Abstract
Background:
Dexmedetomidine, an α2 adrenergic agonist, has been found to be a useful adjuvant to local anesthetics. It has been found to produce satisfactory block with lower doses of spinal bupivacaine.
Aim:
The aim of this study is to compare the difference in spinal block characteristics and hemodynamic effects of 7, 8, and 9 mg hyperbaric bupivacaine combined with 5 μg dexmedetomidine and to find out the optimum dose that would provide satisfactory block and hemodynamic stability for lower limb orthopedic surgeries.
Settings and Study Design:
This was a prospective, observational study.
Materials and Methods:
Ninety patients undergoing lower limb orthopedic surgeries were allocated to three groups of thirty each. Group A received 7 mg, Group B 8 mg and Group C 9 mg 0.5% hyperbaric bupivacaine along with dexmedetomidine 5 μg. The spinal block characteristics, hemodynamic stability, and side effects were compared.
Statistical Analysis:
The quantitative variables were compared using ANOVA test and the qualitative variables using Chi-square test.
Results:
All three groups had satisfactory anesthesia and analgesia. The onset of analgesia was slower and peak sensory level lower in Group A. The onset of motor block, time to attain peak sensory levels, duration of analgesia, maximum pain scores, and requirement of rescue analgesics were comparable among groups. Duration of motor block and time of regression of sensory level were more in Group C. Hemodynamics and sedation scores were comparable.
Conclusion:
Dexmedetomidine with lower doses of bupivacaine produces satisfactory anesthesia without hemodynamic instability. A dose of 7 mg bupivacaine with 5 μg dexmedetomidine may be sufficient for orthopedic surgeries.
Keywords: Adjuvant, dexmedetomidine, low-dose bupivacaine, orthopedic surgery, spinal anesthesia
INTRODUCTION
Dexmedetomidine, with its high α2 adrenergic agonism, has been found to be a useful adjuvant to intrathecal bupivacaine in prolonging sensory and motor block and reducing local anesthetic requirement.[1,2] A few studies have attempted to compare the effects of additives with varying doses of bupivacaine for spinal anesthesia in an attempt to arrive at an optimum dose with minimum adverse effects.[3,4,5] Dexmedetomidine has been found to be effective for urological and orthopedic surgeries with low-dose bupivacaine.[6,7]
In this study, we used combination of 5 μg dexmedetomidine with 7 mg, 8 mg, and 9 mg of hyperbaric bupivacaine, respectively. We wanted to find out whether minor alterations in the dose of bupivacaine would produce changes in spinal block characteristics and hemodynamic effects and if we could arrive at an optimum lower dose of bupivacaine, which in combination with dexmedetomidine would provide satisfactory block without hemodynamic instability. This would be beneficial in the orthopedic population who are mostly elderly with various comorbidities.
MATERIALS AND METHODS
A prospective, observational study was conducted on ninety American Society of Anesthesiologists (ASA) physical status Classes I and II patients aged 20–60 years and height 150–180 cm, posted for lower limb orthopedic surgery.
After Institutional Ethics Committee approval and informed consent, patients were allocated into three Groups, A, B, and C. Patients with a history of allergy to either dexmedetomidine or bupivacaine, infection at the puncture site, coagulopathy, history of arrhythmias, and labile hypertension were excluded from the study. In the operation theater, electrocardiography, peripheral oxygen saturation, and noninvasive blood pressure (BP) were connected, and basal parameters were recorded. Intravenous (IV) access was obtained on the nondominant hand with 18-gauge cannula, and crystalloid infusion started. Oxygen was administered by a face mask.
Under strict asepsis, spinal anesthesia was performed at L3–L4 interspace with a 25-gauge Quincke needle by a midline approach with the patient in the lateral position and operated side down. Group A received 1.4 ml (7 mg) 0.5% bupivacaine heavy with 5 μg dexmedetomidine, Group B received 1.6 ml (8 mg) 0.5% bupivacaine heavy with 5 μg dexmedetomidine, and Group C received 1.8 ml (9 mg) 0.5%bupivacaine heavy with 5 μg dexmedetomidine. The study medication was prepared and administered by an anesthesiologist not involved in the collection of data. The completion of injection was taken as the time zero for induction of anesthesia.
The patients were then turned supine. Heart rate (HR), BP, and oxygen saturation were monitored every 2 min for the first 20 min and every 5 min thereafter. Sensory level was assessed by loss of sensation to pinprick in the midclavicular line bilaterally. Time to reach sensory level of T10 on the operated side was taken as the time of onset of analgesia, and surgery was commenced. The maximum sensory level and time to achieve it was noted. Motor block was assessed according to modified Bromage scale (0: No motor block, 1: Inability to raise extended legs, 2: Inability to flex knees, and 3: Inability to flex ankle joints). Time taken to reach Bromage 3 was taken as the time of onset of motor block.
Hypotension was defined as systolic BP <90 mmHg or fall in mean arterial pressure more than 30% from baseline and was treated with injection ephedrine 6 mg boluses. Bradycardia was defined as fall in HR below 50 beats/min and treated with injection atropine 0.6 mg. Sedation scores was assessed using Ramsay sedation score (1: Anxious or restless or both, 2: Cooperative, oriented and tranquil, 3: Responding to commands, 4: Asleep, brisk response to light, glabellar tap or auditory stimuli, 5: Asleep, sluggish response, and 6: Asleep, unarousable).
Duration of surgery was noted. Postoperative analgesia was assessed hourly using numerical rating scale. Patients were asked to choose a number between 0 and 10 to rate their pain with 8 - no pain and 10 - worst imaginable pain. Rescue analgesia (injection tramadol 1 mg/kg IV) was given when the pain score was 4 and above. The total number of rescue analgesic doses required in 24 h was noted.
Statistical analysis
The observations made were tabulated and analyzed using SPSS software version 16 (SPSS Inc., IL, Chicago, USA). To calculate the sample size, a power analysis of = 0.05 and = 1.00 showed that 30 patients were needed in each group to detect a 30 min difference between the median duration of analgesia among the groups. For qualitative variables, Chi-square test was used with P value reported at 95% confidence interval. ANOVA test was used to analyze the quantitative variables after ascertaining normal distribution of data, and statistical significance was assumed for values of P < 0.05. Tukey's honestly significant difference post hoc test was used to compare between groups if P was statistically significant.
RESULTS
All the enrolled patients completed the study. Demographic data were comparable in the three groups [Table 1].
Table 1.
Demographic data

The block characteristics and requirement of rescue analgesics are summarized in Table 2. The onset of analgesia (time to reach T10 sensory level) was slower in Group A (2.33 ± 0.568 min) compared to Group B (2.10 ± 0.305 min) and C (2.00 ± 0.025 min). Time to reach peak sensory level and time to attain Bromage 3 were comparable in the three groups.
Table 2.
Outcome variables
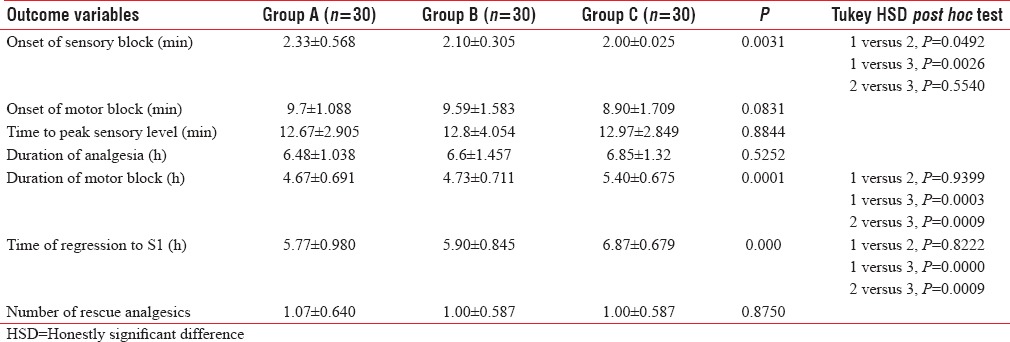
The peak sensory levels are shown in Figure 1. The peak sensory level was higher in Group C (median T4) than Group B (median T6), which was higher than Group A (median T8).
Figure 1.
Peak sensory levels
Duration of postoperative analgesia was comparable in the three groups (P = 0.5252). The requirement of rescue analgesics was also comparable between the groups (P = 0.8750).
Time of regression to S1 dermatome was more in Group C (6.87 ± 0.679 h) than in Groups B (5.90 ± 0.845 h) and A (5.77 ± 0.980 h). Time of regression to Bromage 0 was also longer in Group C (5.40 ± 0.675 h) than in Groups B (4.73 ± 0.711 h) and A (4.67 ± 0.691 h).
There was a gradual decrease in the mean HR intraoperatively, but none of the patients had significant bradycardia that required treatment. Changes in HR were comparable between three groups [Figure 2]. The preoperative mean arterial pressure was lower in Group C, and there was a decrease in BP after around 30 min of subarachnoid block. Hypotension was more seen in Group C, but the difference was statistically not significant [Figure 3]. Hypotension developed was manageable with Inj. Ephedrine 6 mg IV. In Group A, 10% of patients required single dose of inj. Ephedrine and 3.3% required 2 doses. In Group B, 16.6% required single dose and 3.33% had double dose. In Group C (1.8 ml), 33% of patients required single dose and 6.6% required two doses (P = 0.1736).
Figure 2.
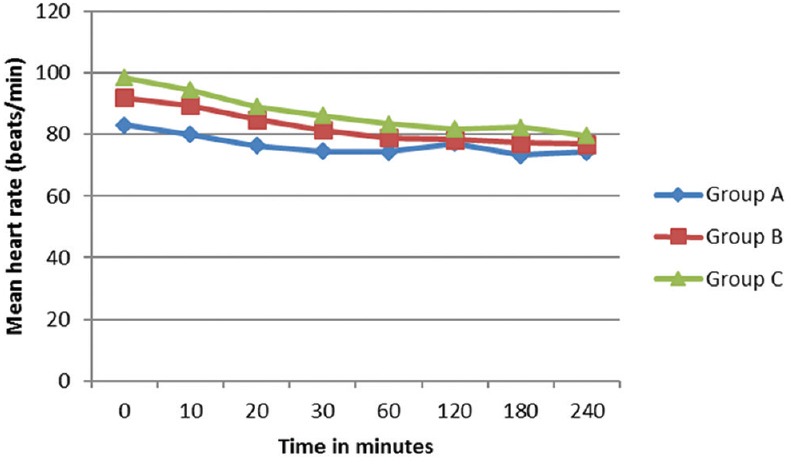
Comparison of mean heart rate between groups
Figure 3.
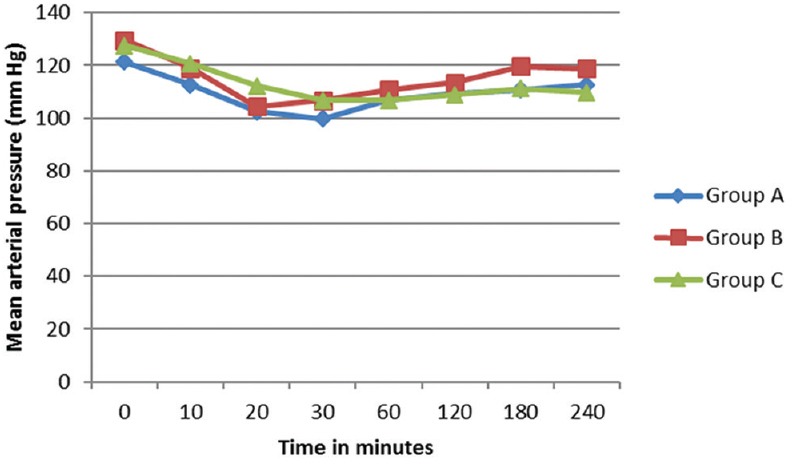
Comparison of mean arterial pressure between groups
Maximum pain score attained was 5 and more in Group A patients, but there was no statistically difference noted among three groups (P = 0.431). In both Groups B and C, maximum pain score attained was 4.
Ramsay sedation score was comparable in all three groups. Most of the patients had a sedation score of 2, and all the patients were comfortable intraoperatively. None of the patients had any other significant side effects such as nausea, vomiting, dry mouth, or respiratory depression.
DISCUSSION
Patients undergoing lower limb orthopedic surgeries are generally elderly with various comorbidities. Hemodynamic stability, perioperative analgesia as well as early ambulation are essential for these patients. Regional anesthesia has many benefits such as staying awake, maintenance of protective reflexes, good postoperative analgesia, and early postoperative ambulation. The addition of various adjuvants helps in reducing the dose of local anesthetic, thus providing more hemodynamic stability, as well as prolonging their duration of action.
A reduced dosage of local anesthetic can limit the extent of block. However, a smaller concentration of drug may be insufficient to provide adequate spinal block. Hence, several additives have been used. However, additives such as opioids can produce side effects such as nausea, vomiting, pruritus, and respiratory depression. Intrathecal α2 agonists potentiates the effects of local anesthetics and allow a decrease in dose without respiratory depression and hemodynamic instability. Dexmedetomidine is believed to act at the spinal[8] and supraspinal receptors.[9] Compared to its counterpart, clonidine, it has 8-fold greater affinity for α2 receptors. As an adjuvant to local anesthetics, it prolongs sensory and motor block and reduces need for analgesic requirements.[10,11] Undesirable side effects such as respiratory depression do not occur.[12] Animal[13] and clinical[1,2] models have shown the safety of intrathecal administration of dexmedetomidine. Dexmedetomidine 3 μg, when added to 6 mg intrathecal bupivacaine, produced faster onset and prolonged duration of analgesia in patients undergoing transurethral prostatectomy. However, duration of motor block was prolonged.[6]
In our study, 5 μg dexmedetomidine was added to 7, 8, and 9 mg of 0.5% hyperbaric bupivacaine, respectively, for spinal anesthesia in patients undergoing lower limb orthopedic surgery. Several studies have attempted to study the effects of adjuvants with varying doses of bupivacaine.[3,4] In a study by Sendhil et al.,[3] fentanyl 25 μg was combined with three different doses of bupivacaine in transurethral resection of prostate surgery to arrive at an optimum dose. We used three different doses of bupivacaine in an attempt to find out whether there was an optimum dose which when combined with 5 μg dexmedetomidine could provide sufficient duration of block as well as hemodynamic stability.
The maximum sensory level obtained in Group A was up to T6, while Group B attained a sensory level of T4, and for Group C, it was up to T2. Low doses of local anesthetic alone can limit the spinal block level with minimum hemodynamic alterations and produce rapid recovery. However, this may not be sufficient to provide adequate anesthesia for the surgery.[13] The addition of dexmedetomidine allowed these lower volumes to be used.
The hemodynamic profile was similar in all the three groups. There was no incidence of hypotension or bradycardia. There was only a slight delay in achieving an adequate sensory level with 7 mg dose of bupivacaine. The motor and sensory block was prolonged with the 9 mg dose, compared to the 7 and 8 mg doses, but complete recovery occurred within 8 h in all three groups. Lower doses provide sufficient analgesia and lesser duration of motor block, thus aiding in early ambulation. The postoperative analgesia and sedation scores were comparable.
Dexmedetomidine has been found to prolong the duration of spinal anesthesia in a dose-dependent manner.[14,15] In a meta-analysis, when used intrathecally, it hastened the sensory block onset by 19%, prolonged motor block duration by 88%, and delayed the time of the first analgesic request by 127% compared with local anesthetic alone.[16] The onset of motor block was not delayed. In our study, onset of sensory block was delayed and the peak level attained lower with the 7 mg dose which is to be expected because of the lower dose. However, the onset of motor block was comparable in the three groups. Dexmedetomidine was found to hasten the onset time of motor block when added to local anesthetic for peripheral nerve block.[17]
α2 agonists have antinociceptive action for both somatic and visceral pain.[18] Mechanisms by which they prolong motor and sensory blocks of local anesthetics are not known. Local anesthetics act by sodium channel blockade. α2 adrenergic agonist binds to presynaptic C fibers and postsynaptic dorsal horn neurons. Hence, the analgesic effect may be due to the depression of release of C-fiber transmitters and hyperpolarization of postsynaptic dorsal horn neurons. Prolonged motor blockade might be caused by direct impairment of excitatory amino acids from the spinal interneurons.[18]
In another study, 2 and 4 μg dexmedetomidine added to 15 mg bupivacaine produced comparable analgesia in patients undergoing inguinal surgeries.[19] In a comparison with 5 and 10 μg intrathecal dexmedetomidine for lower limb surgeries, 10 μg gave faster onset and longer duration of block as well as postoperative analgesia.[14] Higher doses of 15 and 20 μg of dexmedetomidine was found to produce hypotension and bradycardia.[15] The longer duration of blockade, adequate postoperative analgesia, and desirable level of sedation makes dexmedetomidine a suitable adjuvant to hyperbaric bupivacaine for spinal anesthesia. This may be beneficial for longer duration surgeries, precluding the need for an epidural, or general anesthesia.
Compared to clonidine and fentanyl, dexmedetomidine significantly prolonged sensory and motor block and reduced postoperative analgesic requirement.[20] Dexmedetomidine 2.5 μg produced similar duration of analgesia as 250 μg of morphine when added to 15 mg of hyperbaric bupivacaine. However, the duration of both sensory and motor block was prolonged, and incidence of hypotension was more with dexmedetomidine.[21] Thus, higher dose of dexmedetomidine as well as hyperbaric bupivacaine produces hemodynamic instability.
In a study by Sudheesh et al., intrathecal dexmedetomidine 3 μg dose did not produce faster ambulation compared to 5 μg dose in combination with 4 mg bupivacaine though it produced comparable duration of analgesia for perianal surgeries. The median block height attained in the two groups were L1 and T11, respectively.[22] In another study, dexmedetomidine 5 μg and fentanyl 25 μg was added to low dose of 4 mg bupivacaine for lower abdominal surgeries; however, they were able to achieve the desired level only by a 5°–10° Trendelenburg position.[23] Hence, we did not choose too low a dose of bupivacaine.
In our study, we used a dose of dexmedetomidine that would produce minimal hemodynamic side effects. Although all three groups fared well with respect to the duration of anesthesia and analgesia and hemodynamic stability, the level of block was higher with 8 and 9 mg doses. Hence, a 7 mg dose with 5 μg dexmedetomidine may be sufficient for orthopedic surgeries which produced a median block level of T8. Minor variations in dose of bupivacaine do not produce marked alterations in block characteristics or hemodynamic effects. Hence, further reduction in dose of bupivacaine may not be beneficial. However, the use of 9 mg dose was associated with a prolonged motor block and delayed sensory regression. Hence, higher doses of bupivacaine may be associated with delayed ambulation.
CONCLUSION
Five-microgram dexmedetomidine added to lower doses of 7, 8, and 9 mg bupivacaine produced satisfactory anesthesia and analgesia for lower limb orthopedic surgeries. There was no difference among the groups with respect to duration of analgesia and requirement of rescue analgesics though duration of motor block and sensory regression was prolonged in the 9 mg bupivacaine group. Since higher levels of block were attained with the 8 and 9 mg doses, a dose of 7 mg intrathecal bupivacaine with 5 μg dexmedetomidine may be sufficient for orthopedic surgeries.
Limitations
A control without adjuvant was not used. However, many studies and meta-analysis have shown that adjuvants prolong the blocks and provide longer duration of postoperative analgesia. We have not included ASA III and IV patients, and hence, the results cannot be applied to them.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gupta R, Bogra J, Verma R, Kohli M, Kushwaha JK, Kumar S. Dexmedetomidine as an intrathecal adjuvant for postoperative analgesia. Indian J Anaesth. 2011;55:347–51. doi: 10.4103/0019-5049.84841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222–7. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 3.Sendhil MM, Krishna KS, Nanthaprabhu M, Anandan H. Randomized clinical comparison of three different doses of bupivacaine with fentanyl for TURP- search for an optimum dose to be used in day care urological procedures. Ann Int Med Dent Res. 2016;2:AN10–4. [Google Scholar]
- 4.Mehta S, Dalwadi H, Shah TD. Comparative study of low dose bupivacaine –Fentanyl vs. conventional dose of bupivacaine in spinal anaesthesia for orthopedic procedures in elderly patients. Gujarat Med J. 2015;70:25–8. [Google Scholar]
- 5.Prajapati P, Parmar H. Low dose bupivacaine and bupivacaine with fentanyl for spinal anesthesia for transurethral resection of prostate. Int Arch Integr Med. 2015;2:11–9. [Google Scholar]
- 6.Kim JE, Kim NY, Lee HS, Kil HK. Effects of intrathecal dexmedetomidine on low-dose bupivacaine spinal anesthesia in elderly patients undergoing transurethral prostatectomy. Biol Pharm Bull. 2013;36:959–65. doi: 10.1248/bpb.b12-01067. [DOI] [PubMed] [Google Scholar]
- 7.Chang YS, Kim JE, Sung TY. Low-dose bupivacaine with dexmedetomidine prevents hypotension after spinal anesthesia. Open Anesthesiol J. 2015;9:39–45. [Google Scholar]
- 8.Kobayashi T, Otsuguro K, Yamaguchi S, Ito S. Contribution of α2A-adrenoceptor subtype to effect of dexmedetomidine and xylazine on spinal synaptic transmission of mice. Eur J Pharmacol. 2015;761:321–9. doi: 10.1016/j.ejphar.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Ma D, Hossain M, Rajakumaraswamy N, Arshad M, Sanders RD, Franks NP, et al. Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharmacol. 2004;502:87–97. doi: 10.1016/j.ejphar.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Li C, Pirrone M, Sun L, Mi W. Comparison of dexmedetomidine and clonidine as adjuvants to local anesthetics for intrathecal anesthesia: A meta-analysis of randomized controlled trials. J Clin Pharmacol. 2016;56:827–34. doi: 10.1002/jcph.666. [DOI] [PubMed] [Google Scholar]
- 11.Sarma J, Narayana PS, Ganapathi P, Shivakumar MC. A comparative study of intrathecal clonidine and dexmedetomidine on characteristics of bupivacaine spinal block for lower limb surgeries. Anesth Essays Res. 2015;9:195–207. doi: 10.4103/0259-1162.153763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patro SS, Deshmukh H, Ramani YR, Das G. Evaluation of dexmedetomidine as an adjuvant to intrathecal bupivacaine in infraumbilical surgeries. J Clin Diagn Res. 2016;10:UC13–6. doi: 10.7860/JCDR/2016/17987.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Zhou F, Li C, Kong M, Liu H, Zhang P, et al. Molecular mechanisms underlying the analgesic property of intrathecal dexmedetomidine and its neurotoxicity evaluation: An in vivo and in vitro experimental study. PLoS One. 2013;8:e55556. doi: 10.1371/journal.pone.0055556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eid HE, Shafie MA, Youssef H. Dose related prolongation of hyperbaric bupivacaine spinal anaesthesia by dexmedetomidine. Ain Shams J Anesthesiol. 2011;4:83–95. [Google Scholar]
- 15.Naaz S, Bandey J, Ozair E, Asghar A. Optimal dose of intrathecal dexmedetomidine in lower abdominal surgeries in average Indian adult. J Clin Diagn Res. 2016;10:UC09–13. doi: 10.7860/JCDR/2016/18008.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdallah FW, Brull R. Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: A systematic review and meta-analysis. Br J Anaesth. 2013;110:915–25. doi: 10.1093/bja/aet066. [DOI] [PubMed] [Google Scholar]
- 17.Marhofer D, Kettner SC, Marhofer P, Pils S, Weber M, Zeitlinger M. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: A volunteer study. Br J Anaesth. 2013;110:438–42. doi: 10.1093/bja/aes400. [DOI] [PubMed] [Google Scholar]
- 18.Oda A, Iida H, Tanahashi S, Osawa Y, Yamaguchi S, Dohi S. Effects of alpha2-adrenoceptor agonists on tetrodotoxin-resistant Na+ channels in rat dorsal root ganglion neurons. Eur J Anaesthesiol. 2007;24:934–41. doi: 10.1017/S0265021507000543. [DOI] [PubMed] [Google Scholar]
- 19.Yektas A, Belli E. The effects of 2 μg and 4 μg doses of dexmedetomidine in combination with intrathecal hyperbaric bupivacaine on spinal anesthesia and its postoperative analgesic characteristics. Pain Res Manag. 2014;19:75–81. doi: 10.1155/2014/956825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahendru V, Tewari A, Katyal S, Grewal A, Singh MR, Katyal R. A comparison of intrathecal dexmedetomidine, clonidine, and fentanyl as adjuvants to hyperbaric bupivacaine for lower limb surgery: A double blind controlled study. J Anaesthesiol Clin Pharmacol. 2013;29:496–502. doi: 10.4103/0970-9185.119151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurhekar P, Kumar SM, Sampath D. Comparative evaluation of intrathecal morphine and intrathecal dexmedetomidine in patients undergoing gynaecological surgeries under spinal anaesthesia: A prospective randomised double blind study. Indian J Anaesth. 2016;60:382–7. doi: 10.4103/0019-5049.183387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sudheesh K, Rao RR, Kavya M, Aarthi J, Rani DD, Nethra SS. Comparative study of two doses of intrathecal dexmedetomidine as adjuvant with low dose hyperbaric bupivacaine in ambulatory perianal surgeries: A prospective randomised controlled study. Indian J Anaesth. 2015;59:648–52. doi: 10.4103/0019-5049.167485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nayagam HA, Singh NR, Singh HS. A prospective randomised double blind study of intrathecal fentanyl and dexmedetomidine added to low dose bupivacaine for spinal anesthesia for lower abdominal surgeries. Indian J Anaesth. 2014;58:430–5. doi: 10.4103/0019-5049.138979. [DOI] [PMC free article] [PubMed] [Google Scholar]


