Abstract
Objective:
The development of atherosclerotic plaques and spontaneous isolated dissection (SID) of the superior mesenteric artery (SMA) was considered to be related to opposite haemodynamics. The purpose of this study was to compare their occurrence sites and the morphology of the SMA to confirm the haemodynamic aetiologies.
Methods:
57 patients with SID and 64 patients with atherosclerotic plaques were compared about patient characteristics, location of SID and plaque, the distance from lesion to the aortic ostia, SMA branching angle and inlet diameter of the SMA.
Results:
The location of SID and plaque was very different (p < 0.001). The anterior wall was the most common entry site of SIDSMA (84.0%) but the least frequent origin site of atherosclerotic plaques (7.8%). The posterior, left and right walls were the frequent origin sites of atherosclerotic plaques (total 92.2%) but not for SIDSMA. Most plaques started from the aortic ostia, and their average distance to the aortic ostia was significantly less than the distance from the entry site to the aortic ostia of SIDSMA (p < 0.001). No significant difference was found between SIDSMA and the plaque groups in the branching angle and inlet diameter of the SMA.
Conclusion:
The vastly different sites of SIDSMA and atherosclerotic plaque indicate their opposite haemodynamic aetiology.
Advances in knowledge:
By comparing the location of the two diseases, we demonstrate their different haemodynamic causes.
INTRODUCTION
Spontaneous isolated dissection (SID) of the superior mesenteric artery (SMA) was once considered to be a rare disease. However, with the increasing use of contrast-enhanced CT, a growing number of cases with SIDSMA have been reported in recent years, especially in East Asian countries.1–5 In our institution, this condition is also not uncommon in clinical practice. SIDSMA is more prevalent in male patients in their 50s. The potential risk factors included hypertension, smoking, gene variation and haemodynamic abnormalities. The entry sites of SIDSMA were always close to the lower margin of the pancreas. Thus, one explanation was the shear stress injury at the transitional zone of the SMA from a fixed to a relatively unfixed segment.6 However, in the study of Park et al,4 the dissection entry was found not consistent with the lower margin of the pancreas. With the constant entry site corresponding to high wall shear stress, SIDSMA was believed to be more likely due to abnormal haemodynamic forces. The site was in accordance with the high wall shear stress area in another computational fluid dynamic model.7 However, Tomita et al2 demonstrated the “dissection origin” was more proximal to the pancreatic edge or the maximum curve of the SMA, therefore the haemodynamic mechanism was still controversial.
Atherosclerotic plaques are the main cause of SMA stenosis and may lead to acute or chronic mesenteric ischaemia.8 It has now been widely accepted that although affected by many factors,9 the development of atherosclerotic plaques was related to haemodynamic forces.10,11 The plaques initially arise in the area of low and oscillatory shear stress and rarely appear in the area of high shear stress. Thus, low and oscillatory shear stress facilitated the development of atherosclerotic plaques, whereas high shear stress was considered as an atheroprotective factor. Plaques of the SMA were frequently close to the aortic ostia.8,12,13 A transient computational fluid dynamic model showed that the site of high oscillatory shear stress in the SMA was consistent with the clinical observed location of atheroma.7 Therefore, if the occurrence of SIDSMA was closely related to high shear stress, the entry site cannot be located in the common site of atherosclerotic plaques. The common occurrence site of SID and atherosclerotic plaques of the SMA should be very different.
The purpose of this study was to retrospectively analyze the contrast-enhanced CT images of SID and atherosclerotic plaques of the SMA and to compare their occurrence sites and the morphology of the SMA to verify the opposite haemodynamic aetiology.
METHODS AND MATERIALS
The local institutional review board approved this retrospective study. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. Cases diagnosed of “superior mesenteric artery dissection” and “superior mesenteric artery plaque/stenosis” were retrieved from the CT database from June 2013 to September 2016. Two radiologists with more than 10 years of experience reviewed the images individually. Disagreements were resolved by discussion or upon consensus by a third author. The diagnosis of SIDSMA was made when: (a) double lumen with intimal flap was seen after contrast enhancement or (b) a crescent-shaped area with higher attenuation than blood was found with no contrast enhancement.14 Patients with combined aortic dissection and trauma- or operation-related SMA dissection were excluded. The atherosclerotic plaques of the SMA were confirmed as calcification or filling defected with low density after contrast agent injection. Patients with two or more plaques or combined with other conditions in SMA, such as thrombosis or aneurysm were also excluded. Finally, a total of 57 patients (55 males and 2 females; mean age, 54.4 years; range, 40–87 years) with SIDSMA and 64 patients (37 males and 27 females; mean age, 74.7 years; range, 54–93 years) with atherosclerotic plaques were included in this study. The demographic characteristics (age and gender) and clinical characteristics (acute abdominal pain, hypertension, diabetes mellitus, smoking history and intra-abdominal cancer) are shown in Table 1.
Table 1.
The demographic and clinical characteristics of patients
| Patient characteristics | SIDSMA (n = 57) | Plaque (n = 64) | p-value |
|---|---|---|---|
| Age (years), mean ± SD | 54.4 ± 9.1 | 74.7 ± 9.1 | <0.001 |
| Male, n (%) | 55 (96.5%) | 37 (57.8%) | <0.001 |
| Acute abdominal pain, n (%) | 51 (89.5%) | 23 (35.9%) | <0.001 |
| Hypertension, n (%) | 16 (28.1%) | 28 (43.8%) | 0.073 |
| Diabetes mellitus, n (%) | 3 (5.3%) | 12 (18.6%) | 0.025 |
| Smoking history, n (%) | 22 (38.6%) | 15 (23.4%) | 0.108 |
| Intra-abdominal cancer, n (%) | 3 (5.3%) | 16 (25%) | 0.003 |
SD, standard deviation; SIDSMA, spontaneous isolated dissection of the superior mesenteric artery.
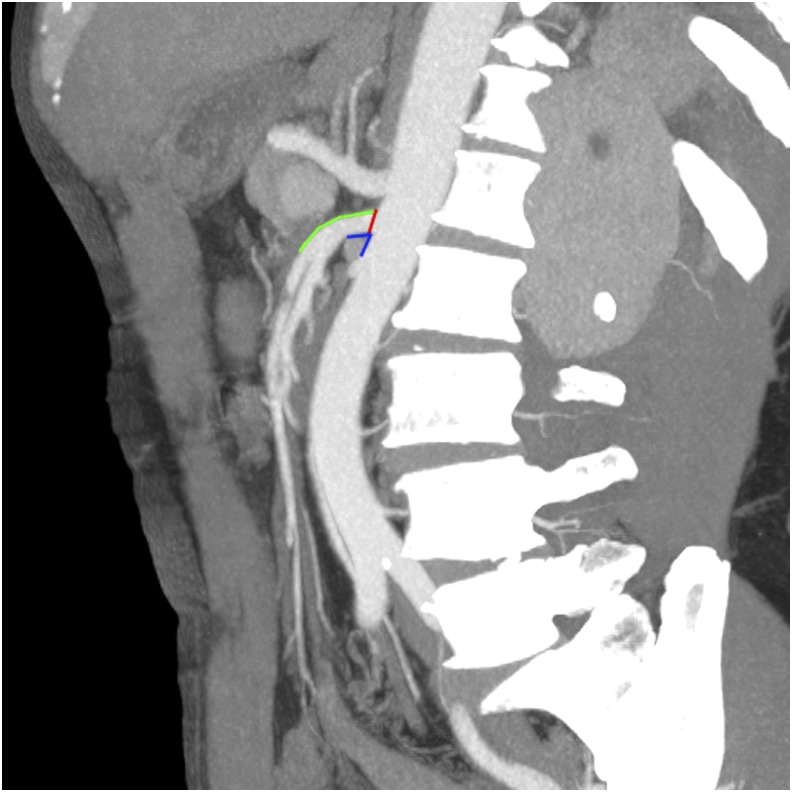
CT imaging was conducted with dual-source 64-slice scanner (Siemens Medical System, Forchheim, Bavaria, Germany). After intravenous injection of 1.5 ml kg−1 of iopromide (Ultravist® 370; Bayer Schering, Berlin, Germany) at a flow rate of 3–5 ml s−1, the contrast-enhanced images were acquired. The scanning parameters were 120 kV, 312 mA, field of view 362 × 362 cm, matrix 512 × 512 and slice thickness 0.75 mm. Raw data were transferred to the local database. Under the supervision of an expert, three authors investigated the lesion characteristics and the morphology of the SMA on the Volume Viewer (AW Suite 2.0; GE Healthcare Milwaukee, WI, USA) with multiplanar reformatting (MPR) and maximum intensity projections. The morphology types of SIDSMA were categorized based on the classification of Yun et al.3 The location of the entry site of SID and origin site of plaques were recorded as anterior, posterior, left or right. The distance from the entry site or origin site to the aortic ostia and the distance from the entry site to the lower margin of the pancreas were measured on the MPR images. The anatomical feature of the SMA, including the SMA branching angle from the aorta and the inlet diameter were measured on sagittal MPR images (Figure 1).
Figure 1.
The distance from the entry site of spontaneous isolated dissection of the superior mesenteric artery (SMA) to the aortic ostia (green), the SMA branching angle (blue) and the inlet diameter (red) were measured on a sagittal multiplanar reconstruction image.
Patient characteristics, lesion characteristics and the morphology measurement of the SMA were compared between the SID and atherosclerotic plaque groups. Pearson's or Spearman's correlation analysis was employed to test the influence of age, sex and clinical characteristics on the occurrence site of SID and atherosclerotic plaques. Numerical data and approximately normally distributed data were compared using the Student's t test. The χ2 or Fisher's exact test (for small samples or highly imbalanced table cells) were used for categorical variables. Statistical analyses were performed using SPSS® software v. 22 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL), with two-sided p < 0.05 indicating a significant difference.
RESULTS
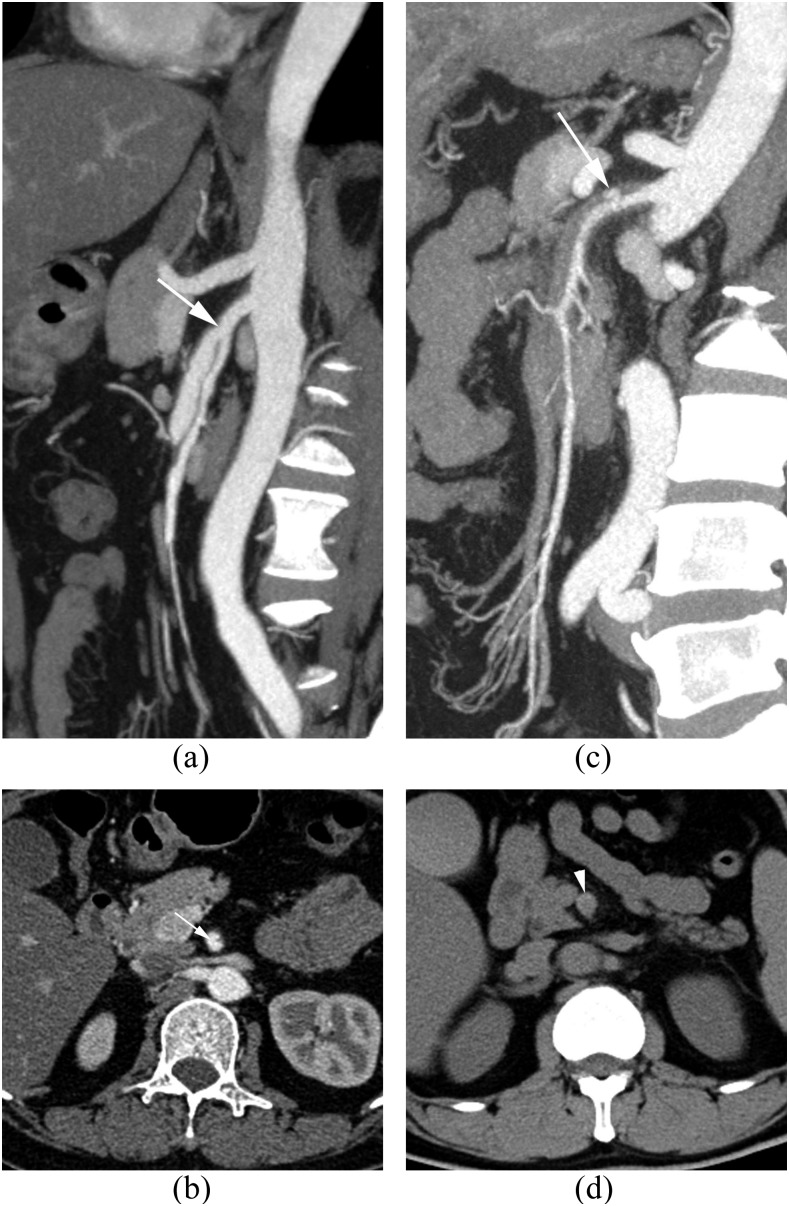
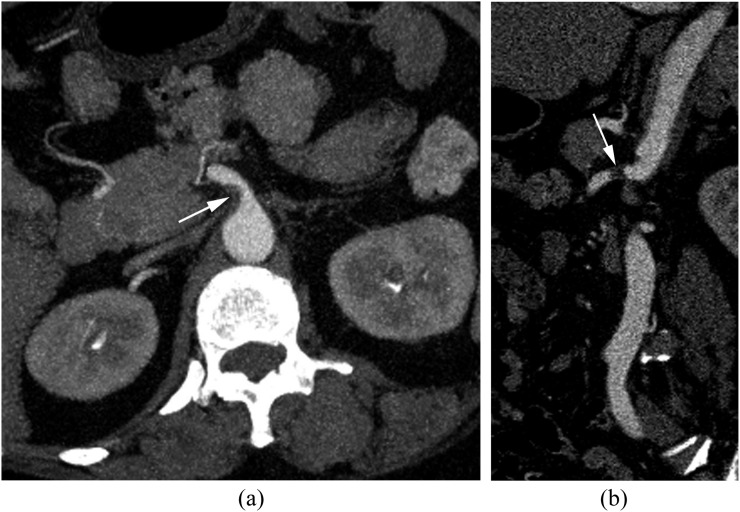
According to the classification of Yun et al, SIDSMAs were classified as 16 Type I (28.1%), 11 Type IIa (19.3%), 23 Type IIb (40.4%) and 7 Type III (12.3%) (Figure 2). Patients with SIDSMA had younger age and higher sex ratio than patients with atherosclerotic plaques (p < 0.001 for both). Acute abdominal pain was more common in patients with SIDSMA (89.5%) than in those with atherosclerotic plaques (35.9%). Diabetes mellitus and intra-abdominal cancer were more frequent in patients with atherosclerotic plaques (Table 1). The location of SID and plaque was very different (p < 0.001). The most common entry site of SIDSMA was the anterior wall (84.0%), which was the least frequent origin site for atherosclerotic plaques (7.8%). Atherosclerotic plaques were equally distributed in the posterior, left and right walls (total 92.2%). There were six SIDSMAs started in the left wall and two in the right wall, with none in the posterior wall (Table 2). 49 (76.6%) of the 64 plaques started from the aortic ostia (Figure 3). The distance from the origin site to the aortic ostia of the atherosclerotic plaques was significantly less than the distance from the entry site to the aortic ostia of SIDSMA (p < 0.001). There was no significant relationship between age, gender, clinical characteristics and site of SIDSMA or plaques. No significant different was found between SIDSMA and plaque groups with regard to SMA branching angle and inlet diameter (Table 3). The distance from the entry site to the lower margin of the pancreas of SIDSMA varied from −27.9 cm (below) to 33.5 cm (higher) (mean ± standard deviation, −1.8 ± 14.7 cm).
Figure 2.
A 47-year-old female patient with Type IIa spontaneous isolated dissection (SID) of the superior mesenteric artery (SMA) (a, b) and a 48-year-old male patient with Type IIb SIDSMA (c, d). Sagittal and transverse views of post-contrast-enhanced images show the entry site (white arrows) of the dissection (a–c) located in the anterior wall of the SMA; the transverse view before contrast enhancement shows the crescent-shaped high attenuation (white arrowhead) in the SMA (d).
Table 2.
The entry site of spontaneous isolated dissection of the superior mesenteric artery (SIDSMA) and the origin site of atherosclerotic plaque
| Anterior, n (%) | Posterior, n (%) | Left, n (%) | Right, n (%) | |
|---|---|---|---|---|
| SID (n = 50) | 42 (84.0%) | 0 | 6 (12.0%) | 2 (4.0%) |
| Plaque (n = 64) | 5 (7.8%) | 21 (32.8%) | 18 (28.1%) | 20 (31.2%) |
SID, spontaneous isolated dissection.
Figure 3.
A 58-year-old female patient with atherosclerotic plaque. Transverse (a) and sagittal (b) views of post-contrast-enhanced images show the plaque (white arrow) located in the right wall of the superior mesenteric artery and started from the aortic ostia.
Table 3.
The comparison of lesion characteristics and the morphology of the superior mesenteric artery between spontaneous isolated dissection of the superior mesenteric artery (SIDSMA) and atherosclerotic group
| SIDSMA, mean ± SD | Plaque, mean ± SD | t | p-value | |
|---|---|---|---|---|
| DEntry (cm) | 26.4 ± 10.7a | 5.7 ± 12.8 | 9.192 | <0.001 |
| Angle (°) | 70.8 ± 29.2 | 61.6 ± 23.4 | 1.892 | 0.061 |
| Diameter (mm) | 9.3 ± 1.3 | 9.2 ± 1.4 | 0.545 | 0.587 |
SD, standard deviation.
Measurement from 50 patients with Type I and Type II. DEntry, distance from the entry site or origin site to the aortic ostia.
DISCUSSION
The exact pathogenic factor of SIDSMA is still elusive. It may be related to hypertension, smoking or gene variation. A new point of view was that the anatomical and haemodynamic characteristics of the SMA may be a causative factor of SIDSMA.4,15 The systemic risk factors of atherosclerotic plaque formation included hypertension, diabetes, high low-density lipoprotein cholesterol and smoking.9 However, haemodynamic factors contribute to the localization of plaque by interplaying with endothelial surfaces at the cellular and biochemical level.10,16 Although both SIDSMA and atherosclerotic plaque might be related to haemodynamic aetiology, their mechanisms were substantially different. Our cases demonstrate that the vast majority of SIDSMA occurred in the convex arc of the anterior wall, which was consistent with the high shear stress area shown on the vascular haemodynamic model of several previous studies.4,7 In addition, a few SIDSMAs appeared in the left and right walls but none in the posterior wall. The mean distance between the entry site of SID and the origin of the SMA in our study was close to that recorded by Park et al4 but larger than that recorded by Tomita et al.2 The possible explanation was that Tomita et al did not measure the distance from the location of the rupture but the origin site of dissection to the aortic ostia, which is closer to the SMA origin. The preference location of the plaque was the posterior, left and right walls near the aortic ostia. The anterior wall was rarely involved. The frequent location was vastly different from the SIDSMA and consistent with the site of high oscillatory shear stress showed by previous haemodynamic models.7 This suggests that the haemodynamic factor plays an important role in their development, especially in the occurrence site. In addition, it is of clinical significance to make clear the different haemodynamic aetiologies for the prevention and treatment of these two diseases. Earlier reporters believed that the occurrence of SIDSMA was related to the inferior borderline of the pancreas and the unfixed mesentery.6 However, the large variation in distance between the entry site and the lower margin of the pancreas ruled out such a probability. There was no significant difference in the diameter of the SMA entrance between the two groups. The SMA branching angle in patients with SIDSMA was higher than that in patients with plaque, but with no statistical difference.
The characteristics of patients with SIDSMA in this study were similar to those in previous studies.4,5,17 The reason of gender and age preference was still unclear.15 A case report showed that SIDSMA may be related to genes. The heterogeneity of a chromosome locus at 5q13-14, which linked to familial ascending aortic aneurysms and dissections, was found in two patients (an uncle and his nephew) and an unaffected female familial member.18 An interesting question was the sparing of the female with the defective gene. By contrast, there was no obvious gender preference for the atherosclerotic plaque in the SMA. The age of patients of atherosclerotic plaque was significantly greater than that of SIDSMA. However, age and gender were not confirmed to be related to the lesion site. Patient numbers for hypertension, diabetes mellitus, smoking history or intra-abdominal cancer in the SIDSMA group were not significantly more than those in the atherosclerotic group. The incidence rate of diabetes and hypertension in this study was not higher than the average value of the similar age group in a population-based survey.19 In the study by Park et al,4 SIDSMA was less common in patients with hypertension than in those with combined aortic and SMA dissection. This suggests that systemic risk factors may not play an important role in the development of SIDSMA.
There are some limitations in this study. Firstly, the hypothesis of haemodynamic distribution came from previous studies. This study did not analyze individual haemodynamics. Individual haemodynamics may vary due to different branching angles, inlet diameters and convex curvatures of the SMA. Further studies are needed to compare the haemodynamic abnormalities with the site of the lesion. In addition, the mechanisms of their formation are quite different, therefore the same factors, including age, gender and clinical characteristics, might play a different role in their formation. In spite of this, the correlation analysis showed these factors had no influence on the occurrence site.
CONCLUSION
The frequent occurrence site of SIDSMA and atherosclerotic plaque had almost no overlap and was consistent with areas of high and low (oscillatory) wall shear stress, respectively. This indicated critical and opposite roles that hemodynamic factors played in their occurrence site.
Acknowledgments
ACKNOWLEDGMENTS
The authors thank the staff of the Department of Vascular Surgery of Yixing Hospital Affiliated of Jiangsu University for their support.
Contributor Information
Zhi-gang Min, Email: minzhigang1980@163.com.
Hai-rong Shan, Email: staff278@yxph.com.
Long Xu, Email: staff754@yxph.com.
Su Yan, Email: staff1612@yxph.com.
Xue-xia Sheng, Email: staff838@yxph.com.
Jian Ji, Email: staff6429@yxph.com.
Zhi-hong Cao, Email: profczh@gmail.com.
REFERENCES
- 1.Zerbib P, Perot C, Lambert M, Seblini M, Pruvot FR, Chambon JP. Management of isolated spontaneous dissection of superior mesenteric artery. Langenbecks Arch Surg 2010; 395: 437–43. doi: https://doi.org/10.1007/s00423-009-0537-1 [DOI] [PubMed] [Google Scholar]
- 2.Tomita K, Obara H, Sekimoto Y, Matsubara K, Watada S, Fujimura N, et al. Evolution of computed tomographic characteristics of spontaneous isolated superior mesenteric artery dissection during conservative management. Circ J 2016; 80: 1452–9. doi: https://doi.org/10.1253/circj.cj-15-1369 [DOI] [PubMed] [Google Scholar]
- 3.Yun WS, Kim YW, Park KB, Cho SK, Do YS, Lee KB, et al. Clinical and angiographic follow-up of spontaneous isolated superior mesenteric artery dissection. Eur J Vasc Endovasc Surg 2009; 37: 572–7. doi: https://doi.org/10.1016/j.ejvs.2008.12.010 [DOI] [PubMed] [Google Scholar]
- 4.Park YJ, Park CW, Park KB, Roh YN, Kim DI, Kim YW. Inference from clinical and fluid dynamic studies about underlying cause of spontaneous isolated superior mesenteric artery dissection. J Vasc Surg 2011; 53: 80–6. doi: https://doi.org/10.1016/j.jvs.2010.07.055 [DOI] [PubMed] [Google Scholar]
- 5.Luan JY, Guan X, Li X, Wang CM, Li TR, Zhang L, et al. Isolated superior mesenteric artery dissection in China. J Vasc Surg 2016; 63: 530–6. doi: https://doi.org/10.1016/j.jvs.2015.09.047 [DOI] [PubMed] [Google Scholar]
- 6.Solis MM, Ranval TJ, McFarland DR, Eidt JF. Surgical treatment of superior mesenteric artery dissecting aneurysm and simultaneous celiac artery compression. Ann Vasc Surg 1993; 7: 457–62. doi: https://doi.org/10.1007/bf02002130 [DOI] [PubMed] [Google Scholar]
- 7.Jeays AD, Lawford PV, Gillott R, Spencer P, Barber DC, Bardhan KD, et al. Characterisation of the haemodynamics of the superior mesenteric artery. J Biomech 2007; 40: 1916–26. doi: https://doi.org/10.1016/j.jbiomech.2006.09.009 [DOI] [PubMed] [Google Scholar]
- 8.Gunenc Beser C, Karcaaltıncaba M, Celik HH, Basar R. The prevalence and distribution of the atherosclerotic plaques in the abdominal aorta and its branches. Folia Morphol (Warsz) 2016; 75: 364–375. doi: https://doi.org/10.5603/FM.a2016.0005 [DOI] [PubMed] [Google Scholar]
- 9.Spence JD. Recent advances in pathogenesis, assessment, and treatment of atherosclerosis. F1000Res 2016; 5: F1000 Faculty Rev-1880. doi: https://doi.org/10.12688/f1000research.8459.1 [Google Scholar]
- 10.Frangos SG, Gahtan V, Sumpio B. Localization of atherosclerosis: role of hemodynamics. Arch Surg 1999; 134: 1142–9. [DOI] [PubMed] [Google Scholar]
- 11.Harloff A. Carotid plaque hemodynamics. Interv Neurol 2012; 1: 44–54. doi: https://doi.org/10.1159/000338360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dick AP, Graff R, Gregg DM, Peters N, Sarner M. An arteriographic study of mesenteric arterial disease. I. Large vessel changes. Gut 1967; 8: 206–20. doi: https://doi.org/10.1136/gut.8.3.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naeem A, Nasim N, Ihsan U, Masood A. A morphological study of celiac, superior mesenteric and inferior mesenteric arteries in atherosclerosis. J Ayub Med Coll Abbottabad 2012; 24: 18–21. [PubMed] [Google Scholar]
- 14.Sakamoto I, Ogawa Y, Sueyoshi E, Fukui K, Murakami T, Uetani M. Imaging appearances and management of isolated spontaneous dissection of the superior mesenteric artery. Eur J Radiol 2007; 64: 103–10. [DOI] [PubMed] [Google Scholar]
- 15.Kim YW. Current understandings of spontaneous isolated superior mesenteric artery dissection. Vasc Specialist Int 2016; 32: 37–43. doi: https://doi.org/10.5758/vsi.2016.32.2.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999; 282: 2035–42. [DOI] [PubMed] [Google Scholar]
- 17.Xiong J, Wu Z, Guo W, Liu X, Wang L, Zhang H, et al. The value of a new image classification system for planning treatment and prognosis of spontaneous isolated superior mesenteric artery dissection. Vascular 2015; 23: 504–12. doi: https://doi.org/10.1177/1708538115589527 [DOI] [PubMed] [Google Scholar]
- 18.Jia Z, Zhang X, Wang W, Tian F, Jiang G, Li M. Spontaneous isolated superior mesenteric artery dissection: genetic heterogeneity of chromosome locus 5q13-14 in 2 male familial cases. Ann Vasc Surg 2015; 29: 1019.e1–5. doi: https://doi.org/10.1016/j.avsg.2015.01.030 [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Zhang W, Tian D, Li L, Deng X, Deng J, et al. Associations of undergoing a routine medical examination or not with prevalence rates of hypertension and diabetes mellitus: a cross-sectional study. Int J Environ Res Public Health 2016; 13: 628. doi: https://doi.org/10.3390/ijerph13070628 [DOI] [PMC free article] [PubMed] [Google Scholar]