Abstract
Background:
In this meta-analysis, we explore the role of repetitive transcranial magnetic stimulation (rTMS), a noninvasive neuromodulation technique in the treatment of chronic pain.
Methods:
Studies comparing rTMS and conventional treatment for chronic pain were searched. The comparison was made for decrease in the pain scores with and without (sham) the use of rTMS after a follow-up interval of 4–8 weeks. All reported pain scores were converted into a common scale ranging from “0” (no pain) to “10” (worst pain).
Results:
Nine trials with 183 patients in each of the groups were included in the analysis. The decrease in pain scores with rTMS was 1.12 (95% confidence interval [CI] being 1.46–0.78) (fixed effects, I2 = 0%, P < 0.001) and in sham-rTMS was 0.28 (95% CI being 0.49–0.07) (Fixed effects, I2 = 0, P = 0.01). The pooled mean drop in pain scores with rTMS therapy was higher by 0.79 (95% CI being 0.26–1.33) (fixed effects, I2 = 0, P < 0.01). The duration and frequency of rTMS were highly variable across trials. Publication bias was unlikely (Egger's test, X-intercept = 0.13, P = 0.75).
Conclusions:
Use of rTMS improves the efficacy of conventional medical treatment in chronic pain patients. This treatment is not associated with any direct adverse effects. However, the duration and frequency of rTMS therapy is presently highly variable and needs standardization.
Keywords: Chronic pain, fibromyalgia, meta-analysis, neuroplasticity, repetitive transcranial magnetic stimulation
INTRODUCTION
It is well recognized that chronic nonmalignant pain is an underestimated epidemiologic health problem.[1] Apart from unimaginable discomfort, the financial burden on the society is enormous. The reported prevalence of chronic pain in the general population is about 27%, which is similar to other common chronic conditions. Among the chronic pain conditions, fibromyalgia has the highest unemployment, claims for incapacity benefit, and greatest number of days of absence from work.[2] Sufficient evidence exists to make the management of chronic pain a top priority for researchers, clinicians, and the governments. Although chronic pain may be a manifestation of an underlying disease, often, no recognizable explanation might be forthcoming, in spite of extensive investigations. As a result, it might be appropriate to consider chronic unexplained pain as a chronic disease in its own right.[3,4] Optimum management of chronic persistent pain does not involve the elimination of the cause alone. It must address both the consequences and contributors that together comprise the disease of persistent pain.
The classic approach to the management of chronic pain involves the identification of the cause and elimination of the primary pathology, if possible. Frequently, the pathology is either unidentifiable or an appropriate cure may not exist. Symptom management takes a top priority even though such an approach has a number of inherent dangers. Nonopioid and opioid analgesics form the backbone of pharmacotherapy. However, prescription opioid misuse in the USA has increased over 3-fold since 1990 to epidemic proportions, with substantial increases in prescription opioid use also reported in other countries, such as Australia and New Zealand.[5] One of the methods for minimizing risks and negative consequences associated with opioid analgesics is to find alternative modalities of chronic pain management.
Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive neuromodulation technique that has been closely examined in a variety of clinical situations such as Parkinson’s, fibromyalgia, treatment-resistant depression, and schizophrenia.[6,7,8,9] Both prospective, randomized controlled trials and misanalysis exist for these indications. It is use in the management of chronic pain is an evolving area. Although many studies exist, a meta-analysis is not available at the time of writing.
METHODS
The present study was conducted based on the recommendations by the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines [Figure 1]. The Population, Intervention, Control, and Outcome Study (PICOS) evaluation strategy was used to enroll the relevant trials into the analysis [Table 1]. Studies fulfilling PICOS criterion for primary outcome goal (rTMS efficacy in comparison to control) were planned for analysis. We included trials comparing sham rTMS in combination with conventional medical treatment for chronic pain conditions. To evaluate comparative efficacy, we included only trials those reported either of the following:
Figure 1.
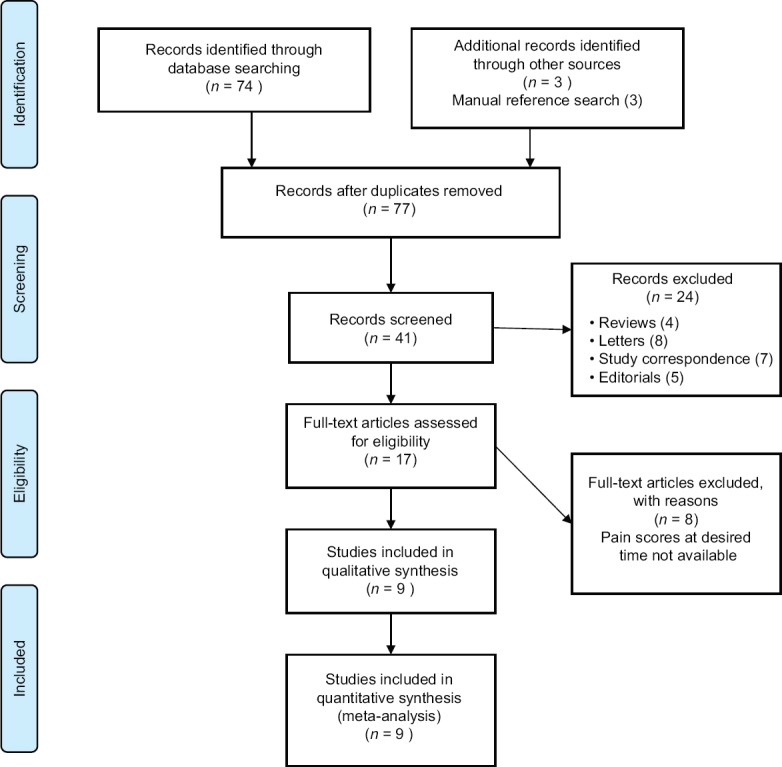
Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram
Table 1.
Population, Interventions, Control, and Outcome Study data extraction framework

Pre- and post-treatment pain scores in both the groups (mean and standard deviation [SD])
Change in mean pain scores in both rTMS and control arms after the intervention.
Search strategy
Two reviewers (AS. and GB.) independently searched the online literature available on MEDLINE, EMBASE, Science Citation Index Expanded, Cochrane Central Register of Controlled Trials, clinical trials registry, Scopus, and metaRegister of controlled trials. The medical subject heading terms included the following terms “rTMS, chronic pain and rTMS, comparative efficacy of rTMS for chronic pain, rTMS analgesic efficacy” on the aforementioned database. Terms used for exclusion during the search included rTMS “major depressive disorders, multiple sclerosis, amyotrophic lateral sclerosis, movement disorders.” Prospective, randomized controlled trials comparing rTMS (in various stimulation frequencies, doses, and duration) with conventionally used analgesic strategies for chronic pain conditions in adult population aged 18 years and older, published either as full articles or meeting abstracts (in peer-reviewed journals), were considered. We manually searched the references in published systematic reviews on similar topics. Our search extended to both the English and non-English language literature. The decision to include a potential study in the analysis was based on the independent assessment of full text by the aforementioned reviewers, working independently. Disagreements were resolved by consensus and if necessary by arbitration by the third independent author (PMS). Quality assessment for bias in the included studies was carried out as per the other published meta-analysis and the guidelines laid by the Cochrane collaboration by another independent researcher.
Data extraction
Data were initially abstracted into a standardized format entered into Microsoft Excel 2016 (Macintosh edition, Microsoft Corporation, USA). The following data from individual studies was extracted: Study design, year and country of publication, nature of pain disorder, participant numbers primary/secondary outcome reported, use of rTMS (duration, number of sittings, frequency used), analgesic strategy being compared, need for additional treatment for pain, specific adverse effect (if mentioned), and single center versus multicenter trials [Table 2]. If data were expressed in terms of median and interquartile range (IQR), authors were contacted for the mean and SD values. However, if authors did not reply, as a last resort we estimated the mean using the validated formula by Hozo et al.:[10] Mean = 2m + a + b/4 where m is the median, and a and b are the 25th and 75th centiles, respectively.[11] The SD was estimated by the formula given by the Cochrane collaboration: IQR = 1.35 SD.[12,13]
Table 2.
Characteristics of included studies
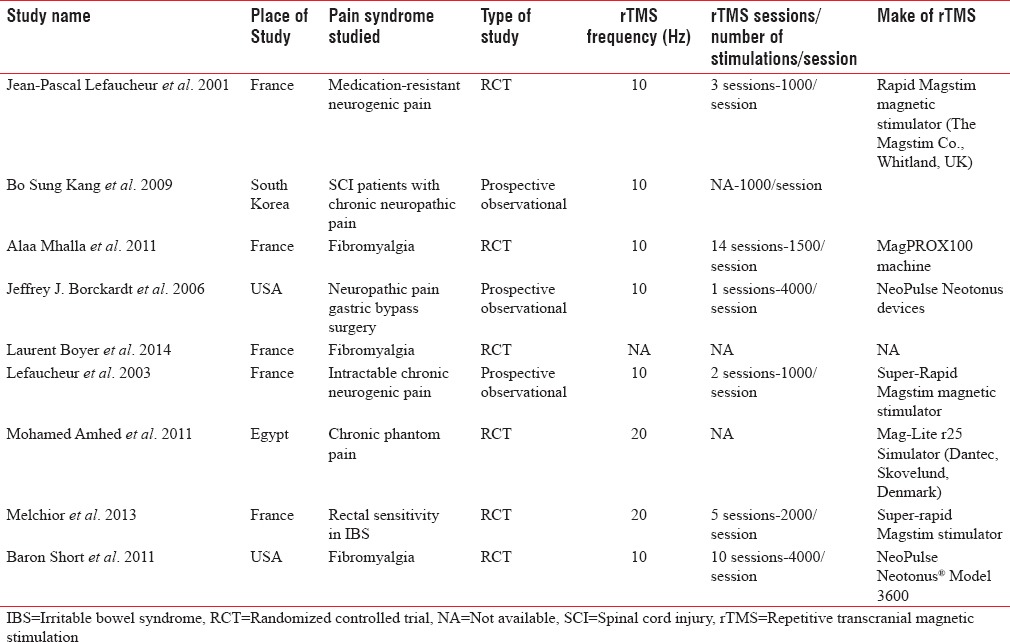
Studies used multiple scores to quantify pain. All visual analog scale (VAS), numeric pain scale scores were converted to a common numeric scale from 0 to 10 using equivalent proportions. For scales such as pain inventory and impact questionnaire (e.g., fibromyalgia impact questionnaire) median scores along with interquartile rage were extracted. These values were proportionated to a scale of 0–10 and Hozo’z method was used to estimate mean and SD for these scores. Trials those reported mean change in values with SD (pre- and post-treatment) were also included in the analysis.
Statistical analysis
The statistical analysis of the pooled data was performed using Comprehensive Meta-analysis-Version 3 (BioStat Inc., USA). Meta-analysis was performed initially using fixed effect modeling and eventually with random-effect methods (after assessment of heterogeneity with fixed modeling). I2 statistic was used to quantify the heterogeneity between the trials. Values of I2 <40% were considered nonsignificant, 40%–60% were considered to represent moderate heterogeneity, 60%–90% was reported as high heterogeneity. Results were expressed as pooled mean values for individual treatment group (pre- and post-treatment). Treatment effect between both treatment modalities was compared using a comparison of drop in pain scores in individual groups. We calculated pooled mean difference between the change in pain scores to compare treatment effect (mean fall in rTMS group-mean fall (or change) in control group). Wherever, heterogeneity was found to be lower than 40% results from “Fixed effect modeling” were used else results from “Random effect modeling” were used. Potential publication bias quantified using the Egger's regression test and it was further evaluated using the funnel plot [Figure 2].
Figure 2.
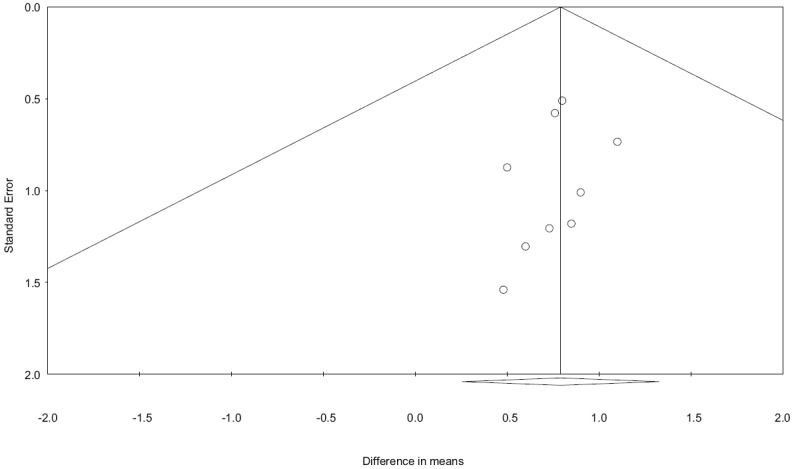
Funnel plot evaluating publication bias across trials. A positive publication bias is unlikely with X-intercept at +0.13 with P value being 0.75 (two-tailed)
RESULTS
The initial search identified 77 articles from the above database. Endnote (Thompson Reuters, USA) was used to remove the duplicates obtained from individual search by different reviewers. Eventually, nine trials were identified that measured/reported the primary outcome (comparative change in pain score on relevant scales).
All included trials were available as full-text manuscripts. None of the published abstracts were found to be relevant for our present analysis. All articles included were in the English language and articles in non-English language were excluded from (reporting of relevant parameters) the analysis. In all nine trials, patients received conventional medical therapy for chronic pain. In addition, patients were randomly divided into two groups where they received either a sham rTMS or therapeutic rTMS. Of the nine trials included, three evaluated fibromyalgia, one phantom limb, one irritable bowel syndrome, one postsurgical chronic pain (postgastric bypass) and three compared heterogeneous chronic pain conditions (neurogenic hand pain, facial pain syndromes etc.) No major adverse effects related to rTMS were reported in the included trials.
Results were evaluated under the following groups:
-
Efficacy of both treatment arms.
We first calculated pooled mean change in pain scores on a scale of ten in both groups (i.e., posttreatment pain scores − pretreatment pain scores)
-
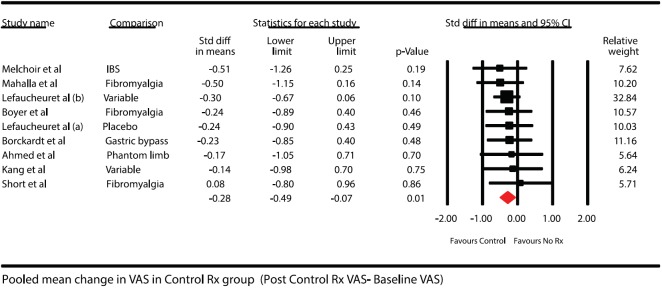
Control group (Sham rTMS groups)Results for 183 patients were available in this subset in all the nine studies included in the study. The mean change in this group along with 95% confidence interval (CI) is shown in Figure 3. The overall heterogeneity for this pooling was 0%
-
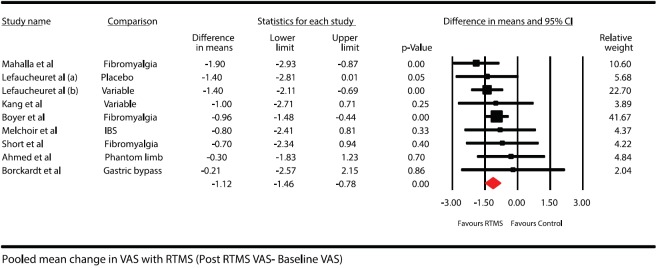
rTMS groupsResults were available for patients from all the 183 included 9 groups. The mean fall in VAS is shown in figure. The overall heterogeneity for above pooled estimate was 0% [Figure 4]
-
Comparative efficacy of addition of rTMS to treatment.
Figure 3.
Forest plot showing pooled mean difference in pain scores after treatment in control group (baseline pain scores – posttreatment pain scores). Solid diamond at the bottom of comparison denotes the final net effect
Figure 4.
Forest plot showing pooled mean difference in pain scores after treatment in repetitive transcranial magnetic stimulation group (baseline pain scores – posttreatment pain scores). Solid diamond at the bottom of comparison denotes the final net effect
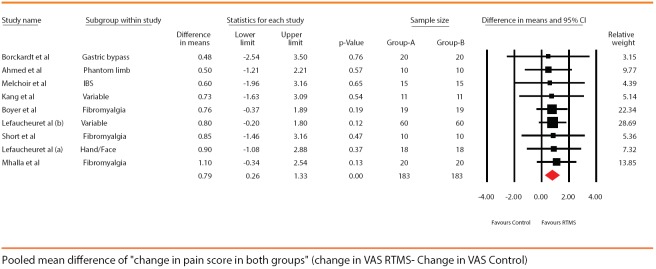
For the comparison, each group had 183 patients in the pooled results. The overall better treatment effect was seen with rTMS compared to sham group. The drop in pain scores was higher by a mean of 0.79 (95% CI being 0.26–1.32). The overall heterogeneity for the comparison was 0% [Figure 5].
Figure 5.
Forest plot showing pooled mean difference in the “change” in pain scores seen in both the groups posttreatment (drop in pain scores in control group – drop of pain scores in repetitive transcranial magnetic stimulation group). Solid diamond at the bottom of comparison denotes the final net effect
Evaluation of the publications bias
A funnel plot was constructed, and distribution of studies was accessed. The publication bias was further estimated using the Egger's test and intercept was found at 0.13 with a P value of 0.75, thus publication bias was unlikely.
DISCUSSION
Our meta-regression clearly demonstrates the therapeutic efficacy of both rTMS and sham treatment in the reduction of pain scores in patients with chronic pain, when compared to no treatment. However, rTMS therapy was significantly more effective than sham treatment in reducing the posttreatment pain scores compared to pretreatment values. To the best our knowledge, this is the first meta-analysis that has quantified the effect of rTMS and sham treatment for chronic pain.
The therapeutic benefit of rTMS is in accordance with the findings noted by O’Connell et al.[14] In their Cochrane review, various noninvasive stimulation techniques used in chronic pain was analyzed. Similar to us, they found a beneficial effect of high-frequency single dose stimulation of motor cortex in pain scores reduction. However, low frequency and multiple dose stimulation rTMS were not found to have any beneficial effect. It should be noted that they reported high heterogeneity and hence bias toward the observed results. In our analysis, eight of the nine studies targeted the motor cortex, and only one had targeted the prefrontal cortex. All of the nine studies analyzed used HFrTMS as either single or repetitive stimulation.
Similar results were observed by Jin et al. in their meta-analysis where they studied the optimal parameters of HFrTMS in the treatment of chronic neuropathic pain.[15] Nevertheless, they too had vast heterogeneity (I2 = 81%), and as a result, the data were further stratified based on the rTMS characteristics. They found that five sessions of HFrTMS provided maximal pain relief lasting up to 1 month. Our meta-regression analysis demonstrated low heterogeneity, and hence, it can be safely concluded that HFrTMS adds to the therapeutic efficacy of treatment of chronic pain.
HFrTMS to contralateral M1 cortex has had therapeutic implications in variety of disorders, including chronic pain, depression, and schizophrenia. However, the exact mechanism of action of rTMS is still unknown. In a recent review, DosSantos et al. critically examined the potential mechanisms supporting the value of motor cortex stimulation to treat chronic pain syndromes. The possibilities include the controls related to excitation of horizontal fibres, modulation of deeper and remote brain structures, and the mediation of various neurotransmitters implicated in the pain pathway.
Among the various postulated theories, glutamate receptors are known to play a significant role in the pain pathways, and the modulation of these receptors has been implicated in therapeutic role for different types of pain.[16] In a study on rats, Lee et al. proposed neuroplasticity as one of the mechanisms of action for rTMS.[17] They found that in the cerebellar cortex of the rats treated with rTMS, the transcription of mGluR1 decreased following a single session of high-frequency rTMS. Synthesis of mGluR, PKC, and GluR2 was reduced after rTMS, especially high-frequency stimulation.
Depression is a common comorbidity in patients with chronic pain.[18] Clearly, pain and depression should be treated concurrently to achieve a positive outcome. Insomnia also frequently occurs with chronic lower back pain. Several studies have proposed hypotheses for TMS pain management. The beneficial effects of rTMS are clearly demonstrated in these case reports.
In addition to rTMS, intravenous ketamine is an emerging therapy for the treatment of chronic pain syndromes, especially those that have a neuropathic component, and cancer pain.[19] Low-dose ketamine produces strong analgesia in neuropathic pain states as a likely result of the N-methyl-D-aspartate receptor receptor inhibition although other mechanisms are possibly involved, including enhancement of descending inhibition and anti-inflammatory effects acting centrally. Nevertheless, the long-term effectiveness of ketamine to treat chronic pain remains controversial as studies often demonstrate contradicting results. In addition, intravenous ketamine has shown promise in the treatment of depression.[20,21,22] Considering that many chronic pain conditions are associated with depression, use of ketamine might act by multiple mechanisms in relieving chronic pain syndrome.
As chronic pain without an organic basis is becoming a major health hazard, it is important to use all modalities its management. rTMS is an important addition to a clinician's armamentarium.
Limitations
Unlike the other meta-analysis, our study did not aim to analyze the various characteristics of rTMS therapy such as its effective dose, duration, repeating frequencies, and targeted brain areas in relief of chronic pain. These factors may play a role in relieving chronic pain. These factors should be studied further once the benefits of rTMS in chronic pain are firmly established. As the trials did not describe any specific adverse effects associated with rTMS, we were unable to make these comparisons. Another inevitable difficulty was conversion of all pain scales to a scale with common denominator. Although intuitively, it may be simple mathematical conversion, exact representation of pain quantified in trial cannot be assured with absolute certainty.
Nevertheless, such limitations exist for almost all meta-analysis where conversion of outcome variables into single common scale is a necessity for pooling the data. Our extensive experience with meta-analysis has proved this point.[23,24,25,26] The included trials varied in terms of the pain syndromes; as a result, we cannot conclude if one type of chronic pain may respond better with rTMS than other. Despite our attempts to subdivide the results based on the type of pain, we were unable to perform such analysis as the number of trials in each of the subgroup turned out to be too small to make any valid statistical inferences. We, however, wish to highlight an important feature of our meta-analysis. The heterogeneity in all our comparisons was “zero.” This could indirectly point that the treatment effect across various trials (different types of chronic pain) did not vary significantly. Therefore, until further research is available, it would be reasonable to state that rTMS did contribute to the reduction of all types of chronic pain.
Availability of limited number of trials examining the usefulness of rTMS is an important drawback of the current meta-analysis. However, our extensive experience with meta-analysis has demonstrated that clinically useful can be obtained with small groups of studies.[24,25]
CONCLUSIONS
The use of rTMS improves the efficacy of conventional medical treatment in chronic pain patients. This treatment is not associated with any direct adverse effects. The duration and frequency of rTMS therapy are presently highly variable and needs standardization.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Latina R, Sansoni J, D’Angelo D, Di Biagio E, De Marinis MG, Tarsitani G. Etiology and prevalence of chronic pain in adults: A narrative review. Prof Inferm. 2013;66:151–8. doi: 10.7429/pi.2013.663151. [DOI] [PubMed] [Google Scholar]
- 2.Leadley RM, Armstrong N, Lee YC, Allen A, Kleijnen J. Chronic diseases in the European Union: The prevalence and health cost implications of chronic pain. J Pain Palliat Care Pharmacother. 2012;26:310–25. doi: 10.3109/15360288.2012.736933. [DOI] [PubMed] [Google Scholar]
- 3.Persistent Pain as a Disease Entity: Implications for Clinical Management. [Last cited on 2016 Nov 14]. Available from: https://www.proxy.library.upenn.edu: 2063/pubmed/15271732 .
- 4.Doleys DM. How neuroimaging studies have challenged us to rethink: Is chronic pain a disease? J Pain. 2010;11:399–400. doi: 10.1016/j.jpain.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Ling W, Mooney L, Hillhouse M. Prescription opioid abuse, pain and addiction: Clinical issues and implications. Drug Alcohol Rev. 2011;30:300–5. doi: 10.1111/j.1465-3362.2010.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou YH, Hickey PT, Sundman M, Song AW, Chen NK. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol. 2015;72:432–40. doi: 10.1001/jamaneurol.2014.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knijnik LM, Dussán-Sarria JA, Rozisky JR, Torres IL. Repetitive transcranial magnetic stimulation for fibromyalgia: Systematic review and meta-analysis. Pain Pract. 2016;16:294–304. doi: 10.1111/papr.12276. [DOI] [PubMed] [Google Scholar]
- 8.Gaynes BN, Lloyd SW, Lux L, Gartlehner G, Hansen RA, Brode S, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: A systematic review and meta-analysis. J Clin Psychiatry. 2014;75:477–89. doi: 10.4088/JCP.13r08815. [DOI] [PubMed] [Google Scholar]
- 9.Cole JC, Green Bernacki C, Helmer A, Pinninti N, O’reardon JP. Efficacy of transcranial magnetic stimulation (TMS) in the treatment of schizophrenia: A review of the literature to date. Innov Clin Neurosci. 2015;12:12–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez AV, Guarnizo M, Miranda Y, Pasupuleti V, Deshpande A, Paico S, et al. Association between insulin resistance and breast carcinoma: A systematic review and meta-analysis. PLoS One. 2014;9:e99317. doi: 10.1371/journal.pone.0099317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi S, Rodseth R, McCartney CJ. Effects of dexamethasone as a local anaesthetic adjuvant for brachial plexus block: A systematic review and meta-analysis of randomized trials. Br J Anaesth. 2014;112:427–39. doi: 10.1093/bja/aet417. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J, Deeks J. Selecting studies and collecting data. Cochrane Handbook for Systematic Reviews of Interventions [Internet]. Ver. 510. The Cochrane Collaboration. 2011. [Last cited on 2017 Feb 14]. Available from: http://handbook.cochrane.org/
- 14.O’Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2014;4:CD008208. doi: 10.1002/14651858.CD008208.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Jin Y, Xing G, Li G, Wang A, Feng S, Tang Q, et al. High frequency repetitive transcranial magnetic stimulation therapy for chronic neuropathic pain: A meta-analysis. Pain Physician. 2015;18:E1029–46. [PubMed] [Google Scholar]
- 16.Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol. 2006;17:592–604. doi: 10.1016/j.semcdb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Lee SA, Oh BM, Kim SJ, Paik NJ. The molecular evidence of neural plasticity induced by cerebellar repetitive transcranial magnetic stimulation in the rat brain: A preliminary report. Neurosci Lett. 2014;575:47–52. doi: 10.1016/j.neulet.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Park EJ, Lee SJ, Koh DY, Han YM. Repetitive transcranial magnetic stimulation to treat depression and insomnia with chronic low back pain. Korean J Pain. 2014;27:285–9. doi: 10.3344/kjp.2014.27.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao M, Rejaei D, Liu H. Ketamine use in current clinical practice. Acta Pharmacol Sin. 2016;37:865–72. doi: 10.1038/aps.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdallah CG, Salas R, Jackowski A, Baldwin P, Sato JR, Mathew SJ. Hippocampal volume and the rapid antidepressant effect of ketamine. J Psychopharmacol. 2015;29:591–5. doi: 10.1177/0269881114544776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niciu MJ, Luckenbaugh DA, Ionescu DF, Richards EM, Vande Voort JL, Ballard ED, et al. Ketamine's antidepressant efficacy is extended for at least four weeks in subjects with a family history of an alcohol use disorder. Int J Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu039. pii: Pyu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 23.Goudra BG, Singh PM, Gouda G, Borle A, Gouda D, Dravida A, et al. Safety of non-anesthesia provider-administered propofol (NAAP) sedation in advanced gastrointestinal endoscopic procedures: Comparative meta-analysis of pooled results. Dig Dis Sci. 2015;60:2612–27. doi: 10.1007/s10620-015-3608-x. [DOI] [PubMed] [Google Scholar]
- 24.Singh PM, Borle A, Shah D, Sinha A, Makkar JK, Trikha A, et al. Optimizing prophylactic CPAP in patients without obstructive sleep apnoea for high-risk abdominal surgeries: A meta-regression analysis. Lung. 2016;194:201–17. doi: 10.1007/s00408-016-9855-6. [DOI] [PubMed] [Google Scholar]
- 25.Singh PM, Arora S, Borle A, Varma P, Trikha A, Goudra BG. Evaluation of etomidate for seizure duration in electroconvulsive therapy: A systematic review and meta-analysis. J ECT. 2015;31:213–25. doi: 10.1097/YCT.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 26.Singh PM, Borle A, Gouda D, Makkar JK, Arora MK, Trikha A, et al. Efficacy of palonosetron in postoperative nausea and vomiting (PONV) – A meta-analysis. J Clin Anesth. 2016;34:459–82. doi: 10.1016/j.jclinane.2016.05.018. [DOI] [PubMed] [Google Scholar]