Abstract
Acute gastrointestinal (GI) bleeding is a common cause of both emergency department visits and hospitalizations in the USA and can have a high morbidity and mortality if not treated rapidly. Imaging is playing an increasing role in both the diagnosis and management of GI bleeding. In particular, CT angiography (CTA) is a promising initial test for acute GI bleeding as it is universally available, can be performed rapidly and may provide diagnostic information to guide management. The purpose of this review was to provide an overview of the uses of imaging in the diagnosis and management of acute GI bleeding, with a focus on CTA.
INTRODUCTION
Acute gastrointestinal (GI) bleeding is a common cause of both emergency department visits and hospital admissions, leading to 375 hospitalizations per 100,000 individuals per year in the USA.1–3 If acute GI bleeding is not diagnosed promptly and adequately treated, morbidity and mortality are high,4 reaching 40% in patients who are haemodynamically unstable.5 Diagnosis and treatment requires a multidisciplinary approach that may involve diagnostic and interventional radiology, emergency medicine, internal medicine, gastroenterology and general surgery. Imaging is playing a growing role in the management of acute GI bleeding by localizing the source of bleeding, differentiating the underlying disease processes and aiding decisions to proceed to endovascular therapies to treat many causes of GI bleeding.
The purpose of this review article is to highlight the increasing role of radiology in the diagnosis and management of acute GI bleeding, with a focus on the emerging role of multidetector CT (MDCT) angiography. We will provide an overview of the clinical diagnosis and common causes of acute GI bleeding and will review the modalities (endoscopy, radionuclide imaging, catheter angiography) traditionally used for diagnosis. We will then provide an in-depth review of the role of CT angiography (CTA), including examples of the CT appearance of many causes of acute GI bleeding, common diagnostic pitfalls and new directions in CT including dose reduction and dual-energy CT (DECT). Finally, we will propose simplified algorithms for the appropriate use of imaging in acute upper and lower GI bleeding.
CLINICAL DIAGNOSIS AND COMMON CAUSES OF ACUTE GASTROINTESTINAL BLEEDING
Clinical assessment and appropriate triage of patients with acute GI bleeding can be challenging. Following haemodynamic stabilization of the patient, the most important diagnostic consideration is whether the source of bleeding is in the upper GI tract (above the ligament of Treitz) or lower GI tract (below the ligament of Treitz), as patients with upper GI tract bleeding will be triaged to endoscopy and those with lower tract bleeding will generally be evaluated with imaging or colonoscopy depending on the clinical scenario. Common causes of upper GI bleeding include peptic ulcer disease (62%), arteriovenous malformations (10%), gastritis or duodenitis (8%) and oesophageal varices (6%)6 (Figure 1). Common causes of lower GI bleeding include diverticular disease (40%), colitis and inflammatory bowel disease (21%), neoplasia (14%), coagulopathic haemorrhage (12%) and angiodysplasia, among others.7
Figure 1.
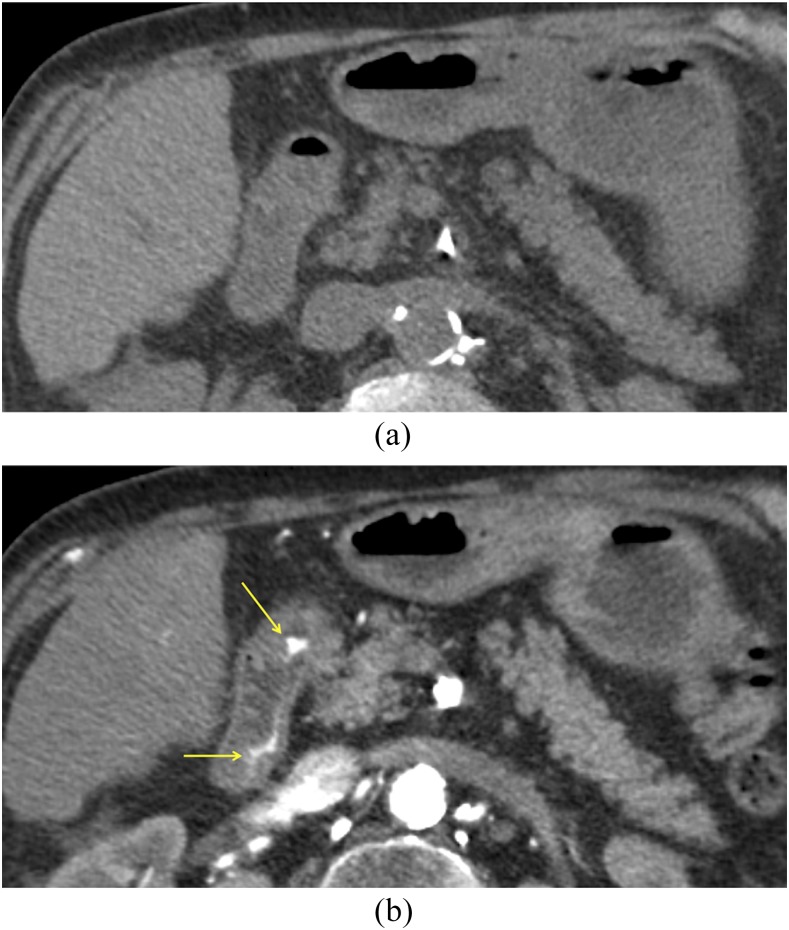
CT angiography in an 82-year-old male with bright red blood per rectum: on axial non-contrast CT (a), there is no hyperattenuating material in the bowel lumen. The axial arterial phase image (b) demonstrates active extravasation of contrast (arrows) into the second portion of the duodenum, which was found on endoscopy to arise from a duodenal ulcer. Ulcer disease is the most common cause of upper gastrointestinal bleeding.
Distinguishing between upper and lower GI bleeding based solely upon clinical history and physical examination is difficult, as there is significant overlap in the clinical presentation. Most (75%) cases of GI bleeding have an upper GI tract source. Patients with upper GI bleeding classically present with haematemesis (vomiting bright red blood), “coffee ground” emesis (vomiting darker blood that has been partially digested) or melena (passage of dark faeces containing digested blood), although patients can present with haematochezia (passage of bright red blood per rectum) in cases of brisk upper GI bleeding with rapid transit time. Conversely, patients with lower GI bleeding classically present with haematochezia, but may also present with melena. Although nasogastric tube aspiration can be assessed for the presence of blood, it is neither sensitive nor specific for diagnosis of an upper GI source;8 so, diagnostic imaging and endoscopy are often relied upon to determine the source of bleeding. In patients presenting acutely to the emergency department with GI bleeding, several clinical factors are typically assessed to determine the appropriate diagnostic and treatment strategy, including the likely location of bleeding, rate of haemorrhage and overall hemodynamic status.1
DIAGNOSTIC MODALITIES
Upper endoscopy and colonoscopy
Endoscopy is often the first-line diagnostic and therapeutic modality in patients with acute GI bleeding, particularly if there is a suspected upper GI source. Endoscopy is highly sensitive and specific for acute upper GI bleeding with sensitivity of up to 98% and specificity of up to 100%.9 Endoscopy and colonoscopy provide direct visualization of the mucosa to identify the source of bleeding, enable application of haemostatic therapy and can be used for tissue sampling in cases of suspected malignancy.10 However, endoscopy and colonoscopy have a number of limitations. They may not be readily available in the emergency room setting. For patients with high-volume bleeding, it may be impossible to adequately visualize the source of haemorrhage with endoscopy. In addition, for those with lower GI tract bleeding, endoscopy is unable to assess the majority of the small bowel distal to the ligament of Treitz and provides limited visualization of the distal duodenum.11 Bowel preparation for colonoscopy can take 3–5 h, which may not be possible in patients with acute colonic haemorrhage who are clinically unstable. Even with adequate bowel preparation, sensitivity and specificity of colonoscopy in acute lower GI bleeding has been shown to be lower than that of MDCT.11
Video capsule endoscopy is a technique that has been shown to be beneficial in evaluation of obscure GI bleeding (persistent bleeding with negative upper and lower endoscopy).12,13 Recently, some researchers have evaluated capsule endoscopy in assessment of acute upper GI bleeding in patients in the emergency department as a method to triage patients.14–16 Although this approach shows promise, capsule endoscopy is not currently considered a suitable substitute for endoscopy.4
Radionuclide imaging
Radionuclide imaging for GI bleeding is generally performed with technetium-99m tagged red blood cells, with initial injection of radiotracer and subsequent gamma camera imaging. GI bleeding can be diagnosed when radiotracer activity is visualized outside of normal areas of blood pool, which either focally intensifies or moves over time in an antegrade or retrograde fashion17 (Figure 2).
Figure 2.
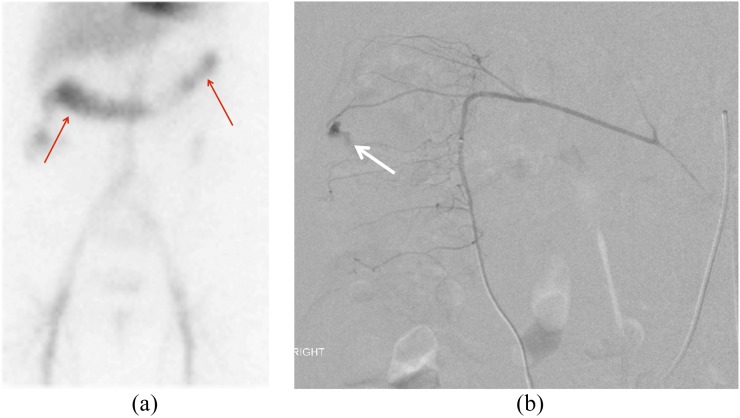
Tagged red blood cell images in a 78-year-old female with bright red blood per rectum demonstrating accumulation of radiotracer within the transverse colon (a, arrows). Selective catheter angiography of a branch of the right colic artery (b) shows brisk contrast extravasation (white arrow) into the lumen of the caecum, which was subsequently treated with coil embolization.
Radionuclide studies have the advantage of being highly sensitive, detecting rates of bleeding as low as 0.05–0.1 ml min−1.18 In addition, scintigraphy can assess for bleeding over a prolonged period of time and can detect both arterial and venous haemorrhage. However, owing to their prolonged imaging times, these studies are not ideal for patients who are clinically unstable. In addition, radionuclide imaging may have limited availability in the acute care setting, particularly overnight. Radionuclide scans also often cannot provide precise anatomic localization of the site of active bleeding;19 this, in combination with their high sensitivity, could potentially lead to some positive radionuclide scans that are followed by negative endoscopy or catheter angiography.20
Catheter angiography
Catheter angiography is considered the first-line imaging and treatment modality for patients who are unstable with lower GI bleeding, patients following a failed upper or lower endoscopy and patients with lower GI bleeding with a source of bleeding visualized on an additional imaging modality.21 For a known source of GI bleeding, selective catheter angiography of the bleeding artery and embolization can be performed (Figure 2). For an unknown bleeding source, angiography is generally performed of the celiac axis, superior mesenteric artery, and inferior mesenteric artery. Bleeding can be diagnosed confidently when active extravasation of contrast material into the bowel lumen is visualized (Figure 2), and embolization can be performed if active extravasation is visualized. In addition, for patients with upper GI bleeding, prophylactic embolization can be performed even if a site of bleeding is not identified, most commonly of the gastroduodenal artery. This has been shown to be effective, particularly in patients with large volume upper GI bleeding refractory to endoscopic treatment.22
Sensitivity and specificity of catheter angiography is highly variable in the literature, with sensitivity averaging 60%,19 and technical success rates ranging from 73–100% for lower GI bleeding and 60–100% for upper GI bleeding.23 Angiography has a high spatial resolution, can detect rates of bleeding as low as 0.5 ml/min24 and has the added major advantage of allowing for treatment of GI bleeding. The primary disadvantage of catheter angiography is that it is an invasive and time-consuming procedure with a potentially high radiation dose. In addition, patients may have falsely negative studies if GI bleeding is intermittent and they are not actively bleeding during the angiogram.
Multidetector CT angiography
Overview
MDCT angiography has increasingly been adopted for the diagnosis of acute GI bleeding. Because CT can be acquired rapidly and is nearly universally available in the acute setting, it may be an ideal initial diagnostic test for patients who are haemodynamically stable with acute GI bleeding, as well as for patients awaiting catheter angiography or endoscopy. CTA has the advantage of being able to precisely localize the source of arterial and venous GI bleeding, and to diagnose underlying pathology that may be the cause of bleeding to direct future management.25 In particular, CTA can uniquely diagnose causes of GI bleeding that are not within the GI tract, such as haemobilia.26 In addition, CTA can delineate the underlying vascular anatomy prior to embolization and characterize any anatomical variants that may impact management. Disadvantages of CTA include decreased sensitivity relative to radionuclide imaging,23 relatively high radiation dose and the need for i.v. contrast. Owing to the short acquisition time, false-negative results can occur if the patient is not bleeding at the time of the scan.
CT technique
Specific CTA protocols in patients with suspected GI bleeding will vary between vendors and institutions. In general, CTA for GI bleeding is performed as a three-phase examination, including non-contrast, arterial and venous phase imaging.11,27,28 For all phases, the scan range should include the complete abdomen and pelvis (from the diaphragm to below the inferior pubic rami). Arterial phase images are obtained with bolus tracking at the abdominal aorta (150 HU threshold), with venous phase imaging obtained at 70–90 s following contrast injection.27,29 100–125 ml i.v. contrast (with an iodine concentration of 300 mg per ml or greater) is administered at a rate of 4–5 ml sec−1, followed by 40–50 ml of saline at the same rate. Oral contrast is not administered, as positive contrast agents would obscure i.v. contrast extravasation into the bowel lumen, and negative or neutral contrast agents would dilute the extravasated i.v. contrast.27 Non-contrast imaging is incorporated to ensure that the pre-existing hyperdense ingested material within the bowel lumen can be differentiated from active haemorrhage during the scan.13,30 Images can be reconstructed at 5-mm thickness for non-contrast images and 1.25 mm for arterial and venous phase images.30 Multiplanar reformation images in the sagittal and coronal planes are reconstructed for all phases. Maximum intensity projection images in the sagittal and coronal planes are also performed for arterial and venous phase images, to increase conspicuity of haemorrhage and delineate vascular anatomy.
Imaging findings and diagnostic pitfalls
The critical imaging finding of GI bleeding is active extravasation of i.v. contrast into the bowel lumen.27,29,31 This can be diagnosed with CTA when an intraluminal focus of high attenuation (>90 HU) is seen on arterial phase images (“contrast blush”) that is not present on non-contrast images (Figure 3). On portal venous phase images, this extravasation should change in appearance and generally moves distally within the bowel lumen. The extravasation itself can have a variety of imaging appearances depending on the underlying physiology, including a linear “jet” of contrast, “cloudlike” morphology, circular configuration or a contrast fluid level.27
Figure 3.
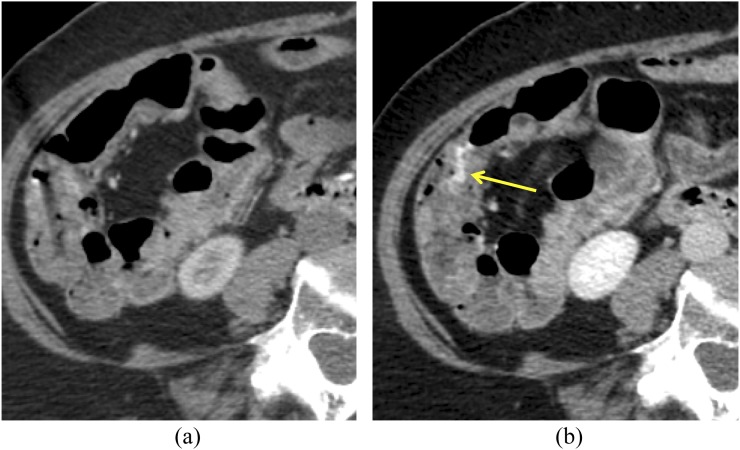
Diverticular haemorrhage in a 77-year-old male: there is no hyperattenuating material within the bowel lumen on the non-contrast scan (a). There is active extravasation of contrast (arrows) into the lumen of the right colon on the arterial phase image (b), which evolves in morphology at the portal venous phase (c).
There are several important variations in the imaging appearance of GI bleeding to be familiar with. In patients with slow or delayed bleeding, extravasation may be present only on the portal venous phase images, as there is greater accumulation of blood within the bowel lumen on delayed images (Figure 4). In patients with recent bleeding who are not actively bleeding at the time of imaging, CT may only reveal hyperdense clot within the bowel lumen without active extravasation (Figure 5). The site of clot of the highest attenuation has been deemed the “sentinel clot”, which is closest to the site of active bleeding.32 For all patients with GI bleeding, maximum intensity projection images must be screened carefully, as they may identify subtle bleeding that can be overlooked on source images, can visualize underlying vascular malformations and can delineate underlying vascular anatomy prior to angiography (Figure 6).
Figure 4.
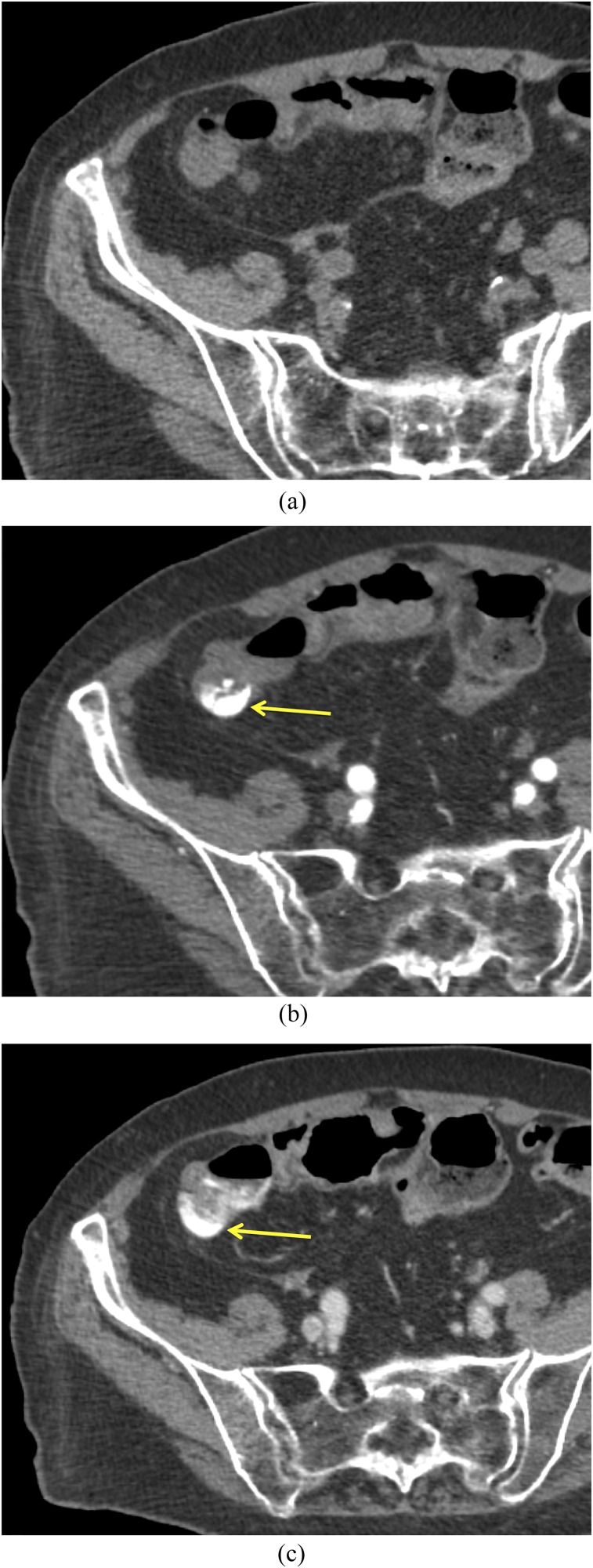
An 83-year-old female with delayed diverticular bleeding: on the axial arterial phase (a) CT image, no hyperattenuating material is present within the bowel lumen. On the subsequent portal venous phase image (b), there is subtle contrast extravasation within the right colon (arrow).
Figure 5.
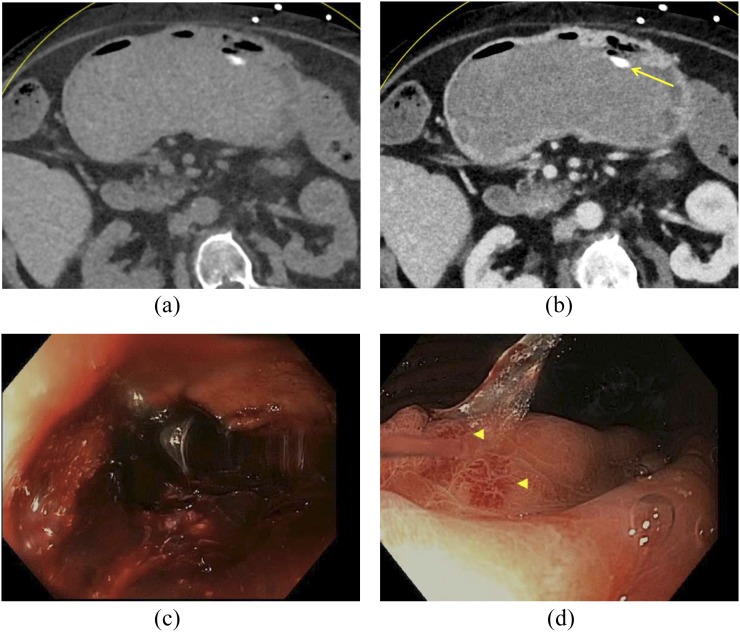
A 73-year-old female with haematemesis and bright red blood per rectum who underwent dual-energy CT: virtual non-contrast (a) and portal venous phase (b) images demonstrate a large volume of hyperdense clot within the gastric lumen. No active extravasation is seen; however, there is an ingested pill within the stomach (arrow). Subsequent endoscopy confirmed a large volume of blood within the stomach (c), as well as multiple bleeding arteriovenous malformations (arrowheads, d).
Figure 6.
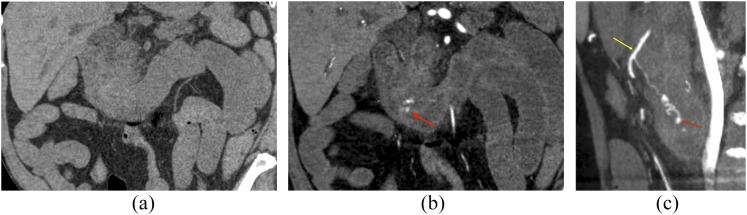
A 70-year-old male with bleeding following endoscopy: on coronal non-contrast image (a), there is no hyperattenuating material within the bowel lumen. On coronal arterial phase image (b), active extravasation of i.v. contrast into the second and third portions of the duodenum is seen (arrow). On sagittal maximum intensity projection images (c), the active extravasation (arrow) can be seen arising from a branch of the gastroduodenal artery (arrow).
Recognition of potential diagnostic pitfalls is important when interpreting CT angiograms for active GI bleeding. Fluid distension of bowel loops may dilute contrast extravasation, potentially causing a false-negative result.33 High-density material within or near the bowel lumen, including surgical clips, ingested material and fecaliths, can be mistaken for haemorrhage without a non-contrast scan (Figures 5 and 7). Cone-beam artefacts can also lead to the false appearance of high density within the bowel lumen.33
Figure 7.
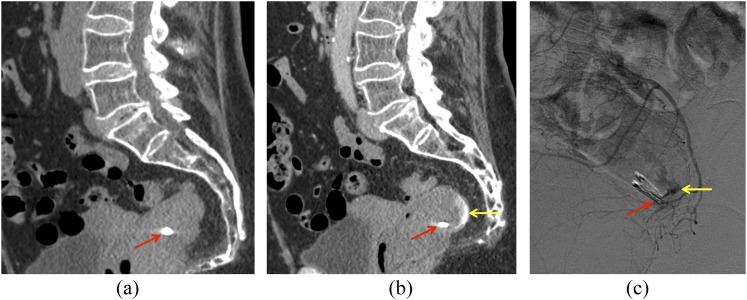
In a 72-year-old female with gastrointestinal bleeding following rectal biopsy, the clip (arrows) can be seen on non-contrast image (a), with active contrast extravasation present in the rectum on portal venous phase image (b, arrow). Catheter angiogram of a rectal branch (c) confirms a site of active extravasation (arrow) adjacent to the rectal clip. Embolization was performed subsequently.
Efficacy of multidetector CT angiography in evaluating acute gastrointestinal bleeding
Several studies have evaluated the rate of bleeding that can be detected with MDCT;34,35 for example, in one study, Dobritz et al35 used an experimental model and showed that rates of bleeding above 0.25 ml min−1 could be detected by MDCT with a sensitivity of 97% and specificity of 100% using arterial and portal venous phase images. A large body of research has examined the diagnostic performance of CTA in the assessment of acute GI bleeding. For example, in a prospective study, Martí et al36 performed CTA in 47 patients with acute lower GI bleeding and found a sensitivity of 100%, specificity of 96% and accuracy of 93%. Kennedy et al28 evaluated 86 CT angiograms in 74 patients and found a sensitivity of 79% and a specificity of 95%. Similar findings have been reported in multiple additional studies assessing CTA for upper and lower GI bleeding.11,25,37–41 While sensitivity and specificity of CTA in GI bleeding vary between studies, García-Blázquez et al42 found that CTA had a sensitivity of 85.2% and specificity of 92.1% in detecting GI bleeding in a systematic review and meta-analysis of 22 studies including 672 patients with acute GI bleeding. The sensitivity of CTA in determining the underlying aetiology of bleeding has also been reported to be above 90% in multiple studies.11,36,39 For patients with lower GI bleeding, those with a negative CTA have been shown to be unlikely to develop recurrent bleeding; therefore, patients with lower GI bleeding and a negative CTA may not need subsequent angiography.43
Dose reduction
As a three-phase CT examination, radiation dose is a potential concern for GI bleeding CTA scans. There are many alterations that can be made to CT protocols to reduce radiation dose. Because non-contrast images for GI bleeding scans are performed to assess for high-density ingested material within the bowel, they can be obtained at a very low dose to accomplish this diagnostic task.27 Advances in CT technology including automatic tube voltage selection and iterative reconstruction have allowed for further radiation dose reductions for abdominal CT imaging.44–47 Recently, some authors have suggested that radiation dose could be further conserved in CTA for GI bleeding by eliminating either the arterial or portal venous phase. For example, in a study performed by Kim et al,48 2 readers evaluated CTA scans in 46 patients with acute GI bleeding by reviewing arterial, portal venous and combined data sets independently and found no added diagnostic value between the 3 data sets. A study by Dobritz et al34 found that in an experimental model, the portal venous phase was more sensitive in detection of bleeding than the arterial phase.
New directions: dual-energy CT
DECT is an emerging technology of promise in the assessment of GI bleeding. While conventional CT imaging uses a single X-ray spectrum, DECT acquires data at two different X-ray spectra: lower energy at 80 kV or 100 kV and higher energy at 140 kV. This allows materials with different kV-dependent X-ray absorption behaviours, such as iodine and calcium, to be differentiated or quantified using DECT post-processing techniques. DECT post-processing can be used to create virtual non-contrast (VNC) images with iodine subtracted from the image, as well as iodine maps to depict iodine content, both of which can be useful in the assessment of GI bleeding (Figure 8). The use of VNC images can obviate the need for a non-contrast phase, substantially reducing patient radiation dose. In a recent study by Sun et al,41 112 consecutive patients with suspected GI bleeding underwent DECT angiography. The authors found that substituting VNC imaging for true non-contrast imaging did not impact diagnostic performance in detection of GI bleeding and allowed for a radiation dose reduction of roughly 30%.
Figure 8.
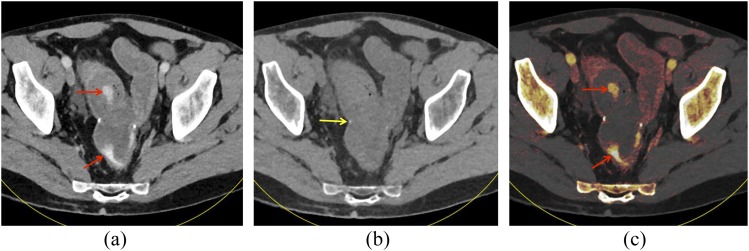
A 51-year-old male with prior colectomy for clostridium difficile colitis presenting with rectal bleeding: “mixed” image from a dual-energy CT scan in the portal venous phase (a) shows hyperattenuating material within the distal small bowel and rectum (arrows). On the virtual non-contrast (VNC) image (b), this material is not present within the bowel lumen, showing that this is not ingested material. The anastomotic suture material remains visible on the VNC image (arrow). On iodine overlay image (c) with iodine content colour-coded in orange, the hyperattenuating material within the bowel lumen demonstrates iodine content, further confirming that this represents active extravasation.
Diagnostic algorithms
A summary of the advantages and disadvantages of the various imaging modalities for acute GI bleeding is displayed in Table 1. Based upon the advantages and disadvantages of each modality, we also propose potential diagnostic algorithms for patients with acute upper and lower GI bleeding (Figures 9 and 10).
Table 1.
Advantages and disadvantages of diagnostic modalities in acute gastrointestinal (GI) bleeding
| Endoscopy | Radionuclide imaging | Catheter angiography | MDCT | |
|---|---|---|---|---|
| Advantages | Highly sensitive and specific in upper GI bleeding Can treat GI bleeding if detected Can sample tissue if suspected malignancy |
Can detect low rates of bleeding Can detect arterial or venous bleeding Non-invasive |
Can treat GI bleeding if detected High spatial resolution Small vessels can be selectively injected |
Readily available Rapid acquisition Precise anatomic localization |
| Disadvantages | May not be universally available Cannot visualize the majority of the small bowel Limited visualization of bleeding with active haemorrhage |
Often cannot precisely localize the site of bleeding Time consuming May not be available in the acute setting |
Requires a high rate of bleeding for detection of extravasation Invasive and time consuming Radiation dose |
May be less sensitive than radionuclide imaging Radiation dose Requires i.v. contrast |
MDCT, multidetector CT.
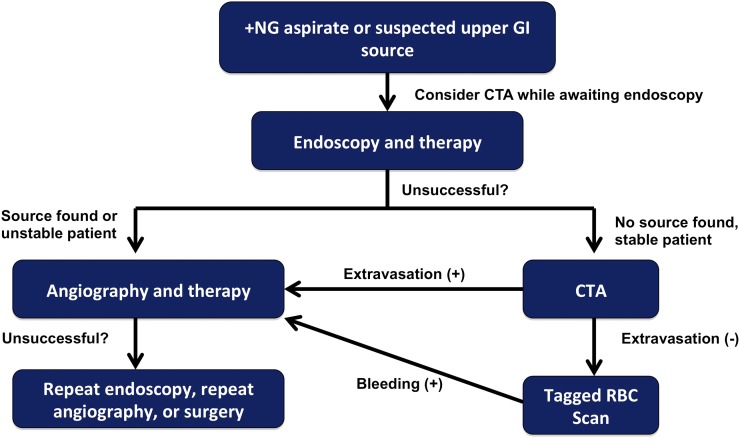
Figure 9.
Proposed diagnostic algorithm for patients with acute upper gastrointestinal (GI) bleeding. CTA, CT angiography; RBC, red blood cell.
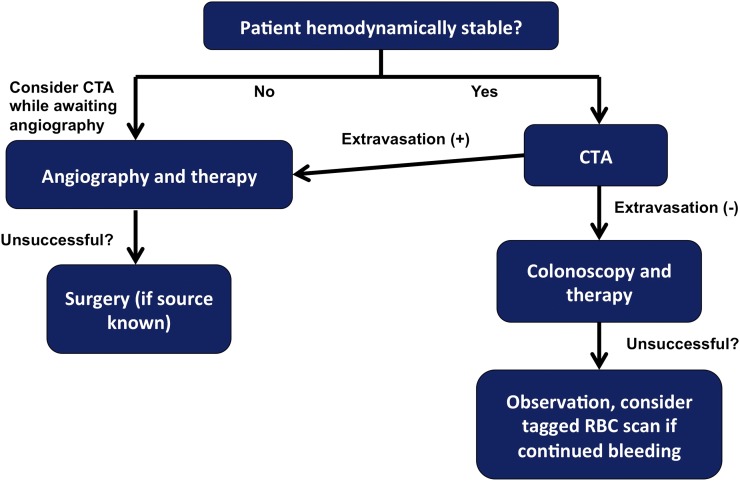
Figure 10.
Proposed diagnostic algorithm for patients with acute lower gastrointestinal (GI) bleeding. CTA, CT angiography; RBC, red blood cell.
CONCLUSION
Imaging is playing a growing role in the diagnosis and management of patients with acute GI bleeding. CTA is promising as a first-line diagnostic modality in many patients in the acute care setting, as it is a test that is widely available and can be performed rapidly. CTA findings can help further guide endoscopic, endovascular or surgical management. Advances in CT technology, including the emergence of DECT, will allow for these scans to be performed at a lower dose. Radiologists should be aware of the appropriate uses of CTA and other imaging modalities in patients with acute GI bleeding, as well as common imaging findings and pitfalls, in order to appropriately diagnose and manage these patients.
Contributor Information
Jeremy R Wortman, Email: jwortman@partners.org.
Wendy Landman, Email: wlandman@partners.org.
Urvi P Fulwadhva, Email: ufulwadhva@partners.org.
Salvatore G Viscomi, Email: sviscomi@partners.org.
Aaron D Sodickson, Email: asodickson@partners.org.
REFERENCES
- 1.Whelan CT, Chen C, Kaboli P, Siddique J, Prochaska M, Meltzer DO. Upper versus lower gastrointestinal bleeding: A direct comparison of clinical presentation, outcomes, and resource utilization. J Hosp Med 2010; 5: 141–7. doi: https://doi.org/10.1002/jhm.606 [DOI] [PubMed] [Google Scholar]
- 2.Manning-Dimmitt LL, Dimmitt SG, Wilson GR. Diagnosis of gastrointestinal bleeding in adults. Am Fam Physician 2005; 71: 1339–46. [PubMed] [Google Scholar]
- 3.Zhao Y, Encinosa W. Hospitalizations for Gastrointestinal Bleeding in 1998 and 2006. December 2008 [cited 29 February 2016]; Available from: http://www-ncbi-nlm-nih-gov.ezp-prod1.hul.harvard.edu/books/NBK54562/ [PubMed]
- 4.Khamaysi I, Gralnek IM. Acute upper gastrointestinal bleeding (UGIB)—initial evaluation and management. Best Pract Res Clin Gastroenterol 2013; 27: 633–8. doi: https://doi.org/10.1016/j.bpg.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman HM, Curfmah KL. Acute gastrointestinal bleeding. AACN Adv Crit Care 1997; 8: 449–58. doi: https://doi.org/10.1097/00044067-199708000-00013 [DOI] [PubMed] [Google Scholar]
- 6.Wilkins T, Khan N, Nabh A, Schade RR. Diagnosis and management of upper gastrointestinal bleeding. Am Fam Physician 2012; 85: 469–76. [PubMed] [Google Scholar]
- 7.Vernava AM, III, Moore BA, Longo WE, Johnson FE. Lower gastrointestinal bleeding. Dis Colon Rectum 1997; 40: 846–58. doi: https://doi.org/10.1007/bf02055445 [DOI] [PubMed] [Google Scholar]
- 8.Cuellar RE, Gavaler JS, Alexander JA, Brouillette DE, Chien MC, Yoo YK, et al. Gastrointestinal tract hemorrhage. The value of a nasogastric aspirate. Arch Intern Med 1990; 150: 1381–4. [DOI] [PubMed] [Google Scholar]
- 9.Lee EW, Laberge JM. Differential diagnosis of gastrointestinal bleeding. Tech Vasc Interv Radiol 2004; 7: 112–22. doi: https://doi.org/10.1053/j.tvir.2004.12.001 [DOI] [PubMed] [Google Scholar]
- 10.Feinman M, Haut ER. Upper gastrointestinal bleeding. Surg Clin North Am 2014; 94: 43–53. doi: https://doi.org/10.1016/j.suc.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 11.Frattaroli FM, Casciani E, Spoletini D, Polettini E, Nunziale A, Bertini L, et al. Prospective study comparing multi-detector row CT and endoscopy in acute gastrointestinal bleeding. World J Surg 2009; 33: 2209–17. doi: https://doi.org/10.1007/s00268-009-0156-6 [DOI] [PubMed] [Google Scholar]
- 12.Leighton JA, Triester SL, Sharma VK. Capsule endoscopy: a meta-analysis for use with obscure gastrointestinal bleeding and Crohn's disease. Gastrointest Endosc Clin N Am 2006; 16: 229–50. doi: https://doi.org/10.1016/j.giec.2006.03.004 [DOI] [PubMed] [Google Scholar]
- 13.Soto JA, Park SH, Fletcher JG, Fidler JL. Gastrointestinal hemorrhage: evaluation with MDCT. Abdom Imaging 2015; 40: 993–1009. doi: https://doi.org/10.1007/s00261-015-0365-4 [DOI] [PubMed] [Google Scholar]
- 14.Rubin M, Hussain SA, Shalomov A, Cortes RA, Smith MS, Kim SH. Live view video capsule endoscopy enables risk stratification of patients with acute upper GI bleeding in the emergency room: a pilot study. Dig Dis Sci 2010; 56: 786–91. doi: https://doi.org/10.1007/s10620-010-1336-9 [DOI] [PubMed] [Google Scholar]
- 15.Gutkin E, Shalomov A, Hussain SA, Kim SH, Cortes R, Gray S, et al. Pillcam ESO® is more accurate than clinical scoring systems in risk stratifying emergency room patients with acute upper gastrointestinal bleeding. Therap Adv Gastroenterol 2013; 6: 193–8. doi: https://doi.org/10.1177/1756283x13481020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meltzer AC, Ali MA, Kresiberg RB, Patel G, Smith JP, Pines JM, et al. Video capsule endoscopy in the emergency department: a prospective study of acute upper gastrointestinal hemorrhage. Ann Emerg Med 2013; 61: 438–43. doi: https://doi.org/10.1016/j.annemergmed.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 17.Zuckier LS. Acute gastrointestinal bleeding. Semin Nucl Med 2003; 33: 297–311. doi: https://doi.org/10.1016/s0001-2998(03)00033-3 [DOI] [PubMed] [Google Scholar]
- 18.Alavi A, Dann RW, Baum S, Biery DN. Scintigraphic detection of acute gastrointestinal bleeding. Radiology 1977; 124: 753–6. doi: https://doi.org/10.1148/124.3.753 [DOI] [PubMed] [Google Scholar]
- 19.Hastings GS. Angiographic localization and transcatheter treatment of gastrointestinal bleeding. Radiographics 2000; 20: 1160–8. doi: https://doi.org/10.1148/radiographics.20.4.g00jl361160 [DOI] [PubMed] [Google Scholar]
- 20.Yi WS, Garg G, Sava JA. Localization and definitive control of lower gastrointestinal bleeding with angiography and embolization. Am Surg 2013; 79: 375–80. [PubMed] [Google Scholar]
- 21.Millward SF. ACR appropriateness criteria® on treatment of acute nonvariceal gastrointestinal tract bleeding. J Am Coll Radiol 2008; 5: 550–4. doi: https://doi.org/10.1016/j.jacr.2008.01.010 [DOI] [PubMed] [Google Scholar]
- 22.Dixon S, Chan V, Shrivastava V, Anthony S, Uberoi R, Bratby M. Is there a role for empiric gastroduodenal artery embolization in the management of patients with active upper GI hemorrhage? Cardiovasc Intervent Radiol 2013; 36: 970–7. doi: https://doi.org/10.1007/s00270-012-0511-0 [DOI] [PubMed] [Google Scholar]
- 23.Schenker MP, Majdalany BS, Funaki BS, Yucel EK, Baum RA, Burke CT, et al. ACR appropriateness criteria® on upper gastrointestinal bleeding. J Am Coll Radiol 2010; 7: 845–53. doi: https://doi.org/10.1016/j.jacr.2010.05.029 [DOI] [PubMed] [Google Scholar]
- 24.Nusbaum M, Baum S, Blakemore WS, Finkelstein AK. Demonstration of intra-abdominal bleeding by selective arteriography. Visualization of celiac and superior mesenteric arteries. JAMA 1965; 191: 389–90. [DOI] [PubMed] [Google Scholar]
- 25.Foley PT, Ganeshan A, Anthony S, Uberoi R. Multi-detector CT angiography for lower gastrointestinal bleeding: can it select patients for endovascular intervention? J Med Imaging Radiat Oncol 2010; 54: 9–16. doi: https://doi.org/10.1111/j.1754-9485.2010.02131.x [DOI] [PubMed] [Google Scholar]
- 26.Mortimer AM, Wallis A, Planner A. Multiphase multidetector CT in the diagnosis of haemobilia: a potentially catastrophic ruptured hepatic artery aneurysm complicating the treatment of a patient with locally advanced rectal cancer. Br J Radiol 2011; 84: e95–8. doi: https://doi.org/10.1259/bjr/20779582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Artigas JM, Martí M, Soto JA, Esteban H, Pinilla I, Guillén E. Multidetector CT angiography for acute gastrointestinal bleeding: technique and findings. RadioGraphics 2013; 33: 1453–70. doi: https://doi.org/10.1148/rg.335125072 [DOI] [PubMed] [Google Scholar]
- 28.Kennedy DW, Laing CJ, Tseng LH, Rosenblum DI, Tamarkin SW. Detection of active gastrointestinal hemorrhage with CT angiography: a 4(1/2)-year retrospective review. J Vasc Interv Radiol 2010; 21: 848–55. doi: https://doi.org/10.1016/j.jvir.2010.01.039 [DOI] [PubMed] [Google Scholar]
- 29.Laing CJ, Tobias T, Rosenblum DI, Banker WL, Tseng L, Tamarkin SW. Acute gastrointestinal bleeding: emerging role of multidetector CT angiography and review of current imaging techniques. RadioGraphics 2007; 27: 1055–70. doi: https://doi.org/10.1148/rg.274065095 [DOI] [PubMed] [Google Scholar]
- 30.Geffroy Y, Rodallec MH, Boulay-Coletta I, Jullès MC, Ridereau-Zins C, Zins M. Multidetector CT angiography in acute gastrointestinal bleeding: why, when, and how. Radiographics 2011; 31: E35–46. doi: https://doi.org/10.1148/rg.313105206 [DOI] [PubMed] [Google Scholar]
- 31.Yoon W, Jeong YY, Kim JK. Acute gastrointestinal bleeding: contrast-enhanced MDCT. Abdom Imaging 2006; 31: 1–8. doi: https://doi.org/10.1007/s00261-005-0367-8 [DOI] [PubMed] [Google Scholar]
- 32.Hamilton JD, Kumaravel M, Censullo ML, Cohen AM, Kievlan DS, West OC. Multidetector CT evaluation of active extravasation in blunt abdominal and pelvic trauma patients. RadioGraphics 2008; 28: 1603–16. doi: https://doi.org/10.1148/rg.286085522 [DOI] [PubMed] [Google Scholar]
- 33.Stuber T, Hoffmann MH, Stuber G, Klass O, Feuerlein S, Aschoff AJ. Pitfalls in detection of acute gastrointestinal bleeding with multi-detector row helical CT. Abdom Imaging 2009; 34: 476–82. doi: https://doi.org/10.1007/s00261-008-9437-z [DOI] [PubMed] [Google Scholar]
- 34.Dobritz M, Engels HP, Schneider A, Wieder H, Feussner H, Rummeny EJ, et al. Evaluation of dual-phase multi-detector-row CT for detection of intestinal bleeding using an experimental bowel model. Eur Radiol 2009; 19: 875–81. doi: https://doi.org/10.1007/s00330-008-1205-5 [DOI] [PubMed] [Google Scholar]
- 35.Dobritz M, Engels HP, Schneider A, Bauer J, Rummeny EJ. Detection of intestinal bleeding with multi-detector row CT in an experimental setup. How many acquisitions are necessary? Eur Radiol 2009; 19: 2862–9. doi: https://doi.org/10.1007/s00330-009-1510-7 [DOI] [PubMed] [Google Scholar]
- 36.Martí M, Artigas JM, Garzón G, Álvarez-Sala R, Soto JA. Acute lower intestinal bleeding: feasibility and diagnostic performance of CT angiography. Radiology 2012; 262: 109–16. doi: https://doi.org/10.1148/radiol.11110326 [DOI] [PubMed] [Google Scholar]
- 37.Yoon W, Jeong YY, Shin SS, Lim HS, Song SG, Jang NG, et al. Acute massive gastrointestinal bleeding: detection and localization with arterial phase multi-detector row helical CT. Radiology 2006; 239: 160–7. doi: https://doi.org/10.1148/radiol.2383050175 [DOI] [PubMed] [Google Scholar]
- 38.Anthony S, Milburn S, Uberoi R. Multi-detector CT: review of its use in acute GI haemorrhage. Clin Radiol 2007; 62: 938–49. doi: https://doi.org/10.1016/j.crad.2007.02.019 [DOI] [PubMed] [Google Scholar]
- 39.Scheffel H, Pfammatter T, Wildi S, Bauerfeind P, Marincek B, Alkadhi H. Acute gastrointestinal bleeding: detection of source and etiology with multi-detector-row CT. Eur Radiol 2007; 17: 1555–65. doi: https://doi.org/10.1007/s00330-006-0514-9 [DOI] [PubMed] [Google Scholar]
- 40.Jaeckle T, Stuber G, Hoffmann MH, Jeltsch M, Schmitz BL, Aschoff AJ. Detection and localization of acute upper and lower gastrointestinal (GI) bleeding with arterial phase multi-detector row helical CT. Eur Radiol 2008; 18: 1406–13. doi: https://doi.org/10.1007/s00330-008-0907-z [DOI] [PubMed] [Google Scholar]
- 41.Sun H, Xue HD, Wang YN, Qian JM, Yu JC, Zhu F, et al. Dual-source dual-energy computed tomography angiography for active gastrointestinal bleeding: a preliminary study. Clin Radiol 2013; 68: 139–47. doi: https://doi.org/10.1016/j.crad.2012.06.106 [DOI] [PubMed] [Google Scholar]
- 42.García-Blázquez V, Vicente-Bártulos A, Olavarria-Delgado A, Plana MN, van der Winden D, Zamora J, et al. ; EBM-Connect Collaboration. Accuracy of CT angiography in the diagnosis of acute gastrointestinal bleeding: systematic review and meta-analysis. Eur Radiol 2013; 23: 1181–90. doi: https://doi.org/10.1007/s00330-012-2721-x [DOI] [PubMed] [Google Scholar]
- 43.Chan V, Tse D, Dixon S, Shrivastava V, Bratby M, Anthony S, et al. Outcome following a negative CT angiogram for gastrointestinal hemorrhage. Cardiovasc Intervent Radiol 2015; 38: 329–35. doi: https://doi.org/10.1007/s00270-014-0928-8 [DOI] [PubMed] [Google Scholar]
- 44.Schindera ST, Winklehner A, Alkadhi H, Goetti R, Fischer M, Gnannt R, et al. Effect of automatic tube voltage selection on image quality and radiation dose in abdominal CT angiography of various body sizes: a phantom study. Clin Radiol 2013; 68: e79–86. doi: https://doi.org/10.1016/j.crad.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 45.Mayer C, Meyer M, Fink C, Schmidt B, Sedlmair M, Schoenberg SO, et al. Potential for radiation dose savings in abdominal and chest CT using automatic tube voltage selection in combination with automatic tube current modulation. AJR Am J Roentgenol 2014; 203: 292–9. doi: https://doi.org/10.2214/ajr.13.11628 [DOI] [PubMed] [Google Scholar]
- 46.Desai GS, Fuentes Orrego JM, Kambadakone AR, Sahani DV. Performance of iterative reconstruction and automated tube voltage selection on the image quality and radiation dose in abdominal CT scans. J Comput Assist Tomogr 2013; 37: 897–903. doi: https://doi.org/10.1097/rct.0b013e3182a73fa6 [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez-Guindalini FD, Ferreira Botelho MP, Töre HG, Ahn RW, Gordon LI, Yaghmai V. MDCT of chest, abdomen, and pelvis using attenuation-based automated tube voltage selection in combination with iterative reconstruction: an intrapatient study of radiation dose and image quality. AJR Am J Roentgenol 2013; 201: 1075–82. doi: https://doi.org/10.2214/AJR.12.10354 [DOI] [PubMed] [Google Scholar]
- 48.Kim JW, Shin SS, Yoon W, Chang NK, Heo SH, Jeong YY, et al. Diagnosis of acute gastrointestinal bleeding: comparison of the arterial, the portal, and the combined set using 64-section computed tomography. J Comput Assist Tomogr 2011; 35: 206–11. doi: https://doi.org/10.1097/rct.0b013e31820a0ac8 [DOI] [PubMed] [Google Scholar]