Abstract
Objective:
The aim of this case–control study was to compare patients with a healthy peri-implant environment and patients affected by peri-implantitis, evaluating the occurrence of antibodies to extracellular matrix (ECM) molecules. The authors hypothesized the presence of ECM autoantibodies in serum of peri-implantitis patients.
Materials and Methods:
Patients were divided into two groups: one with dental implants with a diagnosis of peri-implantitis and one control group with implants classified as being “healthy.” Enzyme-linked immunosorbent assay was performed on patients’ sera to detect human antibodies to type I, III, IV, and V collagens, laminin, and fibronectin. Fisher exact test was performed to evaluate statistical association, with a significant P < 0.05.
Results:
Forty-two patients were enrolled in this study, 27 females (64.28%) and 15 males (35.72%) with a mean age of 53 ± 29.69 years (age range 32–74). The presence of antibodies to CIII was recorded in 6/21 (28.57%) patients of test group, compared to just 2/21 (9.52%) for the control group, showing a statistically significant difference (P < 0.05). Other antibodies tested were found to be not statistically significant or absent.
Conclusions:
Within the limitations of this study, it can be concluded that further studies, with larger sample and different design, are necessary to address the research purpose, evaluating possible associations between anti-ECM antibodies and peri-implantitis.
Keywords: Collagen, enzyme-linked immunosorbent assay, extracellular matrix, peri-implantitis
INTRODUCTION
Implant treatment is a successful and effective procedure for replacing missing teeth;[1,2] over the years, numerous surfaces and coatings have been utilized to try to maximize on growth potential and secondary stability, increasing bone to implant contact values.[3,4,5,6]
A direct relationship between oral health and quality of life has been suggested in the majority of people, and a positive functional, social, and psychological impact of implant-supported rehabilitation on patient's oral health-related quality of life was assessed.[7]
In a recent systematic review, Doornewaard et al. reported an implant survival rate of 97.3% after at least 5 years of functional loading with a peri-implant bone loss >3 mm occurring in just 5% of dental implants.[8]
Several authors reported implant survival rate between 91.96% and 95.6% after a long-term follow-up of 15 years, highlighting the reliability of modern implant dentistry procedures.[9,10]
However, nowadays, peri-implantitis is considered as an emerging disease with pathogenic mechanisms still unknown and without long-term follow-up successful treatment strategies.[11,12,13]
The prevalence of peri-implant diseases is controversial, depending mostly on study design and population;[14] Derks and Tomasi in 2015, reported 43% for mucositis and 22% for peri-implantitis.[15]
Peri-implantitis and periodontal disease share similarities in etiology and clinical features. Microbiota associated are very similar, such as the species of the red and orange complexes, Prevotella nigrescens, Campylobacter rectus, and Aggregatibacter actinomycetemcomitans (Aa), as well as Staphylococcus aureus, enteric bacilli, and Candida albicans.[16,17,18]
The extracellular matrix (ECM) plays an important role on bacterial adhesion to titanium surface, most notably Porphyromonas gingivalis.[19]
ECM is a biologically active tissue and is one of the main components of gingival tissue; it is composed by a mixture of collagens, proteoglycans, and glycoproteins.[20,21]
Degradation of ECM is caused by the acute and chronic inflammation induced by peri-implantitis pathogens, with the activation of matrix metalloproteinases (MMPs), especially MMP-1-3-8-13, and inflammatory cytokines (tumor necrosis factor alpha [TNF-α], interleukin-1 [IL-1], IL-6).[22,23]
Over the years, several authors have reported autoimmunity and the presence of autoantibodies against ECM constituents in patients affected by peri-implantitis.[24,25,26,27]
The aim of this study is to compare patients with a healthy peri-implant environment and patients affected by peri-implantitis, evaluating the occurrence of antibodies to ECM molecules.
MATERIALS AND METHODS
Study design
To address the research purpose, the authors designed and implemented a case–control study.
The study sample was composed by a population of participants presenting at the university's department for peri-implantitis treatment. Recruitment started in January 2016 and ended in July 2016.
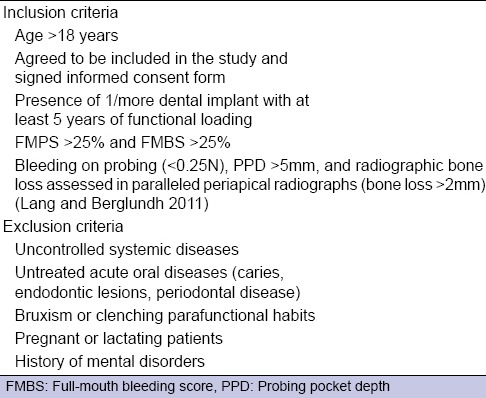
To be included in the study sample, patients had to meet specific inclusion and exclusion criteria [Tables 1 and 2]. All patients signed the informed consent form according to the World Medical Declaration of Helsinki.
Table 1.
Inclusion and exclusion criteria of control group (patients with healthy dental implants)

Table 2.
Inclusion and exclusion criteria of test group (patients with peri-implantitis)
The study was approved by the Institution Review Board.
Peri-implantitis assessment
Peri-implantitis was assessed through five clinical and radiological parameters:
Probing pocket depth (PPD)
Modified sulcus bleeding index (m SBI) (Mombelli et al. 1987)
Modified gingival index (m GI) (Mombelli et al. 1987)
Modified plaque index (m PI) (Mombelli et al. 1987)
Peri-implant bone loss.
Probing was recorded with a 15 UNC Color-Coded Probe (Hu-Friedy, Chicago, IL, USA) in 6 sites around each implant: Buccal, Mesio-Buccal, Disto-Buccal, Palatal, Mesio-Palatal, and Disto-Palatal.
A standardized (Rinn, Dentsply, York, PA, USA) periapical radiograph was taken for each implant to evaluate peri-implant bone loss levels. As for m SBI, m GI, and m PI, they were evaluated in 4 sites around implant and the mean value was calculated getting implant's score.
In detail, the diagnosis was established for dental implants having PPD values >5 mm, bleeding at probing or suppuration, and peri-implant bone loss >2 mm. Dental implants were considered as being “healthy” in the presence of PPD <5 mm, the absence of bleeding or suppuration, and peri-implant bone loss <2 mm.
Enzyme-linked immunosorbent assay
Serum of patients included in the study was collected and stored at −20°C until use.
Purified human type I, III, IV, and V collagens (CI/CIII/CIV/CV) and fibronectin (FN) were obtained from Chemicon International (Temecula, CA, USA).
Purified laminin (LM) was obtained from Sigma-Aldrich (Saint Louis, Missouri, USA). Mouse monoclonal antibodies antihuman CI/CIII/CIV/CV were obtained from Chemicon International (Temecula, CA, USA); mouse monoclonal antibodies antihuman FN and rabbit polyclonal antibodies antihuman IgG and IgM were obtained from Sigma-Aldrich (Saint Louis, Missouri, USA).
ECM antigens and bovine serum albumin (BSA) were diluted at 1–2 μg/mL; 100 mL of each mixture were incubated overnight at 37°C in polyvinyl chloride microtiter plates (Dynatech, Chantilly, VA, USA).
Antigen-coated wells were then blocked with 5% nonfat dry milk in PBS for 1 h at 37°C and incubated with human sera.
Sera were initially assayed at 1:25, 1:50, and 1:100 dilutions; each serum was assayed in duplicate for reactivity to ECM or BSA. Anti-ECM antibodies diluted at 1 μg/mL were used as positive controls.
After an overnight incubation at 4°C, the plates were washed 5 times with 1% nonfat dry milk in PBS, and goat antihuman IgG, IgM or goat anti-mouse or anti-rabbit IgG peroxidase-conjugated antibodies were added and incubated for 1 h at 37°C.
The plates were washed and wells were layered with a solution containing o-Phenylenediamine dihydrochloride in the presence of H2O2.
The reaction was blocked with 50 μL of H2SO4 (4N) and absorbance of samples was read at 492 nm. Any serum producing an OD exceeding mean of control sera OD values ± 3 standard deviation (SD) was considered positive for autoantibodies presence.
Statistical analysis
Descriptive statistics (mean, frequency, range, SDs) was computed; the Fisher exact test was performed to evaluate statistical association.
P < 0.05 was considered statistically significant.
A specific statistical software (IBM SPSS V10 Statistics, IBM, Armonk, USA) was used to analyze the data.
RESULTS
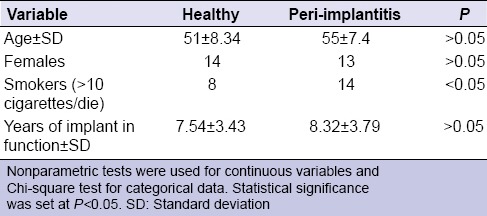
Forty-two patients were enrolled in this study, 27 females (64.28%) and 15 males (35.72%) with a mean age of 53 ± 29.69 years (age range 32–74).
Twenty-one patients had a diagnosis of peri-implantitis in at least one dental implant and constituted the test group, while 21 participants represented the control group, with dental implants classified as being healthy [Table 3].
Table 3.
Patients’ characteristics of study and control groups
Dental implants of different brands were used, all implants had at least 5 years of functional loading.
The presence of immunoglobulins directed toward conformational epitopes of ECM collagens and glycoproteins in patient's sera was assessed by enzyme-linked immunosorbent assay.
The presence of antibodies to CIII was recorded in 6/21 (28.57%) patients of test group, compared to just 2/21 (9.52%) for the control group, showing a statistically significant difference (P < 0.05).
Just two patients showed antibodies to CIV in the test group, while none was observed in the control group (P > 0.05).
None of the patients in both groups showed antibodies to CV and FN.
Antibodies anti-CI and anti-LM were found in either group; anti-CI: 3/21 in test group and 2/21 in control group, anti-LM: 2/21 in test group and 2/21 in control group; however, their values were not statistically significant (P > 0.05).
DISCUSSION
The authors hypothesized the presence of ECM autoantibodies in serum of peri-implantitis patients; according to our results, only anti-CIII antibodies presented statistically significant values compared to control group.
Peri-implantitis was defined as a chronic inflammatory lesion, characterized by peri-implant bone loss, bleeding at probing and suppuration.[28]
Albrektsson et al. in 2017, highlighted how lack of consensus on definition and diagnosis may affect peri-implantitis prevalence.[29]
Papathanasiou et al. in 2016, founded eight different definitions of peri-implantitis, based on combination of several marginal bone loss values and PPD considered as threshold.[30]
Peri-implantitis represents an emerging disease and like periodontitis, occurs mainly as a result of an overwhelming bacterial insult and subsequent host immune response, with a spontaneous progression if left untreated.[31,32]
In particular, several authors have reported that bacterial species associated with periodontitis and peri-implantitis are similar, including mainly Gram-negative anaerobes such as P. gingivalis, Prevotella intermedia, and Aa.[33,34]
Furthermore, proinflammatory cytokines (e.g. IL-1, IL-6, IL-8, and TNF-α) are upregulated in peri-implantitis and several authors suggested a possible role of autoimmunity as one of the pathogenic mechanisms of development of peri-implantitis.[35,36]
Autoantibodies to CI, FN, and LN have been detected in periodontitis patients, associated to an high level of cytokines and chemical mediators of inflammation.[37,38]
It is well established and documented that an abnormal activation of inflammation may lead to the development of autoimmune phenomena with the production of autoantibodies, which may contribute to damage caused by local inflammation.[39,40]
In this study, we demonstrated the presence of anti-CIII antibodies in peri-implantitis patients, thus suggesting that they may have a pathogenic role in the development of peri-implantitis.
Other antibodies tested were found to be not statistically significant or absent.
Autoantibodies directed toward ECM constituents may mediate bacterial adhesion to the implant surface, therefore, promoting biofilm formation and propagation.
The small size of the sample and absence of several ECM constituents, tenascin and vitronectin among others, in the group of antibodies tested, represented major limitations for this study.
Furthermore, due to the simultaneous presence of anti-CI/LM in either group, these antibodies might not influence final outcome of implant rehabilitation.
CONCLUSIONS
Within the limitations of this study, it can be concluded that further studies, with larger sample and different design, are necessary to address the research purpose, evaluating possible associations between anti-ECM antibodies and peri-implantitis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Albrektsson T, Donos N Working Group. Implant survival and complications. The Third EAO consensus conference 2012. Clin Oral Implants Res. 2012;23(Suppl 6):63–5. doi: 10.1111/j.1600-0501.2012.02557.x. [DOI] [PubMed] [Google Scholar]
- 2.Shibli JA, Pires JT, Piattelli A, Iezzi G, Mangano C, Mangano F, et al. Impact of different implant surfaces topographies on peri-implant tissues: An update of current available data on dental implants retrieved from human jaws. Curr Pharm Biotechnol. 2017;18:76–84. doi: 10.2174/1389201017666161221120618. [DOI] [PubMed] [Google Scholar]
- 3.Brauner E, Guarino G, Jamshir S, Papi P, Valentini V, Pompa V, et al. Evaluation of highly porous dental implants in postablative oral and maxillofacial cancer patients: A prospective pilot clinical case series report. Implant Dent. 2015;24:631–7. doi: 10.1097/ID.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 4.Eick S, Kindblom C, Mizgalska D, Magdon A, Jurczyk K, Sculean A, et al. Adhesion of Porphyromonas gingivalis and Tannerella forsythia to dentin and titanium with sandblasted and acid etched surface coated with serum and serum proteins-An in vitro study. Arch Oral Biol. 2017;75:81–8. doi: 10.1016/j.archoralbio.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Peixoto CD, Almas K. The implant surface characteristics and peri-implantitis. An evidence-based update. Odontostomatol Trop. 2016;39:23–35. [PubMed] [Google Scholar]
- 6.Koodaryan R, Hafezeqoran A. Evaluation of implant collar surfaces for marginal bone loss: A systematic review and meta-analysis. Biomed Res Int 2016. 2016 doi: 10.1155/2016/4987526. 4987526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cicciù M, Matacena G, Signorino F, Brugaletta A, Cicciù A, Bramanti E. Relationship between oral health and its impact on the quality life of Alzheimer's disease patients: A supportive care trial. Int J Clin Exp Med. 2013;6:766–72. [PMC free article] [PubMed] [Google Scholar]
- 8.Doornewaard R, Christiaens V, De Bruyn H, Jacobsson M, Cosyn J, Vervaeke S, et al. Long-Term effect of surface roughness and patients’ factors on crestal bone loss at dental implants. A systematic review and meta-analysis. Clin Implant Dent Relat Res. 2017;19:372–99. doi: 10.1111/cid.12457. [DOI] [PubMed] [Google Scholar]
- 9.Benic GI, Bernasconi M, Jung RE, Hämmerle CH. Clinical and radiographic intra-subject comparison of implants placed with or without guided bone regeneration: 15-year results. J Clin Periodontol. 2017;44:315–25. doi: 10.1111/jcpe.12665. [DOI] [PubMed] [Google Scholar]
- 10.De Angelis F, Papi P, Mencio F, Rosella D, Di Carlo S, Pompa G. Implant survival and success rates in patients with risk factors: Results from a long-term retrospective study with a 10 to 18 years follow-up. Eur Rev Med Pharmacol Sci. 2017;21:433–7. [PubMed] [Google Scholar]
- 11.Sendyk DI, Rovai ES, Pannuti CM, Deboni MC, Sendyk WR, Wennerberg A. Dental implant loss in older versus younger patients: A systematic review and meta-analysis of prospective studies. J Oral Rehabil. 2017;44:229–36. doi: 10.1111/joor.12465. [DOI] [PubMed] [Google Scholar]
- 12.Salvi GE, Cosgarea R, Sculean A. Prevalence and mechanisms of peri-implant diseases. J Dent Res. 2017;96:31–7. doi: 10.1177/0022034516667484. [DOI] [PubMed] [Google Scholar]
- 13.Lindhe J, Meyle J Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8 Suppl):282–5. doi: 10.1111/j.1600-051X.2008.01283.x. [DOI] [PubMed] [Google Scholar]
- 14.Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35(8 Suppl):286–91. doi: 10.1111/j.1600-051X.2008.01274.x. [DOI] [PubMed] [Google Scholar]
- 15.Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42(Suppl 16):S158–71. doi: 10.1111/jcpe.12334. [DOI] [PubMed] [Google Scholar]
- 16.Mencio F, De Angelis F, Papi P, Rosella D, Pompa G, Di Carlo S. A randomized clinical trial about presence of pathogenic microflora and risk of peri-implantitis: Comparison of two different types of implant-abutment connections. Eur Rev Med Pharmacol Sci. 2017;21:1443–51. [PubMed] [Google Scholar]
- 17.Ata-Ali J, Candel-Marti ME, Flichy-Fernández AJ, Peñarrocha-Oltra D, Balaguer-Martinez JF, Peñarrocha Diago M. Peri-implantitis: Associated microbiota and treatment. Med Oral Patol Oral Cir Bucal. 2011;16:e937–43. doi: 10.4317/medoral.17227. [DOI] [PubMed] [Google Scholar]
- 18.Mencio F, Papi P, Di Carlo S, Pompa G. Salivary bacterial leakage into implant-abutment connections: Preliminary results of an in vivo study. Eur Rev Med Pharmacol Sci. 2016;20:2476–83. [PubMed] [Google Scholar]
- 19.Mahmoud H, Williams DW, Hannigan A, Lynch CD. Influence of extracellular matrix proteins in enhancing bacterial adhesion to titanium surfaces. J Biomed Mater Res B Appl Biomater. 2012;100:1319–27. doi: 10.1002/jbm.b.32698. [DOI] [PubMed] [Google Scholar]
- 20.Germano F, Bramanti E, Arcuri C, Cecchetti F, Cicciù M. Atomic force microscopy of bacteria from periodontal subgingival biofilm: Preliminary study results. Eur J Dent. 2013;7:152–8. doi: 10.4103/1305-7456.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang YC, Yang SF, Lai CC, Liu JY, Hsieh YS. Regulation of matrix metalloproteinase production by cytokines, pharmacological agents and periodontal pathogens in human periodontal ligament fibroblast cultures. J Periodontal Res. 2002;37:196–203. doi: 10.1034/j.1600-0765.2002.00663.x. [DOI] [PubMed] [Google Scholar]
- 22.McKnight H, Kelsey WP, Hooper DA, Hart TC, Mariotti A. Proteomic analyses of human gingival and periodontal ligament fibroblasts. J Periodontol. 2014;85:810–8. doi: 10.1902/jop.2013.130161. [DOI] [PubMed] [Google Scholar]
- 23.Cavalla F, Osorio C, Paredes R, Valenzuela MA, García-Sesnich J, Sorsa T, et al. Matrix metalloproteinases regulate extracellular levels of SDF-1/CXCL12, IL-6 and VEGF in hydrogen peroxide-stimulated human periodontal ligament fibroblasts. Cytokine. 2015;73:114–21. doi: 10.1016/j.cyto.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Maciejczyk M, Pietrzykowska A, Zalewska A, Knas M, Daniszewska I. The significance of matrix metalloproteinases in oral diseases. Adv Clin Exp Med. 2016;25:383–90. doi: 10.17219/acem/30428. [DOI] [PubMed] [Google Scholar]
- 25.Masuelli L, Pompa G, Fabrizi M, Quaranta A, Vozza I, Piccoli L, et al. Patients with peri-implantitis, unlike those with a healthy periimplant microenvironment, display antibodies to more than one heat shock protein (HSP 27, HSP 65 and HSP 90) linear epitope. Eur J Inflamm. 2011;9:257–67. [Google Scholar]
- 26.Quaranta A, Piattelli A, Scarano A, Quaranta M, Pompa G, Iezzi G. Light-microscopic evaluation of the dimensions of peri-implant mucosa around immediately loaded and submerged titanium implants in monkeys. J Periodontol. 2008;79:1697–703. doi: 10.1902/jop.2008.070631. [DOI] [PubMed] [Google Scholar]
- 27.Candel-Martí ME, Flichy-Fernández AJ, Alegre-Domingo T, Ata-Ali J, Peñarrocha-Diago MA. Interleukins IL-6, IL-8, IL-10, IL-12 and periimplant disease. An update. Med Oral Patol Oral Cir Bucal. 2011;16:e518–21. doi: 10.4317/medoral.16.e518. [DOI] [PubMed] [Google Scholar]
- 28.Sanz M, Chapple IL Working Group of the VIII European Workshop on Periodontology. Clinical research on peri-implant diseases: Consensus report of Working Group 4. J Clin Periodontol. 2012;39(Suppl 12):202–6. doi: 10.1111/j.1600-051X.2011.01837.x. [DOI] [PubMed] [Google Scholar]
- 29.Albrektsson T, Chrcanovic B, Östman PO, Sennerby L. Initial and long-term crestal bone responses to modern dental implants. Periodontol 2000. 2017;73:41–50. doi: 10.1111/prd.12176. [DOI] [PubMed] [Google Scholar]
- 30.Papathanasiou E, Finkelman M, Hanley J, Parashis AO. Prevalence, etiology and treatment of peri-implant mucositis and peri-implantitis: A survey of Periodontists in the united states. J Periodontol. 2016;87:493–501. doi: 10.1902/jop.2015.150476. [DOI] [PubMed] [Google Scholar]
- 31.Schminke B, Vom Orde F, Gruber R, Schliephake H, Bürgers R, Miosge N. The pathology of bone tissue during peri-implantitis. J Dent Res. 2015;94:354–61. doi: 10.1177/0022034514559128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klinge B, Meyle J Working Group. Peri-implant tissue destruction. The Third EAO Consensus Conference 2012. Clin Oral Implants Res. 2012;23(Suppl 6):108–10. doi: 10.1111/j.1600-0501.2012.02555.x. [DOI] [PubMed] [Google Scholar]
- 33.Rakic M, Grusovin MG, Canullo L. The microbiologic profile associated with peri-implantitis in humans: A systematic review. Int J Oral Maxillofac Implants. 2016;31:359–68. doi: 10.11607/jomi.4150. [DOI] [PubMed] [Google Scholar]
- 34.Tonetti MS, Chapple IL, Jepsen S, Sanz M. Primary and secondary prevention of periodontal and peri-implant diseases: Introduction to, and objectives of the 11th European Workshop on Periodontology consensus conference. J Clin Periodontol. 2015;42(Suppl 16):S1–4. doi: 10.1111/jcpe.12382. [DOI] [PubMed] [Google Scholar]
- 35.De-Gennaro LA, Lopes JD, Mariano M. Autoantibodies directed to extracellular matrix components in patients with different clinical forms of periodontitis. J Periodontol. 2006;77:2025–30. doi: 10.1902/jop.2006.060104. [DOI] [PubMed] [Google Scholar]
- 36.Ejeil AL, Igondjo-Tchen S, Ghomrasseni S, Pellat B, Godeau G, Gogly B. Expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in healthy and diseased human gingiva. J Periodontol. 2003;74:188–95. doi: 10.1902/jop.2003.74.2.188. [DOI] [PubMed] [Google Scholar]
- 37.Cicciù M, Herford AS, Cervino G, Troiano G, Lauritano F, Laino L. Tissue fluorescence imaging (VELscope) for quick non-invasive diagnosis in oral pathology. J Craniofac Surg. 2017;28:e112–5. doi: 10.1097/SCS.0000000000003210. [DOI] [PubMed] [Google Scholar]
- 38.Govze Y, Herzberg MC. Serum and gingival crevicular fluid anti-desmosomal antibodies in periodontitis. J Periodontol. 1993;64:603–8. doi: 10.1902/jop.1993.64.7.603. [DOI] [PubMed] [Google Scholar]
- 39.Novo E, Viera N. Antineutrophil cytoplasmic antibodies: A missing link in the pathogenesis of periodontal disease? J Periodontal Res. 1996;31:365–8. doi: 10.1111/j.1600-0765.1996.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 40.Séguier S, Gogly B, Bodineau A, Godeau G, Brousse N. Is collagen breakdown during periodontitis linked to inflammatory cells and expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human gingival tissue? J Periodontol. 2001;72:1398–406. doi: 10.1902/jop.2001.72.10.1398. [DOI] [PubMed] [Google Scholar]


