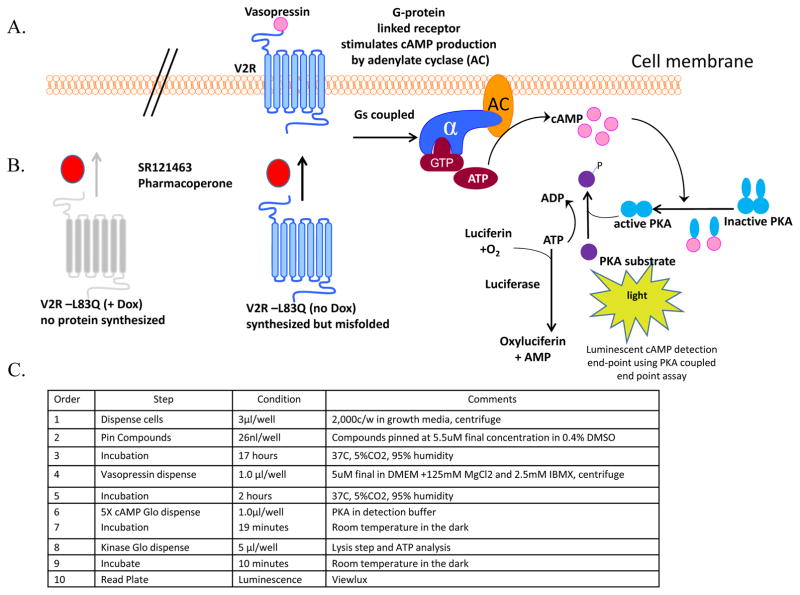
The final HTS protocol is summarized in Figure 1.
Figure 1. Schematic diagram of the V2R cAMP detection luminescence reporter assay.
A. Representation of a cell-based assay to monitor pharmacoperone rescue of mutant vasopressin receptor function. In wild type V2R responses, vasopressin initiates Gαs activation of adenylate cyclase which increases the concentration of cAMP. In this detection format cAMP binds to protein kinase A, and the regulatory subunits undergo a conformational change to release the catalytic subunits. The free catalytic subunits then catalyze the transfer of the terminal phosphate of ATP to a protein kinase A substrate, consuming ATP in the process. The level of remaining ATP is determined using the luciferase-based ATP detection reagent. Luminescence is inversely proportional to cAMP levels. Thus, as cAMP concentration increases, luminescence decreases. B. Untreated cells “no Dox” have an active tet promoter resulting in expression of misfolded mutant protein. Upon treatment of the cells with vasopressin the pharmacoperone (SR121463) receptor folding is corrected and function is rescued, resulting in a Gs-coupled response which is readily monitored. In “+ Dox” cells, the Tet minus promoter is inactive and no protein is made, thus cells are non-responsive to SR121463 and V2R; i.e., the counterscreen. C. V2R uHTS assay protocol in 1,536 well plate format.
Screening Library
The Scripps Diversity Drug Library (SDDL), used for this HTS campaign, currently consists of 644,951 unique compounds, representing a diversity of drug-like small molecules that are chemically relevant to traditional and non-traditional drug-discovery biology.7 The SDDL has been curated from over 20 commercial and academic sources and contains more than 40,000 compounds unique to Scripps. SDDL compounds are selected based on scaffold novelty, physical properties and spatial connectivity. In its current state, the SDDL has several focused sub-libraries for screening popular drug-discovery target classes (e.g., kinases/transferases, GPCRs, ion channels, nuclear receptors, hydrolases, transporters), with diverse chemistries (e.g., click-chemistry, PAINS-free collections, Fsp3 enriched, covalent inhibitors and natural product collections) and with desirable physical properties (“rule-of-five”, “rule-of-three”, polar surface area, etc.)8–13. All samples in the SDDL were confirmed for purity via LC-MS and/or NMR to provide adequate QA/QC after completion of an HTS campaign.
Screening data acquisition, normalization, representation and analysis
All data files were uploaded into the Scripps institutional HTS database (Symyx Technologies, Santa Clara, CA) for plate QC and hit identification. Activity for each well was normalized on a per-plate basis using the following equation:
| Eq (1) |
Where “High Control” represent wells containing cells with SR121463 at EC100 and a 5 μM vasopressin stimulus; while “Low Control” represents wells containing just DMSO with the 5 μM vasopressin stimulus and finally the “Data Wells” contain test compounds with same vasopressin stimulus dose. The Z′ and S:B were calculated using the High Control and Low Control wells. In each case, 24 wells per control were used to generate these values and a Z′ value greater than 0.5 was required for a plate to be consider acceptable (Supplemental Figure 3).14
Results
Assay principle and screening strategy
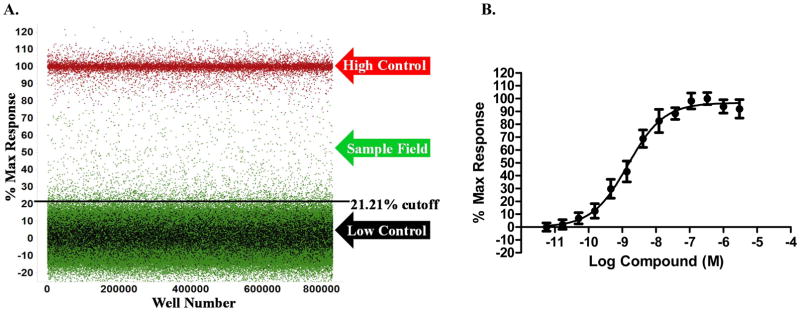
The V2R HTS assay was miniaturized into 1,536-well plates and read using the ViewLux to measure the level of luminescence detected correlating to the amount of cAMP generated upon addition of the potential pharmacoperone. Pharmacoperones will allow V2R to fold properly and shuttle to the surface, allowing the vasopressin ligand to bind, driving the production of cAMP, which then activates protein kinase A (PKA). These events will reduce the ATP to ADP ratio within the cell. Using the pathway where cAMP activates PKA, the cAMP Glo Max luminescent detection system detects cAMP by using PKA as a substrate. The activated PKA is phosphorylated by the ATP and converts it to ADP. The Kinase Glo detects the ATP levels remaining in the well (Figure 1). This results in an inverse relationship between the amount of cAMP present and the luminescent signal. The control pharmacoperone SR121463 displays a dose-dependent response in the cAMP Glo Max system and was used to optimize the conditions for the HTS campaign (Figure 2). Based on data in other reports, the EC50 for SR121463 was in the expected range for the entire campaign (−8.99 ± −9.08M; N=22 separate curves).5
Figure 2.
A. V2R primary HTS scattergram of 644,951 compounds screened in singlicate at ~5.22μM; compound wells (green dots), and control wells (red and black dots). Data is shown normalized to % Max Response of SR-121463. The hit cutoff is shown as the dark line at 21.21% B. An example CRC of the control compound SR121463 as tested during HTS normalized to % Max response vs. log molar concentration of SR-121463. The average EC50 is equal to −8.99 ± −9.08M; N=22 separate curves with 16 replicates per dilution.
The fully-automated Kalypsys/GNF robotic platform was utilized in this HTS campaign to accomplish the tasks of dispensing cells and detection reagents, transfer of test compounds, and control the necessary incubation periods prior to plate measurements by the ViewLux (Supplemental Figure 3B).15 Each plate contained 24 control wells that were used either for response normalization purposes or to verify appropriate pharmacoperone response which ultimately helps to accurately identify hit compounds. All wells contained 0.4% DMSO. Following optimization and robotic implementation we were able to complete 10 plates/h and >100,000 compound wells/day. The entire primary HTS against the 645K compound library, as tested in singlicate with control plates, was completed in 8 business days.
1536-well format assay optimization
The first step in 1,536 well assay development was to optimize the cell number per well. The signal to basal ratio (S/B) as calculated by the high control response (EC100 SR121463) divided by low control response (DMSO only), was not significantly affected by the number of cells plated per well for most seeding densities. However, increasing the cell concentration resulted in sub-optimal Z′ values (Supplemental Figure 1). Once the optimal cell number was obtained (2,000 cells per well), the incubation time and vasopressin concentration were then optimized. The incubation time affected both the Z′ and S:B. The longer the incubation, the better the Z′ statistics; a 2 h incubation was found to be best (Supplemental Figure 1). Finally, the optimal concentration of vasopressin stimulus was determined to maximize the overall agonist response and to ensure this was not a limiting factor within the assay. The S:B and Z′ improved with increasing vasopressin. Although 10 μM vasopressin appeared optimal, a 5 μM vasopressin dose gave similar results, being less cost prohibitive, and thus was chosen for the HTS effort (Supplemental Figure 1).
Primary HTS
In the primary screen, 644,951 SDDL compounds were tested in singlicate at a final nominal test concentration of 5.22 μM using the optimized conditions described in Figure 1. The results of the primary screen are shown in Figure 2 and the statistics can be found in Table 1. In summary, 3,472 compounds were found to be active, that is, they exhibited statistically significant activity, deemed at a cutoff >21.2%; which was determined using an interval based mathematical algorithm described previously.7 Briefly, samples with activity greater than three standard deviations above the high control or less than three standard deviations below the low control were excluded from use only in further calculations; i.e. they aren’t removed from consideration as hits. Two values were then calculated: (1) the average percent activation of all compounds tested in the screen, and (2) three times their standard deviation. The sum of these two values was used as the cutoff parameter, i.e., any compound that exhibited greater percent activation than the cutoff parameter was declared active.9, 10 Day to day reproducibility was monitored using the SR121463 dose response curve. Z′ for the primary screen was found to be 0.73±0.09 with a S:B of 4.8 ±0.8. The log molar pEC50 of the SR121463 was stable at −8.99±−9.08 over 22 plates throughout the primary screen.
Table 1.
V2R uHTS campaign summary and results
| Step | Screen type | Target | Number of compounds tested | Selection criteria | Number of selected compounds (hit rate) | Assay statistics
|
|
|---|---|---|---|---|---|---|---|
| Z′ | S/B | ||||||
| 1a | Primary screen | V2R | 644,951 | 21.21% a | 3,472 (0.54%) | 0.73 ±0.09 | 4.83 ±0.84 |
| 2a | Confirmation screen | V2R | 3,734 | 21.21%b | 1,930 (51.69%) | 0.80 ±0.03 | 5.75 ±0.14 |
| 2b | Counterscreen screen | V2R + DOX | 3,734 | 16.35 %c | 256 (6.86%) | 0.84 ±0.03 | 7.08 ±0.32 |
| 3a | Dose Response Screen | V2R | 664 | EC50<5μM | 147 (22.14%) | 0.84 ±0.02 | 7.64 ±1.42 |
| 3b | Dose Response Counterscreen | V2R + DOX | 664 | EC50<5μM | 0 (0%) | 0.79 ±0.04 | 5.86 ±0.99 |
| 4a | Powders Dose Response | V2R | 96 | EC50<5μM | 83 (86%) | 0.79 ±0.04 | 8.28 ±0.22 |
| 4b | Powders Dose Response | V2R + DOX | 96 | EC50<5μM | 0 (0%) | 0.81 ±0.01 | 8.38 ±0.64 |
The primary screen hit-cutoff was calculated using the Interval based cutoff.
The primary hit cutoff was used
The counterscreen hit cutoff was calculated by using the average + 3 standard deviations of the DMSO sample field plates, n=6 plates.
Confirmation and Counterscreen Assays
Active compounds were selected for confirmation screening, which used the same reagents and detection system as the primary screening assay, but tested each of the 3,734 compounds at a single concentration (nominally 5.22 μM) in triplicate. The V2R cAMP pharmacoperone confirmation assay performance yielded an average Z′ of 0.80±0.03 and a S:B of 5.75±0.14. Using the primary HTS assay cut-off, 1,930 hits were confirmed with activity equal to or above 21.2% equating to a reasonable confirmation rate of 51.7%.
A counterscreen assay, similar in format to the V2R cAMP pharmacoperone assay, was then performed. This assay exploits the tetracycline responsive promoter and use of the same cell line as the primary assay but treated with doxycycline (Figure 1). In this way, the assay can identify sundry “off-target” hits and compounds that modify cAMP production by other means. The V2R + Dox counterscreen assay performance had an average Z′ of 0.84±0.03 and a S:B of 7.08±0.32. Using a standard method for the counterscreen hit cut-off (the average of the DMSO samples + 3SD), which equates to 16.35%, 256 hits were identified. 16 These 256 hits presumably activated an off target pathway and were not of further interest.
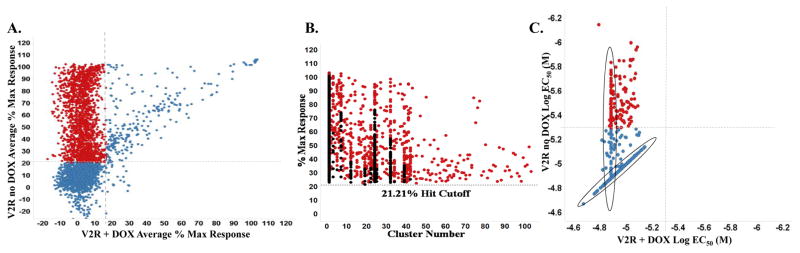
A correlation plot of average maximum percent response for the confirmation screen and counterscreen is shown in Figure 3A. Highlighted in red are compounds with activity only in the V2R expressing system. A total of 1,694 compounds found active in the primary assay and not in the counterscreen were further investigated using structural cluster analysis. Clustering was based on their maximum common sub-structures and ranked based on highest activity in each cluster. The top 640 compounds from this cluster ranking is indicated in red (Figure 3B). Using well established and previously utilized in-silico tools such as PAINs (Pan-assay interference compounds) and promiscuity filtering, we identified 666 actives that have low promiscuity and were free of PAINs structures.13,17 These 666 compounds were selected to be tested in dose response studies, of which 664 were available (Supplemental Figure 2). A summary of the secondary assay data is shown in Table 1.
Figure 3.
A. Correlation plot of the V2R confirmation and counter screen data analysis of 3,734 compounds tested in triplicate at ~5.22μM plotted as the average % Max Response for the counterscreen on the X-axis (V2R+ DOX) vs. the confirmation assay on the Y-axis (V2R no DOX). Respective hit cutoffs are represented as dashed lines. Active and confirmed compounds that are not hits in the counterscreen are shown as red dots; unconfirmed or active also in the counterscreen are shown in blue. B. 1,694 compounds active only in V2R the “no DOX” assay format (red dots). These were clustered based on maximum common sub-structure similarity and ranked across all clusters to identify the top 640 most active and diverse compounds; highlighted as red dots. C. Correlation plot of primary and counterscreen EC50 data for 664 compounds tested in 10 point 1:3 dilution titration assays. Dashed lines represent activity cutoff parameters for each assay tested. Selectivity toward the primary V2R assay is demonstrated (red dots). Some compounds (circled) do not reach EC50 even at highest concentration tested (nominally 13.4μM).
Titration and Counterscreen Assays
The titration assays were run using the confirmation and counterscreening assay protocols, but with 664 compounds tested at 10 dose levels (3-fold dilutions) in triplicate. The V2R cAMP pharmacoperone titration assay performance had an average Z′ of 0.84±0.02 and a S:B of 7.64±1.42 and the counterscreen titration assay had an average Z′ of 0.79±0.04 and a S:B of 5.86±0.99. For each tested compound, percent activation was plotted against compound concentration. A four parameter equation describing a sigmoidal dose-response curve was then fitted with adjustable baseline using Assay Explorer software (Symyx Technologies Inc.). The reported EC50 values were generated from fitted curves by solving for the X-intercept value at the 50% activity level of the Y-intercept value. The following rule was used to declare a compound as “active” or “inactive”: Compounds with EC50 >5 μM were considered inactive. Compounds with EC50 ≤ 5 μM were considered active. 147 compounds were confirmed active in the V2R cAMP pharmacoperone assay while being inactive in the V2R + Dox cAMP counterscreen (Figure 3C). Later the same 664 samples tested in the titration assays were submitted for LC-MS purity analysis. 647 samples (97.4%) were validated for molecular identity and acceptable purity. As determined by nominal methods (UV-VIS spectroscopy, MS and ELSD), 625 samples demonstrated purity of >80%.
Of the 147 confirmed active and counterscreen-negative compounds, 96 were available from vendors and de novo powder samples were obtained. These samples were solvated in DMSO and subjected to the same assays in CRC format. Of the 96 compounds evaluated, 83 (86%) were confirmed active in the primary assay exhibiting an EC50 ≤ 5 μM, while none were active in the counterscreen. The most active compound identified was SR-01000443595 which, upon testing as de-novo powder, yielded an EC50 of 70nM.
Discussion
The HTS campaign was successful at identifying potential V2R pharmacoperones leads. During assay optimization several issues were resolved. For example, high cell concentrations resulted in higher variability and lower Z’s (Supplemental Figure 1A). This appeared to be due to the cells clumping and limiting our ability to properly dispense reproducible cell number per well. Keeping the cells at lower densities removes the effect, and thus, a condition of 2,000 cells/well was selected. During the initial robotic assay validation a 1 μM vasopressin stimulus was used, which we subsequently found to give us variable day-to-day responses. An optimal 5 μM vasopressin stimulus applied for 2 h, as shown in supplemental figure 1C and 1D, gave much more consistent responses.
In summary, the primary screen tested 644,951 compounds, of which 3,472 (0.5%) were found to be active. This hit rate is moderately low but not out of a normal range (0.5 – 1.5%) as found across >280 HTS campaigns tested at the SRIMSC. In this assay, using the cAMP-Glo detection technology, compounds with activity in the negative direction would be the result of more luminescent signal. In this case these negative effectors would likely be kinase inhibitors which effect the PKA coupled detection assay format or ATP analogs which effect the luciferase activity. Further investigation of the primary screen activity for false negative rate found 1,398 compounds (0.2%) that exhibited activity in the negative direction below 3 standard deviations plus average of the sample field (<−21.89%). Analysis of the compounds with the most negative activity, i.e. at or below −50% (190 in total), found they were all known kinase inhibitors including staurosporine. While these impacted the overall primary hit rate, they should also be ruled out based on lack of V2R target directed activity. It should be noted that the confirmation and counterscreen stage of the HTS campaign tested 3,734 compounds; which is greater than the primary actives found. We had increased our library size with additional compounds and added the new compounds to the confirmation and counterscreen assays to determine if any of those compounds could also be active.
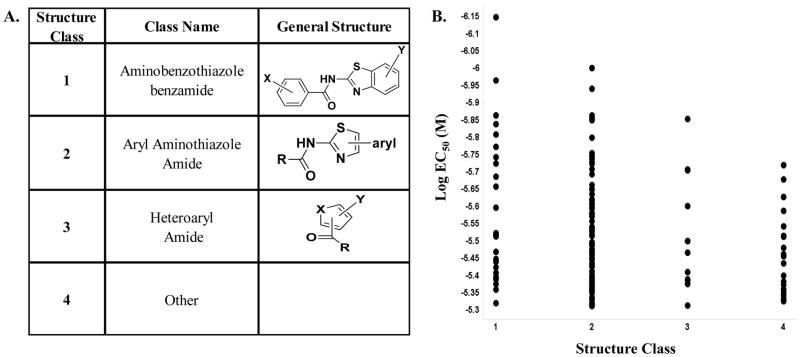
At the completion of the titration assays, 147 compounds were identified to selectively rescue the effect of vasopressin on the accumulation of cAMP in cells containing the mutated V2R gene at EC50 < 5 μM. These 147 compounds were further analyzed by their sub-structures (Figure 4). Other considerations, such as assay-promiscuity, undesirable PAIN moieties, and rule-of-5 non-compliance were used to narrow this set of selectively active compounds (supplemental table 1). The remaining compounds were broken down into 3 main structural classes, with a 4th “other” grouping. The two most-represented series were the related aminobenzothiazole benzamides and the related aryl aminothiazazole amides (see general structures in Figure 4). A third series is termed the heteroaryl amides, in which the core heterocycle was neither a thiazole nor a benzothiazole. These three related structure classes comprise about 80% of the most potent and selective hits. 96 compounds were selected based upon structure, activity, and availability. De novo powder samples of these compounds were obtained and tested in the titration assays. Doing so afforded us the opportunity to test at a higher starting concentration as compared to the original titration assays; 52μM vs 13μM. 86% (83 compounds) confirmed activity (Table 1) using the same procedure tested during the HTS campaign. Eight of these improved their EC50 by more than fivefold presumably due to use of new powders instead of former liquid samples (Supplemental Table 1). While most compounds retained activity within 5 fold of their original result, from these data, it would appear that class 2 (Figure 4) has a little more potency and consistency in the data which will help direct future chemistry efforts. An example was SR-01000443595, an aryl aminothiazole amide which, upon testing as de-novo powder, was the most active compound with an improvement of ~41 fold in efficacy. Of the 13 compounds that didn’t confirm as powders, most (9) were marginally above the cut-off. Notably, 5 of the 13 were tested for the first time as powders as they were readily available from commercial sources and are proposed substructure class 1 analogs. The lack of activity associated with these particular compounds (ex. SR-01000392254; SR-01000405773, SR-01000442043; SR-01000461570 and SR-01000393329) will also help define future chemistry initiatives.
Figure 4.
Comparing EC50 to compound structural classes. 147 compounds were confirmed as activators in the V2R pharmacoperone HTS campaign and were inactive in the counterscreen. A. Generally, these compounds fall into 3 related structure classes, plus a smaller “other” group for structural outliers. The common substructure is depicted for each of the 3 most-represented classes, along with substitution positions and allowed groups. B. The EC50 data plotted vs. the structure class for all 147 compounds. The Y-axis is plotted as decreasing Log EC50 (M), such that more potent compounds rise further on the Y-axis.
We are unaware of any other reported structures in these chemical classes known to restore activity to misfolded proteins, including in this instance, rescuing activity of a V2R mutant. To the best of our knowledge, these efforts represent the first large-scale pharmacoperone HTS assay of any kind completed to date. Lessons learned will serve as a guide for other pharmacoperone screening projects, including gonadotropin releasing hormone receptor (GnRHR); a target we wish to pursue in a future effort.
Conclusion
We have successfully completed an HTS campaign, identifying potential pharmacoperones capable of rescuing V2R activity in cells expressing a mutated V2R misfolded protein. We have shown that in this system we can restore the function of the V2R by demonstrating vasopressin stimulation of cAMP production in these mutant cell lines in the presence of a pharmacoperone (with multiple test compounds showing efficacy). Leads identified fall into three chemical clusters and appear to set a basis for medicinal chemistry efforts in probe development. Further efforts are underway to determine whether these compounds act as rote agonists or as antagonists in cells expressing the wild-type V2R protein. Those that show no antagonist characteristics could be interesting leads for therapeutic intervention. Through the appropriate selection and application of assays, HTS has proven to be productive in identify potential pharmacoperones which may be useful in the treatment of diseases caused by protein misfolding and misrouting, including diabetes insipidus.
Supplementary Material
Acknowledgments
We thank Pierre Baillargeon and Lina DeLuca (Lead Identification, Scripps Florida) for compound management.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health grant 5R01DK099090-04
Footnotes
Declaration of Conflicting Interests
The are no conflicts of interest amongst any of the authors and the work pertained in this manuscript.
References
- 1.Bernier V, Morello JP, Zarruk A, et al. Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. Journal of the American Society of Nephrology : JASN. 2006;17(1):232–43. doi: 10.1681/ASN.2005080854. [DOI] [PubMed] [Google Scholar]
- 2.Conn PM, Ulloa-Aguirre A, Ito J, et al. G protein-coupled receptor trafficking in health and disease: lessons learned to prepare for therapeutic mutant rescue in vivo. Pharmacological reviews. 2007;59(3):225–50. doi: 10.1124/pr.59.3.2. [DOI] [PubMed] [Google Scholar]
- 3.Kirchlechner V, Koller D, Seidl R, et al. Treatment of nephrogenic diabetes insipidus with hydrochlorothiazide and amiloride. Archives of Disease in Childhood. 1999;80(6):548–552. doi: 10.1136/adc.80.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serradeil-Le Gal C. An overview of SR121463, a selective non-peptide vasopressin V(2) receptor antagonist. Cardiovascular drug reviews. 2001;19(3):201–14. doi: 10.1111/j.1527-3466.2001.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 5.Conn PM, Smith E, Hodder P, et al. High-throughput screen for pharmacoperones of the vasopressin type 2 receptor. Journal of biomolecular screening. 2013;18(8):930–7. doi: 10.1177/1087057113483559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morello JP, Salahpour A, Laperriere A, et al. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest. 2000;105(7):887–95. doi: 10.1172/JCI8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jambrina E, Cerne R, Smith E, et al. An Integrated Approach for Screening and Identification of Positive Allosteric Modulators of N-Methyl-D-Aspartate Receptors. Journal of biomolecular screening. 2016 doi: 10.1177/1087057116628437. [DOI] [PubMed] [Google Scholar]
- 8.Lovering F, Bikker J, Humblet C. Escape from flatland: increasing saturation as an approach to improving clinical success. Journal of medicinal chemistry. 2009;52(21):6752–6. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- 9.Lipinski CA, Lombardo F, Dominy BW, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced drug delivery reviews. 2001;46(1–3):3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 10.Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug discovery today. 2003;8(24):1128–37. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 11.Ding S, Gray NS, Wu X, et al. A combinatorial scaffold approach toward kinase-directed heterocycle libraries. Journal of the American Chemical Society. 2002;124(8):1594–6. doi: 10.1021/ja0170302. [DOI] [PubMed] [Google Scholar]
- 12.Congreve M, Carr R, Murray C, et al. A ‘rule of three’ for fragment-based lead discovery? Drug discovery today. 2003;8(19):876–7. doi: 10.1016/s1359-6446(03)02831-9. [DOI] [PubMed] [Google Scholar]
- 13.Baell JB, Holloway GA. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. Journal of medicinal chemistry. 2010;53(7):2719–40. doi: 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- 14.Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. Journal of biomolecular screening. 1999;4(2):67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 15.Michael S, Auld D, Klumpp C, et al. A robotic platform for quantitative high-throughput screening. Assay Drug Dev Technol. 2008;6(5):637–57. doi: 10.1089/adt.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spicer T, Fernandez-Vega V, Chase P, et al. Identification of Potent and Selective Inhibitors of the Plasmodium falciparum M18 Aspartyl Aminopeptidase (PfM18AAP) of Human Malaria via High-Throughput Screening. Journal of biomolecular screening. 2014;19(7):1107–1115. doi: 10.1177/1087057114525852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith E, Chase P, Niswender CM, et al. Application of Parallel Multiparametric Cell-Based FLIPR Detection Assays for the Identification of Modulators of the Muscarinic Acetylcholine Receptor 4 (M4) Journal of biomolecular screening. 2015;20(7):858–68. doi: 10.1177/1087057115581770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.