Abstract
Objective:
Passive smoking leads to melanin pigmentation on gingiva. However, documentation of gingival pigmentation and salivary amylase activity in passive smokers relative to the duration of exposure to environmental tobacco smoke (ETS), is incomplete and requires further investigation. Thus, this study aimed to assess effects of ETS exposure on gingival pigmentation in young adults. In addition, to correlate a number of years of exposure to an extent, the intensity of gingival pigmentation and salivary amylase activity.
Materials and Methods:
A total of 200 nonsmokers aged 18–35 years with a positive history of ETS exposure were recruited for the study. Duration and source of ETS were assessed using a questionnaire. Gingival pigmentation was assessed using gingival pigmentation index for the extent and Dummett oral pigmentation index for intensity. The skin color of all patients was also assessed. Pearson Chi-square test and one-way ANOVA was used to statistically analyze the data.
Results:
Number of years of exposure to passive smoking was highly significant to the extent and intensity of gingival pigmentation (P < 0.001). ETS from home environment was highly significant to the intensity of pigmentation. Environmental sources of ETS contributed to pigmentation as the majority of patients reported exposure from vehicles and workplace. The salivary amylase levels were inversely proportional to the duration of exposure to ETS.
Conclusion:
Within limitations of this cross-sectional observational study, it was concluded that there was a strong correlation between ETS exposure and gingival pigmentation. Duration of exposure was significant to an extent, the intensity of pigmentation and salivary amylase activity.
Keywords: Environmental tobacco smoke, gingiva, melanin, passive smoking, young adults
INTRODUCTION
The World Health Organization has indicated that chronic environmental tobacco smoke (ETS) exposure, especially among young adults increases the risk of serious health hazards such as impaired lung function and increased incidence of subsequent lung cancer.[1] Brownish or black pigmentation in human gingiva has been reported in several countries.[2] The prevalence rate of gingival pigmentation is diverse according to race and country.[3] Physiological gingival pigmentation is more in dark skinned individuals compared to light-skinned patients.[4] About 15% of Europeans have oral pigmentation, and this rate reaches 80% in the Asian population.[5] Pigmentation in human gingiva derives from melanin granules, which are synthesized in melanosomes of melanocytes.[6]
The prevalence of gingival pigmentation in smokers increases and reaches maximum levels even on slight exposure to smoking in minimal categories of duration of smoking and number of cigarettes smoked.[7] The increase in gingival pigmentation was reported in 21.5% of smokers and intensity of pigmentation was related to a number of cigarettes consumed.[8] Various studies have observed a positive association of ETS exposure from parents on gingival pigmentation in children.[9,10,11] Further, the extent of gingival pigmentation has been observed to be higher in adolescents who are exposed to ETS at home compared to those who are not exposed.[12] A relationship between second-hand smoke and gingival pigmentation in women has been observed, and this effect was magnified when residing in smaller houses.[13] These findings are significant when applied to India; since the majority of the urban population are from a lower socio-economic status and reside in small households. Apart from that, the other sources of ETS for young adults could be workplace and vehicles. There remains a need to quantify the dose from passive smoking in more representative samples of the population to estimate the burden that passive smoking may impose on the whole community. Human whole saliva is an important body fluid that contains a highly complex mixture of substances similar to other body fluids in many aspects.[14] It has been observed that salivary alpha-amylase levels may be influenced by smoking and exposure to ETS in children.[15] Gingival pigmentation could be an easy method to assess as well as educate patients in terms of their oral health.[12] There have been no studies assessing the effect of ETS exposure from various environmental sources, its relationship with gingival pigmentation and salivary amylase activity in young adults. Thus, the aim of this study was to assess the effects of ETS exposure from various sources and its relationship to gingival pigmentation in young adults. In addition, to correlate a number of years of exposure with extent, the intensity of gingival pigmentation and salivary alpha amylase activity.
MATERIALS AND METHODS
A total of 200 nonsmoking systemically healthy subjects aged 18–35 years were randomly selected from patients reporting to Department of Periodontics, SRM Dental College, Ramapuram, Chennai. The inclusion criteria were subjects with a positive history of exposure to ETS. Subjects with long-term use of minocycline, naevi, antimalarial drugs, Kaposi's sarcoma, melanomas, Addison's disease, or amalgam tattoos were excluded from the study. Informed consent from all patients was obtained and the study was approved by the ethical committee of the institute. All the measurements were carried out by a single examiner who had been calibrated. ETS exposure was assessed through a questionnaire regarding ETS exposure at home from parents, spouse for married individuals and cohabitants for unmarried individuals. Environmental sources included college, workplace, and vehicles. Unstimulated whole saliva samples were collected from all subjects 1 h after breakfast. The collected samples were immediately centrifuged at 3000 rpm for 10 min at 4°C to remove cell debris and stored frozen until time of assay. The Quantichrom™ α-amylase kit DAMY 100 (Bioassay Systems San Francisco, California, USA) was used for the assessment of salivary α-amylase. The quantitative alpha-amylase activity was assessed by a colorimeter assay (595 nm). The intensity of color produced was proportional to the activity of the enzyme in the saliva samples.[16]
The subjects were then examined for the presence of melanin pigmentation on the gingiva clinically, and the extent of pigmentation was correlated with digital photographs which were then reproduced on a computer display. The extent of brownish or black pigmentation units on the gingiva of labial aspect of anterior teeth was classified according to a modification of melanin index categories - gingival pigmentation index (GPI)[17] as follows: 0 = no pigmentation; 1 = solitary units of pigmentation in papillary gingiva without the formation of a continuous ribbon between solitary units; and 2 = 1 unit of formation of a continuous ribbon extending from two neighboring solitary units.
The intensity of pigmentation was recorded using the Dummett oral pigmentation index (DOPI)[18] as follows 0 = Pink tissue (no clinical pigmentation); 1 = Mild, light brown tissue (mild clinical pigmentation); 2 = Medium brown or mixed pink and brown tissue (moderate clinical pigmentation); 3 = deep brown or blue/black tissue (heavy clinical pigmentation). To compare three or more mean values one-way ANOVA was applied and to compare proportions. Chi-square test was applied. SPSS version 22.0 (IBM, United States) was used to analyze the data. Significance level was fixed as 5% (α = 0.05).
RESULTS
The mean age of participants was 23.7 ± 3.1 years. 78% were females, and 22% were males. None of the participants was active smokers and the average years of exposure to ETS were 4.31 ± 2.8 years with a maximum of 15 years. The mean exposure to passive smoke per day was 13.1 ± 14.4 min. The extent of gingival pigmentation according to GPI index; 51.5% of patients exhibited a continuous ribbon extending from neighboring solitary units (score 2). The intensity of pigmentation was found to be medium brown in 53% and deep brown in 8.5% of patients as assessed by DOPI [Figure 1]. The number of years of exposure to passive smoking was highly significant when compared to the extent and intensity of gingival pigmentation (P < 0.001) [Tables 1 and 2]. The intensity of pigmentation was highly significant to skin color as well (P = 0.001). ETS exposure from home was assessed in this study, and it was observed that 39% of patients reported to have at least 1 smoking parent and out of that 69% exhibited formation of continuous ribbon [Table 3]. The ETS exposure from parents and spouse was not significant to the extent of the pigmentation (P = 0.375) and (P = 0.062), respectively [Figures 2 and 3]. However, the intensity of pigmentation exhibited a moderate significance when the source was from parents (P = 0.003) and highly significant when the source was a spouse (P < 0.001). In the present study, 99% of patients reported positive exposure to ETS from environmental sources, namely, vehicles and workplace. The intensity of pigmentation was highly significant to ETS exposure from workplace, namely, colleagues and co-workers (P = 0.018).
Figure 1.
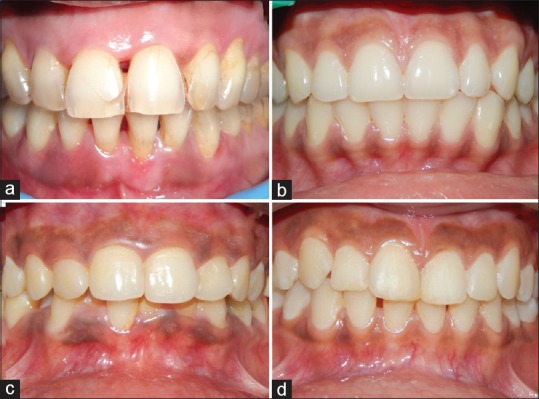
Depicting the intensity of gingival pigmentation (dummett oral pigmentation index) compared to years of exposure (a) no clinical pigmentation in <1 year of environmental tobacco smoke. (b) Mild clinical pigmentation in 5 years of environmental tobacco smoke. (c) Moderate clinical pigmentation in 10 years of environmental tobacco smoke. (d) Heavy clinical pigmentation in 15 years of environmental tobacco smoke
Table 1.
Comparison of number of years of environmental tobacco smoke exposure to gingival pigmentation index (extent of gingival pigmentation)
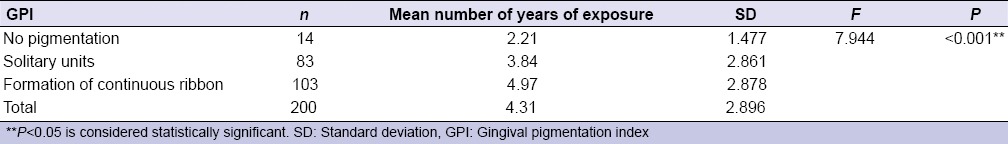
Table 2.
Comparison of number of years of environmental tobacco smoke exposure to Dummett oral pigmentation index (intensity of gingival pigmentation)
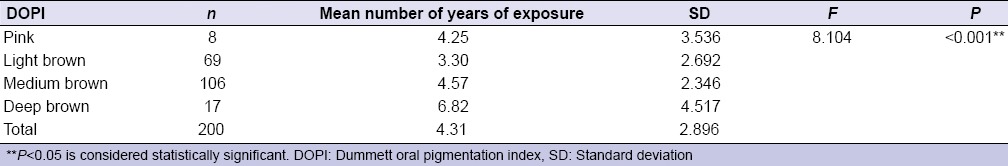
Table 3.
Comparison of environmental tobacco smoke from parent to Dummett oral pigmentation index (intensity of gingival pigmentation)
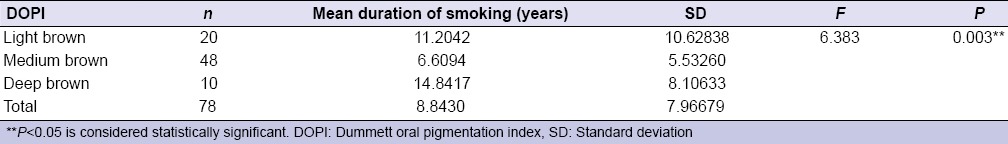
Figure 2.
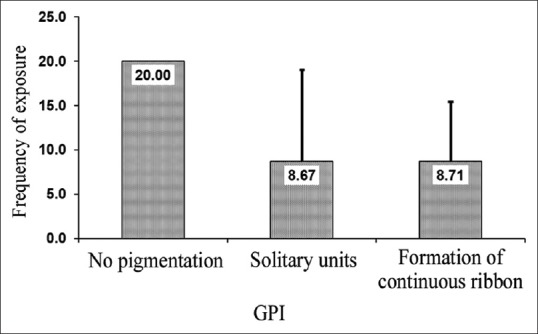
Representation of correlation of environmental tobacco smoke from parent to gingival pigmentation index (extent of gingival pigmentation)
Figure 3.
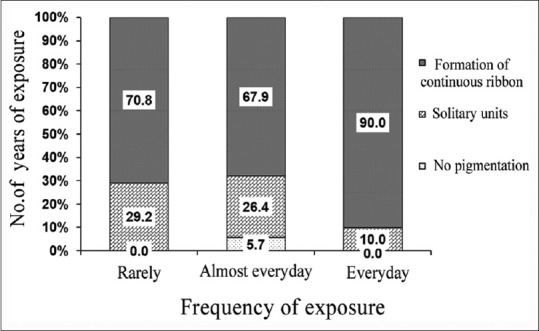
Representation of correlation between environmental tobacco smoke exposure from spouse to gingival pigmentation index (extent of gingival pigmentation)
Salivary alpha-amylase activity was assessed in all 200 patients. The levels were assessed in three subgroups according to number of years of exposure to ETS. Group 1 (1–5 years), Group 2 (6–10 years), and Group 3 (11–15 years). Amylase activity was expressed as mol maltose/mg protein. It was observed that subjects in Group 3 had a mean value of 10.07 (7.1–12.62), Group 2 had 11.10 (7.42–12.66), and Group 1 had the highest value of 14.40 (9.45–22.04). The amylase levels when compared between group 1 and 3 were highly significant (P < 0.05).
DISCUSSION
The results of this study revealed that prevalence of gingival pigmentation in young adults was directly proportional to the amount of ETS exposure. The previous studies did not take into consideration the environmental sources of ETS which add to the ETS burden. Apart from that the exact amount of time per day patients were exposed to passive smoking was also not assessed.[3,9,10,11,12] This study was unique in this aspect since it evaluated the ETS exposure from home and environmental sources while also accounting for the time exposed to passive smoking per day. The association between a number of years of exposure to ETS with extent and intensity of gingival pigmentation was highly significant. The greater the number of smoking years, the more was the extent of pigmentation in the present study. Madhani and Thomas, similarly observed that ETS from smoker parents causes more prevalence of gingival pigmentation in children which was statistically significant to a number of years exposed to ETS.[12]
It has been established that skin color is associated with the intensity of gingival pigmentation.[19] The extent of gingival pigmentation was more in fair skin patients. These findings are similar to a study done by Hajifattahi et al., in 2010 who observed that passive smoking leads to more pigmentation in the fair skin than patients with dark skin color.[10]
In the present study, the ETS exposure from parents was highly significant to the intensity of gingival pigmentation. Studies evaluating, association of melanin pigmentation of gingiva in children with parents who smoke observed a positive correlation to ETS exposure from parents to the prevalence of gingival pigmentation. Further, it was seen that percentage of smoking parents was higher in children who exhibited greater pigmentation than those who did not.[8,9] Similarly, Sreeja et al. 2015, observed that gingival pigmentation in children has been linked to passive smoking from parents and other adults who smoke.[20]
In the present study, the extent of gingival pigmentation was not significant to the severity of exposure from spouse, but the intensity of pigmentation was highly significant (P < 0.001). These findings are similar to a recent study in which relationship of gingival pigmentation in women exposed to second-hand smoke from their husbands was observed. The odds ratio of gingival pigmentation in women exposed to second-hand smoke from husbands was 3 and this effect was magnified in smaller houses.[14]
A factor that can skew the determination of true effects of ETS from parents and spouse is the impact of ETS from additional environmental sources. The ETS exposure from environmental sources such as vehicles and workplace was also assessed, and it was seen that 99% of patients reported positive ETS exposure from vehicles. Similarly, Aurrekoetxea et al. 2016 observed exposure to second-hand tobacco smoke in 4-year-old children in Spain and found that based on parental reports, more than half of children were exposed to ETS out of which 21.6% were exposed at home, whereas 41.7% were exposed elsewhere. Highlighting the environmental sources contributing to ETS exposure. They also found that children whose parents were from a lower educational level had a higher odds of exposure to ETS.[21]
In this study, ETS exposure from workplace was highly significant to the intensity of gingival pigmentation with 37.5% of subjects having positive exposure from workplace exhibiting medium brown and deep brown color (P = 0.018). The gingival pigmentation due to ETS exposure can be used as a visible tool for patient education to inform the ill effects of passive smoking on oral health and developing precancerous lesions. Cicciù et al., 2017 studied the use of an autofluorescence examination handheld device the Visually Enhanced Lesion Scope (VEL scope) system to delineate between benign, dysplastic, and malignant oral mucosa lesions and found it to be noninvasive. They noted that it can be used as a screening tool to educate patients since any pigmented or precancerous oral and gingival lesions can be screened easily and aid in the detection and diagnosis.[22]
The activity of amylase was decreased in patients with the highest exposure to ETS (11–15 years) compared to least exposure (1–5 years) which was statistically significant (P < 0.05). Similarly, Granger et al. 2007 reported lower salivary amylase activity for mothers, but not for infants as a result of exposure to tobacco smoke.[14] However, this is in contrast to Avsar et al., 2009 who observed higher salivary amylase activity in children with passive smoking.[15] This could be attributed to differences in age of subjects. In the present study, the low levels of amylase in subjects with high exposure to ETS can be explained by inhibition of salivary amylase by cigarette smoke may be due to the interaction between smoke aldehydes and–SH groups of the enzyme molecules.[15]
CONCLUSION
There is a correlation between ETS and gingival melanin pigmentation. Duration of ETS exposure is highly significant to both extent and intensity of pigmentation. Environmental sources of ETS significantly contributed to the gingival pigmentation. The salivary amylase levels were inversely proportional to the duration of exposure. The findings of the present study are helpful in understanding the impact of ETS exposure from various sources in the environment. In a country like India where no stringent smoke-free legislation exists, the social norm from smoking in home or car will shift to formal smoking restrictions or bans once the awareness about the impact of ETS exposure on oral health is educated to the general population. In addition, gingival pigmentation can be used as a visible tool for patient education.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jarvis M, Tunstall-Pedoe H, Feyerabend C, Vesey C, Salloojee Y. Biochemical markers of smoke absorption and self reported exposure to passive smoking. J Epidemiol Community Health. 1984;38:335–9. doi: 10.1136/jech.38.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Multani S. Interrelationship of smoking, lip and gingival melanin pigmentation, and periodontal status. Addict Health. 2013;5:57–65. [PMC free article] [PubMed] [Google Scholar]
- 3.Hanioka T, Tanaka K, Ojima M, Yuuki K. Association of melanin pigmentation in the gingiva of children with parents who smoke. Pediatrics. 2005;116:e186–90. doi: 10.1542/peds.2004-2628. [DOI] [PubMed] [Google Scholar]
- 4.Eisen D. Disorders of pigmentation in the oral cavity. Clin Dermatol. 2000;18:579–87. doi: 10.1016/s0738-081x(00)00148-6. [DOI] [PubMed] [Google Scholar]
- 5.Unsal E, Paksoy C, Soykan E, Elhan AH, Sahin M. Oral melanin pigmentation related to smoking in a Turkish population. Community Dent Oral Epidemiol. 2001;29:272–7. doi: 10.1034/j.1600-0528.2001.290406.x. [DOI] [PubMed] [Google Scholar]
- 6.Hedin CA, Larsson A. The ultrastructure of the gingival epithelium in smokers’ melanosis. J Periodontal Res. 1984;19:177–90. doi: 10.1111/j.1600-0765.1984.tb00806.x. [DOI] [PubMed] [Google Scholar]
- 7.Araki S, Murata K, Ushio K, Sakai R. Dose-response relationship between tobacco consumption and melanin pigmentation in the attached gingiva. Arch Environ Health. 1983;38:375–8. doi: 10.1080/00039896.1983.10545823. [DOI] [PubMed] [Google Scholar]
- 8.Tadakamadla J, Kumar S, Nagori A, Tibdewal H, Duraiswamy P, Kulkarni S. Effect of smoking on oral pigmentation and its relationship with periodontal status. Dent Res J (Isfahan) 2012;9(Suppl 1):S112–4. [PMC free article] [PubMed] [Google Scholar]
- 9.Haresaku S, Hanioka T, Tsutsui A, Watanabe T. Association of lip pigmentation with smoking and gingival melanin pigmentation. Oral Dis. 2007;13:71–6. doi: 10.1111/j.1601-0825.2006.01249.x. [DOI] [PubMed] [Google Scholar]
- 10.Hajifattahi F, Azarshab M, Haghgoo R, Lesan S. Evaluation of the relationship between passive smoking and oral pigmentation in children. J Dent (Tehran) 2010;7:119–23. [PMC free article] [PubMed] [Google Scholar]
- 11.Sridharan S, Ganiger K, Satyanarayana A, Rahul A, Shetty S. Effect of environmental tobacco smoke from smoker parents on gingival pigmentation in children and young adults: A cross-sectional study. J Periodontol. 2011;82:956–62. doi: 10.1902/jop.2010.100479. [DOI] [PubMed] [Google Scholar]
- 12.Madhani SM, Thomas B. Evaluation of gingival pigmentation in children exposed to and not exposed to environmental tobacco smoke. SRM J Res Dent Sci. 2014;5:21–5. [Google Scholar]
- 13.Moravej-Salehi E, Moravej-Salehi E, Hajifattahi F. Relationship of gingival pigmentation with passive smoking in women. Tanaffos. 2015;14:107–14. [PMC free article] [PubMed] [Google Scholar]
- 14.Granger DA, Blair C, Willoughby M, Kivlighan KT, Hibel LC, Fortunato CK, et al. Individual differences in salivary cortisol and alpha-amylase in mothers and their infants: Relation to tobacco smoke exposure. Dev Psychobiol. 2007;49:692–701. doi: 10.1002/dev.20247. [DOI] [PubMed] [Google Scholar]
- 15.Avsar A, Darka O, Bodrumlu EH, Bek Y. Evaluation of the relationship between passive smoking and salivary electrolytes, protein, secretory IgA, sialic acid and amylase in young children. Arc Oral Biol. 2009;54:457–63. doi: 10.1016/j.archoralbio.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Rinderknecht H, Wilding P, Haverback BJ. A new method for the determination of alpha-amylase. Experientia. 1967;23:805. doi: 10.1007/BF02146851. [DOI] [PubMed] [Google Scholar]
- 17.Hedin CA. Smokers’ melanosis. Occurrence and localization in the attached gingiva. Arch Dermatol. 1977;113:1533–8. doi: 10.1001/archderm.113.11.1533. [DOI] [PubMed] [Google Scholar]
- 18.Dummett CO, Gupta OP. The DOPI assessment in gingival pigmentation. J Dent Res. 1966;45:122. [Google Scholar]
- 19.Ponnaiyan D, Jegadeesan V, Perumal G, Anusha A. Correlating skin color with gingival pigmentation patterns in South Indians – A cross sectional study. Oral Health Dent Manag. 2014;13:132–6. [PubMed] [Google Scholar]
- 20.Sreeja C, Ramakrishnan K, Vijayalakshmi D, Devi M, Aesha I, Vijayabanu B. Oral pigmentation: A review. J Pharm Bioallied Sci. 2015;7(Suppl 2):S403–8. doi: 10.4103/0975-7406.163471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aurrekoetxea JJ, Murcia M, Rebagliato M, Guxens M, Fernández-Somoano A, López MJ, et al. Second-hand smoke exposure in 4-year-old children in Spain: Sources, associated factors and urinary cotinine. Environ Res. 2016;145:116–25. doi: 10.1016/j.envres.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 22.Cicciù M, Herford AS, Cervino G, Troiano G, Lauritano F, Laino L. Tissue fluorescence imaging (VELscope) for quick non-invasive diagnosis in oral pathology. J Craniofac Surg. 2017;28:e112–5. doi: 10.1097/SCS.0000000000003210. [DOI] [PubMed] [Google Scholar]


