Abstract
During development, microglial progenitor cells migrate into the brain from the periphery, a process critical to the maturation of the developing brain. Although they perform functions similar to mature, adult microglia, immature microglia are distinct from mature microglia. Activation of immature microglia, via an early-life immune challenge, can lead to persistent changes in microglial function, resulting in long-term neuronal and cognitive dysfunction. Early-life immune activation is associated with multiple neurodevelopmental disorders, including autism, ADHD, schizophrenia, and cerebral palsy – disorders with known or suspected immune etiologies, and strong sex biases for males. Activation of immature microglia requires further examination to determine its potential role in these neurodevelopmental disorders. More work is also necessary to better understand the relationship between developing microglia and other developing neural cells during this critical period of development. Thus, we treated freshly isolated, sex-specific microglia from the rat hippocampus with lipopolysaccharide (LPS) on P4, in either the presence or absence of other neural cells. Mixed and microglial-specific cultures were analyzed for inflammatory gene expression to determine whether immature microglia exhibited a sex-specific response to immune activation, and if the presence of all other neural cells influenced that response. We found that the microglial response to an LPS-induced immune activation differed depending on the presence of other neural cells in the culture. We found very few sex differences in the cytokine response, except that the microglial expression of IL-6 following immune activation was more robust in male microglia that were in the presence of other neural cells than female microglia in the same condition.
Keywords: microglia, cell culture, lipopolysaccharide, sex differences, neurodevelopmental disorders
1. Introduction
Microglia are the immune cells of the brain. They migrate into the central nervous system (CNS) from the periphery during embryonic development. There, they play a role in many important neurodevelopmental processes, including synaptic pruning and the removal of naturally occurring apoptotic cells [1]. Upon arrival in the CNS, immature microglia exhibit a round or amoeboid morphology. As they mature, microglia extend long processes into their surrounding environments and undertake a ramified appearance [2]. Following immune activation, microglia in the adult brain can retract their processes and resume an amoeboid shape, responding to infection with a robust increase in cytokine production and the phagocytosis of pathogens or cellular debris – a process that results in a transient impairment in microglial functions [3]. Importantly, immature, amoeboid microglia are distinct from mature, amoeboid microglia. Unlike the transient changes in microglial function that result from an immune challenge in adulthood, activation of neonatal microglia can result in persistent changes in immature microglial function, making early development a sensitive period for microglial activation [4]. Still, further examination of neonatal microglial activation is necessary to better understand the changes in cytokine production that result from immune activation during this sensitive period of development.
Immune activation during early development has been associated with the onset of multiple neurodevelopmental disorders, including autism, ADHD, schizophrenia, and cerebral palsy [5,6]. Given that stimulation of neonatal microglia, via immune activation, can lead to long-term neuronal and cognitive dysfunction [3,5], it’s important we understand the relationship between neonatal or immature microglia and other developing neural cells during an immune challenge. Importantly, the neurodevelopmental disorders listed above also exhibit a striking sex bias in that the presentation and diagnosis of these early-emerging disorders are greater in males than in females [7]. Modeling this bias, female rats did not show the same long-term behavioral deficits that male rats showed, following an infection on postnatal day 4 (P4). Moreover, there are more microglia in the developing amygdala, hippocampus, and cortex of male rats compared to female rats on P4 [8]. Together, these data demonstrate that neonatal microglia may be influenced by sex-specific factors, subsequently impacting how they respond to early-life insults.
To examine how immature microglia respond to an immune challenge and to determine whether there are sex differences in that response, we treated sex-specific, microglial cells from the hippocampus of P4 rat pups with lipopolysaccharide (LPS). This time point was chosen because there are more microglia in the developing hippocampus of male as compared to females rats on P4; and, it is presumed that this male-biased sex difference in microglia number may contribute to the long-term sex-specific deficits associated with neonatal microglial activation. By standardizing microglial number across the sexes, we were able to examine whether there were inherent sex differences in microglial function even in the absence of sex differences in the total number of microglia. We hypothesized that developing male microglia would produce a more exaggerated cytokine response to an immune challenge. In contrast, we found no sex differences in the response of neonatal microglia to LPS, except in the expression of IL-6, which was up-regulated significantly more in male microglia, but only in the presence of other neural cells. Moreover, we found that neonatal microglia did not possess estrogen receptor α, β, or the androgen receptor, perhaps precluding them from responding to these sex-specific factors. Importantly, however, we found that the immune response of cultured microglia was greatly influenced by the presence of other neural cells, which has important implications for the use of cell-type specific cultures in experimental models.
2. Materials and Methods
2.1 Animals
Adult male and female Sprague Dawley rats were ordered from Envigo Laboratories (Indianapolis, IN) and housed in same sex pairs in clear, polyethylene cages (45 cm x 20.5 cm x 24 cm). The colony room was maintained at 22°C on a 12:12 light/dark cycle (lights on from 07:00 to 19:00 h), with food and water available ad-libitum. Mature males and females were paired for five days and monitored daily for sperm plugs. Litters were not manipulated for size or sex ratio at the time of birth. Sentinel rats were housed in the same colony room and regularly examined for the presence of common rodent diseases. All tests were negative. All experiments were approved by the University of Delaware Institutional Animal Care and Use Committee.
2.2 Cell Culture
On P4, male and female pups were euthanized and their hippocampi were collected for dissociation. Whole hippocampi from three same-sex pups were pooled into one sample (see Supplementary Figure 1 for experimental design). Four samples were generated from each culture and plated in replicates that were treated with either saline or LPS. At least three independent cultures were represented within each experiment. Using the Neural Tissue Dissociation Kit and magnetic sorting CD11b/c MicroBeads (Miltenyi Biotec), three populations of cells could be acquired from each sample: 1) all neural cells, not sorted using the CD11b/c MicroBeads – “unsorted cells”; 2) microglia, selected by the CD11b/c Microbeads – “microglia”; and 3) cells not selected by the CD11b/c Microbeads – “microglia-depleted/all other neural cells.”
Cell populations were seeded to a 96-well culture plate at 30,000 cells per well and fed with 300 μL of MACS Neuro Medium (Miltenyi Biotec). Each well was treated with vehicle (MACS Neuro Media; 3 μL/30,000 cells) or LPS (doses below). The LPS was derived from E.coli 0111:B4 and diluted in pyrogen-free DPBS (Sigma Aldrich). The cells were maintained in a CO2 incubator at 37°C, 5% CO2, for four hours. Following incubation, cells were collected in Isol-RNA Lysis Reagent (VWR) and flash frozen at −80°C.
2.2.1 Experiment 1: A Dose Response Curve to LPS
Relative gene expression of IL-1β was analyzed in the microglia population following a 4-hour exposure to either 10, 500, or 1,000 ng/mL of LPS. These doses were effective in elucidating dose-dependent responses to LPS in primary astrocyte cultures [9]. LPS doses falling within this range also significantly increase IL-1β gene expression in cortical microglia from P0/P1 rats in a sex-specific manner [10]. The goal of the dose response curve was to determine an appropriate dose of LPS to robustly stimulate neonatal microglia from the hippocampus.
2.2.2 Experiment 2: Relative Gene Expression of IL-1β, IL-6, and TLR-4
Relative gene expression data for IL-1β, IL-6, and TLR-4 were analyzed in the microglia population, the microglia-depleted/all other neural cells population, and the unsorted cells population, following a 4-hour exposure to either a 500 ng/mL or 1,000 ng/mL LPS dose. The goal of this experiment was to determine if the microglial response to an immune challenge would be sex-dependent and if the presence of other neural cells within the culture would impact this response.
2.2.3 Experiment 3: Relative Gene Expression of Esr-1, Esr-2, and AR
Relative gene expression data for the estrogen receptor α and β (Esr-1 & Esr-2) and the androgen receptor (AR) were analyzed in the microglia population and the microglia-depleted/all other neural cells population, following a 4-hour exposure to a 1,000 ng/mL LPS dose. The objective of this experiment was to determine if neonatal microglia have the ability to respond to sex hormones, possibly influencing cell function in a sex-dependent manner. At the same time, this analysis allowed us to determine whether critical steroid hormone receptors were modulated in the presence of a neonatal immune challenge.
2.3 Quantitative Real-Time PCR
RNA was extracted from the cell populations using Isol-RNA Lysis Reagent. Genomic DNA was removed and cDNA was synthesized from 100 ng of extracted RNA, per sample, using the QuantiTect® Reverse Transcription Kit (Qiagen). IL-1β (Forward: GAAGTCAAGACCAAAGTGG, Reverse: TGAAGTCAACTATGTCCCG) and TLR-4 (Forward: CAGAGGAAGAACAAGAAGC, Reverse: CCAGATGAACTGTAGCATTC) primers were ordered from Integrated DNA Technologies and diluted to a final concentration of 0.13 μM for qPCR reactions. IL-6, Esr-1, Esr-2, AR, and RPLP1 were ordered from Qiagen and diluted according to QuantiTect® Primer Assays specifications. qPCR was performed using the RealMasterMix™ Fast SYBR Kit (5 Prime) in 10 μL reactions on a CFX96Touch real-time PCR machine. The 2ΔΔCq method was used to calculate the relative gene expression of each gene of interest. RPLP1 was used as the housekeeping gene. RPLP1 expression significantly differed across the three cell populations (F2,136 = 23.444, p < 0.000, η2= 0.256). Thus, rather than calculating the relative gene expression across the three cell populations, we determined relative gene expression levels across sex and treatment groups, within each cell population.
2.4 Statistical Analysis
Statistical software program SPSS (IBM) was used for all data analyses. In Experiment 1, we used a 2x2x3 ANOVA, with Sex (male vs. female), Treatment (vehicle vs. LPS), and LPS Dose (10 vs. 500 vs. 1,000 ng/mL) as between-subjects variables. In Experiments 2 and 3, relative gene expression for each gene of interest was analyzed using a 2x2 ANOVA with Sex (male vs. female) and Treatment (vehicle vs. LPS) as between-subjects variables. No more than one sample was removed from an analysis as an outlier. When significant main effects or interactions were found, the Fisher’s Least Significant Difference (LSD) post hoc test was used to examine between-group differences. The recognized significance level for all analyses was p < 0.05. Cell population-specific gene expression levels for all three cells populations were plotted on the same axis for each gene of interest in order to visualize how LPS treatment and/or sex impacted fold-changes in relative gene expression within and across the different cell populations. Data in the graphs represent the mean ± standard error of the mean (SEM).
3. Results
3.1 Experiment 1: A Dose Response Curve to LPS
We found a significant main effect of treatment (F3,42 = 41.836, p < 0.001, η2= 0.749), indicating that LPS increased IL-1β expression in microglial cells in a dose-dependent manner (Supplementary Figure 2). Post hoc tests revealed that IL-1β expression did not significantly differ between microglia receiving the 10 ng/mL dose of LPS (M = 1.785, SE = 0.196) and microglia receiving vehicle (M = 1.238, SE = 0.191; p = 0.056). IL-1β expression did significantly increase in microglia receiving either the 500 ng/mL (M = 3.352, SE = 0.161; p < 0.001) or 1,000 ng/mL LPS dose (M = 3.608, SE = 0.161; p < 0.001), compared to vehicle-treated microglia. IL-1β relative gene expression did not significantly differ between microglia receiving the 500 ng/mL LPS dose and those receiving the 1,000 ng/mL dose (p = 0.268). No significant sex (F1,42 = 1.274, p = 0.076, η2= 0.073) or sex x treatment effects (F3,42 = 1.085, p = 0.366, η2= 0.072) were found. LPS doses of 500 or 1,000 ng/mL were used for subsequent cell-type specific experiments.
3.2 Experiment 2: Relative Gene Expression of IL-1β, IL-6, and TLR-4
3.2.1 IL-1β
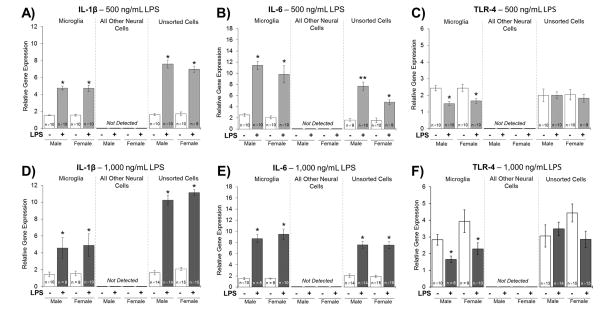
Treatment of microglia with LPS at 500 or 1,000 ng/mL significantly increased IL-1β gene expression relative to vehicle-treated microglia (F1,36 = 215.440, p < 0.001, η2= 0.857, Figure 1A; F1,33 = 124.839, p < 0.001, η2= 0.791, Figure 1D). IL-1β expression was not detectable in the microglia-depleted/all other neural cells population (Figure 1A and 1D). Treatment of unsorted cells with LPS at 500 or 1,000 ng/mL significantly increased IL-1β gene expression relative to vehicle-treated unsorted cells (F1,34 = 298.435, p < 0.001, η2= 0.895, Figure 1A; F1,54 = 455.803, p < 0.001, η2= 0.894, Figure 1D). No significant sex or sex x treatment interactions were found. The presence of all other neural cells influenced the relative gene expression of IL-1β from microglia. Specifically, the fold-change in IL-1β relative gene expression, induced by LPS (at both doses), was more robust in the unsorted cell population compared to the microglial cell population (Figure 1A and 1D).
Figure 1. Experiment 2: Relative Gene Expression of IL-1β, IL-6, and TLR-4.
A and D) Relative gene expression of IL-1β significantly increased in microglia treated with LPS compared to vehicle-treated microglia. IL-1β expression was not detected in the microglia-depleted/all other neural cell population. IL-1β expression significantly increased in unsorted cells treated with LPS compared to vehicle-treated unsorted cells. B) Relative gene expression of IL-6 significantly increased in microglia treated with a 500 ng/mL LPS dose compared to vehicle-treated microglia. IL-6 expression was not detected in the microglia-depleted/all other neural cell population. IL-6 expression significantly increased in unsorted cells treated with a 500 ng/mL LPS dose compared to vehicle-treated unsorted cells; and, the LPS-facilitated increase in IL-6 expression was greater in the male unsorted cells compared to the female unsorted cells. C and F) Relative gene expression of TLR-4 significantly decreased in microglia treated with LPS compared to vehicle-treated microglia. TLR-4 expression was not detected in the microglia-depleted/all other neural cell population. No significant differences were found in TLR-4 expression in the unsorted cells. E) Relative gene expression of IL-6 significantly increased in microglia treated with a 1,000 ng/mL LPS dose compared to vehicle-treated microglia. IL-6 expression was not detected in the microglia-depleted/all other neural cell population. IL-6 expression significantly increased in unsorted cells treated with a 1,000 ng/mL LPS dose compared to vehicle-treated unsorted cells. *p < 0.05
3.2.2 IL-6
Treatment of microglia with LPS at 500 or 1,000 ng/mL significantly increased IL-6 gene expression relative to vehicle-treated microglia (F1,36 = 92.256, p < 0.001, η2= 0.719, Figure 1B; F1,33 = 152.237, p < 0.001, η2= 0.822, Figure 1E). IL-6 expression was not detectable in the microglia-depleted/all other neural cells population (Figure 1B and 1E). In the unsorted cell population, the 500 ng/mL LPS dose increased IL-6 expression compared to vehicle-treated unsorted cells in a sex-specific manner (F1,34 = 7.447, p < 0.010, η2= 0.180, Figure 1B). Post hoc analyses indicated that the LPS-induced increase in IL-6 expression was greater in the male unsorted cells compared to the female unsorted cells. Treatment of unsorted cells with the 1,000 ng/mL LPS dose significantly increased IL-6 relative gene expression compared to vehicle-treated unsorted cells in both sexes equally (F1,53 = 152.339, p < 0.001, η2= 0.742, Figure 1E). The presence of all other neural cells influenced the relative gene expression of IL-6 from microglia. Specifically, the fold-change in IL-6 relative gene expression, induced by LPS (at both doses), was less robust in the unsorted cell population compared to the microglial cell population (Figure 1B and 1E).
3.2.3 TLR-4
Treatment of microglia with LPS at 500 or 1,000 ng/mL significantly decreased TLR-4 relative gene expression compared to vehicle-treated microglia (F1,36 = 24.534, p < 0.001, η2= 0.405, Figure 1C; F1,33 = 10.692, p = 0.003, η2= 0.245, Figure 1F). TLR-4 relative gene expression was not detectable in the microglia-depleted/all other neural cells population (Figure 1C and 1F). No significant effects were found when unsorted cells were treated with 500 ng/mL or 1000 ng/mL LPS (Figure 1C and 1F).
3.3 Experiment 3: Relative Gene Expression of Hormone Receptors
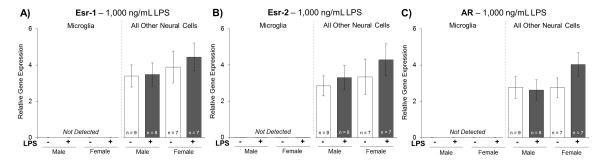
Esr-1, Esr-2, and AR relative gene expression were not detectable in the microglial cell population (Figure 2A, 2B, and 2C). Relative gene expression values for Esr-1, Esr-2, and AR were detectable in the microglia-depleted/all other neural cells population; but, no significant main effects or interactions were found in the expression of these hormone receptors (Figure 2A, 2B, and 2C).
Figure 2. Experiment 3: Relative Gene Expression of Hormone Receptors.
A, B, and C) Relative gene expression of Esr-1, Esr-2, and AR was not detected in microglia. No significant differences were found in Esr-1, Esr-2, or AR expression in the microglia-depleted/all other neural cell populat ion. *p < 0.05
4. Discussion
In the current experiments, we identified neonatal microglia as the primary source of IL-1β, IL-6, and TLR-4 mRNA in the developing hippocampus. The microglial expression of these molecules in response to an immune challenge differed depending on the presence or absence of other neural cells within the culture. We also found that the expression of Esr-1, Esr-2, and AR was confined to other neural cells and not microglia, keeping these sex-specific factors from influencing neonatal microglia directly. Lastly, we found that the increase in IL-6 produced by an immune activation with 500 ng/mL LPS was more robust in male microglia than female microglia, but only when other neural cells were present in the culture.
4.1 Microglia are the Major Source of IL-1β, IL-6, and TLR-4 mRNA in the Neonatal Hippocampus
When microglia were not present in the culture, IL-1β, IL-6, and TLR-4 mRNA were undetectable, even in the presence of an immune challenge. Given that this population only differed from the unsorted cell population by the exclusion of microglia, and provided that detectable levels of these molecules were observed in the unsorted cell population (microglia inclusive), we conclude that microglia appear to be the predominant source of IL-1β and IL-6, and TLR-4 mRNA in the developing hippocampus (still to be confirmed for IL-1β and IL-6 protein). Chen et al. (2015) also identified neonatal microglia as the major source of two cytokines, TNF-α and nitric oxide, following LPS stimulation. Moreover, they found that while TNF-α and nitric oxide mRNA and protein were undetectable in an astroglial pure population, they became detectable once the enriched astroglial populations were “contaminated” with as little as 0.5% microglial cells. In the same way, IL-1β and IL-6 mRNA were not detectable in microglia-depleted/all other neural cells in the current study; but, when microglia were present, they became detectable. Also, Lehnardt et al. (2002) showed that TLR-4 expression in the neonatal rat CNS was confined solely to microglia; neither astrocytes nor oligodendrocytes expressed TLR-4 mRNA in culture. Taken together, these results indicate that neonatal microglia are the primary responders to bacteria or LPS in the brain, which subsequently leads to the detectable expression of important cytokines, including IL-1β and IL-6.
It is important to note that these conclusions are drawn from in vitro analyses of cytokine expression, as this is the most direct way of determining the specific source of these molecules. But, it must be considered that in a living organism, these cytokines may also find their way into the brain from the periphery, following immune activation.
4.2 IL-1β, IL-6, and TLR-4 Expression in Neonatal Microglia is influenced by Other Neural Cells
We found that the magnitude of the microglial response to LPS depended on the presence of all other neural cells within the culture. Specifically, the fold-change in IL-1β relative gene expression in the unsorted cell population following LPS treatment was greater than that observed in the microglia cultured alone. Microglia only constitute about 10 percent of the overall neural cell population; but, in response to an immune challenge, microglia increased IL-1β expression to a greater extent when functioning as a subgroup of the overall neural cell population compared to when they were concentrated in a population of their own. Interestingly, the opposite trend was observed in IL-6 relative gene expression. Therefore, we conclude that the communication between microglia and other neural cells results in different inflammatory profiles, following immune activation. Previous work has indicated that different types of neuronal activity can regulate microglia behavior variably. For example, neuronal GABA signaling can inhibit the microglial release of IL-6, while neuronal glutamate and serotonin signaling can increase the microglial release of IL-6 [13]. Additionally, cross talk between microglia and astrocytes can impact the microglial response to an immune challenge. Stimulated microglia can directly activate astrocytes, which go on to generate a calcium wave that spreads to local and distant microglia to further excite or inhibit microglial activity [14]. These data indicate that other neural cells can interact with microglia to directly contribute to the cytokine response resulting from an immune challenge.
We also found that the presence of other neural cells could regulate microglial expression of TLR-4, following an immune challenge. LPS significantly down-regulated the expression of TLR-4 on microglia that were cultured alone. We hypothesize this response is due to the down-regulation of the receptor following robust immune activation. In contrast, LPS treatment did not decrease TLR-4 expression on microglia that were cultured in the presence of other neural cells, even though the microglia received the same dose of LPS. We hypothesize that modulation of TLR-4 receptor expression on microglia is a mechanism by which other neural cells interact with microglia to indirectly contribute to the cytokine response resulting from an LPS-specific immune challenge. The data presented here indicate that the presence of other neural cells in a microglial culture can significantly influence the microglial response to an LPS immune challenge.
4.3 A Sex Difference in the Microglial Expression of IL-6
The LPS-facilitated increase in IL-6 expression was greater in the male unsorted cells compared to the female unsorted cells. In contrast, this was not true at the 1,000 ng/mL LPS dose, perhaps due to a “ceiling” effect produced by the higher concentration. This significant sex x treatment interaction was only observed when microglia were in the presence of other neural cells. Therefore, based on the collection of evidence presented above, we hypothesize that the communication between microglia and other neural cells differed between the sexes, resulting in a more robust increase in IL-6 expression from neonatal male microglia at a 500 ng/mL LPS dose.
Notably, we did not observe a significant sex effect or sex x treatment interaction on IL-1β expression in any cell populations, or at any of the LPS doses tested here. In 2012, Loram et al. reported a significant sex difference in IL-1β gene expression in neonatal cortical microglia, following activation with a 100 ng/mL dose of LPS for four hours. The microglia used by Loram et al. (2012) differed from those used in the current study on a number of factors, one of which being the brain region from which they were collected. Importantly, however, the microglia also differed by how they were prepared and isolated, factors that can impact both the “age” and the basal gene expression profiles of the isolated cells. These results suggest that the neonatal microglial response to a specific immune challenge can vary by sex, but also largely depends on microglial preparation and the presence of other neural cells within the microglial culture – two methodological features that are often overlooked when examining neonatal, microglial activation.
Lastly, previous work has indicated that males may be more susceptible to certain neuropsychiatric disorders due to a greater number of “activated” microglia in the male brain, compared to the female brain, during early development – a sex difference that was linked to the surge in testosterone that occurs in males during early development [8]. The hormone receptor expression data in the current study, and that reported by Lenz, Nugent, Haliyur, & McCarthy (2013), indicate that Esr-1 (and also AR) is not present on neonatal microglia from the hippocampus of neonatal rats. But, Esr-1, Esr-2, and AR are present on other neural cells, as seen in the current study, where they may still contribute to the sexual differentiation of the developing brain. Therefore, the mechanism by which these critical hormones interact with microglia to impact immune activation requires other neural cell “translators,” demonstrating the complexity of the sex-specific communication between microglia and all other neural cells during this critical period of development.
4.5 Conclusions
The data in the current study reveal some of the many intricacies of neonatal microglial activation, following an LPS immune challenge. These findings necessitate further investigation into the distinct signaling cascades involved in neonatal microglial activation by LPS and how these cascades may differ between the sexes. We have also brought to attention the importance of including other neural cells when using in vitro models of microglial activation, as well as the need for delineating “freshly-isolated” microglia from microglia in culture for an extended period of time. By characterizing these intricate microglial activation profiles in males and females during early development, we may increase our opportunities for identifying more sites of intervention to treat or even prevent sex-biased neuropsychiatric disorders with known immune etiologies.
Supplementary Material
Highlights.
Activation of neonatal microglia is influenced by other developing neural cells.
Microglia are the primary source of cytokines in the neonatal hippocampus.
Few sex differences were found in the microglial cytokine response to LPS.
LPS causes a sex-specific increase in IL-6 from microglia with other neural cells.
Acknowledgments
Funding: This work was supported by the National Institutes of Health [NIH R21MH104280 and R01MH106553] and a University of Delaware Research Foundation Grant.
Abbreviations
- P
postnatal day
- LPS
lipopolysaccharide
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- TLR-4
Toll-like receptor 4
- CD11b/c
cluster differentiation factor 11b/c
- E.coli
Escherichia coli
- DPBS
Dulbecco’s phosphate buffered saline
- qPCR
quantitative real-time polymerase chain reaction
- Esr-1
estrogen receptor α
- Esr-2
estrogen receptor β
- AR
androgen receptor
- RPLP1
Ribosomal Protein Lateral Stalk Subunit P1
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boulanger LM. Immune Proteins in Brain Development and Synaptic Plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Cuadros Ma, Navascues J. The Origin and Differentiation of Microglial Cells During Development. Prog Neurobiol. 1998;56:173–189. doi: 10.1016/s0301-0082(98)00035-5. [DOI] [PubMed] [Google Scholar]
- 3.Harry GJ, Kraft AD. Neuroinflammation and Microglia: Considerations and approaches for neurotoxicity assessment. Expert Opin Drug Metab Toxicol. 2008;4:1265–1277. doi: 10.1517/17425255.4.10.1265.Neuroinflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry VH, Newman Ta, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- 5.Garay PA, McAllister AK. Novel roles for immune molecules in neural development: Implications for neurodevelopmental disorders. Front Synaptic Neurosci. 2010;2:1–16. doi: 10.3389/fnsyn.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson PH. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Bao A-M, Swaab DF. Sex differences in the brain, behavior, and neuropsychiatric disorders. Neurosci. 2010;16:550–565. doi: 10.1177/1073858410377005. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012;120:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerciat M, Unkila M, Garcia-Segura LM, Arevalo MA. Selective estrogen receptor modulators decrease the production of interleukin-6 and interferon-γ-inducible protein-10 by astrocytes exposed to inflammatory challenge in vitro. Glia. 2010;58:93–102. doi: 10.1002/glia.20904. [DOI] [PubMed] [Google Scholar]
- 10.Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HEW, Maier SF, Watkins LR. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology. 2012;37:1688–1699. doi: 10.1016/j.psyneuen.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen SH, Oyarzabal EA, Sung YF, Chu CH, Wang Q, Chen SL, Lu RB, Hong JS. Microglial regulation of immunological and neuroprotective functions of astroglia. Glia. 2015;63:118–131. doi: 10.1002/glia.22738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The Toll-Like Receptor TLR4 Is Necessary for Lipopolysaccharide-Induced Oligodendrocyte Injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. 20026268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung J, Chansard M, Ousman SS, Nguyen MD, Colicos MA. Activation of microglia by neuronal activity: Results from a new in vitro paradigm based on neuronal-silicon interfacing technology, Brain. Behav Immun. 2010;24:31–40. doi: 10.1016/j.bbi.2009.06.150. [DOI] [PubMed] [Google Scholar]
- 14.Liu W, Tang Y, Feng J. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life Sci. 2011;89:141–146. doi: 10.1016/j.lfs.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Microglia are essential to masculinization of brain and behavior. J Neurosci. 2013;33:2761–2772. doi: 10.1523/JNEUROSCI.1268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.