Abstract
Objective:
The treatment of locally advanced unresectable pancreatic cancer remains extremely challenging, particularly as the efficacy of concurrent chemoradiotherapy (CRT) remains unclear.
Methods:
We studied 93 patients (8.0%) with locally advanced unresectable pancreatic cancer without distant metastases from among a total group of 1168 patients who were diagnosed with pancreatic cancer from March 2005 to November 2015 at the Kochi Health Sciences Center, Kochi, Japan. We therefore evaluated the clinical efficacy of CRT in patients with locally advanced unresectable pancreatic cancer.
Results:
Of the 93 patients with locally advanced unresectable pancreatic cancer, 35 patients (37.6%) were subsequently classified as having resectable disease following CRT. The median overall survival of patients who received CRT alone for locally advanced unresectable pancreatic cancer was 8.0 months, and all died within 3 years. On the other hand, the overall 1-, 3- and 5-year survival rates in patients who were reclassified as having resectable tumour after CRT were 71.3%, 39.2% and 23.5%, respectively. Our pathological assessments after surgical resection suggested that CRT might be associated with a significant reduction in the risk of lymph node metastases in patients with locally advanced unresectable pancreatic cancer.
Conclusion:
The results of this study suggested that CRT is clinically effective in improving survival, particularly in association with the resultant possibility of curative resection.
Advances in knowledge:
The best treatment strategy for patients with locally advanced unresectable pancreatic cancer is the subject of considerable debate, and CRT is only recommended if cancer has only grown around the pancreas without any distant metastases.
INTRODUCTION
Pancreatic cancer is a major public health problem worldwide.1,2 Affected patients have a dismal prognosis with 5-year overall survival rates of 8%, predominantly because patients do not usually experience symptoms during the early stages of this cancer and the disease is generally advanced by the time it is diagnosed.1 Indeed, 30–35% of patients are diagnosed with locally advanced pancreatic cancer; 80–85% of detected pancreatic cancers are classified as clinically unresectable disease;3,4 and few cases of metastatic pancreatic cancer demonstrate a sustained response to chemotherapy (CT) or radiation therapy (RT).5 Despite such statistics, complete surgical resection of the affected tissue remains the only hope of cure for patients with pancreatic cancer, and macroscopic or, ideally, microscopic margin-free tumour resection is considered a prerequisite for favourable survival in pancreatic cancer.6–8 The major current challenge in the field of pancreatic cancer is, therefore, to cure patients with locally advanced unresectable pancreatic cancer because locoregional control of the disease is increasingly difficult once distant metastatic disease becomes evident.
Treatment options for patients with locally advanced pancreatic cancer include CT and RT, with the latter long considered the standard of care in such cases.9 However, the role of RT is now controversial because of differing conclusions drawn by clinical trials over the past decades comparing concurrent chemoradiotherapy (CRT) with CT alone in patients with locally advanced unresectable pancreatic cancer.10–14 Furthermore, in most studies, the treatment focus for patients with locally advanced disease shifted to extending length of life, predictive investigation or the sharing of risk information.10–14
Regardless of outcomes, patients with locally advanced pancreatic cancer experience reduced quality of life during treatment, mainly as a result of pain and obstruction,15 and RT still might have an important role in the management of their disease. In the present study, therefore, we retrospectively evaluated the clinical effects of CRT in cases of advanced unresectable pancreatic cancer.
METHODS AND MATERIALS
Patients
We retrospectively reviewed clinical databases of the Kochi Health Sciences Center, Kochi, Japan, to identify patients who underwent treatment for locally advanced unresectable pancreatic cancer from March 2005 to November 2015. The diagnosis of pancreatic cancer was made initially following imaging and then confirmed by pathological analysis. Locally advanced unresectable pancreatic cancer was then defined according to any of the following criteria: tumour invasion to the plexus around the superior mesenteric artery that exceeded 180°, spread to the hepatic artery or celiac abutment.16,17 Evaluated clinical characteristics included age, gender, part of the pancreas affected by tumour, tumour size and pathological findings. Our department followed the prognosis of each case and obtained accurate details regarding outcomes. The ethics committees of the Kochi Health Sciences Center approved the study.
Chemoradiotherapy regimen
The initial diagnosis of pancreatic cancer was made following imaging studies. Some cases failed to perform endoscopic ultrasound-guided fine-needle aspiration to diagnose pancreatic cancer before CRT, although histological diagnosis was mandate before CRT. CRT was performed in patients with locally advanced unresectable pancreatic cancer at the Kochi Health Sciences Center. Patients who were allocated to the gemcitabine-alone group received gemcitabine intravenously at a dose of 800 mg m−2 over 30 min on Days 1, 8 and 15 of a 28-day cycle. Patients who were allocated to the S-1-alone group received S-1 orally twice daily at a dose calculated according to the body–surface area (<1.25 m2, 60 mg day−1; ≥1.25 to <1.5 m2, 80 mg day−1; ≥1.5 m2, 100 mg day−1) on Days 1 through 14 of a 21-day cycle.18 Intensity-modulated radiotherapy was administered with three-dimensional treatment planning using 10- or 15-MV photons. The total dose was 50 Gy delivered in 25 fractions over 5 weeks. The gross tumour volume was defined as the area of solid macroscopic tumours that was enhanced with contrast on CT imaging. The gross tumour volume plus a margin of at least 5 mm, including any areas of microscopic spread and the regional lymph nodes, was defined as the clinical target volume. The clinical target volume plus a 10-mm margin in the craniocaudal direction and a 5-mm margin in the lateral direction to account for daily set-up error and respiratory organ motion was defined as the planning target volume.
Assessment
Physical examinations, complete blood cell counts and biochemistry tests were routinely conducted at 2-week intervals. Objective tumour response was evaluated every 4–6 weeks by CT according to the Response Evaluation Criteria in Solid Tumors v. 1.0.19 An established team of medical experts at the Kochi Health Sciences Center, including gastroenterologists, surgeons, radiologists, oncologists and pathologists, evaluated whether locally advanced unresectable pancreatic cancer could be reclassified as resectable following CRT treatment. In cases of intent-to-cure surgery, the margin status obtained from a surgically resected specimen was assessed by two expert pathologists at the Kochi Health Sciences Center.20 Overall survival was calculated from the date of treatment initiation for locally advanced unresectable pancreatic cancer until the date of death in patients with or without recurrent disease. The primary end point of this study was overall survival in patients with locally advanced unresectable pancreatic cancer following CRT. The secondary end point was pathological features of pancreatic carcinoma after CRT compared with features of specimens of pancreatic carcinomas resected at our clinic without any neoadjuvant treatment. Using subgroup analysis by assessing the therapeutic response and evaluating trends in levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), we also evaluated which types of locally advanced unresectable pancreatic cancer were reclassified after CRT as potentially treatable via surgical resection with the intent to cure.
Statistical analyses
Patients alive in July 2016 were censored at the time of follow-up. Qualitative variables were compared using the χ2 test or Fisher's test while quantitative variables were analyzed using Student's t-test or a non-parametric test, and survival data were determined using a stratified log-rank test. All tests were two-sided, with a p-value of <0.05 considered to indicate statistical significance. All analyses were performed using SPSS® (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). The overall survival and disease-free survival were estimated using the Kaplan–Meier method.21
RESULTS
We examined a total of 1168 patients who were diagnosed with pancreatic cancer from March 2005 to November 2015 at the Kochi Health Sciences Center. Among these patients, 804 patients (68.8%) had already been diagnosed with unresectable pancreatic cancer with distant metastases, 271 (23.2%) had received diagnoses of resectable pancreatic cancer (RPC) and 93 patients (8.0%) had been classified with locally advanced unresectable pancreatic cancer without any distant metastases. Of these 93 patients with locally advanced unresectable pancreatic cancer, 55 were males and 38 were females, ranging in age from 48 to 83 years (median, 68 years) (Table 1). There were no significant differences between the study participants with respect to gender, body mass index or tumour size. There were also no significant differences between the groups with locally advanced unresectable pancreatic cancer and RPC with respect to the levels of pre-treatment tumour markers including CEA and CA19-9. Interestingly, however, the mean age of patients with locally advanced unresectable pancreatic cancer was significantly higher than that of patients with RPC at the time of presentation. Furthermore, as expected, the incidence of the situation of locally advanced unresectable pancreatic cancer was significantly greater in the pancreatic body because of the anatomical complex (Table 1). The major causes of unresectability prior to CRT were tumour invasion to the plexus around the superior mesenteric artery that exceeded 180°, invasion to the hepatic artery or celiac abutment (Table 1).
Table 1.
Characteristics of patients who underwent treatment for locally advanced pancreatic cancer
| Characteristics | LAPC (n = 93) | RPC (n = 271) | p-value |
|---|---|---|---|
| Age (years, mean ± SD) | 67.9 ± 8.5 | 71.2 ± 10.3 | 0.003 |
| Gender (male/female) | 55/38 | 145/126 | 0.346 |
| Body mass index (kg m2, mean ± SD) | 21.2 ± 2.9 | 22.0 ± 3.2 | 0.066 |
| Pre-treatment TM (median, range) | |||
| CEA (ng ml−1) | 3.6 (1.0–198.1) | 3.5 (0.5–307.0) | 0.681 |
| CA19-9 (U ml−1) | 207.3 (0.1–16,406.0) | 281.0 (0.1–44,188.0) | 0.891 |
| Pre-treatment demographics | |||
| Tumour size (cm, median ± SD) | 3.2 ± 1.2 | 3.6 ± 1.8 | 0.447 |
| Location (Ph/Pb/Pt) | 57/33/3 | 194/25/51 | <0.001 |
| Pre-treatment unresectable factors | |||
| SMA | 43 | – | – |
| SMA + CHA + CeA | 27 | – | – |
| CHA | 16 | – | – |
| CHA + CeA | 4 | – | – |
| SMA + CeA | 3 | – | – |
| Radiotherapy plus | |||
| Gemcitabine | 23 | – | – |
| S-1 | 70 | – | – |
CA19-9, pre-treatment blood chemistry serum carbohydrate antigen 19-9 level; CeA, celiac artery; CEA, pre-treatment blood chemistry serum carcinoembryonic antigen level; CHA, common hepatic artery; LAPC, locally advanced pancreatic cancer; Pb, pancreas body; Ph, pancreas head; Pt, pancreas tail; RPC, resectable pancreatic cancer; SD, standard deviation; SMA, superior mesenteric artery; TM, tumour marker.
Fortunately, of 93 patients with locally advanced unresectable pancreatic cancer, 35 patients (37.6%) were reclassified as having resectable disease following CRT. None of Grade 3 or 4 leucopenia, neutropenia, aspartate aminotransferase and alanine aminotransferase was observed in the patients during CRT, whereas stomatitis was experienced in two patients who received CRT (2.2%). In the remaining 58 patients, the major factors accounting for unresectability after CRT were liver metastases (31 patients), peritoneal dissemination (26 patients), lymph node metastases (6 patients), local growth (4 patients) and lung metastases (2 patients). Table 2 details the operation-related characteristics and pathological features in patients who underwent radical surgical resection after CRT for pancreatic cancer diagnosed as locally advanced unresectable pancreatic cancer without any distant metastases (n = 35) compared with those in patients with RPC (n = 271). The operation time was significantly longer in the CRT group than in the RPC group [median operation time, 330 min in the CRT group vs 263 min in the RPC group, respectively (p = 0.001)]. Post-operative morbidities, examined by abdominal ultrasonography and/or CT, were not significantly different between the groups in terms of post-operative complications including post-operative pancreatic fistula after pancreatic resection based on the classification system of the International Study Group of Pancreatic Surgery and the Clavien–Dindo classification of surgical complications. There were no significant differences in tumour size or pathological differentiation between the patients with locally advanced unresectable pancreatic cancer who were reclassified as having RPC after CRT and the patients with RPC at the time of presentation (Figure 1). Interestingly, the rates of both lymphatic permeation and lymph node metastases were significantly reduced in patients who underwent CRT compared with patients who received no neoadjuvant treatment, although the incidence of plexus-nerve invasion in patients with CRT was significantly higher than in patients who underwent surgery first (Table 2). Our pathological assessments after surgical resection with the intent to cure for pancreatic carcinoma suggested that CRT might be associated with a significant improvement in the risk of lymph node metastases in patients with locally advanced unresectable pancreatic cancer.
Table 2.
Operation-related characteristics and pathological features in patients who underwent radical surgical resection for pancreatic cancer after chemoradiotherapy (CRT) diagnosed as locally advanced unresectable pancreatic cancer without any distant metastases (n = 35) compared with those in resectable pancreatic cancer patients
| Characteristics | Resectablea (n = 35) | RPC (n = 271) | p-value |
|---|---|---|---|
| Operation time (min)b | 330 (205–817) | 263 (55–822) | 0.001 |
| Blood loss volume (ml)b | 510 (30–15,017) | 400 (10–15,330) | 0.090 |
| Post-operative morbidities | |||
| Pancreatic fistula >grade B/C (%) | 2 (5.7) | 29 (10.7) | 0.534 |
| C–D classification >III | 3 (8.6) | 31 (11.4) | 0.824 |
| Tumour size (cm, median ± SD) | 4.0 ± 1.7 | 3.5 ± 1.7 | 0.157 |
| Differentiation (well/mod/por) | 12/14/9 | 74/158/39 | 0.086 |
| Pathological demographics (%) | |||
| Adjacent organ invasion | 17 (48.6) | 143 (52.8) | 0.640 |
| Portal vain invasion | 14 (40.0) | 91 (33.6) | 0.451 |
| Plexus-nerve invasion | 18 (51.4) | 43 (15.9) | <0.001 |
| Lymphatic permeation | 13 (37.1) | 179 (66.1) | 0.001 |
| Microvascular involvement | 20 (57.1) | 147 (54.2) | 0.746 |
| Perineural invasion | 30 (85.7) | 215 (79.3) | 0.507 |
| Retroperitoneal invasion | 27 (77.1) | 197 (71.6) | 0.576 |
| Serosal invasion | 13 (37.1) | 126 (46.5) | 0.296 |
| Lymph node metastasis | 13 (37.1) | 187 (69.0) | <0.001 |
| Positive surgical margin | 5 (14.3) | 55 (20.3) | 0.538 |
mod, moderately differentiated adenocarcinoma; por, poorly differentiated adenocarcinoma; RPC, resectable pancreatic cancer; SD, standard deviation; well, well differentiated adenocarcinoma.
Post-operative complications were graded according to the Clavien–Dindo classification, which was validated in pancreatic surgery. Complications requiring surgical, endoscopic or radiological intervention, requiring intensive care admission or causing death were considered as major (grade III–V). Pancreatic fistula is defined according to the International Study Group on Pancreatic Fistula criteria.
Locally advanced unresectable pancreatic cancer could shift to be considered as resectable following the CRT treatment.
Data presented as median (range).
Figure 1.
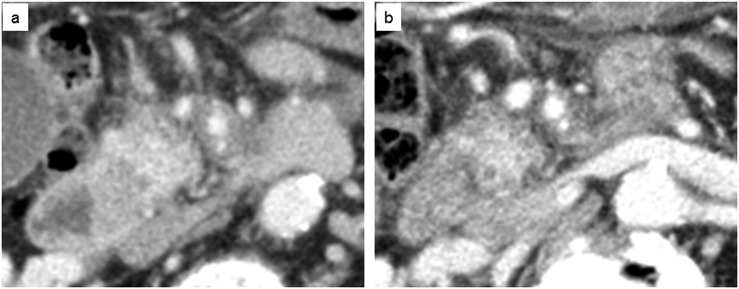
Typical CT of locally advanced pancreatic cancer. (a) Enhanced CT demonstrated involvement of superior mesenteric vein as well as total encasement of superior mesenteric artery by tumour before chemoradiotherapy. (b) Enhanced CT revealed improvement in the tissue changes around the superior mesenteric artery and vein with radiological response or resolution of peritumoural inflammatory change after chemoradiotherapy.
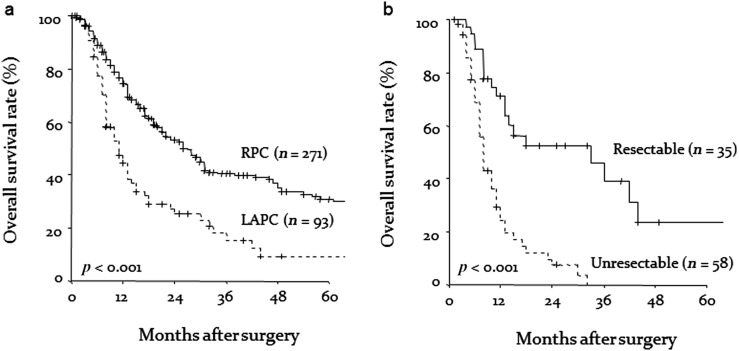
The analysis of overall survival was based on 238 deaths (65.4%) among the 364 patients. The duration of patient follow-up as of July 2016 ranged from 1 to 125 months with a median of 13.0 months (mean 19.1 months). Although overall 1-, 3- and 5-year survival rates in patients diagnosed with RPC at the time of presentation were 76.7%, 40.4% and 30.9%, respectively, the overall 1-, 3- and 5-year survival rates in patients with locally advanced unresectable pancreatic cancer were 47.2%, 15.5% and 9.3%, respectively (p < 0.001) (Figure 2a). Surgical resection of locally advanced unresectable pancreatic cancer was performed after CRT in 30 patients with margin-negative histology (85.7%) and in only 5 patients showing microscopic tumour residue (total, n = 35) (Table 2). The median overall survival of patients who received CRT alone for locally advanced unresectable pancreatic cancer was 8.0 months, and all died within 3 years (Figure 2b). On the other hand, the overall 1-, 3- and 5-year survival rates in patients who were reclassified as having resectable tumour after CRT and received curative surgical management were 71.3%, 39.2% and 23.5%, respectively (Figure 2b). Patients undergoing curative resection in this study showed a significant survival advantage over those whose tumours were not amenable to surgical treatment (p < 0.001). Surprisingly, analyses of survival according to surgically treated patients showed no significant differences in post-operative survival rate between patients with locally advanced unresectable pancreatic cancer who underwent surgery after CRT and patients who underwent surgery first (p = 0.639). Our clinicopathological data after surgical resection suggested that prognosis for patients with locally advanced unresectable pancreatic cancer might be improved after CRT, if the pancreatic adenocarcinoma could be reclassified as resectable disease and be surgically removed with the intent to cure. The major sites or forms of recurrence observed were liver (83 patients), local recurrence (59 patients), peritoneal dissemination (51 patients), lung metastases (35 patients) and lymph nodes (25 patients) (Table 3). There was no significant association between the patient groups with respect to the incidence of peritoneal dissemination, liver metastases, lymph node recurrence or local recurrence (Table 3).
Figure 2.
(a) Kaplan–Meier estimates of overall survival rates after curative procedure for resectable pancreatic cancer (RPC) at the time of presentation and overall survival rates for locally advanced unresectable pancreatic cancer (LAPC). (b) The actual survival rate (%) compared with treatment options in patients with unresectable pancreatic cancer.
Table 3.
Recurrence pattern after surgery
| Characteristics | Resectablea (n = 35) | RPC (n = 271) | p-value |
|---|---|---|---|
| Liver | 12 | 71 | 0.311 |
| Local | 3 | 56 | 0.139 |
| Peritoneal dissemination | 6 | 45 | 0.872 |
| Lymph nodes | 2 | 23 | 0.814 |
| Lung | 6 | 29 | 0.398 |
| Remnant pancreas | 1 | 4 | 0.919 |
| Brain | 1 | 0 | 0.225 |
| Bone | 2 | 4 | 0.292 |
RPC, resectable pancreatic cancer.
Locally advanced unresectable pancreatic cancer could shift to be considered as resectable following the CRT treatment.
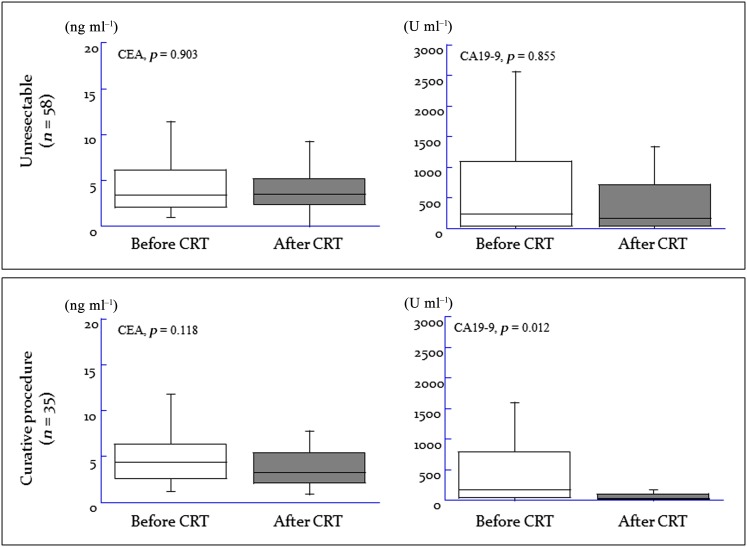
Our subgroup analyses evaluated whether the tumours of patients with locally advanced unresectable pancreatic cancer could be rendered completely resectable after CRT, thus providing a peri-treatment predictive estimate. Monitoring the serum levels of tumour markers during the course of CRT revealed no significant difference in CEA levels between patients with still unresectable disease and patients who underwent curative surgery during CRT (Figure 3); however, there was a significant reduction in serum CA19-9 levels during CRT in patients who underwent curative surgical management (mean reduction rate, 57.8%) compared with patients with unresectable disease (mean reduction rate, −48.7%) (p = 0.012).
Figure 3.
Changing trends in serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels during the course of treatment.
DISCUSSION
In the current study, we observed that 38% of cases of locally advanced unresectable pancreatic cancer could be reclassified as resectable following CRT treatment. Importantly, sufficient curative treatment was achieved for patients with locally advanced unresectable pancreatic cancer after CRT, and there were no statistically significant differences in 3-year (40% vs 39%) and 5-year overall survival (31% vs 24%) between patients who first underwent surgery for pancreatic cancer diagnosed as resectable at the time of presentation and those with locally advanced unresectable pancreatic cancer receiving surgical management after CRT. A few similar studies have cited median overall survival time after surgical treatment for locally advanced unresectable pancreatic cancer following CRT, and the current reports range widely from 19 to 32 months, as does the reported incidence of surgical treatment after CRT for locally advanced unresectable pancreatic cancer, at 14–29%.22–25 These wide ranges probably result from the lack of a general consensus regarding the clinical definition of locally advanced unresectable pancreatic cancer prior to 2014.16,17 In the current study, the initial diagnosis of locally advanced unresectable pancreatic cancer was made according to National Comprehensive Cancer Network guidelines.16 We believe that our report may be valuable as a landmark in the understanding of post-operative outcomes and clinical effects of CRT in the pursuit of optimal treatment of locally advanced unresectable pancreatic cancer.
CRT was historically used as the primary and then initial treatment for patients with locally advanced pancreatic cancer. In this context, some studies have argued that conversion to resectability represents the ultimate goal of treatment in locally advanced disease, and actual downstaging of tumours that encase or obliterate the celiac or superior mesenteric vessels is extremely uncommon with current treatment strategies.4 Several studies have suggested that CRT may enhance resectability and inhibit lymph node metastases.26,27 A Phase II trial published in 1993 demonstrated a significant reduction in the incidence of positive margins and lymph nodes after pre-operative CRT and concluded that low rate of nodal metastasis might be attributable to neoadjuvant treatment.28 Interestingly, our series clearly demonstrated a significant reduction in the incidence of positive lymph node metastases in tumours treated with CRT. Thus, a definitive study that randomizes patients to treatment with surgery first or CRT at the point of diagnosis of pancreatic cancer is needed to provide effective evaluation strategies for determining whether pancreatic cancer has progressed to systemic disease; however, the current state of clinical equilibrium in the community makes such evidenced-based research unlikely.20
Because of minimal survival benefit provided by surgery alone, the standard treatment of pancreatic cancer had entered the era of post-operative adjuvant chemotherapy using gemcitabine with the results of large clinical studies.29,30 Current major questions that warrant clinical research and co-operation include the impact of surgical indications on overall survival after curative surgical management, accepting the proven impact on both local and distant metastatic recurrences and whether CRT in the neoadjuvant setting improves outcomes. Of note, results of previous research support our study by including extensive heterogeneity in the regimens of chemotherapy and CRT used, as well as in the criteria for surgical resection.10–14,22–25 On the other hand, negative data were reported about CRT using 5-fluorouracil and radiation for pancreatic cancer.31 A previous author showed that adjuvant CRT not only fails to benefit patients but also reduces survival when it is given before CT.31 Our study has several potential limitations and is a retrospective cohort review of patients undergoing pancreatic resection that included only patients who underwent a resection. Clearly, our results warrant further investigation that addresses the adequacy of neoadjuvant regimens. Indeed, the pancreatic cancer community throughout the world is anticipating the results of a large randomized clinical study with more aggressive CRT regimens such as FOLFIRINOX, gemcitabine-based or capecitabine-based CRT, and gemcitabine plus nab-paclitaxel that might show a survival benefit in locally advanced unresectable pancreatic cancer.28,32,33
Importantly, in the group of patients who received CRT in the current study, the 35 patients who completed the entire therapeutic regimen, including curative surgery, experienced significantly better clinical outcomes than the remaining 58 patients who did not undergo resection. From the view point of surgery-related demographics, although median operative time after CRT was longer in patients with locally advanced pancreatic cancer, our results suggested that incidence of severe complications were not affected by neoadjuvant CRT, even when CRT causes injury of not only cancerous but also normal tissues.34 Unfortunately, adequate evaluation of resectability has historically been vague, and considerable debate and controversy remain regarding which patients are deemed truly resectable. Factors that contribute to this confusion are multiple and include subjective interpretation of cross-sectional imaging, technical/surgical ability and overall institutional experience, because imaging no longer predicts unresectability after neoadjuvant therapy.28,35,36 To address these issues, the current study highlighted altered CA19-9 status as an independent factor associated with resectability in the evaluation of patients with locally advanced unresectable pancreatic cancer during CRT, although, surprisingly, serum CA19-9 levels in the five patients who underwent pancreatectomy after CRT and survived for more than 3 years were within the normal range. These findings suggested that patients who develop distant metastases on reassessment might not show a CA19-9 reduction rate >60%, further suggesting that the CA19-9 reduction rate is associated with the systemic progression of locally advanced unresectable pancreatic cancer.37 Accordingly, we believe that an indicated shift in locally advanced unresectable pancreatic cancer to resectability following CRT is not a contraindication to surgical management with a curative intent and should be routinely offered to suitable patients in high-volume specialized centres.
In conclusion, the treatment of locally advanced unresectable pancreatic cancer remains extremely challenging, and few patients are ultimately cured of their disease. This study specifically focused on the role of CRT treatment with respect to disease management in patients with locally advanced unresectable pancreatic cancer compared with patients with RPC at the time of presentation. Our results suggested that CRT shows some clinical efficacy in prolonging survival, possibly by affecting resectability. Accordingly, we recommend that the relevant surgeons, oncologists and radiologists engage in robust academic and clinical co-operation to optimize therapeutic management for patients with locally advanced unresectable pancreatic cancer.
FUNDING
This work was supported by the Kochi Organization for Medical Reformation and Renewal grants.
Contributor Information
Kenta Sui, Email: kenta_sui@khsc.or.jp.
Takehiro Okabayashi, Email: tokabaya@gmail.com.
Yasuo Shima, Email: yasuo_shima@khsc.or.jp.
Sojiro Morita, Email: sojiro_morita@khsc.or.jp.
Jun Iwata, Email: jun_iwata@khsc.or.jp.
Tatsuaki Sumiyoshi, Email: tasu050520@yahoo.co.jp.
Yuichi Saisaka, Email: you1sai@gmail.com.
Yasuhiro Hata, Email: hatapw@yahoo.co.jp.
Yoshihiro Noda, Email: yoshihiro_noda@khsc.or.jp.
Manabu Matsumoto, Email: manabu_matumoto@khsc.or.jp.
Akihito Nishioka, Email: akihito_nishioka@khsc.or.jp.
Tastuo Iiyama, Email: iiyamat@kochi-u.ac.jp.
Yasuhiro Shimada, Email: yasuhiro_shimada@khsc.or.jp.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014; 371: 1039–49. doi: https://doi.org/10.1056/nejmra1404198 [DOI] [PubMed] [Google Scholar]
- 3.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol 2010; 7: 163–72. doi: https://doi.org/10.1038/nrclinonc.2009.236 [DOI] [PubMed] [Google Scholar]
- 4.Heestand GM, Murphy JD, Lowy AM. Approach to patients with pancreatic cancer without detectable metastases. J Clin Oncol 2015; 33: 1770–8. doi: https://doi.org/10.1200/jco.2014.59.7930 [DOI] [PubMed] [Google Scholar]
- 5.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet 2011; 378: 607–20. doi: https://doi.org/10.1016/s0140-6736(10)62307-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartwig W, Werner J, Jäger D, Debus J, Büchler MW. Improvement of surgical results for pancreatic cancer. Lancet Oncol 2013; 14: e476–485. doi: https://doi.org/10.1016/s1470-2045(13)70172-4 [DOI] [PubMed] [Google Scholar]
- 7.Hartwig W, Hackert T, Hinz U, Gluth A, Bergmann F, Strobel O, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg 2011; 254: 311–19. doi: https://doi.org/10.1097/sla.0b013e31821fd334 [DOI] [PubMed] [Google Scholar]
- 8.Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo CF, Deshpande V, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg 2013; 257: 731–6. doi: https://doi.org/10.1097/sla.0b013e318263da2f [DOI] [PubMed] [Google Scholar]
- 9.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74: 2913–21. doi: https://doi.org/10.1158/0008-5472.can-14-0155 [DOI] [PubMed] [Google Scholar]
- 10.Loehrer PJ, Sr, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol 2011; 29: 4105–12. doi: https://doi.org/10.1200/jco.2011.34.8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouché O, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol 2008; 19: 1592–9. doi: https://doi.org/10.1093/annonc/mdn281 [DOI] [PubMed] [Google Scholar]
- 12.Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst 1988; 80: 751–5. [PubMed] [Google Scholar]
- 13.Klaassen DJ, MacIntyre JM, Catton GE, Engstrom PF, Moertel CG. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil—an Eastern Cooperative Oncology Group study. J Clin Oncol 1985; 3: 373–8. doi: https://doi.org/10.1200/jco.1985.3.3.373 [DOI] [PubMed] [Google Scholar]
- 14.Hazel JJ, Thirlwell MP, Huggins M, Maksymiuk A, MacFarlane JK. Multi-drug chemotherapy with and without radiation for carcinoma of the stomach and pancreas: a prospective randomized trial. J Can Assoc Radiol 1981; 32: 164–5. [PubMed] [Google Scholar]
- 15.Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009; 27: 1806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tempero MA, Malafa MP, Behrman SW, Benson AB, 3rd, Casper ES, Chiorean EG, et al. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2014; 12: 1083–93. [DOI] [PubMed] [Google Scholar]
- 17.Bockhorn M, Uzunoglu FG, Adham M; International Study Group of Pancreatic Surgery. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014; 155: 977–88. doi: https://doi.org/10.1016/j.surg.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 18.Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013; 31: 1640–8. doi: https://doi.org/10.1200/jco.2012.43.3680 [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. doi: https://doi.org/10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- 20.Ravikumar R, Sabin C, Abu Hilal M; UK Vascular Resection in Pancreatic Cancer Study Group. Portal vein resection in borderline resectable pancreatic cancer: a United Kingdom multicenter study. J Am Coll Surg 2014; 218: 401–11. doi: https://doi.org/10.1016/j.jamcollsurg.2013.11.017 [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457. [Google Scholar]
- 22.Arvold ND, Ryan DP, Niemierko A, Blaszkowsky LS, Kwak EL, Wo JY, et al. Long-term outcomes of neoadjuvant chemotherapy before chemoradiation for locally advanced pancreatic cancer. Cancer 2012; 118: 3026–35. doi: https://doi.org/10.1002/cncr.26633 [DOI] [PubMed] [Google Scholar]
- 23.Turrini O, Viret F, Moureau-Zabotto L, Guiramand J, Moutardier V, Lelong B, et al. Neoadjuvant chemoradiation and pancreaticoduodenectomy for initially locally advanced head pancreatic adenocarcinoma. Eur J Surg Oncol 2009; 35: 1306–11. doi: https://doi.org/10.1016/j.ejso.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 24.Sa Cunha A, Rault A, Laurent C, Adhoute X, Vendrely V, Béllannée G, et al. Surgical resection after radiochemotherapy in patients with unresectable adenocarcinoma of the pancreas. J Am Coll Surg 2005; 201: 359–65. doi: https://doi.org/10.1016/j.jamcollsurg.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 25.Snady H, Bruckner H, Cooperman A, Paradiso J, Kiefer L. Survival advantage of combined chemoradiotherapy compared with resection as the initial treatment of patients with regional pancreatic carcinoma. An outcomes trial. Cancer 2000; 89: 314–27. doi: https://doi.org/10.1002/1097-0142(20000715)89:2<314::aid-cncr16>3.0.co;2-v [DOI] [PubMed] [Google Scholar]
- 26.Spitz FR, Abbruzzese JL, Lee JE. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol 1997; 15: 928–37. doi: https://doi.org/10.1200/jco.1997.15.3.928 [DOI] [PubMed] [Google Scholar]
- 27.Yeung RS, Weese JL, Hoffman JP, Pisters PW, Lowy AM, Fenoglio CJ, et al. Neoadjuvant chemoradiation in pancreatic and duodenal carcinoma. A Phase II study. Cancer 1993; 72: 2124–33. doi: https://doi.org/10.1002/1097-0142(19931001)72:7<2124::aid-cncr2820720711>3.0.co;2-c [DOI] [PubMed] [Google Scholar]
- 28.Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015; 261: 12–17. doi: https://doi.org/10.1097/sla.0000000000000867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017; 389: 1011–24. [DOI] [PubMed] [Google Scholar]
- 30.Uesaka K, Boku N, Fukutomi A. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016; 388: 248–57. doi: https://doi.org/10.1016/s0140-6736(16)30583-9 [DOI] [PubMed] [Google Scholar]
- 31.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004; 350: 1200–10. doi: https://doi.org/10.1056/nejmoa032295 [DOI] [PubMed] [Google Scholar]
- 32.Alvarez R, Musteanu M, Garcia-Garcia E, Lopez-Casas PP, Megias D, Guerra C, et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br J Cancer 2013; 109: 926–33. doi: https://doi.org/10.1038/bjc.2013.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee S, Hurt CN, Bridgewater J, Falk S, Cummins S, Wasan H, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol 2013; 14: 317–26. doi: https://doi.org/10.1016/s1470-2045(13)70021-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho SW, Tzeng CW, Johnston WC, Cassera MA, Newell PH, Hammill CW, et al. Neoadjuvant radiation therapy and its impact on complications after pancreaticoduodenectomy for pancreatic cancer: analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). HPB (Oxford) 2014; 16: 350–6. doi: https://doi.org/10.1111/hpb.12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silvestris N, Longo V, Cellini F, Reni M, Bittoni A, Cataldo I, et al. Neoadjuvant multimodal treatment of pancreatic ductal adenocarcinoma. Crit Rev Oncol Hematol 2016; 98: 309–24. doi: https://doi.org/10.1016/j.critrevonc.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 36.Schorn S, Demir IE, Reyes CM, Saricaoglu C, Samm N, Schirren R, et al. The impact of neoadjuvant therapy on the histopathological features of pancreatic ductal adenocarcinoma—a systematic review and meta-analysis. Cancer Treat Rev 2017; 55: 96–106. doi: https://doi.org/10.1016/j.ctrv.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 37.Hess V, Glimelius B, Grawe P, Dietrich D, Bodoky G, Ruhstaller T, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol 2008; 9: 132–8. doi: https://doi.org/10.1016/s1470-2045(08)70001-9 [DOI] [PubMed] [Google Scholar]