Abstract
Gingival enlargements are frequently encountered in dental practice. There are different types of gingival enlargements and they vary according to the etiologic factors and pathologic processes that produce them. The exact diagnosis of the enlargement is important as some gingival enlargements can cause extensive morbidity or even mortality. Oral cancers especially squamous cell carcinomas present with variations in clinical presentation and the sites affected. A detailed medical history, clinical examination and radiographic evaluation will help identify the lesion. A biopsy will help provide a definitive diagnosis. An early diagnosis and treatment of squamous cell carcinomas is important as these tumours have a propensity for invasion of adjacent tissues and distant lymphatic metastasis which leads to a worsened prognosis. In this case report, the diagnosis and management of squamous cell carcinoma masquerading as a gingival overgrowth in the mandibular anterior region in a renal patient is reported. Dentists need to be aware and alert of the possibility of squamous cell carcinoma presenting on sites such as gingiva thereby preventing extensive morbidity and even mortality in these patients.
Keywords: Gingival overgrowth, oral cancer, renal patient, squamous cell carcinoma
INTRODUCTION
Oral cancer represented mainly by oral squamous cell carcinoma (SCC) is the eighth most common cancer worldwide accounting for more than 300,000 new cases and 145,000 deaths in 2012.[1] Despite recent advances in cancer detection and therapy, oral cancer remains a public health problem, especially in developing countries. A recent report of the GLOBOCAN Global Burden of Cancer study 2012 shows that the WHO southeast Asia region comprising mainly India, Sri Lanka, Pakistan, and Taiwan has the highest incidence and mortality rates of oral cancer worldwide. The GLOBOCAN data reveals that the crude rate and age-standardized incidence rate are higher in more developed regions, but the mortality rates are higher in less developed areas.[2]
SCC is the most common malignant neoplasm of the oral cavity (90%).[3] Among all cancers in men, SCC accounts for 4%, while in women it is 2%, the prognosis of the tumor depends on the stage at diagnosis.[4] The gingiva is not a frequent site of oral malignancy. Oral cancer accounts for 5% of all malignant tumors in the body of which 6% occur in the gingiva.[5] The most common malignant tumor of the gingiva is SCC. The majority (70%) of gingival SCCs (GSCCs) occur in the mandible.[6] Females are more affected by GSCC than males.[7] GSCC has been reported with varied clinical appearance, mainly as an exophytic mass with either a granular, papillary, or verrucous surface or as an ulcerative lesion. It is often a slow growing, asymptomatic lesion which is often misdiagnosed as chronic inflammatory enlargement, pyogenic granuloma, or a fibroma. The initial symptoms are usually an intraoral mass or swelling, ulceration, pain, ill-fitting denture, mobility of teeth, or unhealed extraction site.
SCC presents with different levels of differentiation and has a high propensity for lymph node metastasis. In spite of the recent advances in diagnosis and treatment modalities, <50% of oral SCC patients survive for 5 years.[8] Late diagnosis, regional lymph node metastasis, and recurrences are the major causes related to the poor prognosis and reduced survival for oral SCC patients.[9]
This case reports the diagnosis of GSCC in a renal patient which presented as a gingival overgrowth involving the lingual gingiva of mandibular anterior teeth.
CASE REPORT
A 49-year-old male patient visited our clinic in December 2015 with a chief complaint of swelling of gums in the lower jaw. An informed consent was obtained from the patient for taking a detailed case history and treatment planning.
The patient had been a smoker (4–5 cigarettes per day) for the past 20 years. He was also using alcohol frequently. He was hypertensive and was under medication (nifedipine) for the past 5 years. He was also under the care of the Nephrology unit of a local hospital for interstitial nephritis for the past 5 years.
His dental history recorded periodic oral prophylaxis visits. He stated that he had noticed a small swelling on the gums on the inner surface of the lower anterior teeth for 3 months. He visited a local dentist who prescribed an antibiotic ointment (Metronidazole gel) for topical application. However, the swelling continued to increase in size and so he decided to consult a specialist and reported to our clinic. The patient did not have any pain which was the reason for the delay in asking for a second opinion about the swelling.
Periodontal evaluation showed that he had chronic generalized periodontitis. The lingual gingiva of mandibular anterior teeth revealed an exophytic growth of size 4 cm × 3 cm, firm in consistency and sessile. The surface of the lesion was irregular, with areas of ulceration [Figure 1]. Deep periodontal pockets of 6–8 mm [lingual aspect] and mobility of mandibular anterior teeth [incisors] were recorded. Bleeding on probing was present.
Figure 1.
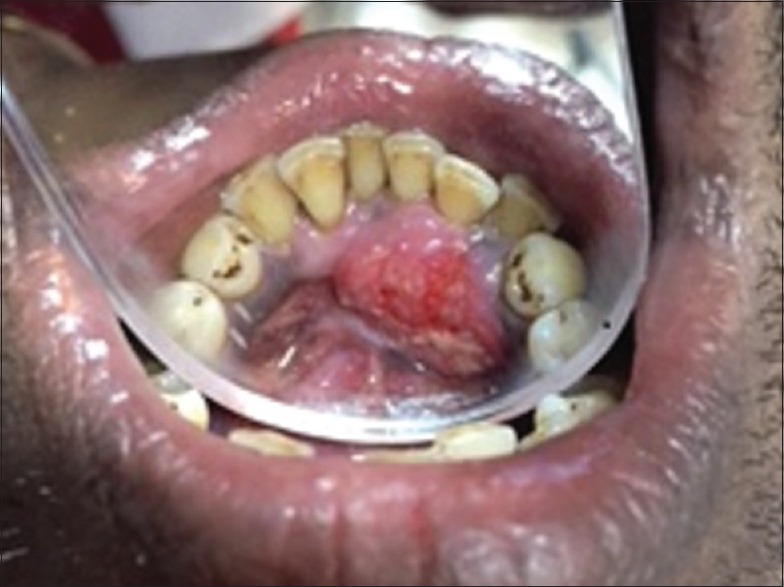
Gingival enlargement in mandibular anterior region lingually
Extraoral examination revealed palpable, firm, mobile, and nodular submandibular lymph nodes on the left side. The patient was initially advised to take an intraoral periapical radiograph. Radiographic evaluation showed extensive bone loss in relation to tooth number 41, 31, 32, and 33 [Figure 2].
Figure 2.
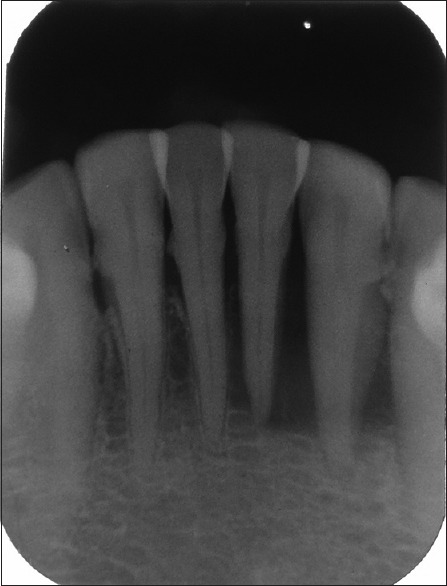
Intraoral periapical radiograph of mandibular anterior teeth showing extensive bone loss
Based on the patient's history, clinical and radiographic evaluation a provisional diagnosis of pyogenic granuloma was made with a differential diagnosis of SCC.
Nonsurgical periodontal therapy was done and chlorhexidine mouthwash was prescribed twice daily for 1 week. However, no change in the gingival enlargement was observed at 1 week recall. The case was discussed with his consultant nephrologist and an excisional biopsy of the lesion was done.
Histopathology
Histopathological examination showed hyperplastic hyperparakeratinized stratified squamous epithelium with features of dysplasia. The surface epithelium showed breach in the continuity of the basement membrane. The underlying connective tissue was densely collagenous and showed numerous islands of neoplastic epithelial cells and abundant keratin pearl formation. Moderate amount of chronic inflammatory infiltrate was also seen composed of lymphocytes, plasma cells, and neutrophils [Figure 3a and b]. Based on these histopathological findings, a diagnosis of well differentiated SCC was made.
Figure 3.
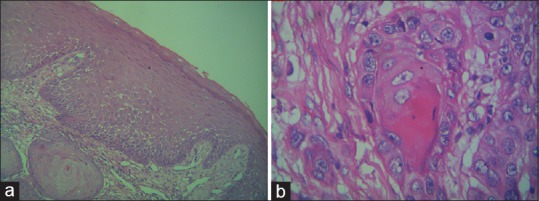
(a) Photomicrograph showing features of well-differentiated squamous cell carcinoma – low magnification (×10). (b) Photomicrograph showing features of well-differentiated squamous cell carcinoma – high magnification (×40)
A cone beam computed tomography scan was done to assess the extent of alveolar bone involvement. Severe bone loss was seen in relation to teeth number 31, 32, 33, and 41 extending up to the apical third giving a floating teeth appearance [Figure 4].
Figure 4.
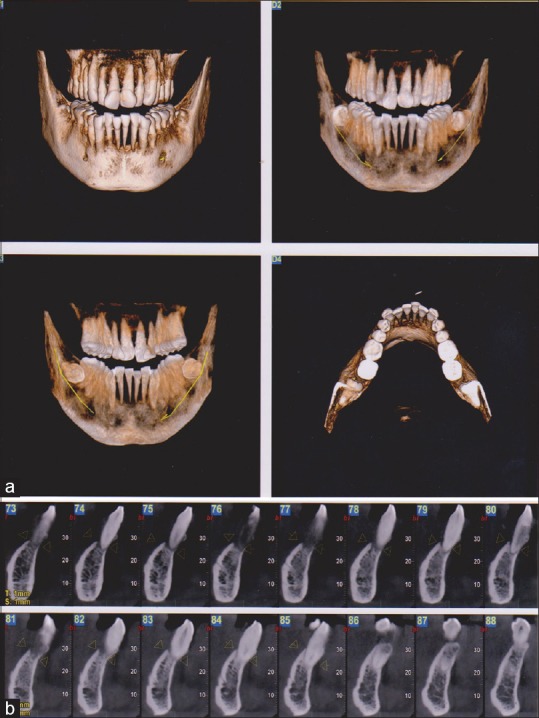
(a) Cone beam computed tomography image (three-dimensional reconstruction) of site of lesion showing extensive destruction of bone in mandibular anterior region. (b) Cone beam computed tomography image (cross-sectional view) showing bone loss in mandibular anterior teeth
To assess the extent of lymph node involvement an ultrasonogram of the head and neck was done. It revealed the presence of a small benign appearing subcutaneous nodule (4.6 mm × 3.1 mm) with central cystic degeneration in the right posterior triangle. A small submandibular lymph nodes measuring 2.8 mm in short axis and a few, small, level two, lymph nodes measuring 2–3 mm which appear reactive were noted on the left side. Echo texture of submandibular glands appeared normal. Bilateral thyroid gland appeared normal.
The patient was immediately referred to Regional Cancer Centre, Trivandrum (a comprehensive cancer hospital) for expert management. A wide excision of the lesion with margins was done followed by bilateral functional neck dissection of regional lymph nodes. This was followed by fractionated radiotherapy sessions. The patient is being followed up for the past 8 months and no signs of recurrence have been detected.
DISCUSSION
SCC is defined as a malignant epithelial neoplasm exhibiting squamous differentiation characterized by the formation of keratin and/or the presence of intercellular bridges.
The appearance of SCC in the gingiva makes its diagnosis confusing. It can be mistaken for other proliferative lesions of the gingiva such as pyogenic granuloma, inflammatory enlargements, drug-induced enlargements, or conditioned enlargement. Epstein et al. in 2012 has shown that clinical oral examination was considered to have poor overall performance as a diagnostic method for predicting dysplasia and oral SCC.
Therefore, it is important for a clinician to combine a thorough intraoral examination and radiographic evaluation to arrive at a provisional diagnosis. A conclusive diagnosis can be arrived only after a biopsy is taken and histopathological examination is done.
SCC of the gingiva more frequently involves the mandible than maxilla and 60% of these are located posterior to the premolars.[5] Other sites of GSCC reported include posterior maxilla, anterior maxilla, and rarely anterior mandible.[10] It is mainly observed in females older than 50 years. However, some studies have shown a male predilection.
The clinical presentation in this case was unique because it was located on the lingual gingiva of anterior teeth in the mandible. GSCC presenting as an aphthous ulcer of lingual gingiva near lingual frenum of mandible has been reported.[11]
The problem with GSCC is that in early stages the lesion may mimic periodontal disease and will be symptom less which could lead to a diagnostic delay. Another important feature of GSCC is its higher degree of aggressiveness with an increased invasion of adjacent tissues and increased chances of lymphatic metastatic spread. It is therefore imperative for the clinician to diagnose and manage the carcinoma early to prevent extensive spread and improve the prognosis of the lesion. In the present case, the confirmatory diagnosis was arrived with the histopathological report which showed the features of a well-differentiated SCC. There was invasion of adjacent bony tissue at the time of diagnosis (T4-primary tumor).
A strong association has been established between the advanced stage of disease and poor prognosis in oral cancer.[12] The presence of bone invasion in GSCC influences the presence of neck metastasis, treatment decisions (type of resection), and prognosis of the tumor. The overall survival rate for GSCC is said to be about 54%.[13]
In the present case, the patient suffered from interstitial nephritis, a form of chronic kidney disease. A search of dental literature shows no reports of renal diseases causing SCC of the gingiva. Therefore, in the present case, the development of GSCC could be purely a coincidental finding with the patient's history of smoking and alcohol use.
Treatment options have ranged from resection of maxilla and mandible, radical neck dissection depending on the extent of invasion, and lymphatic node spread. If diagnosed early, lesions have been treated with radiation therapy. Total diagnostic delay in oral cancer is defined as the time from the first onset of signs and symptoms up to the definitive diagnosis. In this case, the total diagnostic delay had been 3 months which is similar to other studies reported.[14]
CONCLUSIONS
General dentists frequently encounter oral lesions that are ambiguous in clinical presentation and behavior. Oral cancer is considered to be a major health concern and SCC is the most frequently associated neoplasm of the oral cavity. GSCCs are more common in the mandible than maxilla and 60% of these occur posterior to the mandible. However, dentists need to be aware and alert of the possibility of SCC presenting on the anterior gingiva as well, rather than traditional sites of presentation. A biopsy will give a definitive diagnosis and prompt treatment will help improve the prognosis. This case report reinforces the importance of taking gingival biopsies for all gingival swellings and submitting these biopsies for histopathological examination thereby decreasing the morbidity and subsequent mortality resulting from GSCC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol. 2015;8:11884–94. [PMC free article] [PubMed] [Google Scholar]
- 3.Casiglia J, Woo SB. A comprehensive review of oral cancer. Gen Dent. 2001;49:72–82. [PubMed] [Google Scholar]
- 4.Hoffman HT, Karnell LH, Funk GF, Robinson RA, Menck HR. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:951–62. doi: 10.1001/archotol.124.9.951. [DOI] [PubMed] [Google Scholar]
- 5.Barasch A, Gofa A, Krutchkoff DJ, Eisenberg E. Squamous cell carcinoma of the gingiva. A case series analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:183–7. doi: 10.1016/s1079-2104(05)80200-8. [DOI] [PubMed] [Google Scholar]
- 6.Soo KC, Spiro RH, King W, Harvey W, Strong EW. Squamous carcinoma of the gums. Am J Surg. 1988;156:281–5. doi: 10.1016/s0002-9610(88)80292-7. [DOI] [PubMed] [Google Scholar]
- 7.Barasch A, Morse DE, Krutchkoff DJ, Eisenberg E. Smoking, gender, and age as risk factors for site-specific intraoral squamous cell carcinoma. A case-series analysis. Cancer. 1994;73:509–13. doi: 10.1002/1097-0142(19940201)73:3<509::aid-cncr2820730303>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2006;63:64–76. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 9.Massano J, Regateiro FS, Januário G, Ferreira A. Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:67–76. doi: 10.1016/j.tripleo.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 10.Misra S, Chaturvedi A, Misra NC. Management of gingivobuccal complex cancer. Ann R Coll Surg Engl. 2008;90:546–53. doi: 10.1308/003588408X301136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumari PS, Kumar GP, Bai YD, Reddy EY. Gingival squamous cell carcinoma masquerading as an aphthous ulcer. J Indian Soc Periodontol. 2013;17:523–6. doi: 10.4103/0972-124X.118329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogura I, Kurabayashi T, Sasaki T, Amagasa T, Okada N, Kaneda T. Maxillary bone invasion by gingival carcinoma as an indicator of cervical metastasis. Dentomaxillofac Radiol. 2003;32:291–4. doi: 10.1259/dmfr/25125369. [DOI] [PubMed] [Google Scholar]
- 13.Kowalski LP, Franco EL, Torloni H, Fava AS, de Andrade Sobrinho J, Ramos G, et al. Lateness of diagnosis of oral and oropharyngeal carcinoma: Factors related to the tumour, the patient and health professionals. Eur J Cancer B Oral Oncol. 1994;30B:167–73. doi: 10.1016/0964-1955(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 14.Dissanayaka WL, Pitiyage G, Kumarasiri PV, Liyanage RL, Dias KD, Tilakaratne WM. Clinical and histopathologic parameters in survival of oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:518–25. doi: 10.1016/j.oooo.2011.11.001. [DOI] [PubMed] [Google Scholar]


