Abstract
Introduction:
Noise exposure may have anatomical, nonauditory, and auditory influences. Considering nonauditory impacts, noise exposure can cause alterations in the automatic nervous system, including increased pulse rates, heightened blood pressure, and abnormal secretion of hormones. The present study aimed at examining the effect of various sound pressure levels (SPLs) on the serum aldosterone level among rats.
Materials and Methods:
A total of 45 adult male rats with an age range of 3 to 4 months and a weight of 200 ± 50 g were randomly divided into 15 groups of three. Three groups were considered as the control groups and the rest (i.e., 12 groups) as the case groups. Rats of the case groups were exposed to SPLs of 85, 95, and 105 dBA. White noise was used as the noise to which the rats were exposed. To measure the level of rats’ serum aldosterone, 3 mL of each rat’s sample blood was directly taken from the heart of anesthetized animals by using syringes. The taken blood samples were put in labeled test tubes that contained anticoagulant Ethylenediaminetetraacetic acid. In the laboratory, the level of aldosterone was assessed through Enzyme-linked immunosorbent assay protocol. The collected data were analyzed by the use of Statistical Package for Social Sciences (SPSS) version 18.
Results:
The results revealed that there was no significant change in the level of rats’ serum aldosterone as a result of exposure to SPLs of 65, 85, and 95 dBA. However, the level of serum aldosterone experienced a remarkable increase after exposure to the SPL of 105 dBA (P < 0.001). Thus, the SPL had a significant impact on the serum aldosterone level (P < 0.001). In contrast, the exposure time and the level of potassium in the used water did not have any measurable influence on the level of serum aldosterone (P = 0.25 and 0.39).
Conclusion:
The findings of this study demonstrated that serum aldosterone can be used as a biomarker in the face of sound exposure.
Keywords: Aldosterone, hormone, noise, rat, sound pressure level
Introduction
Exposure to too much noise is a profession-related health problem in the world which may have a wide range of social and physiological consequences, for example, anatomical, nonauditory and auditory impacts.[1] Contact with excessive noise can cause noise-induced hearing loss (NIHL), an auditory effect that results in hearing threshold shifts and speech perception deterioration.[2,3] Noise exposure can also affect workers’ health in nonauditory ways. These nonauditory damages hurt the autonomic nervous system, causing increased skin temperature and pulse rate, higher blood pressure, constriction of blood vessels, abnormal secretion of hormones, and tense muscles.[2,4] The inner ear is the body part that is mainly influenced by noise exposure, causing mechanical, and metabolic changes that gradually lead to NIHL. These anatomical and physiological impacts, which stem from the overstimulation of the inner ear through high energy transfer, lead to mechanical damages to the sensitive parts of the OHCs in the cochlea.[5,6] Aldosterone is a mineralocorticoid (MC) released from the zone glomerulus of the adrenal cortex.[7] The MC receptor of the inner ear is in charge of controlling the homeostasis of the endolymph, a process that is exercised through ion channels and transporters which exist in the cochlear duct cells.[8,9] Aldosterone is responsible for increasing sodium reabsorption mainly through regulating the enzyme Na, K-ATPase. This enzyme has been identified in the inner ear epithelium. This regulatory process is indicative of possible hormonal modulation of endolymph secretion and fluid homeostasis, despite the fact that the action on spiral ganglion cells is unknown. Lack of circulating adrenal hormones does not result in any electrophysiological changes in inner ear fluids; in contrast, it decreases endolymph volume.[10,11,12] Aldosterone has a crucial function in maintaining the homeostasis of the inner ear.[13,14] According to the results of some studies, the decline of serum aldosterone level may lead to hearing loss.[14,15] Although no justification has been provided, the decreased serum aldosterone concentration, which influences the expression of Na, K-adenosine triphosphatase (ATPase), and Na–K–Cl contransporter on the cell membranes of the inner ear cells, has to do with disrupted homeostasis of endolymph because of abnormal K+ regeneration in the cochlea.[16] In pathological conditions, changes in the level of serum potassium can lead to more damages to the outer hair cells rather than inner hair cells and auditory nerve fibers.[2,17] Aldosterone is the cause of improvement in the cochlear ion cycle, especially in vascular strip.[18] In mice with autoimmune hearing loss, aldosterone treatment’s effect on improving hearing thresholds and reversal of the pathology in the stria vascularis has been similar to that of prednisolone.[19] Tadros et al. have demonstrated that older people with lower levels of aldosterone (but still in the clinically normal range) have worse hearing thresholds compared to individuals whose aldosterone levels are in the upper middle of the normal range. Therefore, aldosterone may have a protective function in the cochlea.[16]
Several research projects have investigated the relationship between age-related hearing loss (presbycusis) and aldosterone levels.[20] Nevertheless, only a handful of studies has concentrated on the possible impacts of noise exposure on the secretion of aldosterone levels.
It is concluded that the sound pressure level (SPL) affects aldosterone level, aldosterone regulates the levels of serum sodium and serum potassium, and the balance of potassium ion is crucial for the safety of outer hair cells. The present study was, therefore, designed to:
-
(1)
Assess and compare the serum aldosterone level of rats in the control group which was exposed to an SPL of 65 dBA and that of the rats in the case group (which was exposed to SPLs of 85, 95, and 105 dB) during various exposure times.
-
(2)
Assess the effect of various potassium levels of the used water (0.1 and 8 mg/L) on the level of rats’ serum aldosterone.
-
(3)
Present a model for determining the level of serum aldosterone in rats based on the combined effects of SPL, exposure time, and the potassium level of the used water.
-
(4)
Investigate the possibility of using serum aldosterone as a feasible biomarker of noise-induced stress in rat.
Materials and methods
Experimental animals
For the purpose of this study, 45 adult male Sprague-Dawley rats were purchased from Pasteur Research Institute. Their ages ranged from 3 to 4 months, and they weighed 200 ± 50 g. They were kept in the animal unit of the School of Health, Tehran University of Medical Sciences, until the study began. The rats were exposed to a photoperiodic cycle of 12 h of light phase and 12 h of dark phase, with the temperature being around 23±2°C. They had free access to water and food for animals. All the principles of the Declaration of Helsinki about conducting experiments on laboratory animals were observed.
Before the study, the health of rats’ auditory system was investigated through two procedures:
-
(1)
Rats’ external auditory canal and eardrum was examined by the use of an otoscope (model 97150-BI, Welch Allyn CO., New York, USA). Rats were excluded from the study if some defects (e.g., excessive secretion of earwax/cerumen) were observed in their ears.
-
(2)
A specific sound was created around the rats’ ear. If the rats had responses as a result of being exposed to the sound, it indicated that their ears were healthy. Conversely, if no response was observed in rats, it was a sign for some defect in their auditory system. Rats with problematic auditory systems were excluded from the study through this screening process.
Instruments for noise exposure
Experiments on animals were conducted in a four-cell chamber with high efficiency. It was an echo chamber with the dimensions of 40 × 50 × 60 cm. In this echo chamber, sound energy was equally distributed in all directions, meaning that animals would receive an equal amount of sound no matter where they were in this chamber. During the exposure time, the room air must be replaced at a rate of 12 times/h (based on recommended conditions for taking care of animals).[21] Therefore, a ventilation with a flow of 24 L/min was installed in the chamber. This ventilation consisted of an environmental pump and a flow meter used to control the flow rate. During the experiment, the chamber’s temperature was 25 ± 2°C, and the moisture content was 50%. It should be noted that in each cell, there were three rats that were equally exposed to noise.
The software and source of noise generation
First of all, Signal was used to produce white noise. Cool Edit Pro (version 1-2, manufactured by Syntrillium Software Corporation in the United States in 1999–2000) was utilized to play the noise files. Two speakers (model PROBIT, BIT PARDAZ CO., Tehran, Iran) were used to generate the noise. The speakers had an input–output resistance of impedance: 4 (ohms) power: 5 (W) that was directly amplified through an amplifier (model ES-2000s, ES-Pro Audio CO., Taipei, Taiwan) made in Taiwan. The speakers were positioned in the chamber ceiling in a way that they were symmetrical compared to the center of the ceiling.
Sample size calculation
Based on previous studies,[14] the following formula was used for calculating the sample size:
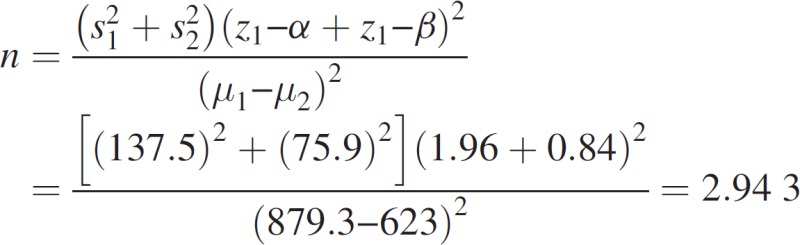
As a result, based on the aims of the study, 15 groups were considered. Three rats were placed in each group, hence having a general sample of 45 rats.
Preparing the water consumed by rats with various levels of potassium
In this study, the same water (consisting of bicarbonate, calcium, sulfate, magnesium, sodium, fluoride, nitrate, chloride, phosphate, and ammonium) was consumed in all groups. The only ingredient of the water that was manipulated based on the aim of the study was the level of potassium. According to the national standard of Iran (Standard 1053, the features of drinking water) and the standards proposed by the World Health Organization, the maximum allowable level of potassium in drinking water is 10 mg/L.[22,23] In the current study, the potassium level of the water was between the minimum (0.1 mg/L) and maximum (8 mg/L) allowable concentrations. To make sure that the rats would drink the water, it was put in rat’s access for 12 h before the beginning of the experiment. The effect of potassium on aldosterone level would be measured through a more reliable procedure.
Organization of experimental groups
In this study, 45 rats were randomly assigned to 15 groups, each one containing three rats. Aldosterone level in each group was measured after the conditions explicated below. The status of each group (control and case) has been explained in the following sections.
Control group
The control groups (which consisted of three groups of rats) were exposed to a background noise of 65 dBA. Also the potassium concentration of the water they used was 0.1 mg/mL. At the beginning of the experiment, the serum aldosterone concentration of rats in control groups was measured. The same measurement was conducted after 3 and 8 h of starting the experiment. It should be noted that the SPL (the background noise of 65 dBA) was created by the ventilation system which had been installed in the place where rats were kept.
Case group
The rats in the case group were exposed to SPLs of 85, 95, and 105 dBA. The sound to which they were exposed was white noise. Also the drinking water had two potassium levels: 0.1 and 8 mg/L. The features of the 12 subgroups of the case group are illustrated in Table 1.
Table 1.
Different subgroups of rats in the case group
| Sound pressure level (dBA) | Potassium level (mg/mL) | Exposure time (h) |
|---|---|---|
| 85 | 0.1 | 3 |
| 8 | ||
| 8 | 3 | |
| 8 | ||
| 95 | 0.1 | 3 |
| 8 | ||
| 8 | 3 | |
| 8 | ||
| 105 | 0.1 | 3 |
| 8 | ||
| 8 | 3 | |
| 8 |
Measurement
Noise
The SPL in the four-cell chamber was measured by the use of a sound level meter (model CEL-440, CASELLA CO., Western New York, USA). This machine is equipped with octave parser and is, thus, able to show the SPL in octave band centers. Before using the machine, we calibrated it by the CEL-282 calibrator (model CEL-440, CASELLA CO., Western New York, USA). In each cell of the chamber, SPL was randomly measured in different spots.
Rats’ serum aldosterone
To measure rats’ serum aldosterone, they must first be anesthetized. To do so, two drugs, namely, ketamine and xylazine, were used. The two drugs were mixed with a ratio of 60 to 40% and were injected into the rats. Blood samples of 3 mL were directly collected from the animal’s heart using the syringes. The collected samples were put in labeled test tubes that contained anticoagulant (EDTA). They were immediately transferred to one of the accredited medical diagnostic laboratories under controlled conditions (ice box). In the laboratory, the level of aldosterone was assessed through the ELISA protocol, which is a reliable, sensitive, replicable, speedy, and specialized procedure.[24]
Statistical analysis
The collected data were analyzed by the use of Statistical Package for Social Sciences (SPSS) version 18 (SPSS, Inc., Chicago, Illinois, USA). Descriptive data analysis procedures, such as mean, standard deviation, and frequency, were used to summarize the data. Furthermore, Shapiro–Wilk test was used to check the normality of distribution. As the data were collected in several occasions over the course of time, repeated measurements analysis of variance (ANOVA) was applied to test the research hypotheses. It should be noted that before using this analytical procedure, we made sure that all its preassumptions were met. To see if there was any significant difference among various groups, both within and between groups one-way ANOVA were run. Subsequently, Tukey, which is a post hoc test, was used to see which mean differences among various groups were statistically significant. The P value was considered to be less than 0.05.
Ethical considerations
The ethical considerations of this research were approved by the Ethics Committee of Tehran University of Medical Sciences) ID: 1394.5). Moreover, all the principles of the Declaration of Helsinki about conducting experiments on laboratory animals were observed.
Results
Results of measuring serum aldosterone level in rats
The average concentration of serum aldestrone among rats in the control group at the beginning of the experiment was 613.33 ± 15.27 pg/mL. The average concentrations of serum aldestrone among these rats were 616.67 ± 10.40 and 603.33 ± 26.18 pg/mL, respectively, after 3 and 8 h of starting the experiment (exposure).
Figure 1 shows changes in the mean level of rats’ serum aldosterone in the case groups as a result of being exposed to white noises of 85, 95, and 105 dBA and consumed water with a 0.1 mL/L level of potassium. The changes were recorded after 3 and 8 h of exposure to noise.
Figure 1.
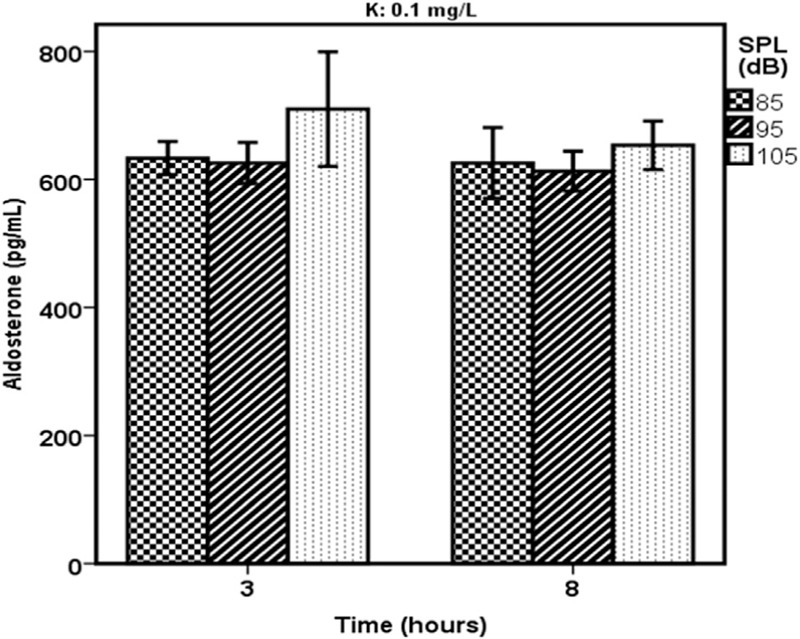
A comparison of different levels of rats’ serum aldosterone in the various sound pressure levels and the water contained 0.1 mg/L of potassium (K)
Figure 2 indicates the variation in the mean level of rats’ serum aldosterone in the case groups as a result of being exposed to white noises of 85, 95, and 105 dBA and consumed water with an 8-mg/L level of potassium. The variation was registered after 3 and 8 h of exposure to noise.
Figure 2.
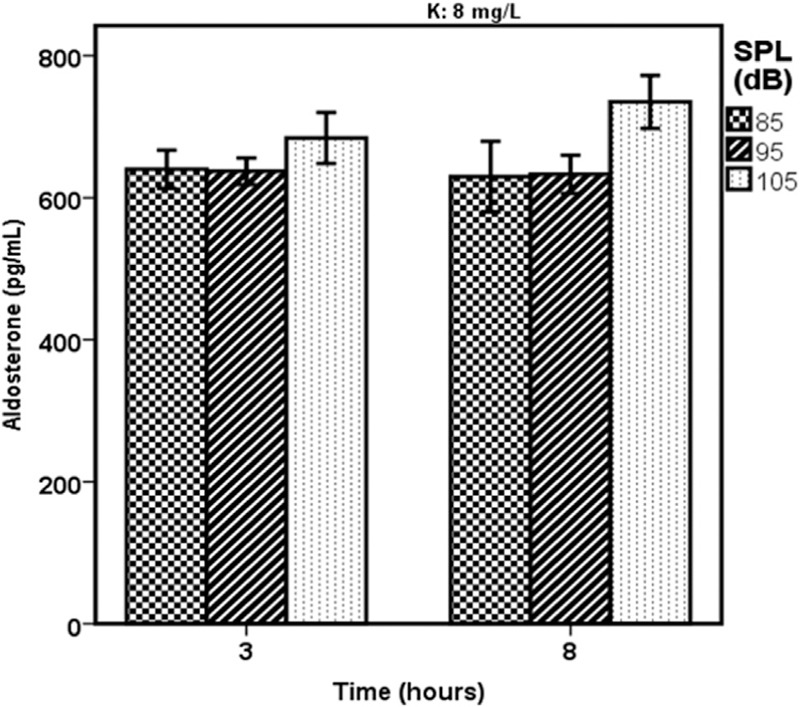
A comparison of different levels of rats’ serum aldosterone in the various sound pressure levels and the water that contained 8 mg/L of potassium (K)
Comparing the relationship between sound pressure level and aldosterone level in various groups
One-way ANOVA was used to investigate the relationship between potassium level and exposure time in various groups based on different SPLs. Table 2 contains the results.
Table 2.
Investigating the relationship between potassium level and exposure time based on various sound pressure levels
| Potassium level (mg/L) | Exposure time (h) | Sum of squares | Degree of freedom | Mean square | F | P value | |
|---|---|---|---|---|---|---|---|
| 0.1 | 3 | Between groups | 13,048.66 | 2 | 6524.33 | 12.43 | 0.007 |
| Within groups | 3149.33 | 6 | 524.88 | ||||
| 8 | Between groups | 2552.66 | 2 | 1276.33 | 4.31 | 0.06 | |
| Within groups | 1773.33 | 6 | 295.55 | ||||
| 8 | 3 | Between groups | 4120.88 | 2 | 2060.44 | 16.21 | 0.004 |
| Within groups | 762.66 | 6 | 127.11 | ||||
| 8 | Between groups | 21,510.22 | 2 | 10,755.11 | 43.40 | <0.001 | |
| Within groups | 1486.66 | 6 | 247.77 | ||||
The results of the Tukey test indicated that there was no statistically significant difference between the three control groups’ mean level of serum aldosterone.
With regard to the case groups, which consumed water with 0.1 mg/L of potassium and were exposed to SPLs of 85, 95 and 105 dBA, Tukey test yielded the following results:
-
(1)
After 3 h of exposure, there was a significant difference in the serum aldosterone mean level of the group exposed to the SPL of 105 dBA and those of the groups exposed to the SPL of 85 and 95 dBA (P = 0.01). However, no significant difference was observed in the mean level of serum aldosterone between 85 and 95 dBA (P = 0.091).
-
(2)
After 8 h of exposure, no significant difference was observed in the mean level of serum aldosterone of the groups exposed to SPLs of 85, 95, and 105 dBA.
In the case groups, which were exposed to SPLs of 85, 95, and 105 dBA and the water with 8 mg/L of potassium, the following results were obtained:
-
(1)
After 3 h of exposure, a significant difference was observed in the mean level of serum aldosterone of the group exposed to the SPL of 105A dB and those of the rats exposed to SPLs of 85 and 95 dBA. However, no significant difference was observed in the mean level of serum aldosterone of the groups exposed to SPLs of 85 and 95 dBA (P = 0.095).
-
(2)
After 8 h of exposure, a significant difference was observed in the mean level of serum aldosterone of the group exposed to the SPL of 105 dBA and those of the groups exposed to SPLs of 85 and 95 dBA. However, no significant difference was observed in the mean level of serum aldosterone of the rats exposed to SPLs of 85 and 95 dBA (P = 0.096).
The results obtained from ANOVA test showed that there was a significant difference between various SPLs in terms of rats’ serum aldosterone level (P < 0.001).
The results of the statistical model of serum aldosterone level in rats
The statistical model of rats’ serum aldosterone when exposed to the water with 0.1 mg/L of potassium as well as 3 and 8 h of noise
Aldosterone (pg/mL) = 382/87 + 2/60 SPL + 25/66 Time 3
For 8 h of exposure, Time 3 = 0, whereas for 3 h of exposure, Time 3 = 1.
Time = exposure time (h)
SPL = sound pressure level (dB)
The statistical model of rats’ serum aldosterone when exposed to the water with 8 mg/L of potassium as well as 3 and 8 h of noise
Aldosterone level (pg/mL) = 311/22 + 3/73 SPL-12/11 Time 3
For 8 h of exposure, Time 3 = 0, whereas for 3 h of exposure, Time 3 = 1.
Time = exposure time (h)
SPL = sound pressure level (dB)
The finalized model of serum aldosterone level based on the combined effect of SPL, exposure time, and potassium level of the used water
Aldosterone level (pg/mL) = 489/26 + 12/14 K + 1/8 SPL−1/99 Time
K = potassium level (mg/L)
SPL = sound pressure level (dB)
Time = exposure time (h)
In this model, the combined effect of these three factors (potassium, SPL, and exposure time) on the level of serum aldosterone is 44.9%.
Discussion
In this study, 45 rats were randomly assigned to 15 groups of four (three control groups and 12 case groups). Aldosterone level was measured in the three control groups which were exposed to the SPL of 65 dBA) and 12 case groups (which were exposed to the SPLs of 85, 95, and 105 dBA). The increase in the potassium level in the extracellular fluid leads to a significant growth of aldosterone secretion.[25] Therefore, rats in the case group were exposed to two types of water with different potassium levels (0.1 and 8 mg/L). We were interested in investigating the effect of potassium level in water at the level of rats’ serum aldosterone.
One-way ANOVA test was used to study the relationship between various SPLs and aldosterone levels in different groups in both between and within groups. The same data analysis procedure was also used to find the relationship between exposure time and aldosterone level in various groups.
The results of the data analysis revealed that various SPLs were significantly different in terms of the level of aldosterone (P < 0.001). Furthermore, the results of the post hoc test (Tukey) showed that there was no significant difference in the serum aldosterone level of SPLs of 65, 85, and 95 dBA. However, a significant discrepancy was detected between the SPL of 105 dBA, on one hand, and the SPLs of 65, 85, and 95 dBA, on the other hand (P < 0.001).
In addition, the results indicated that SPL had a significant effect on aldosterone level when rats drank the water with the potassium levels of 0.1 and 8 mg/L. The exposure time, in contrast, had no measurable influence on aldosterone level. This lack of significant impact of exposure time may be partially explained in the light of rats’ sound conditioning. On the other hand, in the final presented model of the level of rats’ serum aldosterone, which is a combination of potassium level, SPL, and exposure time, it was found that these three factors (potassium, SPL, and exposure time) in total had an effect of 44.9% on the level of serum aldosterone. Moreover, SPL had a significant influence on serum aldosterone level in the finalized model (P < 0.001). On the contrary, potassium and exposure time did not have any considerable impact in the finalized model (P = 0.25, 0.39).
The findings of the present study are in line with those of Ising et al. [26] who found that noise results in aldosterone secretion because it is a source of stress.
The examination of the obtained models in this study showed that the results of the predicted models for measuring rats’ aldosterone level are very much close to the real values; that is, the values obtained from these models are very similar to the ones obtained in the laboratory.
Song et al. [14] studied the relationship between serum aldosterone level and exposure to the noise of 120 dBA among rats. In their study, they divided the rats into three groups (one control and two case groups), with each group consisting of three rats. The control group was not exposed to noise, whereas the case groups were exposed to the noise of 120 dBA. The rats in the case groups were exposed to noise for 3 h each day. The level of aldosterone in the three groups (one control and two case groups) was measured at the beginning of the experiment, 3 h later, and 3 days later. The results displayed that there was a significant difference in the aldosterone level before and after exposure to noise.[14] The results of the current study revealed that there is no change in the level of serum aldosterone after exposure to SPLs of 65, 85, and 95 dBA. However, this level significantly goes up as a result of exposure to the noise of 105 dBA. In addition, exposure time has no significant influence on aldosterone level. As the level of serum aldosterone is significantly influenced by SPL, serum aldosterone can be regarded as a biomarker against exposure to noise. The current study has three innovations:
-
(1)
Investigating the effect of various SPLs (65, 85, 95, and 105 dBA) on the level of serum aldosterone.
-
(2)
Investigating the effect of the level of potassium in the used water (0.1 and 8 mg/L) on the level of rats’ serum aldosterone.
-
(3)
Presenting a statistical model for measuring the level of serum aldosterone based on the combined effect of SPL, exposure time, and the potassium level of the used water.
Financial support and sponsorship
This paper was extracted from the results of a registered research project (registration no. 24455) supported by Tehran University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors wish to thank Tehran University of Medical Sciences and Gol Gohar Mining and Industrial Company for their kind assistance.
REFERENCES
- 1.Nelson DI, Nelson RY, Concha-Barrientos M, Fingerhut M. The global burden of occupational noise-induced hearing loss. Am J Ind Med. 2005;48:446–58. doi: 10.1002/ajim.20223. [DOI] [PubMed] [Google Scholar]
- 2.Zare S, Nassiri P, Monazzam MR, Pourbakht A, Azam K, Golmohammadi T. Evaluation of distortion product otoacoustic emissions (DPOAEs) among workers at an industrial company exposed to different industrial noise levels in2014. Electron Physician. 2015;7:1126. doi: 10.14661/2015.1126-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nassiri P, Zare S, Monazzam MR, Pourbakht A, Azam K, Golmohammadi T. Modeling signal-to-noise ratio of otoacoustic emissions in workers exposed to different industrial noise levels. Noise Health. 2016;18:391. doi: 10.4103/1463-1741.195808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nassiri P, Zare S, Monazzam MR, Pourbakht A. A model to determine the level of serum aldosterone in the workers attributed to the combined effects of sound pressure level, exposure time and serum potassium level: a field-based study. Jundishapur J Health Sci. 2017;9:1–7. [Google Scholar]
- 5.Bonfils P, Avan P, Faulcon P. Distorted odorant perception analysis of a series of 56 patients with Parosmia. Arch Otolaryngol Head Neck Surg. 2005;131:107–12. doi: 10.1001/archotol.131.2.107. [DOI] [PubMed] [Google Scholar]
- 6.Balatsouras DG, Tsimpiris N, Korres S, Karapantzos I, Papadimitriou N, Danielidis V. The effect of impulse noise on distortion product otoacoustic emissions. Int J Audiol. 2005;44:540–9. doi: 10.1080/14992020500190201. [DOI] [PubMed] [Google Scholar]
- 7.Rarey KE, Luttge WG. Presence of type I and type II/IB receptors for adrenocorticosteroid hormones in the inner ear. Hear Res. 1989;41:217–21. doi: 10.1016/0378-5955(89)90013-0. [DOI] [PubMed] [Google Scholar]
- 8.Wangemann P. K+ cycling and the endocochlear potential. Hear Res. 2002;165:1–9. doi: 10.1016/s0378-5955(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 9.Weber PC, Cunningham CD, 3rd, Schulte BA. Potassium recycling pathways in the human cochlea. Laryngoscope. 2001;111:1156–65. doi: 10.1097/00005537-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Zare S, Nassiri P, Monazzam MR, Pourbakht A, Azam K, Golmohammadi T. Evaluation of the effects of occupational noise exposure on serum aldosterone and potassium among industrial workers. Noise Health. 2016;18:1–6. doi: 10.4103/1463-1741.174358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrary E, Sterkers O. Mechanisms of endolymph secretion. Kidney Int Suppl. 1998;65:S98–103. [PubMed] [Google Scholar]
- 12.Lohuis PJ, Borjesson PK, Klis SF, Smoorenburg GF. The rat cochlea in the absence of circulating adrenal hormones: An electrophysiological and morphological study. Hear Res. 2000;143:189–96. doi: 10.1016/s0378-5955(00)00043-5. [DOI] [PubMed] [Google Scholar]
- 13.Shin JE, Kang HH, Chung JW. The effect of pretreatment of CoCl2 on the prevention of noise-induced hearing loss. Korean J Otorhinolaryngol Head Neck Surg. 2007;50:743–9. [Google Scholar]
- 14.Song HM, Lim GC, Lim HW, Kim MJ, Choi SH, Chung JW. Changes of serum aldosterone concentration after noise exposure in mice. Korean J Audiol. 2011;15:137–40. [Google Scholar]
- 15.Wada J, Kambayashi J, Marcus D, Thalmann R. Vascular perfusion of the cochlea: Effect of potassium-free and rubidium-substituted media. Acta Otolaryngol. 1979;225:79–81. doi: 10.1007/BF00455206. [DOI] [PubMed] [Google Scholar]
- 16.Tadros SF, Frisina ST, Mapes F, Frisina DR, Frisina RD. Higher serum aldosterone correlates with lower hearing thresholds: A possible protective hormone against presbycusis. Hear Res. 2005;209:10–8. doi: 10.1016/j.heares.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Marcon S, Patuzzi R. Changes in cochlear responses in guinea pig with changes in perilymphatic K+ Part I: Summating potentials, compound action potentials and DPOAEs. Hearing Res. 2008;237:76–89. doi: 10.1016/j.heares.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Caminos E, Vale C, Lujan R, Martinez-Galan JR, Juiz JM. Developmental regulation and adult maintenance of potassium channel proteins (Kv1. 1 and Kv1. 2) in the cochlear nucleus of the rat. Brain Res. 2005;1056:118–31. doi: 10.1016/j.brainres.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 19.Trune DR, Kempton JB, Kessi M. Aldosterone (mineralocorticoid) equivalent to prednisolone (glucocorticoid) in reversing hearing loss in MRL/MPJ-Fas1pr autoimmune mice. Laryngoscope. 2000;110:1902–6. doi: 10.1097/00005537-200011000-00025. [DOI] [PubMed] [Google Scholar]
- 20.Salehi N, Akbari M, Kashani M, Haghani H. Protective effect of N-acetylcysteine on the hearing of rabbits exposed to noise and carbon monoxide. Audiology. 2011;20:36–46. [Google Scholar]
- 21.Huang Q, Tang J. Age-related hearing loss or presbycusis. Eur Arch Otorhinolaryngol. 2010;267:1179–91. doi: 10.1007/s00405-010-1270-7. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Standards and Industrial Research of Iran (ISIRI) Drinking water: Physical and chemical specifications. 5th. 2009. Standard No. 1053. [Google Scholar]
- 23.Health Canada. Guidance for Potassium from Water Softeners. Ottawa, Ontario: Health Canada, Healthy Environments and Consumer Safety Branch, Water, Air and Climate Change Bureau; 2008. [Google Scholar]
- 24.Ahmadi R, Seifi Nahavandi B, Ghasemi A, Seif A. The effects of Iranian Shisheh (containing methamphetamine) on serum levels of ACTH, cortisol and aldosterone in male rats. JBUMS. 2014;16:49–55. [Google Scholar]
- 25.Connell JM, Davies E. The new biology of aldosterone. J Endocrinol. 2005;186:1–20. doi: 10.1677/joe.1.06017. [DOI] [PubMed] [Google Scholar]
- 26.Ising H, Babisch W, Kruppa B. Noise-induced endocrine effects and cardiovascular risk. Noise Health. 1999;1:37–48. [PubMed] [Google Scholar]


