Abstract
Objective:
To evaluate the feasibility of using contrast medium (CM) of low and ultra-low volumes and injection rates in aortic CT angiography (CTA) through the joint application of single-source dual-energy CT (ssDECT) and adaptive statistical iterative reconstruction (ASIR).
Methods:
120 patients with known or suspected aortic dissection underwent aortic CTA and were equally divided into 3 groups. Conventional 120-kVp scan with a CM volume of 70 ml and an injection rate of 5 ml s−1 was performed on Group A. Groups B and C underwent ssDECT scan with CM volumes of 0.6 and 0.4 ml kg−1, respectively. 40% and 50% ASIR algorithms were applied for Groups B and C, respectively. A five-point grading scheme was utilized to subjectively evaluate the image quality, and the CT value and contrast-to-noise ratio were recorded as objective measures. The radiation dose was also evaluated.
Results:
Groups B and C had equivalent subjective scores and CT values as Group A, whereas they had higher or equivalent contrast-to-noise ratios. Group B had 40.1% and 30% reductions on CM volume and injection rate, respectively, than Group A. Group C further resulted in 19.2% and 22% lesser CM volume and injection rate than Group B. The average effective radiation doses for the study groups were 22.5–24.5% lower than the control group.
Conclusion:
With the aid of ASIR and ssDECT for aortic CTA, it is feasible to adopt low and ultra-low CM volumes and injection rates while obtaining good quality images.
Advances in knowledge:
Low and ultra-low CM volumes and injection rates are feasible in CTA through the joint application of ssDECT and ASIR.
INTRODUCTION
The multislice spiral CT angiography (CTA) plays an important role in the diagnosis of aortic diseases.1–6 CTA is associated with high contrast medium (CM) volumes and injection rates. In addition, because of the requirements of CTA, including fast scan times and high spatial resolution, the resulting radiation doses can be high. A close relationship between the CM volume and occurrence of contrast-induced nephropathy has been demonstrated in the literature,7,8 and the CM injection rate is noted to be positively correlated to the risk of intravenous access failure, particularly for patients with poor peripheral venous status.9 Moreover, the increasing potential of radiation-induced malignancies from CT strongly motivate the reduction of radiation exposure in clinical practices.10–12 Hence, minimal volume and injection rate of CM with low radiation dose is preferred for patients. However, conventional 120-kVp scans with reduced CM volumes tend to lead to poor opacification of the aortic lumen, which limits the diagnostic information from the scan. Improvements in the areas of low tube voltage, high pitch mode,13–15 volume acquisitions16 and single-source dual-energy CT (ssDECT) have further enabled reductions in CM volumes and radiation dose in CTA.
Recently, a 64-slice CT scanner (Discovery CT, GE Healthcare, WI) that supports ssDECT in the latest gemstone spectral imaging (GSI) mode was developed. The new ssDECT could reconstruct any monochromatic image with a broad range of energy levels from 40 to 140 keV. When applying low energy levels to improve CT attenuations, image noise tends to increase due to the reduced number of photons in the X-ray spectrum.17,18 Iterative reconstruction algorithms such as adaptive statistical iterative reconstruction (ASIR) have also been shown to be effective in reducing image noise and improving the image quality.19–22 However, previous GSI modes only allow the joint application of ASIR and ssDECT with energy levels of 65 and 70 keV. The new GSI version removes the restriction on the energy level in the joint utilization and thus provides valuable chances of applying ASIR and ssDECT to reduce the CM volumes, CM injection rates and radiation doses.23,24 Analyses on such topics are still rare. To our knowledge, the only exception lies in abdominal CT, which investigates the feasibility of reducing CM volume and radiation dose by 14% and 41%, respectively, as compared with the conventional scan protocol.23
The purpose of this prospective study is to investigate the effectiveness of supplementing the ssDECT with ASIR in order to reduce iodine CM volume and injection rate, as well as the radiation dose for aortic CTA.
METHODS AND MATERIALS
Patient population
From June 2014 to May 2015, 145 patients with known or suspected aortic dissection underwent thoracoabdominal aortic CTA. The exclusion criteria were heart failure, heart rate >100 bpm, compromised renal function with estimated glomerular filtration rate (eGFR) <30 ml min−1/1.73 m2; documented hypersensitivity to iodine-containing CM and aortic aneurysm. 25 (17.2%) of the 145 patients were excluded as follows: 4 patients had heart rate >100 bpm, there was a technical failure for 2 patients during the contrast injection and 19 patients had an aortic aneurysm. As a result, 120 patients referred to aortic CTA were included (86 males, 34 females; mean age, 55.9 ± 13.7 years). The patients were assigned into three groups with 40 patients each: Group A (the control group) and Groups B and C (study groups). The assignment process was based on the study period. Groups A, B and C comprised patients from the first, middle and last third of the study period, respectively. This prospective study was approved by the institutional review board of First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China, with informed consents of all patients obtained.
Image acquisition
All CT examinations were performed on a spectral CT scanner (Discovery CT, GE Healthcare) that supports both ssDECT mode and standard 120-kVp scan mode. The patients in Group A received conventional single-energy CTA with the following parameters: tube voltage 120 kVp, tube current was modulated to attain a noise index of 12, an automatic tube current modulation set to a maximum value of 600 mA, gantry rotation time 0.6 s and pitch 1.375 : 1. The patients in the study Groups B and C underwent ssDECT scan with the following parameters: helical scanning, tube voltage of dynamic switching between 80 and 140 kVp within 0.5 ms, a fixed tube current 360 mA, a 0.6-s tube rotation time, a 40-mm detector coverage, a pitch factor of 1.375 : 1 and a 35-cm display field of view. The images of all groups were reconstructed with a slice thickness of 1.25 mm and interval of 1.25 mm. The scan range was from 2 cm above the aortic arch to the pubic symphysis level. The imaging delay was determined by the automatic image-triggering software (SmartPrep™; GE Healthcare). Image acquisition started automatically after 5.6 s since the pre-determined threshold (set at 100 HU for the control group and 50 HU for the study groups following extant literature25 and our clinical experience) was reached in the region of interest (ROI) placed at the region of the ascending aorta.
The non-ionic intravenous CM (Iohexol, Omnipaque™ 350 mgI ml−1; GE Healthcare, WI) was injected using a power injector (Envision CT injector, Medrad®, Indianola, PA) through a 20-G catheter inserted into the median cubital vein. In Group A, 70 ml of CM followed by 40 ml of saline tracer was injected at a rate of 5 ml s−1. In the study groups, we consider to reduce the CM volume to a low level and an ultra-low level, respectively. The volume of CM was determined by the body weight of each patient: Group B, the low-volume group, 0.6 ml kg−1 (210 mgI kg−1) of patient weight; Group C, ultra-low-volume group, 0.4 ml kg−1 (140 mgI kg−1). The injection rates for the study groups were calculated as: (CM volume)/(delay time + exposure time).9 The volume of follow-up saline tracer for Groups B and C was selected as 30, 40 or 50 ml, whichever is the closest to the applied CM volume. The injection rate of the saline tracer was the same as that of the CM.
Image evaluation
A radiologist, with over 10 years' experience and a fellowship training in aortic CTA imaging performed all the measurements using a spectral imaging viewer (GSI Viewer; GE Healthcare, WI) and reviewed all the images. Images in Group A were reconstructed using the traditional filtered back projection algorithm. To determine the optimal energy levels for the study Groups B and C, we first measured the mean CT attenuation value of the ascending aorta for the polychromatic images in Group A (120 kVp). The optimal energy levels were then selected to reconstruct the monochromatic images of Groups B and C so that the images were enhanced to the equivalent brightness in the ascending aorta region to the images of Group A. In the process, 40% and 50% ASIRs were applied to reduce image noise for Groups B and C.
For all images, the CT attenuations and standard deviations (SDs) of the vessels were measured by placing ROIs at the ascending aorta, descending aorta, celiac trunk, renal artery and iliac artery. The ROIs were maintained large enough with calcifications or soft plaques avoided. In case of aortic dissection images, ROIs were placed on the true lumen. The attenuations and SDs of the paraspinal muscles at the region of the renal artery were determined as baselines to calculate the contrast-to-noise ratio (CNR) according to (vessel attenuation − muscle attenuation)/muscle SD.
Two cardiovascular radiologists with 5 years' experience performed a blind CTA quantitative evaluation for the randomly ordered images in the three groups. The raters utilized a five-point ordinal scale to grade the image noise level as 1, severe and unacceptable; 2, interfering with the depiction of aortic arteries—just acceptable; 3, moderate—not interfering with their depiction; 4, low—less than average; and 5, minimal or absent.26 Vessel sharpness and detectability of the third order and higher aortic branches were assessed by evaluating the visible branches of the renal artery as 1, blurry and unacceptable; 2, poorer than average; 3, average; 4, better than average (between Scores 3 and 5); and 5, sharpest.27 The overall image quality was also given as 1, unacceptable; 2, poor; 3, average; 4, good; and 5, excellent.28
Radiation dose and CM volume evaluation
The volume CT dose index (CTDIvol) and dose–length product (DLP) were recorded from the CT console after each examination. The effective dose (ED) was calculated through multiplying DLP by a conversion factor of 0.0186, following the recommendation of International Commission on Radiological Protection publication 103.16,29,30 The CM volumes and the injection rates were also recorded for the three groups.
Statistical analysis
The statistical analysis was performed using SPSS® v. 22.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). The numerical values were expressed as mean ± SD. One-way analysis of variance followed by the Bonferroni correction was utilized for multiple comparisons on the continuous variables such as CM volumes and injection rates, radiation doses, CT attenuation values and CNRs among the three groups. Comparisons of the subjective image scores were conducted using the Kruskal–Wallis non-parametric test. Significance level at 0.05 was set for the p-values. Interobserver agreement on the image quality was evaluated through the Cohen's kappa coefficient.
RESULTS
As a representative region, the ascending aorta in Group A had an average CT attenuation value of 374 ± 31 HU. To obtain equivalent CT values in the ascending aorta region, the energy levels for the imaging reconstruction in Groups B and C were, respectively, set as 58–61 keV (average: 60 keV) and 50–60 keV (average: 55 keV).
Study population
Various characteristics of 120 patients in different groups were listed in Table 1. There was no significant difference among groups in patient's age (p = 0.963), weight (p = 0.594) or body mass index (p = 0.880).
Table 1.
Patient characteristics, contrast medium (CM) volumes and radiation exposures
| Characteristic | Group A | Group B | Group C | p-value |
|---|---|---|---|---|
| Number of patients | 40 | 40 | 40 | – |
| Age (years) | 55.7 ± 11.2 | 55.6 ± 17.7 | 54.9 ± 11.6 | 0.963 |
| Body weight (kg) | 73.0 ± 9.9 | 71.0 ± 10.2 | 70.7 ± 12.0 | 0.594 |
| BMI (kg m−2) | 25.1 ± 3.1 | 25.3 ± 2.9 | 25.4 ± 3.5 | 0.880 |
| CM volume (ml) | 70 | 41.9 ± 6.3 | 28.5 ± 4.7 | <0.001 |
| CM injection rate (ml s−1) | 5.0 | 3.5 ± 0.4 | 2.4 ± 0.3 | <0.001 |
| CTDIvol (mGy) | 9.3 ± 2.8 | 7.4 | 7.4 | <0.001 |
| DLP (mGy cm) | 653.0 ± 219.1 | 505.8 ± 22.9 | 490.3 ± 26.3 | <0.001 |
| ED (mSv) | 12.1 ± 4.1 | 9.4 ± 0.4 | 9.1 ± 0.5 | <0.001 |
BMI, body mass index; CTDIvol, volume CT dose index; DLP, dose–length product; ED, effective dose.
Quantitative image quality
The CT attenuations in ascending aorta, descending aorta, celiac trunk, renal artery and iliac artery were listed in Table 2. All >300 HU, the CT values were considered as sufficient for CTA examinations. The differences in terms of attenuation among the three groups were not significant in the ascending aorta, descending aorta, celiac trunk and renal artery (p > 0.05) but were significant in the iliac artery (p = 0.001).
Table 2.
Measured mean attenuation in Hounsfield units (mean ± standard deviation)
| Item | Case | Ascending aorta | Descending aorta | Celiac trunk | Renal artery | Iliac arterya |
|---|---|---|---|---|---|---|
| Group A | 40 | 374 ± 31 | 355 ± 29 | 351 ± 32 | 354 ± 31 | 364 ± 35 |
| Group B | 40 | 379 ± 62 | 359 ± 50 | 339 ± 46 | 334 ± 51 | 331 ± 48 |
| Group C | 40 | 359 ± 77 | 373 ± 48 | 349 ± 41 | 346 ± 39 | 341 ± 34 |
| F-value | – | 1.159 | 1.803 | 1.052 | 2.346 | 7.088 |
| p-value | – | 0.317 | 0.169 | 0.353 | 0.100 | 0.001 |
The detailed multiple comparison results were A vs B, p = 0.001; A vs C, p = 0.032; and B vs C, p = 0.870.
The CNRs and the post hoc multiple comparison results among groups were depicted in Table 3. The CNRs in the entire aorta and its branches were significantly higher in study Group B than in study Group C and control Group A (p < 0.001). While for study Group C, the mean CNRs of all regions were higher than (descending aorta, p = 0.026) or equivalent to (ascending aorta, p = 0.229; celiac trunk, p = 0.245; renal artery, p = 0.183; iliac artery, p = 0.051) those of control Group A.
Table 3.
Contrast-to-noise ratio in the aorta and its branches
| Item | Group A | Group B | Group C |
p-value |
||
|---|---|---|---|---|---|---|
| A vs B | B vs C | A vs C | ||||
| Ascending aorta | 14.6 ± 2.7 | 24.3 ± 8.2 | 16.8 ± 3.5 | <0.001 | <0.001 | 0.229 |
| Descending aorta | 13.9 ± 2.9 | 22.8 ± 7.5 | 17.0 ± 4.0 | <0.001 | <0.001 | 0.026 |
| Celiac trunk | 14.2 ± 3.0 | 22.1 ± 6.9 | 16.0 ± 2.9 | <0.001 | <0.001 | 0.245 |
| Renal artery | 14.5 ± 3.3 | 21.9 ± 6.5 | 16.5 ± 3.3 | <0.001 | <0.001 | 0.183 |
| Iliac artery | 14.3 ± 3.1 | 23.5 ± 7.0 | 16.9 ± 3.5 | <0.001 | <0.001 | 0.051 |
The subjective image quality assessment results are shown in Table 4. The interobserver agreement for image noise, vessel sharpness and the overall image quality was good with all the three kappa coefficients >0.6. With respect to all image quality parameters (image noise, vessel sharpness and overall image quality), no significant differences were detected among groups (p > 0.05). All the examinations were evaluated to be diagnostic (scores ≥3). Sample images from the three groups were shown in Figure 1, demonstrating good image quality for all the three groups.
Table 4.
Subjective evaluation of image quality
| Item | Group A | Group B | Group C | p-value | Kappa |
|---|---|---|---|---|---|
| Image noise | 4.08 ± 0.35 | 4.09 ± 0.32 | 4.03 ± 0.36 | 0.808 | 0.665 |
| Vessel sharpness | 4.05 ± 0.39 | 4.08 ± 0.39 | 4.00 ± 0.39 | 0.516 | 0.715 |
| Overall image quality | 4.18 ± 0.40 | 4.18 ± 0.39 | 4.13 ± 0.42 | 0.738 | 0.645 |
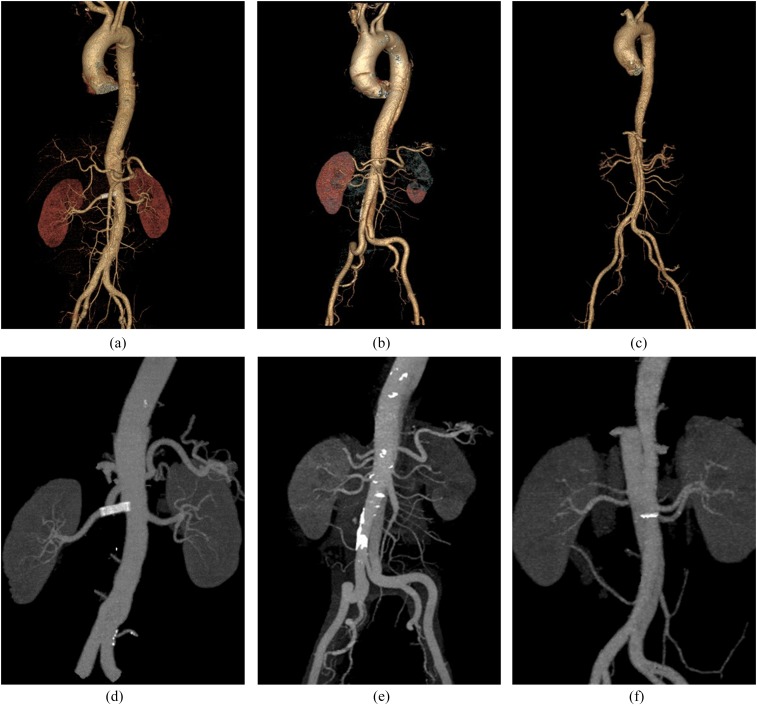
Figure 1.
Three-dimensional reconstructions using the images generated by the conventional and dual-energy scans. For all the volume-rendering (a–c) and maximum-intensity projection (d–f) images, the whole aorta is sufficiently opacified, the renal artery is clearly depicted with two accessory renal arteries and the aortic dissections, as well as the endovascular repairs of the dissections with stent graft placement, are clearly shown. (a, d) Representatives of control Group A, with the patient having a body weight of 85 kg, height of 175 cm and body mass index (BMI) of 27.7 kg m−2. 70 ml of CM with 5.0 ml s−1 injection rate was applied. (b, e) The low-CM-volume Group B, with the contributor of body weight 85 kg, height 172 cm and BMI 28.7 kg m−2. The utilized CM volume was 51 ml with 4.5 ml s−1 injection rate. (c, f) The ultra-low-CM-volume Group C. The scanned patient had a body weight of 85 kg, height of 170 cm and BMI of 29.4 kg m−2. The applied CM volume was 34 ml with 2.9 ml s−1 injection rate.
Contrast medium volume and injection rate
Shown in Table 1, the CM volumes for patients in Groups A, B and C were 70, 41.9 ± 6.3 and 28.5 ± 4.7 ml, respectively. Significant differences in CM volumes were evident among the three groups (p < 0.001). The CM injection rates were 3.5 ± 0.4 ml s−1 and 2.4 ± 0.3 ml s−1 in study Groups B and C, respectively, which were significantly lower than the rate 5.0 ml s−1 used in control Group A (p < 0.001).
Radiation dose
Table 1 depicts the average values of CTDIvol, DLP and ED for the three groups. The CTDIvol, DLP and ED values were significantly greater in Group A (p < 0.001). The study groups (Groups B and C) exhibited reductions in CTDIvol, DLP and ED of 20%, 22.5–24.5% and 22.5–24.5%, respectively.
DISCUSSION
In this study, we evaluated the feasibility of combining monochromatic imaging of ssDECT in the newly developed GSI mode with ASIR to reduce the CM volume, CM injection rate and radiation dose. This study revealed that compared with the conventional aortic CTA method, the joint application of ssDECT and the ASIR technique could reduce the CM volume, CM injection rate and radiation dose by 40.1% (Group B)–59.3% (Group C), 30% (Group B)–52% (Group C) and 22.5–24.5% (Groups B and C), respectively. Although with much reduced CM volume, CM injection rate and radiation dose, the image quality in terms of CNRs and the subjective scores were retained for the ultra-low-CM-volume Group C and even improved for the low-CM-volume Group B.
Compared with the conventional polychromatic imaging, monochromatic images reconstructed with adjusted energy levels provide improved beam-hardening correction, CT attenuation and CNR.31,32 Theoretically, dual-energy CT with low energy levels can improve the contrast for vessels via exploiting the K-edge phenomenon when using CM, and the lowest energy level yields the “brightest” iodine and greatest image contrast.33 The low-energy range has demonstrated promise to reduce the amount of CM because of the high contrast but is expected to be accompanied by excessive image noise due to the lack of photon flux.17,18 In this study, we selected the optimal energy levels through matching the enhancement of the ascending aorta to that for conventional CTA. The selected energy levels in study Groups B and C were, respectively, 58–61 keV (average: 60 keV) and 50–60 keV (average: 55 keV), which provide good overall image quality and are not the lowest energy levels available.
To compensate for the increased image noise, ASIR algorithms that consider a precise modelling of the X-ray photon statistics and electronic noise were incorporated to preserve the image quality.19–22,25 It has been demonstrated in literature that ASIR of 40–50% is appropriate for general applications,34–36 and the ASIR of <40% percentage provides limited reduction of image noise.34 This study selected ASIR of percentages 40% and 50% for the joint application with dual-energy CT in the low-CM-volume group and the ultra-low-CM-volume group, respectively. The percentages of ASIR were determined according to the energy levels used in the image reconstruction, and ASIR of higher percentage was used to accommodate the higher level of image noise for the ultra-low-dose CM group. A typical example from the ultra-low-CM-volume group is shown in Figure 2. Although with very-low CM volume and injection rate, the obtained images feature good quality.
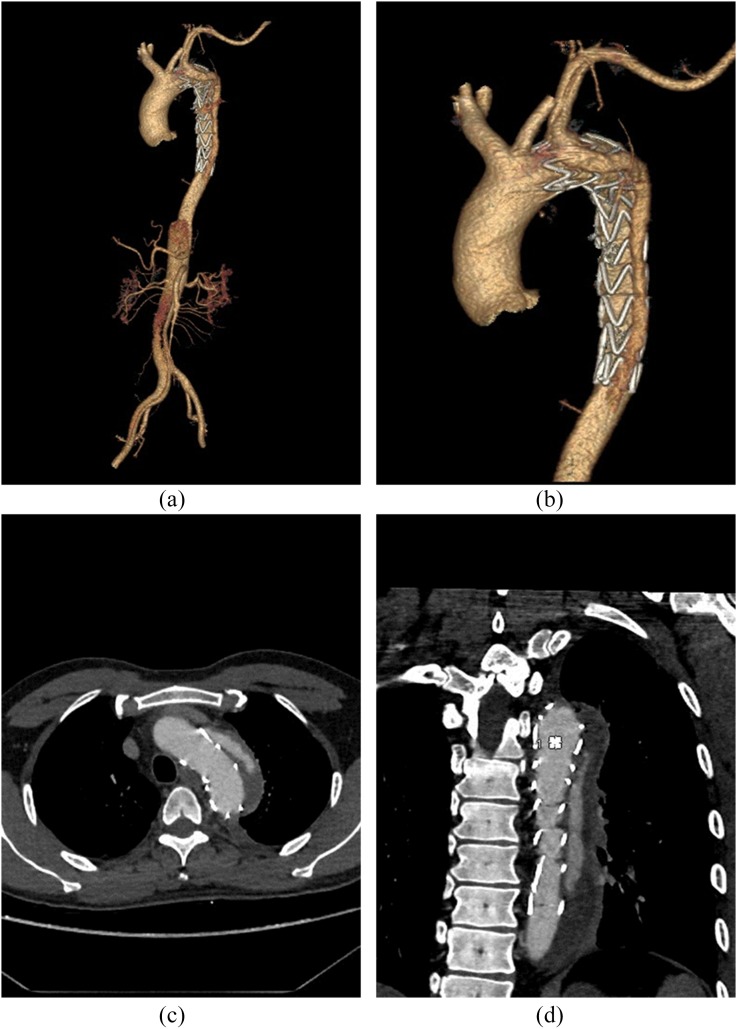
Figure 2.
Two- and three-dimensional reconstructions with images from the ultra-low-CM-volume Group C. The volume-rendering and multiplanar reformation images (a–d) clearly depict the whole aorta, the endovascular repair of aortic dissection with stent graft placed just below the left subclavian artery and the extravasation of contrast out of the stent graft. The patient has a body weight of 65 kg, height of 163 cm and body mass index of 24.4 kg m−2 and was scanned using a CM volume of 26 ml and 2.0 ml s−1 injection rate.
Previous studies revealed that ssDECT in earlier GSI versions suffers from increased radiation dose compared with conventional CT.25,37,38 This is due to the unchangeable pre-defined high tube current to make sure the intensity of X-rays is sufficient for different kV levels.25 The utilized new generation GSI supports several choices of tube currents as a pre-defined parameter for the dual-energy scan, and the selected low tube current (360 mA) for both study groups was shown to be sufficient. The resulting radiation doses were measured as 9.1–9.4 mSv, which are significantly lower than those (12.1 mSv) of the control group.
The CM volume and injection rate were individually adapted by the patient's weight in this study. The mean CM volumes in the study groups were measured as 0.6 ml kg−1 (210 mgI kg−1) in the low-CM-volume group and 0.4 ml kg−1 (140 mgI kg−1) in the ultra-low-CM-volume group, and they were lower than those in the previously studied CM protocols.9,15 The injection rates were also significantly reduced to 3.5 and 2.4 ml s−1 in the study groups, as compared with the conventional choice of 4–5 ml s−1.14,39,40 As suggested by Davenport et al,41 a patient with eGFR <45 ml min−1/1.73 m2 tends to suffer from high risk of contrast-induced nephropathy. In addition, patients such as those with cancer undergoing chemotherapy may be in poor peripheral venous status and require the use of small-bore cannulas for CT scans.42 The proposed ultra-low-CM protocol can thus be applied to patients with poor renal (30 ≤ eGFR < 45 ml min−1/1.73 m2) and peripheral venous conditions to minimize the side effects of CM while keeping the image quality. For patients of relatively good renal function and peripheral venous status, the protocol of low CM volume and injection rate can be utilized to obtain improved image quality.
Low CM protocols in aortic CTA were also feasible by applying low kVp setting, high pitch mode and volume acquisitions.14–16,43 Beeres et al14 applied the CM volume of 90 ml in high-pitch dual-source aortic CTA and obtained good image quality. Using low kVp settings in high pitch mode, Shen et al15 and Zhang et al43 attempted to reduce CM dose to the weight-adapted volume of 1.0 ml kg−1 (270 mgI kg−1) and the fixed volume of 60 ml, respectively. As for volume acquisitions, Chen et al16 proposed the joint application of wide-volume CT and iterative reconstruction algorithm to lower the CM volume to 40 ml in aortic CTA. We may notice that the mentioned CM protocols are all higher than the proposed ultra-low CM protocol in this study. In addition, all the above technologies belong to single-energy CT, and the fixed energy level provides less information than spectral CT.44
This study has limitations. Firstly, single ASIR level was used for each study group. A comprehensive analysis on the effects of different percentages of ASIR is of importance. Secondly, ASIR algorithm is not applied on the reconstructed images of conventional 120-kVp scans in this study and a further study will be performed for a more complete comparison. Thirdly, patients with aortic aneurysm were excluded from this study. It is of importance to investigate the feasibility of reducing CM dose at CTA for aortic aneurysm in a future study. Lastly, the patients in this study are Asians with small body mass indices (<30 kg m−2), which slightly limits the application scope of the results.
In conclusion, the joint application of dual-energy CT and ASIR algorithm under the new GSI mode enables the CM protocols of significantly reduced volumes and injection rates in aortic CTA, as compared with the protocol in the conventional scan. The ultra-low-dose CM protocol with a volume of 0.4 ml kg−1 and an injection rate of 2.4 ml s−1 was sufficient to obtain satisfactory image quality, and the low CM dose of 0.6 ml kg−1 at an injection rate of 3.5 ml s−1 improves image quality.
Contributor Information
Ping Hou, Email: houping1221@163.com.
Xiangnan Feng, Email: fengxiangnan123@gmail.com.
Jie Liu, Email: liujie069@126.com.
Yue Zhou, Email: zhouyue565@163.com.
Yaojun Jiang, Email: jiangyaojun223@126.com.
Xiaochen Jiang, Email: gaorui426@163.com.
Jianbo Gao, Email: cjr_gaojianbo@sina.com.
REFERENCES
- 1.Willoteaux S, Lions C, Gaxotte V, Neqaiwi Z, Bereqi JP. Imaging of aortic dissection by helical computed tomography (CT). Eur Radiol 2004; 14: 1999–2008. doi: https://doi.org/10.1007/s00330-004-2441-y [DOI] [PubMed] [Google Scholar]
- 2.Fujioka C, Horiguchi J, Kiguchi M, Yamamoto H, Kitaqawa T, Ito K. Survey of aorta and coronary arteries with prospective ECG-triggered 100-kV 64-MDCT angiography. AJR Am J Roentgenol 2009; 193: 227–33. doi: https://doi.org/10.2214/ajr.08.1722 [DOI] [PubMed] [Google Scholar]
- 3.Westermann Y, Geigenmüller A, Elgeti T, Wagner M, Dushe S, Borges AC, et al. Planimetry of the aortic valve orifice area: comparison of multislice spiral computed tomography and magnetic resonance imaging. Eur J Radiol 2011; 77: 426–35. doi: https://doi.org/10.1016/j.ejrad.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 4.Duquette AA, Jodoin PM, Bouchot O, Lalande A. 3D segmentation of abdominal aorta from CT-scan and MR images. Comput Med Imaging Graph 2012; 36: 294–303. doi: https://doi.org/10.1016/j.compmedimag.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 5.Nayeemuddin M, Pherwani AD, Asquith JR. Imaging and management of complications of open surgical repair of abdominal aortic aneurysms. Clin Radiol 2012; 67: 802–14. doi: https://doi.org/10.1016/j.crad.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 6.Tsang W, Bateman MG, Weinert L. Accuracy of aortic annular measurements obtained from three-dimensional echocardiography, CT and MRI: human in vitro and in vivo studies. Heart 2012; 98: 1146–52. doi: https://doi.org/10.1136/heartjnl-2012-302074 [DOI] [PubMed] [Google Scholar]
- 7.Katzberg RW, Barrett BJ. Risk of iodinated contrast material-induced nephropathy with intravenous administration. Radiology 2007; 243: 622–8. doi: https://doi.org/10.1148/radiol.2433061411 [DOI] [PubMed] [Google Scholar]
- 8.Zheng M, Liu Y, Wei M, Wu Y, Zhao H, Li J. Low concentration contrast medium for dual-source computed tomography coronary angiography by a combination of iterative reconstruction and low-tube-voltage technique: feasibility study. Eur J Radiol 2014; 83: e92–9. doi: https://doi.org/10.1016/j.ejrad.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Lv P, Wu R, Zhang Y, Hu L, Hou P, et al. Aortic dual-energy CT angiography with low contrast medium injection rate. J Xray Sci Technol 2014; 22: 689–96. [DOI] [PubMed] [Google Scholar]
- 10.Sodickson A, Baeyens PF, Andriole KP, Prevedello LM, Nawfel RD, Hanson R, et al. Recurrent CT, cumulative radiation exposure, and associated radiation-induced cancer risks from CT of adults. Radiology 2009; 251: 175–84. doi: https://doi.org/10.1148/radiol.2511081296 [DOI] [PubMed] [Google Scholar]
- 11.Brenner DJ, Hall EJ. Computed tomography-an increasing source of radiation exposure. N Engl J Med 2007; 357: 2277–84. doi: https://doi.org/10.1056/nejmra072149 [DOI] [PubMed] [Google Scholar]
- 12.Mettler FA, Jr, Bhargavan M, Faulkner K, Gilley DB, Gray JE, Ibbott GS, et al. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources—1950–2007. Radiology 2009; 253: 520–31. doi: https://doi.org/10.1148/radiol.2532082010 [DOI] [PubMed] [Google Scholar]
- 13.Bolen MA, Popovic ZB, Tandon N, Flamm SD, Schoenhagen P, Halliburton SS. Image quality, contrast enhancement, and radiation dose of ECG-triggered high-pitch CT versus non-ECG-triggered standard-pitch CT of the thoracoabdominal aorta. AJR Am J Roentgenol 2012; 198: 931–8. doi: https://doi.org/10.2214/ajr.11.6921 [DOI] [PubMed] [Google Scholar]
- 14.Beeres M, Schell B, Mastragelopoulos A, Herrmann E, Kerl JM, Lee C, et al. High-pitch dual-source CT angiography of the whole aorta without ECG synchronisation: initial experience. Eur Radiol 2012; 22: 129–37. doi: https://doi.org/10.1007/s00330-011-2257-5 [DOI] [PubMed] [Google Scholar]
- 15.Shen Y, Sun S, Xu L, Li Y, Zhang N, Yan Z, et al. High-pitch, low-voltage and low-iodine-concentration CT angiography of aorta: assessment of image quality and radiation dose with iterative reconstruction. PLoS One 2015; 10: e0117469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CM, Chu SY, Hsu MY, Liao YL, Tsai HY. Low-tube-voltage (80 kVp) CT aortography using 320-row volume CT with adaptive iterative reconstruction: lower contrast medium and radiation dose. Eur Radiol 2014; 24: 460–8. doi: https://doi.org/10.1007/s00330-013-3027-3 [DOI] [PubMed] [Google Scholar]
- 17.Lv PJ, Lin XZ, Chen K, Gao J. Spectral CT in patients with small HCC: investigation of image quality and diagnostic accuracy. Eur Radiol 2012; 22: 2117–24. doi: https://doi.org/10.1007/s00330-012-2485-3 [DOI] [PubMed] [Google Scholar]
- 18.Gao SY, Zhang XP, Cui Y, Sun YS, Tang L, Li XT, et al. Fused monochromatic imaging acquired by single source dual energy CT in hepatocellular carcinoma during arterial phase: an initial experience. Chin J Cancer Res 2014; 26: 437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funama Y, Awai K, Nakayama Y, Kakei K, Naqasue N, Sato N, et al. Radiation dose reduction without degradation of low-contrast detectability at abdominal multisection CT with a low-tube voltage technique: phantom study. Radiology 2005; 237: 905–10. doi: https://doi.org/10.1148/radiol.2373041643 [DOI] [PubMed] [Google Scholar]
- 20.Korn A, Fenchel M, Bender B, Danz S, Hauser TK, Ketelsen D, et al. Iterative reconstruction in head CT: image quality of routine and low-dose protocols in comparison with standard filtered back-projection. AJNR Am J Neuroradiol 2012; 33: 218–24. doi: https://doi.org/10.3174/ajnr.a2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pontana F, Pagniez J, Flohr T, Faivre JB, Duhamel A, Remy J, et al. Chest computed tomography using iterative reconstruction vs filtered back projection (Part 1): evaluation of image noise reduction in 32 patients. Eur Radiol 2011; 21: 627–35. doi: https://doi.org/10.1007/s00330-010-1990-5 [DOI] [PubMed] [Google Scholar]
- 22.Sing S, Kalra MK, Gilman MD, Hsieh J, Pien HH, Diqumarthy SR, et al. Adaptive statistical iterative reconstruction technique for radiation dose reduction in chest CT: a pilot study. Radiology 2011; 259: 565–73. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Z, Zhao XM, Zhao YF, Wang XY, Zhou CW. Feasibility study of using gemstone spectral imaging (GSI) and adaptive statistical iterative reconstruction (ASIR) for reducing radiation and iodine contrast dose in abdominal CT patients with high BMI values. PLoS One 2015; 10: e0129201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lv PJ, Liu J, Chai YR, Yan XP, Gao JB, Dong JQ. Automatic spectral imaging protocol selection and iterative reconstruction in abdominal CT with reduced contrast agent dose: initial experience. Eur Radiol 2017; 27: 374–83. doi: https://doi.org/10.1007/s00330-016-4349-8 [DOI] [PubMed] [Google Scholar]
- 25.Ohana M, Labani A, Jeung MY, Ghannudi SE, Gaertner S, Roy C. Iterative reconstruction in single source dual-energy CT pulmonary angiography: is it sufficient to achieve a radiation dose as low as state-of-the-art single-energy CTPA? Eur J Radiol 2015; 84: 2314–20. doi: https://doi.org/10.1016/j.ejrad.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 26.Rizzo S, Kalra M, Schmidt B, Dalal T, Suess C, Flohr T, et al. Comparison of angular and combined automatic tube current modulation techniques with constant tube current CT of the abdomen and pelvis. AJR Am J Roentgenol 2006; 186: 673–9. doi: https://doi.org/10.2214/ajr.04.1513 [DOI] [PubMed] [Google Scholar]
- 27.Noël PB, Köhler T, Fingerle AA, Brown KM, Zabic S, Münzel D, et al. Evaluation of an iterative model-based reconstruction algorithm for low-tube-voltage (80 kVp) computed tomography angiography. J Med Imaging 2014; 1: 033501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lv P, Liu J, Wu R, Hou P, Hu L, Gao J. Use of non-linear image blending with dual-energy CT improves vascular visualization in abdominal angiography. Clin Radiol 2014; 69: e93–9. doi: https://doi.org/10.1016/j.crad.2013.09.019 [DOI] [PubMed] [Google Scholar]
- 29.International Commission on Commission on Radiological Protection (ICRP). The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP 2007; 37: 1–332. [DOI] [PubMed] [Google Scholar]
- 30.Huda W, Magill D, He W. CT effective dose per dose length product using ICRP 103 weighting factors. Med Phys 2011; 38: 1261–5. doi: https://doi.org/10.1118/1.3544350 [DOI] [PubMed] [Google Scholar]
- 31.Yamada M, Jinzaki M, Kuribayashi S, Imanishi N, Funato K, Aiso S. Beam-hardening correction for virtual monochromatic imaging of myocardial perfusion via fast-switching dual-kVp 64-slice computed tomography: a pilot study using a human heart specimen. Circ J 2012; 76: 1799–801. doi: https://doi.org/10.1253/circj.cj-12-0463 [DOI] [PubMed] [Google Scholar]
- 32.Pessis E, Campagna R, Sverzut JM, Bach F, Rodallec M, Guerini H, et al. Virtual monochromatic spectral imaging with fast kilovoltage switching: reduction of metal artifacts at CT. Radiographics 2013; 33: 573–83. doi: https://doi.org/10.1148/rg.332125124 [DOI] [PubMed] [Google Scholar]
- 33.Maturen KE, Kaza PK, Liu PS, Quint LE, Khalatbari SH, Platt JF. “Sweet spot” for endoleak detection: optimizing contras to noise using low keV reconstructions from fast-switch kVp dual-energy CT. J Comput Assist Tomogr 2012; 36: 83–7. doi: https://doi.org/10.1097/rct.0b013e31824258cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marin D, Choudhury KR, Gupta RT, Ho LM, Allen BC, Schindera ST, et al. Clinical impact of an adaptive statistical iterative reconstruction algorithm for detection of hypervascular liver tumours using a low tube voltage, high tube current MDCT technique. Eur Radiol 2013; 23: 3325–35. doi: https://doi.org/10.1007/s00330-013-2964-1 [DOI] [PubMed] [Google Scholar]
- 35.Cornfeld D, Israel G, Detroy E, Bokhari J, Mojibian H. Impact of adaptive statistical iterative reconstruction (ASIR) on radiation dose and image quality in aortic dissection studies: a qualitative and quantitative analysis. AJR Am J Roentgenol 2011; 196: W336–40. doi: https://doi.org/10.2214/ajr.10.4573 [DOI] [PubMed] [Google Scholar]
- 36.Brady S, Kaufman R. Image contrast dependent spatial resolution differences for varying levels of ASiR implementation. Med Phys 2012; 39: 4015. doi: https://doi.org/10.1118/1.4736377 [Google Scholar]
- 37.Ho LM, Yoshizumi TT, Hurwitz LM, Nelson RC, Marin D, Toncheva G, et al. Dual energy versus single energy MDCT: measurement of radiation dose using adult abdominal imaging protocols. Acad Radiol 2009; 16: 1400–7. doi: https://doi.org/10.1016/j.acra.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 38.Agrawal MD, Oliveira GR, Kalva SP, Pinho DF, Arellano RS, Sahani DV. Prospective comparison of reduced-iodine-dose virtual monochromatic imaging dataset from dual-energy CT angiography with standard-iodine-dose single-energy CT angiography for abdominal aortic aneurysm. AJR Am J Roentgenol 2016; 207: W125–32. doi: https://doi.org/10.2214/ajr.15.15814 [DOI] [PubMed] [Google Scholar]
- 39.Napoli A, Catalano C, Francone M, Sciacca V, Varbone I, Greco C, et al. Imaging coronary and extracoronary atherosclerosis: feasibility and impact of whole-body computed tomography angiography. Eur Radiol 2009; 19: 1704–14. doi: https://doi.org/10.1007/s00330-009-1342-5 [DOI] [PubMed] [Google Scholar]
- 40.Ippolito D, Talei Franzesi C, Fior D, Bonaffini PA, Minutolo O, Sironi S. Low kV settings CT angiography (CTA) with low dose contrast medium volume protocol in the assessment of thoracic and abdominal aorta disease: a feasibility study. Br J Radiol 2015; 88: 20140140. doi: https://doi.org/10.1259/bjr.20140140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology 2013; 268: 719–28. doi: https://doi.org/10.1148/radiol.13122276 [DOI] [PubMed] [Google Scholar]
- 42.Gossner J. Feasibility of computed tomography pulmonary angiography with low flow rates. J Clin Imaging Sci 2012; 2: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang LJ, Ba XL, Schoepf UJ, Wichmann JL, Tang CX, Zhou CS, et al. Non-electrocardiogram-triggered 70-kvp high-pitch computed tomography angiography of the whole aorta with iterative reconstruction: initial results. J Comput Assist Tomogr 2016; 40: 109–17. doi: https://doi.org/10.1097/rct.0000000000000329 [DOI] [PubMed] [Google Scholar]
- 44.Simons D, Kachelriess M, Schlemmer HP. Recent developments of dual-energy CT in oncology. Eur Radiol 2014; 24: 930–9. doi: https://doi.org/10.1007/s00330-013-3087-4 [DOI] [PubMed] [Google Scholar]