Summary
Background
Konzo is an irreversible upper-motor neuron disorder affecting children dependent on bitter cassava for food. The neurocognitive ability of children with konzo over time has yet to be fully documented.
Methods
We did a longitudinal study in a konzo outbreak zone continuously affected by konzo since 1990, in the district of Kahemba, southern Bandundu Province, Congo. We enrolled children with a record of neurological diagnosis of konzo in Kahemba town. For all study children with konzo enrolled in the final sample for the baseline assessment, a neurological exam was done by neurologists to confirm konzo diagnosis using the 1996 WHO criteria at 2 years and 4 years. In the initial baseline sample for each child with konzo, we attempted to get consent from a comparison child without konzo (1996 WHO criteria) within 2 years of age, from a neighbouring household who met inclusion criteria. The neuropsychological assessments were the Kaufman Assessment Battery for Children, second edition (KABC-II), and the Bruininks-Oseretsky Test of Motor Proficiency, second edition (BOT-2).
Findings
Data collection occurred between Oct 12, 2011, and Aug 14, 2015, in the town of Kahemba. 123 children from the Congo with konzo and 87 presumably healthy children without konzo from neighbouring households were enrolled. The planned assessments were completed by 76 children with konzo and 82 children without konzo at 2-year follow-up, and by 55 children with konzo and 33 children without konzo at 4-year follow-up. Boys with konzo did worse than those without konzo on the KABC-II Learning (p=0·0424) and on the Mental Processing Index (MPI; p=0·0111) assessments at 2-year follow-up, but girls did not. These differences observed in boys might have been caused by stunting. At 4-year follow-up, the difference in KABC-II MPI score between boys or girls with or without konzo was not significant. Both boys and girls with konzo had lower scores on BOT-2 than children without konzo at both follow-up times (p<0·0001). These differences were not attenuated when controlling for physical growth. Boys with and without konzo declined on BOT-2 fine motor proficiency at 2-year follow-up (boys with konzo p=0·0076; boys without konzo p=0·0224) and KABC-II MPI performance at 2-year follow-up and 4-year follow-up (2 years: boys with konzo p<0·0001, boys without konzo p=0·0213; 4 years: boys with konzo p=0·0256, boys without konzo p=0·10), but that was not the case for the girls with scores remaining stable regardless of konzo status. For boys, increases in urinary thiocyanate concentration was significantly associated with reductions in BOT-2 motor proficiency (p=0·0321), but was not significantly associated in girls and urinary thiocyanate concentration was not associated with KABC-II MPI score for either boys or girls.
Interpretation
Motor and cognitive performance continues to be significantly impaired in boys with konzo at 2-year follow-up compared with boys without konzo. Because these impairments are associated in part with exposure to poorly processed cassava as measured by urinary thiocyanate, interventions are urgently needed to ensure improved processing of cassava to detoxify this food source.
Funding
US National Institutes of Health.
Introduction
Konzo is defined by WHO as a visible symmetric spastic abnormality of gait while walking or running in a formerly healthy person with a history of onset of less than 1 week. After onset, a non-progressive course follows and bilaterally exaggerated knee or ankle jerks without signs of disease of the spine.1 The disease results from subsisting on poorly processed cassava, which has a high toxicity from its cyanogenic glycosidic content.2 Because konzo appears to be a pure upper-motor neuron disorder, cognitive effects were originally deemed absent or minimal.1,3
We contradicted the assumption that cognitive effects were absent or minimal in our 2013 study4 when we documented children with konzo, from a konzo outbreak zone in the Congo, did more poorly than children without konzo on cognitive tests of visual–spatial analysis and problem solving.4 This observation was especially true for the boys in the sample. However, boys with konzo were also less likely to be enrolled in school, perhaps because of social stigma associated with the disorder, and this factor could also contribute to their worse cognitive performance. Both boys and girls with and without konzo from households with konzo did worse on memory tests than children without konzo from an area in that region without a konzo outbreak.
Konzo occurs primarily in extremely impoverished communities who subsist on high-yield varieties of poorly processed bitter cassava with little protein in their diet in times of food insecurity and near-famine conditions.2 Therefore, in comparing both children with and without konzo overall from outbreak and non-outbreak zones dependent on cassava, neurocognitive differences due to extreme poverty might overshadow the effects of cyanogenic toxic exposure itself in poorly processed cassava. In their review, Tylleskar and Tshala-Katumbay2 provide a thorough list of studies of the epidemiology, causes, progression, and prognosis of konzo disease. One study5 did a follow-up evaluation with patients with konso in Mozambique, and concluded that the paralysis was not worsening and that there were some improvements in mobility during the 14 years of follow-up after konzo onset. However, the improved mobility did not seem to be the result of an actual improvement in the neurological condition of the patients. Rather, improvements in mobility were probably the result of patients adapting to their paresis. A follow-up study on the mobility of patients with konzo is not the same as a follow-up on disease progression, and studies have typically not been able to meaningfully evaluate the neuropathophysiological progression of konzo disease with human beings in the field. Therefore, changes in the neurocognitive and neuromotor proficiency of children with konzo are unknown, and the pathophysiological and neurological progression of the disease itself in such children over time is completely unknown.
This study is a follow-up evaluation of cognitive and motor proficiency in the same cohorts of children with and without konzo observed in our 2013 study,4 2 years and 4 years after the baseline evaluations. In these follow-up assessments, we continued to monitor the cognitive and motor proficiency differences between the cohorts with and without konzo, and to see whether decline in these proficiencies is observable over time for either or both groups. Furthermore, we have stratified the cohorts by gender to verify whether boys with konzo are still at greater risk than their counterparts without konzo, as compared with the girls. Finally, we further assessed the overall effect of their socioeconomic conditions and their quality of home environment on the cognitive and motor proficiency outcomes.
Methods
Study design and participants
The study site was a konzo outbreak zone continuously affected by konzo since 1990,6 in the district of Kahemba, southern Bandundu Province, Congo. The data collection occurred between Oct 12, 2011, and Aug 14, 2015, in the town of Kahemba.
From earlier outbreaks in Kahemba, 5000 participants in Kahemba town had been included on a WHO surveillance list, of which about half were children (younger than 18 years of age). From this list, we were able to locate 308 children with a record of neurological diagnosis of konzo in Kahemba town, of which 123 children with konzo were randomly selected for study participation (65 boys, 58 girls; mean age 8·7 years, range 4–17).4 For all study children with konzo enrolled in the final sample for the baseline assessment, a neurological exam was again performed by neurologists on our assessment team (DO, BM-M, and M-TS) to confirm konzo diagnosis using the 1996 WHO criteria at 2 years and 4 years.1 In the initial baseline sample for each child with konzo, we attempted to get consent from a comparison child without konzo (1996 WHO criteria) within 2 years of age from a neighbouring household who met inclusion criteria.4
The Ministry of Health for the Congo and the institutional review board (IRB) for the ethical conduct of human participant research for Oregon Health and Sciences University provided study approval. Caregivers of study children gave oral consent in the local language and with signature or thumbprint for those who were illiterate.
Procedures
All the following measures in the present follow-up assessments were used in the original testing of the study children at baseline.4 All cognitive, motor proficiency, and neurological assessments at all three timepoints (baseline, 2 years, 4 years) were done by the same three co-authors who are neurologists (DO, BM-M, M-TS) trained in the Kaufman Assessment Battery for Children, second edition (KABC-II) and Bruininks/Oseretsky Test of Motor Proficiency (second edition; BOT-2), reducing the interrater variability. Community health workers (nurses) from the Ministry of Health were trained to complete the Caldwell Home Observation for the Measurement of the Environment (HOME)7 and socioeconomic status measures in the home after the consent process,8–10 and these were completed before the other cognitive and motor assessments. Details of the HOME, KABC-II, and BOT-2 and how they were applied are in the appendix. Both the KABC-II and BOT-2 were used in this follow-up assessment because they also proved sensitive to metabolic biomarkers of cyanide exposure from poorly processed cassava in the baseline assessment.11–13
Urinary thiocyanate (Bradbury kit, Australian Centre for International Agricultural Research, Australian National University, Acton, ACT, Australia) was also measured in all study children at baseline and again at 4-year follow-up as a toxin exposure biomarker for recent exposure (in the past week or so) to poorly processed cassava. As such, this measure is only a general indication of cassava processing practices in the household at that time, and cannot be considered a sensitive biomarker to past exposure or cumulative exposure to poorly processed cassava.13,14 Baseline urinary thiocyanate was assessed for the groups with konzo (mean 519 µmol/L, SD 354) and without konzo (mean 389 µmol/L, SD 224). These concentrations represent high cyanogenic exposure in both groups for this time of year, based on earlier studies by Banea and colleagues15,16 of thiocyanate levels among konzo-affected populations in this region. This indicates that exposure to poorly processed cassava in general for cohort households with or without konzo was high, suggesting that all the children in this community could be at risk for konzo.
Outcomes
The main outcomes of the study were motor proficiency as measured by the BOT-2 Total Motor performance score and scales (fine manual, manual coordination, body coordination, strength, and agility), and cognitive ability as measured by the KABC-II mental processing index (MPI) score and scales (sequential processing, simultaneous processing, learning, planning, and delayed recall).
Statistical analysis
We used the Kruskal–Wallis one-way ANOVA, χ2, or Fisher’s exact tests for cross-sectional comparisons of participants with konzo versus without konzo. Three repeated measures of BOT-2 and KABC-II scores were analysed using generalised linear mixed effects models. Generalised linear mixed effects modelling generalises classical analysis of repeated measures and allows for data missing at random, structured covariance matrix, and non-Gaussian error distribution. Non-independence of observations due to repeated measures is accounted for by including the corresponding random effect in the model. Because the distributions of outcomes were highly skewed to the right, we specified log link function and gamma distribution in generalised linear mixed effects models. Based on previously published results,4 we stratified the analyses by a child’s sex. Adjusted means of the logarithms of outcome variables and 95% CIs for them were output from generalised linear mixed effects models. The means were back transformed to the original scale by exponentiation. To gauge clinical significance of findings, we computed standardised mean differences (SMDs) as differences between adjusted means of the outcomes for children with konzo versus without konzo divided by the standard deviation of the children without konzo at baseline, separately for each sex. We used SAS version 9.4 for statistical analyses.
Because konzo is a disease that arises out of extreme poverty, we repeated the analyses comparing children with konzo to those without konzo, controlling for physical growth that could be affected by malnutrition, so that the effect of konzo disease itself could be better evaluated. We added standardised height-for-age Z scores and weight-for age Z scores at baseline to the generalised linear mixed effects models, and evaluated the effects of their addition on konzo versus non-konzo group differences. The significance of the association between these physical growth measures with motor and cognitive outcomes, and the attenuation of konzo versus nonkonzo group differences when controlling for height-for-age z-scores and weight-for age z-scores were used to evaluate the mediational role of stunting and wasting on the BOT-2 motor proficiency and KABC-II cognitive performance principal outcomes.
Because the Caldwell HOME scale and socioeconomic status are strongly correlated, inclusion of only one of them avoids the problem of collinearity. Maternal literacy is included in the socioeconomic status measure, which is strongly associated with the Caldwell HOME score. In the present analyses, we adjusted for Caldwell HOME score because it differed at baseline between the children with and without konzo, whereas socioeconomic status score did not (table 1). Likewise, the number of children with konzo lost to follow-up differed significantly from those we were able to assess in terms of HOME score, so this was another reason it was important to adjust for it, rather than adjust for socioeconomic score.
Table 1.
Patients characteristic
| With konzo | Without konzo | p value | |
|---|---|---|---|
| Baseline | |||
|
| |||
| Number of patients | 123 | 82 | ·· |
| Age (years) | 8·7 (2·5) | 9·1 (2·6) | 0·21 |
| Sex (female) | 58 (41%) | 34 (47%) | 0·47* |
| Enrolled in school | 73 (59%) | 73 (89%) | 0·0001* |
| School grade level | 1·3 (1·6) | 2·5 (1·9) | 0·0010 |
| Caregiver with konzo | 99 (81%) | 58 (66%) | 0·0216* |
| Other family with konzo | 85 (85%) | 61 (70%) | 0·0075* |
| Weight for age (CDC Z score†) | −2·9 (−1·0) | −2·1 (1·5) | <0·0001 |
| Height for Age (CDC Z score†) | −3·4 (1·4) | −2·3 (1·8) | <0·0001 |
| Caldwell HOME score total‡ | 43·5 (8·1) | 47·2 (10·4) | 0·0040 |
| Socioeconomic status score§ | 2·2 (1·1) | 2·8 (3·2) | 0·07 |
| Neurological index Score¶ | 11·4 (4·4) | 2·0 (1·7) | <0·0001 |
| Urinary thiocyanate (µmol/mL) | 519 (354) | 389 (224) | 0·0060 |
|
| |||
| 2 years | |||
|
| |||
| Number of patients | 76 | 36 | ·· |
| Age (years) | 10·2 (2·4) | 10·8 (2·4) | 0·44 |
| Sex (female) | 35 (46%) | 12 (33%) | 0·22 |
| Weight for age (WHO Z score†) | −3·8 (1·3) | −2·3 (1·4) | <0·0001 |
| Height for age (WHO Z score†) | −3·1 (1·4) | −1·7 (1·5) | <0·0001 |
| Caldwell HOME score total‡ | 45·2 (7·2) | 49·2 (11·8) | 0·06 |
| Neurological index score¶ | 12·4 (4·8) | 1·3 (1·9) | <0·0001 |
|
| |||
| 4 years | |||
|
| |||
| Number of patients | 55 | 33 | ·· |
| Age (years) | 11·9 (2·1) | 12·0 (2·2) | 0·85 |
| Sex (female) | 26 (47%) | 14 (42%) | 0·83 |
| Weight for age (WHO Z score†) | −3·8 (1·6) | −2·0 (0·7) | <0·0001 |
| Height for age (WHO Z score†) | −3·7 (1·8) | −2·2 (1·7) | <0·0001 |
| Caldwell HOME score total‡ | 45·5 (8·0) | 49·5 (10·8) | 0·05 |
| Urinary thiocyanate (µmol/mL) | 1056 (577) | 1098 (572) | 0·44 |
Data are mean (SD) or n (%). p values are based on Kruskal-Wallis ANOVA test unless otherwise specified. CDC=Centers for Disease Control and Prevention.
p values for χ2 statistic between categories (gender, enrolled in school).
Z score values are from CDC 2000 norms from Epi Info at baseline and from WHO at 2 years and 4 years.
Based on the Caldwell Home Observation for the Measurement of the Environment (HOME) total score.
The socioeconomic status score is measured by assessing the physical characteristics of home dwelling and material and animal possessions during a visit to the home.
Children with konzo and children without konzo were assessed for upper-motor neuron signs, which are characteristic of konzo. From this exam we computed a total score reflecting the neurological impairment pertaining to konzo. For each of the neurological signs listed in table 1 index, children were scored as either normal (0 points), mildly abnormal (1 point), or moderately or severely abnormal (2 points).
Role of the funding source
The study sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The lead author (MJB) and study statistician (AS) have complete access to the study data and are responsible for the reported study findings, and along with the study principal investigator (DT-K) made the decision to submit for publication.
Results
210 children (median age 8·38 years, IQR 6·92–10·58) completed the first neurocognitive assessment, 117 completed a follow-up assessment approximately 2 years later (median age 10·09 years, IQR 8·67–12·27), and 89 completed another follow-up assessment 2 years later (median age 11·97 years, IQR 10·55–14·14). Results of a diagnostic neurological exam for konzo at 2-year follow-up were available for 116 (99%) of 117 children. Four children (three boys, one girl) without konzo at baseline developed the disease by 2-year follow-up. Another male child developed konzo between the 2-year follow-up and 4-year follow-up. These five children were excluded from the longitudinal analysis that focused on the effects of konzo over time. Table 1 presents characteristics of children who were analysed (123 children with konzo, 87 children without konzo). Baseline outcome scores or physical growth measures (height-for-age Z scores and weight-for age Z scores) of those who did not complete later assessments were not different from those who did (appendix).
As previously reported,4 at intake and throughout follow-up, children with konzo and without konzo were similar in age, gender composition, and socioeconomic status (table 1). Among children without konzo, 91% of girls and 88% of boys were enrolled in school at baseline. The corresponding percentages of children with konzo enrolled in school were 59% of girls and 60% of boys, with no differences by sex. Children with konzo were significantly below those without konzo in terms of height and weight, and height and weight for children with konzo was also less than the normal 95% CI normative range provided by the Centers for Disease Control and Prevention (CDC). Children with konzo also scored lower on the Caldwell HOME scale (measuring quality of caregiving) at baseline and at 2-year and 4-year follow-ups.
For boys (table 2) and girls (table 3), children with konzo had significantly lower scores on all scales of motor proficiency assessed by BOT-2 scale than did children without konzo at baseline, at 2-year follow-up, and at 4-year follow-up, except on the fine manual scale. SMDs of more than 1 indicated a difference of more than 1 SD between the children with and without konzo at baseline. For KABC-II, boys with konzo had significantly lower MPI scores than boys without konzo (p=0·0111) at 2-year follow-up, but not at baseline or 4-year follow-up (table 4), and the SMD for MPI reduced between 2-year follow-up and 4-year follow-up but remained more than a third of the SD, a threshold sometimes used for clinical significance.17 Among girls, the SMD for MPI was low at baseline and 2-year follow-up but increased to 0·54 at 4-year follow-up (table 5). Boys with konzo scored significantly lower on the KABC-II learning domain (p=0·04424) and the KABC-II Planning domain (p=0.0056) at 2-year follow-up, than boys without konzo, but not at 4 years. KABC-II scores were not significantly different between girls with and without konzo at all three timepoints, with SMDs also not indicating clinically meaningful differences except for the planning domain at both follow-up times (table 5).
Table 2.
Comparison of boys with and without konzo on the BOT-2 scale of motor proficiency at baseline, 2 years, and 4 year
| Baseline | 2 years | 4 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Without konzo | With konzo | p value | SMD | Without konzo | With konzo | p value | SMD | Without konzo | With konzo | p value | SMD | |
| Total motor | 37·03 (34·91–39·28) | 26·87 (25·43–28·38) | <0·0001 | 1·46 | 37·09 (34·31–40·09) | 25·44 (23·90–27·07) | <0·0001 | 1·67 | 36·15 (32·94–39·67) | 25·99 (24·11–28·01) | <0·0001 | 1·46 |
|
| ||||||||||||
| Fine manual | 37·91 (36·62–40·35) | 34·41 (32·48–36·46) | 0·0239 | 0·38 | 34·09 (31·31–37·12) | 31·02 (28·99, 33·20) | 0·0405 | 0·33 | 35·22 (31·83–38·98) | 31·24 (28·79–33·91) | 0·06 | 0·43 |
|
| ||||||||||||
| Manual coordination | 38·60 (36·10–41·26) | 29·88 (28·07–31·80) | <0·0001 | 1·15 | 38·09 (34·93–41·55) | 28·15 (26·26–30·19) | <0·0001 | 1·31 | 42·77 (38·56–47·45) | 31·57 (29·04–34·33) | <0·0001 | 1·48 |
|
| ||||||||||||
| Body coordination | 42·12 (39·45– 44·97) | 27·69 (26·06–29·43) | <0·0001 | 1·71 | 42·85 (39·22–46·82) | 25·39 (23·66–27·25) | <0·0001 | 2·07 | 41·57 (37·41–46·19) | 25·15 (23·10–27·38) | <0·0001 | 1·95 |
|
| ||||||||||||
| Strength agility | 39·76 (37·34–42·35) | 27·13 (25·58–28·76) | <0·0001 | 1·83 | 42·65 (39·24–46·36) | 26·33 (24·63–28·15) | <0·0001 | 2·37 | 37·94 (34·35–41·91) | 25·7 (23·75–27·89) | <0·0001 | 1·77 |
Data are means adjusted for child’s age and Caldwell HOME scale quality of caregiving environment, presented with 95% CI below the adjusted mean and SMDs after the p value of the ANCOVA between-group comparison. SMDs of more than 1 indicated a difference of more than 1 SD between the children with and without konzo at baseline. BOT-2=Bruininks-Oseretsky Test of Motor Proficiency, 2nd edition. HOME=Home Observation for the Measurement of the Environment. SMD=standardised mean differences.
Table 3.
Comparison of girls with and without konzo on the BOT-2 scale of motor proficiency at baseline, 2 years, and 4 years
| Baseline | 2 years | 4 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Without konzo | With konzo | p value | SMD | Without konzo | With konzo | p value | SMD | Without konzo | With konzo | p value | SMD | |
| Total motor | 32·42 (30·31–34·69) | 22·68 (31·83–38·82) | <0·0001 | 1·35 | 35·15 (31·83–38·82) | 22·40 (21·06–23·83) | <0·0001 | 1·77 | 33·07 (29·63–36·92) | 22·99 (21·19–24·93) | <0·0001 | 1·4 |
|
| ||||||||||||
| Fine manual | 28·69 (26·60–30·94) | 26·20 (24·69–27·80) | 0·05 | 0·33 | 30·22 (27·24–33·52) | 25·80 (24·14–27·57) | 0·0125 | 0·59 | 28·74 (25·68–32·15) | 26·65 (24·46–29·04) | 0·25 | 0·28 |
|
| ||||||||||||
| Manual coordination | 33·66 (31·14–36·39) | 25·24 (23·75–26·83) | <0·0001 | 1·16 | 36·36 (32·56–40·60) | 25·19 (23·49–27·01) | <0·0001 | 1·55 | 37·92 (33·68–42·71) | 27·99 (25·58–30·63) | <0·0001 | 1·38 |
|
| ||||||||||||
| Body coordination | 38·77 (36·18–41·54) | 24·18 (22·90–25·52) | <0·0001 | 1·92 | 41·14 (37·08–45·64) | 22·75 (21·34–24·25) | <0·0001 | 2·22 | 41·46 (37·11–46·32) | 23·22 (21·37–25·22) | <0·0001 | 2·21 |
|
| ||||||||||||
| Strength agility | 37·69 (35·02–40·57) | 24·36 (23·00–25·81) | <0·0001 | 1·79 | 42·73 (38·18–47·83) | 24·32 (22·69–26·05) | <0·0001 | 2·47 | 39·70 (35·21–44·76) | 23·36 (21·37–25·55) | <0·0001 | 2·19 |
Data are means adjusted for child’s age and Caldwell HOME scale quality of caregiving environment, presented with 95% CI below the adjusted mean and SMDs after the p value of the ANCOVA between-group comparison. BOT-2=Bruininks-Oseretsky Test of Motor Proficiency, 2nd edition. HOME=Home Observation for the Measurement of the Environment. SMD=standardised mean differences.
Table 4.
Comparison of boys with and without konzo on the KABC-II Learning and Mental Processing Index scale at baseline, 2 years, and 4 years
| Baseline | 2years | 4 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Without konzo | With konzo | p value | SMD | Without konzo | With konzo | p value | SMD | Without konzo | With konzo | p value | SMD | |
| Mental processing index | 64·13 (61·68–66·68) | 61·04 (58·87–63·28) | 0·06 | 0·34 | 60·07 (56·98–63·32) | 55·08 (52·90–57·34) | 0·0111 | 0·56 | 60·74 (57·12–64·58) | 57·23 (54·48–60·13) | 0·11 | 0·39 |
|
| ||||||||||||
| Sequential processing | 75·23 (71·94–78·66) | 72·86 (69·91–75·95) | 0·29 | 0·18 | 69·56 (65·52–73·85) | 65·56 (62·62–68·63) | 0·12 | 0·31 | 74·17 (69·19–79·52) | 69·35 (65·61–73·31) | 0·11 | 0·37 |
|
| ||||||||||||
| Simultaneous processing | 69·17 (65·52–73·01) | 64·73 (61·58–68·04) | 0·08 | 0·41 | 64·28 (59·75–69·15) | 61·14 (57·81–64·65) | 0·28 | 0·29 | 67·17 (61·38–73·51) | 64·03 (59·47–68·94) | 0·39 | 0·29 |
|
| ||||||||||||
| Learning | 70·48 (67·33–73·79) | 69·75 (66·84–72·78) | 0·74 | 0·07 | 69·78 (66·84–72·78) | 64·40 (61·45–67·50) | 0·0424 | 0·52 | 66·16 (61·60–71·04) | 65·60 (61·97–69·44) | 0·84 | 0·05 |
|
| ||||||||||||
| Planning | 61·53 (58·74–64·46) | 56·19 (53·81–58·69) | 0·0056 | 0·48 | 57·41 (54·24–60·76) | 55·07 (52·63–57·62) | 0·26 | 0·21 | 59·06 (55·36–63·01) | 55·98 (53·00–59·13) | 0·20 | 0·28 |
|
| ||||||||||||
| Delayed recall | 73·01 (70·09–76·05) | 74·47 (71·71–77·33) | 0·47 | 0·06 | 70·28 (66·39, 74·41) | 69·32 (66·46, 72·31) | 0·70 | 0·10 | 70·57 (65·88–75·59) | 68·45 (64·88–72·23) | 0·47 | 0·23 |
Data are means adjusted for child’s age and Caldwell HOME scale quality of caregiving environment, presented with 95% CI below the adjusted mean and SMDs after the p value of the ANCOVA between-group comparison. HOME=Home Observation for the Measurement of the Environment. KABC-II=Kaufman Assessment Battery for Children, second edition. SMD=standardised mean differences.
Table 5.
Comparison of girls with and without konzo on the KABC-II Learning and Mental Processing Index scale at baseline, 2 years, and 4 years
| Baseline | 2 years | 4 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Without konzo | With konzo | p value | SMD | Without konzo | With konzo | p value | SMD | Without konzo | With konzo | p value | SMD | |
| Mental processing index | 56·45 (53·85–59·17) | 55·37 (53·37–57·44) | 0·51 | 0·13 | 55·71 (51·72–60·00) | 55·22 (52·83–57·72) | 0·84 | 0·06 | 63·58 (59·11–68·39) | 59·18 (56·01–62·54) | 0·10 | 0·54 |
|
| ||||||||||||
| Sequential processing | 70·85 (66·81–75·13) | 67·02 (64·01–70·17) | 0·13 | 0·26 | 65·55 (60·02–71·60) | 67·68 (64·01–70·17) | 0·54 | 0·15 | 75·63 (69·24–82·61) | 73·80 (68·88–79·08) | 0·64 | 0·13 |
|
| ||||||||||||
| Simultaneous processing | 55·81 (52·25–59·62) | 55·10 (52·36–57·98) | 0·76 | 0·08 | 56·80 (50·97–63·30) | 57·70 (54·09–61·56) | 0·80 | 0·10 | 59·54 (51·60– 68·70) | 57·70 (52·44–63·50) | 0·71 | 0·19 |
|
| ||||||||||||
| Learning | 68·51 (64·94–72·28) | 66·54 (63·82–69·38) | 0·37 | 0·20 | 62·74 (57·76–68·16) | 63·81 (60·70–67·07) | 0·73 | 0·11 | 69·49 (64·02–75·43) | 66·74 (62·60–71·17) | 0·41 | 0·28 |
|
| ||||||||||||
| Planning | 54·82 (52·51–57·22) | 54·36 (52·57–56·21) | 0·76 | 0·07 | 57·53 (54·05– 61·23) | 53·92 (51·95–55·96) | 0·08 | 0·57 | 60·43 (56·77–64·32) | 57·64 (54·94–60·48) | 0·22 | 0·44 |
|
| ||||||||||||
| Delayed recall | 71·17 (67·76–74·76) | 69·65 (67·04–72·36) | 0·48 | 0·16 | 69·45 (64·28–75·03) | 67·88 (64·84–71·08) | 0·61 | 0·16 | 69·81 (64·40–75·68) | 67·93 (63·81–72·32) | 0·57 | 0·20 |
Data are means adjusted for child’s age and Caldwell HOME scale quality of caregiving environment, presented with 95% CI below the adjusted mean and SMDs after the p value of the ANCOVA between-group comparison. HOME=Home Observation for the Measurement of the Environment. KABC-II=Kaufman Assessment Battery for Children, second edition. SMD=standardised mean differences.
Examination of the adjusted means reported in tables 2–5 within each group over time indicates that at 2-year follow-up boys with konzo significantly declined from baseline on BOT-2 fine manual scores (p=0·0076) and body coordination scores (p=0·0277). Whereas, boys without konzo declined only on fine manual scores (p=0·0224). Boys with konzo also declined significantly on KABC-II MPI (p<0·0001), sequential processing (p<0·0001), learning (p=0·0023), and delayed recall scores (p=0·0037). The boys without konzo declined significantly on KABC-II MPI (p=0·0213), sequential processing (p<0·0001), and simultaneous processing (p=0·05), although these findings should not be given a strong interpretation due to the large number of tests, which could increase the chance of finding a false positive. No significant BOT-2 or KABC-II changes were apparent between baseline and 2-year follow-up for girls with and without konzo.
At 4-year follow-up, boys with konzo significantly declined from baseline on fine manual (p=0·0088) and body coordination (p=0·0432) BOT-2 scales, and the KABC-II MPI composite (p=0·0256) and delayed (p=0·0037) recall global measures. No significant declines were evident for boys without konzo, nor were there significant declines for the girls with and without konzo from baseline to 4-year follow-up. In fact, girls improved on some of the KABC-II measures, but this finding might not be reliable due to a relatively small sample size at 4-year follow-up in terms of statistical power.
Urinary thiocyanate concentration was significantly higher in children with konzo than children without konzo at baseline, but did not differ significantly at 4-year follow-up (table 1). However, urinary thiocyanate concentration significantly increased from baseline to 4-year follow-up in children with and without konzo (p=0·0080).
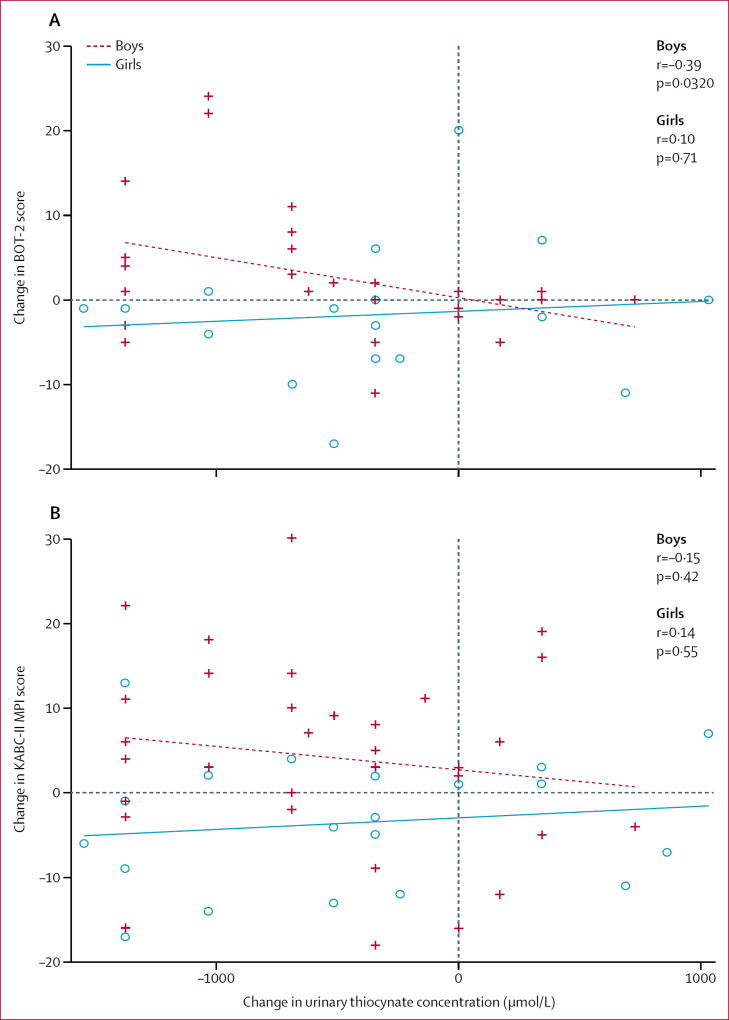
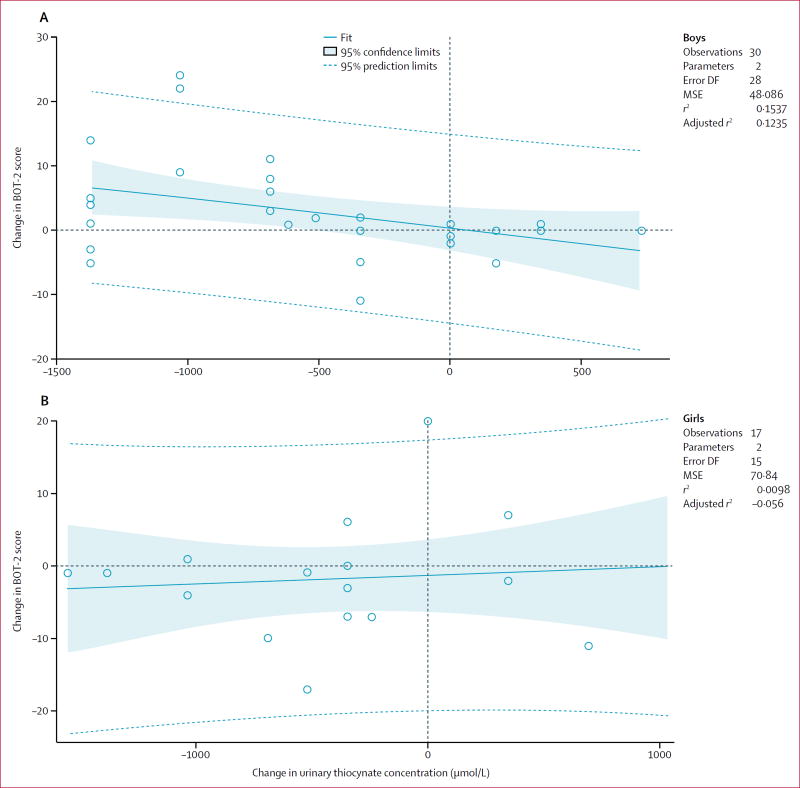
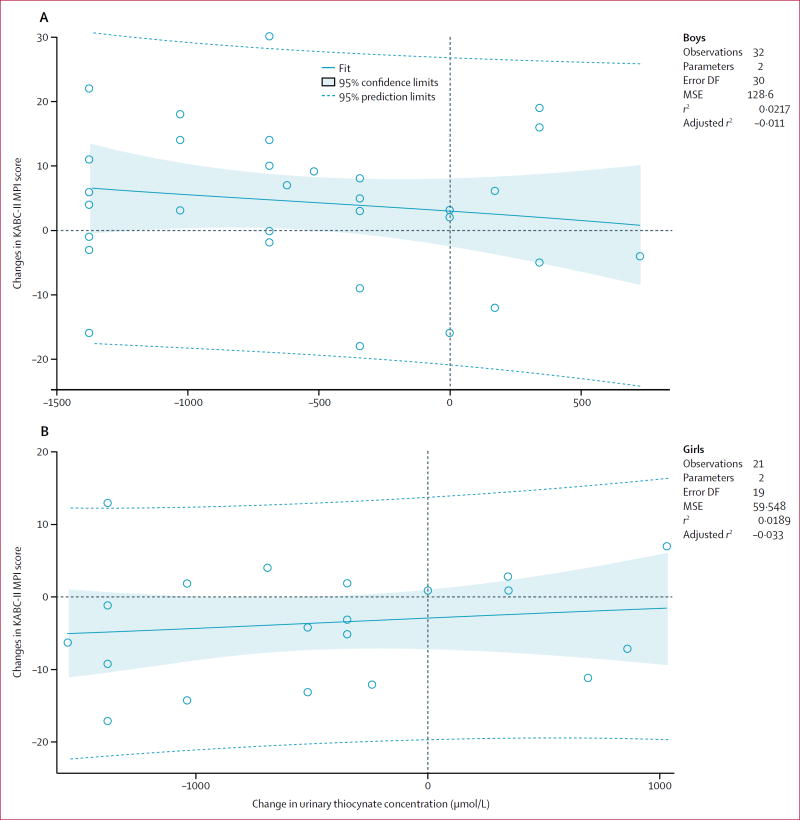
The greater the increase in thiocyanate exposure, the poorer the motor proficiency (figure 1). Change in urinary thiocyanate concentration in boys (with and without konzo) from baseline to 4-year follow-up was significantly associated with change in BOT-2 total motor proficiency score from baseline to 4-year follow-up (r=−0·39, n=47, p=0·0321; figure 2). There was not a significant regression association in girls (r=0·10, n=33, p=0·71; figure 2). Change in urinary thiocyanate concentration was not significantly associated with KABC-II MPI score for boys (r=−0·15, n=50, p=0·42; figure 3), or girls (r=0·14, n=41, p=0·55; figure 3).
Figure 1. Change in urinary thiocyanate and (A) change in BOT-2 of motor proficiency standard total score and (B) change in KABC-II MPI score for children with konzo from baseline to 4-year follow-up.
Girls are depicted in blue along with the linear least square fit line and function, and boys are depicted in red. BOT-2=Bruininks-Oseretsky Test, second edition. KABC-II=Kaufman Assessment Battery for Children, second edition. MPI=Mental Processing Index.
Figure 2. Change in BOT-2 total motor proficiency and change in urinary thiocyanate between baseline and 4-year follow up.
Change in urinary thiocyanate and change in BOT-2 of motor proficiency standard total score in (A) boys with konzo and (B) girls with konzo. BOT-2=Bruininks-Oseretsky Test, seconnd edition. DF=degrees of freedom. MSE=mean square error.
Figure 3. Change in KABC-II MPI cognitive performance and change in urinary thiocyanate between baseline and 4-year followup.
Change in urinary thiocyanate and change in KABC-II MPI score for (A) boys with konzo and (B) girls with konzo. KABC-II=Kaufman Assessment Battery for Children, second edition. MPI=Mental Processing Index. DF=degrees of freedom. MSE=mean square error.
The evaluation of the potential mediational roles of height-for-age Z scores and weight-for-age Z scores for BOT-2 and KABC-II principal outcomes is in the appendix. In brief, when adding weight-for age z-scores as a covariate, the findings presented in tables 2–5 in terms of the differences between children with and without konzo did not change in any appreciable way. However, in terms of its covariate main effect across both konzo groups and across all three time points, weight-for-age z-score was significantly associated with BOT-2 fine manual motor proficiency scores for both sexes (boys: β=0·034, SE=0·013, t(108)=2·52, p=0·0130; girls: β=0·044, SE=0·022, t(87)=2·03, p=0·0463) and significantly correlated with KABC-II simultaneous processing (visual–spatial analysis and problem solving) for boys (boys: β=0·026, SE=0·012, t(108)=2·18, p=0·0321). Controlling for height-for-age Z scores at baseline did not change the differences between children with and without konzo for BOT-2 outcomes (table 2 and 3), despite the fact that height-for-age Z score itself was significantly associated with all BOT-2 outcomes. Controlling for height-for-age Z scores when we analysed KABC-II scores resulted in the attenuation of the few significant between-group differences for the boys in table 4 (appendix).
Discussion
On the BOT-2 assessment of motor proficiency, both boys and girls with konzo did significantly worse than children without konzo at 2-year and 4-year follow-up. The mean differences between the groups were consistent with those apparent at baseline, with SMD exceeding 1. This effect was not mediated by wasting (weight-for-age Z score) or stunting (height-for-age Z score), but seemed to be primarily the result of konzo status. The only exception was the BOT-2 fine manual motor scale, which is consistent with the well-documented effects of konzo primarily affecting the lower limbs, as opposed to the arms and hands.2 However, in terms of decline from baseline to 2-year follow-up, the largest declines for boys both with and without konzo were for BOT-2 fine manual motor proficiency. Notably, wasting, which can be taken as an indicator of more recent nutritional hardship,18 was significantly correlated with BOT-2 fine manual motor proficiency for both sexes. This result suggests that changes in this motor proficiency domain over time might be the result of malnutrition, rather than the direct results of konzo disease progression per se.
Although some reviews of konzo19 have reported neurological relapse in patients with konzo, our reported declines in proficiency as measured by BOT-2 motor proficiency cannot be interpreted as the result of relapse, which would be characterised by more abrupt neurological re-emergence and onset. In statistically evaluating these comparisons, we used generalised linear mixed effects modelling to account for the non-independence of the BOT-2 repeated measure outcomes, as well as to control for the baseline between-group HOME differences among children with and without konzo, and those retained and those lost to follow-up via the missing at random mechanism. Although not directly testable, the missing at random mechanism was supported by the attrition analyses.
BOT-2 motor proficiency decline from baseline to 4-year follow-up was associated with increases in urinary thiocyanate concentration from baseline to 4-year follow-up in boys with and without konzo, but not in girls. Furthermore, increases in urinary thiocyanate concentration in boys and girls with and without konzo were not associated with changes in KABC-II MPI performance (a composite of overall cognitive ability). The absence of statistical significance at 4-year follow-up might be due to a smaller sample size at this point, especially for the children without konzo: a serious limitation in our present study. However, notably, although urinary thiocyanate concentration is useful as a biomarker of recent exposure to poorly processed cassava, it is not a sensitive biomarker for past acute toxic exposure that might have resulted in the abrupt onset of neurological konzo disease, or cumulative exposure that could have long-term neurocognitive and neuromotor effects. Serum-based biomarkers are needed that are sensitive to metabolic processes that might in fact mediate the neuropathic pathways of cyanogenic toxicity in the brain, especially biomarkers that measure cumulative past exposure over time.
Mean differences in KABC-II cognitive ability performance between boys with and without konzo were also consistent from baseline to 2-year and 4-year follow-up, between a third to just more than half of the standard deviation of children without konzo at baseline. The boys without konzo also exhibited significant decline in this measure from baseline to 2-year follow-up, especially on KABC-II sequential processing, which is a measure of visual and auditory working memory. The original baseline findings also documented significant KABC-II cognitive deficits for this global domain for children without konzo, suggesting the possibility that this disease might be associated with a subclinical neurocognitive deficit.4 Although it is generally known how konzo progresses neurologically,2 it is unknown whether the discernable neurological upper-motor neuron disease is directly associated neurocognitive impairment. In the present study we have documented continuing neurocognitive disability in boys with konzo compared with their counterparts without konzo in follow-up. However, it is important to qualify our findings by stating that we do not have field-worthy sensitive and specific biomarkers predicting neurological, motor proficiency, and neurocognitive impairment in a congruent manner over time. Therefore, we cannot conclusively establish that these processes are all associated with the same common mechanism that causes konzo, together comprising the syndrome of konzo. Elucidation of this mechanism is an important objective for future research characterising konzo in children.
Our baseline and present follow-up findings that boys with konzo (but not the girls) have worse cognitive proficiency than their counterparts without konzo is in contrast with a previously published report with a subgroup of our baseline cohorts.12 This previous report modelled cognitive performance (KABC-II scores) and motor proficiency (BOT-2) to serum levels of free thyroxine (free T4), thyroid-stimulating hormone (TSH), and albumin in 40 children with and without konzo, and concluded that girls were more at risk for cognitive deficits.12 Data in that study indicated that girls had lower KABC-II overall cognitive performance (MPI) than boys. This finding does not contradict our present study findings because in Bumoko and colleagues’ study,12 only the main effects of sex and konzo status were presented. Interactions are mentioned in the analysis section but not in the results. As evidenced in tables 4 and 5 in the present study, the pattern of means is consistent with Bumoko and colleagues’ study;12 at intake girls had mean KABC-II MPI overall cognitive performance scores in the range of 50–60, whereas boys had mean scores in the range of 60–70. Thus, girls have a lower performance and are also at heightened risk for neurocognitive disability from konzo when not adjusting for other factors such as socioeconomic status, HOME score, and education. In fact, a “basement” effect might be present in KABC-II assessment of the Kahemba girls with konzo that obscures the true difference with their counterparts without konzo, which could be a psychometric study limitation. Regardless, konzo affected KABC-II overall cognitive performance score differently according to gender—the effects of konzo were greater among boys. Despite the fact that boys with and without konzo overall have higher scores than the girls, there is a greater difference between the groups of boys with and without konzo compared with that difference among girls.
In terms of accounting for this gender difference in neurocognitive risk from konzo, we previously noted that boys with konzo were also less likely to be enrolled in school, perhaps because of social stigma associated with the disorder.4 This might have further contributed to their poorer neurocognitive performance as compared with their counterparts without konzo, and those findings are supported in the present follow-up study as well. We also verified in our follow-up assessments that both the children with and without konzo were significantly below KABC cognitive ability normative performance of children from an area without konzo. These differences were especially apparent on sequential processing (memory) subtests. Another significant limitation in the present follow-up study, was that at 2-year follow-up when performance differences between children with or without konzo as well as decline from baseline were strongest, we did not have samples of urinary thiocyanate from the study children, as were reported in the baseline study and at 4-year follow-up.
In our present follow-up study, children affected by konzo also had significantly lower anthropometric measures (weight for age, height for age), indicative of wasting and stunting. This could be because of the poor nutritional intake contributing to risk for konzo,20,21,22 and nutritional malabsorption from the direct effects of cassava toxicity on intestinal microbiota.5,23 In the present analyses, controlling for stunting (height-for-age Z score) resulted in non-significant between-group differences in the KABC-II outcomes of learning and overall performance (MPI) for the boys at 2-year follow-up. This suggests that stunting, often taken to be an indicator of more long-term chronic nutritional hardship (or malabsorption),19 might potentially mediate these cognitive differences between boys with and without konzo. This effect was not seen in the KABC-II domain of planning (reasoning), reported in an earlier study14 as a particularly robust cognitive outcome in a case study of more severely affected children with konzo.
The effects of dietary micronutrient deficiency should also be considered, such as thiamine deficiency, which has been associated with CNS signs similar to what is seen in konzo and warrants further pathogenic explorations. However, in view of what is known from the evidence about the probable neuropathogenic mechanisms of brain toxicity from cyanogenic toxins in poorly processed cassava, the neurocognitive effects of thiamine and other vitamin deficiencies in konzo are likely to be secondary to these.2,13 Regardless, the role of such micronutrient deficiencies as they relate to the direct neuropathogenic processes from cassava cyanogen exposure in nutritionally at-risk children with konzo is a key priority for future research, particularly because these factors relate to gender differences for konzo risk and progression.
As was the case in our baseline paper,4 most of the caregivers for both the konzo and control groups in our follow-up were affected by konzo, but a higher percentage of caregivers of children with konzo were affected. Caregivers affected by konzo would also be less able to provide for a quality caregiving environment for children with konzo, explaining the significantly reduced HOME scores for this group compared with the group without konzo. This is a further significant neurocognitive risk factor for children with konzo, not directly associated with the neuropathogenesis of toxic cassava.
Much of sub-Saharan Africa is becoming increasingly dependent on high-yield varieties of cassava that thrive in ecologically degraded zones and in conflict zones.24 Therefore, continued brain or behaviour research that will help us better understand its neurotoxic properties is critical. The ability to document the more subtle CNS effects of cyanogenic toxicity with comprehensive and sensitive neuropsychological batteries is of major public health importance globally and more specifically, in African regions dependent on bitter cassava. Notably, boys with konzo significantly declined from baseline on KABC-II overall cognitive performance from baseline to 2-year and even 4-year follow-up. This continued decline in boys could be the result of gender-specific effects of konzo disease noted in the previously published baseline study. The decline could also be the result of a poor neurocognitive trajectory of development and poor nutrition as compounded by konzo-induced disabilities.4 Worse KABC cognitive performance and tactile-based learning over time has been documented in previous studies8,9,25,26 in this general region of Congo. These neuropsychological trajectories over time have been significantly associated with risk factors such as poor nutrition, physical growth, chronic helminths and malaria infection, anaemia, and ethnic groups living in village areas particularly at risk for these developmental impediments.9,27 When these factors are compounded by the functional neurodisabilities induced by konzo disease, the community-wide economic and ecological effects on households can be devastating.28 Interventions to prevent further exposure to poorly processed cassava for children at risk of konzo are urgently needed, so as to prevent continued neuromotor and neurocognitive decline, such as the “wetting method” described by Bradbury and colleagues.29
To our knowledge, this study is the first attempt to characterise the cognitive and motor proficiency decline in children as they continue to subsist on bitter varieties of cassava as the main source of food. We have not only documented specific domains of continuing neurocognitive deficits in children with konzo, especially boys, but have presented evidence for more pervasive neurocognitive effects (eg, memory) for both children with and without konzo in a similar nutritional environment with pervasive risk factors for konzo onset. Although we have documented changes in the neurocognitive and neuromotor proficiency of children with konzo and children at-risk without konzo, the pathophysiological and neurological progression of the disease itself in such children over time remains poorly understood. This is an important next-step in future research. However, what is clear is that in view of the ongoing neuropsychological risk from toxic cassava exposure in children in konzo-outbreak areas, interventions are urgently needed. Such interventions can use a peer-training model for simple and sustainable methods to detoxify the cassava flour (eg, wetting method), and thereby reduce the potential cumulative effects of toxin exposure on neurocognitive and neuromotor development over the long term. In view of the neurocognitive evidence in the present study, these public health benefits can occur even in the absence of abrupt neurological disease onset in at-risk children, as well as when confronted by chronic and possibly progressive neuromotor disability in children with konzo in communities dependent on toxic cassava.
Supplementary Material
Research in context.
Evidence before this study
Our preceding study provided the baseline assessment to the present one, and was the only study that we could find that evaluated the neuropsychological, neurodevelopmental, cognitive, or motor proficiency effects of konzo. This was confirmed by doing a keyword search for these terms in relation to konzo using PubMed and MEDLINE with no date or language restrictions. Previous electrophysiological studies of konzo disease patients using electroencephalogram (EEG), evoked potential EEG, and electromyography (EMG) implicated higher brain centres as being involved in the disease. Again, the only study of the brain effects of konzo was in a few adult Tanzanian patients with konzo brought to Sweden for MRI imaging. However, no clear evidence emerged for konzo effects in terms of brain structure.
Added value of this study
The present study documents the presence of a possible subclinical condition in children without konzo, seemingly on the threshold of neuropsychological impairment from ongoing exposure to poorly processed cassava in affected communities. In a longitudinal assessment over 4 years, we provide the first evidence that there is continued neurocognitive and neuromotor decline in children with and without konzo, especially boys, in affected households.
Implications of all the available evidence
Cassava is a crop of subsistence for more than 600 million people around the world and particularly in many tropical regions. Much of the Congo basin in central Africa will continue to rely on cyanogenic cassava as an important source of food, thus putting millions of children at risk for neurodevelopmental disabilities from exposure to toxic cassava. Therefore, useful tools for monitoring pathophysiological exposure to toxic cassava and risk for konzo are of vital importance. Urinary thiocyanate, used in the present study, is a useful and practical indicator of recent exposure to poorly processed cassava in the field setting. However, its validity and utility for documenting past acute exposure and cumulative exposure over time are very limited. Sensitive and specific biomarkers for the disease process in konzo that are feasible for field-surveillance programmes are desperately needed. Such sensitive and practical biomarker tests for konzo risk should be coupled with community-wide household-based health education and promotion for the safe processing of cassava, such as the wetting method presently being implemented in especially at-risk health zones in the western Congo. Prevention is still the key for an effective public health response to konzo in Africa.
Acknowledgments
All phases of this study were supported by an National Institutes of Health (NIH) grant, R01ES019841 (Principal Investigator: Tshala-Katumbay). The efforts of the anonymous reviewers were of great benefit in improving this manuscript and are very much appreciated. Matthew Boivin has made available a short documentary video on this project, which can be viewed at http://vimeo.com/42317386. His efforts in producing this video are much appreciated. We are grateful to the Kahemba community and participating families for their support in this study.
Footnotes
See Online for appendix
For more on Epi Info see https://www.cdc.gov.epiinfo/index.html
Contributors
MJB participated in the design of the study, oversight of the neuropsychological exam, analysis of the data, drafting of the manuscript, was the corresponding author for the manuscript, and approved the final manuscript as submitted. DO participated in the field testing of the children, drafting of the manuscript, and approved the final manuscript as submitted. BM-M participated in the field testing of the children, drafting of the manuscript, and approved the final manuscript as submitted. M-TS participated in the field testing of the children, drafting of the manuscript, and approved the final manuscript as submitted. DM participated in the study design, konzo diagnosis, clinical analysis, and approved the final manuscript as submitted. AS participated in the statistical analysis and interpretation of study findings, drafting of the manuscript, and approved the final manuscript as submitted. BM had oversight of the in-country study approval process and for the epidemiological surveillance of our konzo study site and population through the Congo Ministry of Health, and approved the final manuscript as submitted. DT-K was the study principal investigator, participated in the design of study, oversight of the neurological exam, and drafting of the manuscript, and approved the final manuscript as submitted.
Declaration of interests
We declare no competing interests.
For the documentary video see http://vimeo.com/42317386
References
- 1.WHO. Konzo, a distinct type of upper motoneuron disease. Wkly Epidemiol Rec. 1996;71:225–32. [Google Scholar]
- 2.Tylleskar T, Tshala-Katumbay D, Konzo . A permanent, non-progressive, motor neuron disease. In: Jagjit Chopra., editor. Neurology in Tropics. 2. India: Elsevier; 2015. pp. 377–86. [Google Scholar]
- 3.Tshala-Katumbay D, Eeg-Olofsson KE, Tylleskar T, Kazadi-Kayembe T. Impairments, disabilities and handicap pattern in konzo—a non-progressive spastic para/tetraparesis of acute onset. Disabil Rehabil. 2001;23:731–36. doi: 10.1080/09638280110055075. [DOI] [PubMed] [Google Scholar]
- 4.Boivin MJ, Okitundu D, Makila-Mabe Bumoko G, et al. Neuropsychological effects of konzo: a neuromotor disease associated with poorly processed cassava. Pediatrics. 2013;131:e1231–39. doi: 10.1542/peds.2012-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cliff J, Nicala D. Long-term follow-up of konzo patients. Trans R Soc Trop Med Hyg. 1997;91:447–49. doi: 10.1016/s0035-9203(97)90279-0. [DOI] [PubMed] [Google Scholar]
- 6.Bonmarin I, Nunga M, Perea WA. Konzo outbreak in the south-west of the Democratic Republic of Congo, 1996. J Trop Pediatr. 2002;48:234–38. doi: 10.1093/tropej/48.4.234. [DOI] [PubMed] [Google Scholar]
- 7.Bradley RH, Caldwell BM. Home observation for measurement of the environment: a revision of the preschool scale. Am J Ment Defic. 1979;84:235–44. [PubMed] [Google Scholar]
- 8.Boivin MJ, Giordani B. Improvements in cognitive performance for schoolchildren in Zaire, Africa, following an iron supplement and treatment for intestinal parasites. J Pediatr Psychol. 1993;18:249–64. doi: 10.1093/jpepsy/18.2.249. [DOI] [PubMed] [Google Scholar]
- 9.Boivin MJ, Giordani B, Ndanga K, Maky MM, Manzeki KM, Ngunu N. Economic advantage and the cognitive ability of rural children in Zaire. J Psychol. 1996;130:95–107. doi: 10.1080/00223980.1996.9914992. [DOI] [PubMed] [Google Scholar]
- 10.Boivin MJ, Giordani B, Ndanga K, et al. Effects of treatment for intestinal parasites and malaria on the cognitive abilities of schoolchildren in Zaire, Africa. Health Psychol. 1993;12:220–26. doi: 10.1037//0278-6133.12.3.220. [DOI] [PubMed] [Google Scholar]
- 11.Bumoko GM, Sadiki NH, Rwatambuga A, et al. Lower serum levels of selenium, copper, and zinc are related to neuromotor impairments in children with konzo. J Neurol Sci. 2015;349:149–53. doi: 10.1016/j.jns.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bumoko GM, Sombo MT, Okitundu LD, et al. Determinants of cognitive performance in children relying on cyanogenic cassava as staple food. Metab Brain Dis. 2014;29:359–66. doi: 10.1007/s11011-014-9492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tshala-Katumbay DD, Ngombe NN, Okitundu D, et al. Cyanide and the human brain: perspectives from a model of food (cassava) poisoning. Ann N Y Acad Sci. 2016;1378:50–57. doi: 10.1111/nyas.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tshala-Katumbay D, Mumba N, Okitundu L, et al. Cassava food toxins, konzo disease, and neurodegeneration in sub-Sahara Africans. Neurology. 2013;80:949–51. doi: 10.1212/WNL.0b013e3182840b81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banea JP, Nahimana G, Kuwa N, Bradbury JH, Denton IC, Mandombi C. Preventive control of konzo in the Democratic Republic of Congo. Food Chem Toxicol. 2012;50:4234–35. doi: 10.1016/j.fct.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Banea JP, Nahimana G, Mandombi C, Bradbury JH, Denton IC, Kuwa N. Control of konzo in DRC using the wetting method on cassava flour. Food Chem Toxicol. 2012;50:1517–23. doi: 10.1016/j.fct.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77:371–83. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 18.Psaki S, Bhutta ZA, Ahmed T, et al. Household food access and child malnutrition: results from the eight-country MAL-ED study. Popul Health Metr. 2012;10:24. doi: 10.1186/1478-7954-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tshala-Katumbay DD, Spencer PS. Toxic disorders of the upper motor neuron system. In: Eisen A, Shaw P, editors. Motor neuron disorders and related diseases. 1. Vol. 82. Amsterdam: Elsevier; 2007. pp. 353–72. [DOI] [PubMed] [Google Scholar]
- 20.Tylleskar T, Banea M, Bikangi N, Nahimana G, Persson LA, Rosling H. Dietary determinants of a non-progressive spastic paraparesis (Konzo): a case-referent study in a high incidence area of Zaire. Int J Epidemiol. 1995;24:949–56. doi: 10.1093/ije/24.5.949. [DOI] [PubMed] [Google Scholar]
- 21.Tylleskar T, Banea M, Bikangi N, Fresco L, Persson LA, Rosling H. Epidemiological evidence from Zaire for a dietary etiology of konzo, an upper motor neuron disease. Bull World Health Organ. 1991;69:581–89. [PMC free article] [PubMed] [Google Scholar]
- 22.Kamalu BP. Pathological changes in growing dogs fed on a balanced cassava (Manihot esculenta Crantz) diet. Br J Nutr. 1993;69:921–34. doi: 10.1079/bjn19930092. [DOI] [PubMed] [Google Scholar]
- 23.Cliff J, Nicala D, Saute F, et al. Konzo associated with war in Mozambique. Trop Med Int Health. 1997;2:1068–74. doi: 10.1046/j.1365-3156.1997.d01-178.x. [DOI] [PubMed] [Google Scholar]
- 24.Banea-Mayambu JP, Tylleskar T, Gitebo N, Matadi N, Gebre-Medhin M, Rosling H. Geographical and seasonal association between linamarin and cyanide exposure from cassava and the upper motor neurone disease konzo in former Zaire. Trop Med Int Health. 1999;2:1143–51. doi: 10.1046/j.1365-3156.1997.d01-215.x. [DOI] [PubMed] [Google Scholar]
- 25.Boivin MJ, Giordani B, Bornefeld B. Use of the Tactual Performance Test for cognitive ability testing with African children. Neuropsychology. 1995;9:409–17. [Google Scholar]
- 26.Giordani B, Boivin MJ, Opel B, Dia Nseyila D, Diawaku N, Lauer RE. Use of the K-ABC with children in Zaire, Africa: an evaluation of the sequential-simultaneous processing distinction within an intercultural context. Int J Disabil Dev Ed. 1996;43:5–24. [Google Scholar]
- 27.Boivin MJ. Community development, health and neuropsychology of African children. In: Sarr A, Makward E, Fofana AT, Frederick C, editors. The histories, languages and cultures of west Africa: interdisciplinary essays. Lewiston. New York: Edwin Mellen Press; 2006. [Google Scholar]
- 28.Boivin MJ. An ecological paradigm for a health behavior analysis of “konzo”, a paralytic disease of Zaire from toxic cassava. Soc Sci Med. 1997;45:1853–62. doi: 10.1016/s0277-9536(97)00123-8. [DOI] [PubMed] [Google Scholar]
- 29.Bradbury JH, Cliff J, Denton IC. Uptake of wetting method in Africa to reduce cyanide poisoning and konzo from cassava. Food Chem Toxicol. 2010;49:539–42. doi: 10.1016/j.fct.2010.04.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.