Abstract
Background
Artemether, originally used for malaria, exhibits potential therapeutic efficacy against several types of cancer, including gastric cancer, hepatocellular carcinoma, and gliomas. In this study, we investigated the role and mechanism of artemether on drug resistance of neuroblastoma cells.
Material/Methods
Cell viability and proliferation were determined by CCK-8 and EdU incorporation assay, respectively. Gene expression was measured by real-time PCR and Western blot analysis.
Results
Our results revealed that artemether treatment remarkably inhibited the proliferation of neuroblastoma cell lines SH-SY5Y, SK-N-SH, and SK-N-BE2. In addition, co-treatment of tumor cells with artemether and doxorubicin significantly reduced cell viability and DNA synthesis compared with doxorubicin-treated cells. On the molecular level, we found that combined treatment with artemether and doxorubicin suppressed the expression of B7-H3 both at the mRNA and protein levels. In addition, artemether failed to sensitize tumor cells to doxorubicin in SH-SY5Y cells overexpressing B7-H3.
Conclusions
Artemether-mediated inhibition of B7-H3 may contribute to doxorubicin sensitivity in neuroblastoma cells, suggesting that artemether could serve as a potential therapeutic option for neuroblastoma.
MeSH Keywords: B7 Antigens, Multidrug Resistance-Associated Proteins, Neuroblastoma
Background
Neuroblastoma is a solid malignant tumor widely diagnosed in children up to 5 years of age [1,2]. It ranks as the second most common pediatric cancer, accounting for approximately 15% of all pediatric tumor mortality [3,4]. Multimodal therapy is available for neuroblastoma including surgery, radiation therapy, chemotherapy, bone marrow transplantation, and even immunotherapy [5]; however, acquired drug resistance to chemotherapeutics has become a major challenge in neuroblastoma treatment, making it an urgent task to develop novel and effective therapy for disease management.
In recent years, various studies have indicated that the anti-malarial drug artemisinin and its derivatives display a significant cytotoxic effect on tumor cells and could increase the drug sensitization of tumors [6,7]. Artemether, the methyl ether derivative of artemisinin, has been also reported to suppress tumor growth, invasion, and migration in several cancers, including gastric cancer, hepatocellular carcinoma, and breast cancer [8–10]. In addition, other researches have demonstrated that artemether exhibits potential therapeutic effects on gliomas, probably through inhibition of vascular cell adhesion molecule-1 [11].
B7-H3, a type I transmembrane protein, is a new member of the B7 family of immune-regulatory molecules [12]. The expression of B7-H3 has been observed in a variety of normal tissues and cells, including dendritic cells, lung, breast, and prostate [13,14]. A previous study has identified B7-H3 as a neuroblastoma-associated molecule that is involved in natural killer cell-mediated lysis [15]. Another study has demonstrated that microRNA-29 directly targets the B7-H3 3′ untranslated region to regulate B7-H3 in neuroblastoma cells, suggesting a potential immune-based therapy for brain tumors [16]. In the present study, we determined the inhibitive function of artemether on neuroblastoma cells and investigated the interaction between this drug and B7-H3 in regulation of anti-cancer effects.
Material and Methods
Cell culture and transfection
Human neuroblastoma cell lines SK-N-SH, SH-SY5Y, and SK-N-BE2 were purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) in an atmosphere containing 5% CO2 at 37°C. Artemether and doxorubicin were purchased from Sigma-Aldrich (St. Louis, MO, USA). For transfection, neuroblastoma cells were transiently transfected with the recombinant plasmid pcDNA(−)3.1-B7-H3 or control pcDNA(−)3.1. At 48 h after transfection, the transfection efficiency was evaluated by real-time PCR and Western blot analysis.
Cell viability assay
Cell viability was measured with CCK-8 assay according to the manufacturer’s protocol. Cells were seeded into 96-well plates at a density of 5.0×103 per well and incubated for 12 h under standard conditions. Then, the medium was replaced with DMEM containing various concentrations of artemether (0, 100, 300, 600, 900, and 1200 μmol/L). After 48 h, 10 μL/well CCK8 solution was added into each well. After incubation for 3 h, the absorbance was recorded at 450 nm using a microplate reader.
EdU incorporation assay
The DNA synthesis was measured using the commercial Click-iT EdU Imaging Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Neuroblastoma cells were exposed to doxorubicin alone (0.5 μg/ml) or in combination with artemether (300 μmol/L) for 48 h. Cells were exposed to EdU for 2 h and fixed for 20 min with 4% paraformaldehyde followed by counting the EdU-positive cells under a fluorescence microscope (Leica, Germany).
Real-time PCR
Total RNAs were isolated using Trizol reagent (Invitrogen, USA) according to the manufacturer’s instructions. RNA was reverse-transcribed with SuperScript III First-Strand Synthesis System (Life Technologies). Expressions were validated using gene-specific primers. Amplification was conducted with SYBR Green PCR Master Mix on the ABI Prism 7500 sequence detection system and data analysis was performed with the ΔΔCT method.
Western blot analysis
Neuroblastoma cells were lysed in cell lysis buffer containing protease inhibitors (Cell Signaling, Danvers, USA). The supernatant was collected, and separated by 10% SDS-PAGE, and proteins were processed for transference to polyvinylidene difluoride (PVDF) membranes (Millipore, USA). The membranes were incubated with anti-B7-H3 and anti-gapdh antibodies (Abcam, Cambridge, USA) at 4°C overnight. The membranes were washed 3 times and then incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. All the protein bands were detected by chemiluminescence (GE Healthcare, Piscataway, USA).
Statistical analysis
All statistical calculations were performed using SPSS statistical software. Each experiment was repeated at least 3 times. Data are represented as mean ±SD. The t test or one-way ANOVA were used to compare groups. A P-value <0.05 was considered statistically significant.
Results
Artemether increased the doxorubicin cytotoxic effects on neuroblastoma cells
We treated neuroblastoma cell lines with different concentrations of artemether alone (0, 100, 300, 600, 900, and 1200 μmol/L) for 48 h to determine its cytotoxic effects on tumor cells. CCK-8 showed that low concentrations of artemether (100 and 300 μmol/L) had little cytotoxic effects on cell growth, but high doses of artemether (600, 900, and 1200 μmol/L) obviously suppressed the proliferation of neuroblastoma cell lines, including SH-SY5Y, SK-N-SH, and SK-N-BE2 (Figure 1A–1C).
Figure 1.
Effects of artemether on neuroblastoma cells. Neuroblastoma cell lines SH-SY5Y (A), SK-N-SH (B), and SK-N-BE2 (C) were subjected to different concentrations of artemether (100, 300, 600, 900, and 1200 μmol/L) for 48 h. CCK-8 assay was performed to determine cell viability. * p<0.05, compared with control.
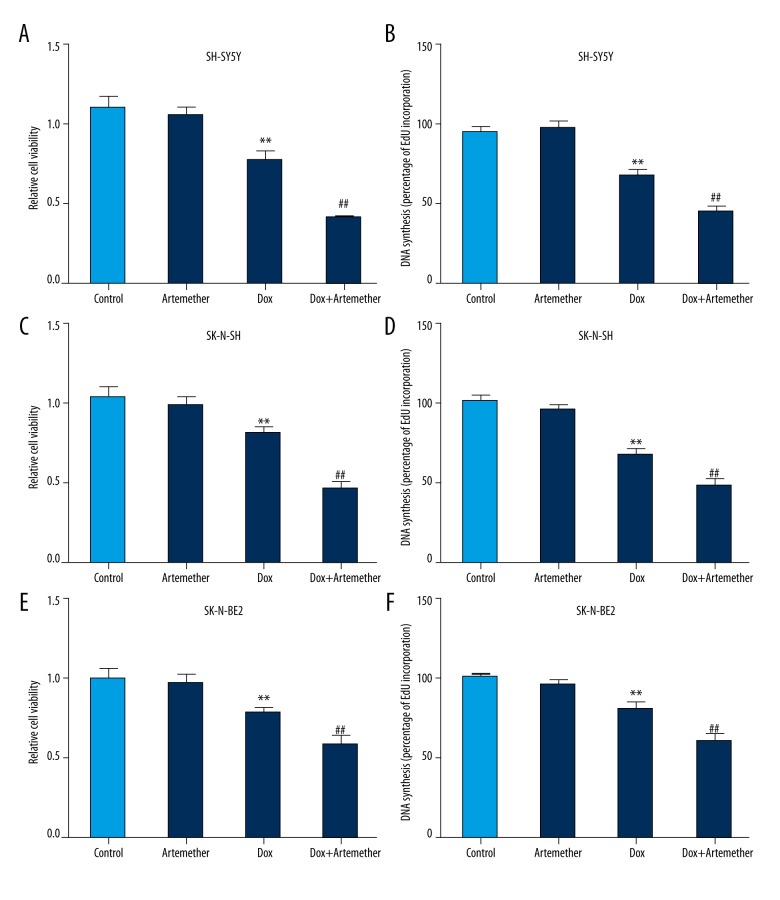
Next, artemether at 300 μmol/L was used to test its chemosensitization effect on neuroblastoma cells. We found that co-treatment of SH-SY5Y cells with artemether (300 μmol/L) and doxorubicin (0.5 μg/ml) significantly inhibited cell viability compared with doxorubicin-treated cells (Figure 2A). In addition, EdU incorporation assay also indicated that artemether suppressed the DNA synthesis of SH-SY5Y cells in the presence of doxorubicin (Figure 2B). Moreover, the cell viability and DNA synthesis were reduced in SK-N-SH (Figure 2C, 2D) and SK-N-BE2 (Figure 2E, 2F) cells in the presence of artemether (300 μmol/L) and doxorubicin (0.5 μg/ml), indicating that artemether could enhance the doxorubicin sensitivity in neuroblastoma cells.
Figure 2.
Artemether increased the doxorubicin cytotoxicity on neuroblastoma cells. Neuroblastoma cell lines SH-SY5Y, SK-N-SH, and SK-N-BE2 were exposed to artemether (300 μmol/L) or doxorubicin (0.5 μg/ml) alone or in combination for 48 h. Cell viability (A, C, E) and DNA synthesis (B, D, F) were measured by CCK-8 assay and EdU incorporation assay, respectively. ** p<0.01, compared with control; ## p<0.01, compared with doxorubicin (Dox)-treated cells.
Artemether inhibits the expression of B7-H3 in neuroblastoma cells
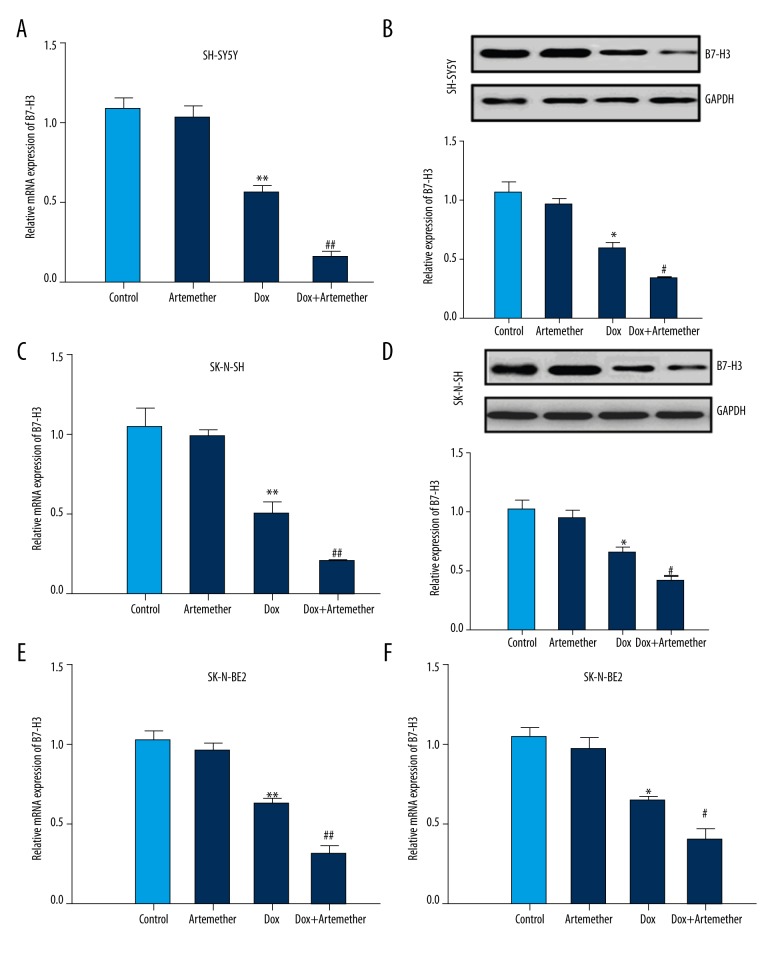
The effects of artemether on B7-H3, a potential oncogene preferentially expressed in tumor tissues, were investigated by the means of real-time PCR and Western blot analysis. Neuroblastoma cell lines SH-SY5Y, SK-N-SH, and SK-N-SH were incubated with doxorubicin alone (0.5 μg/ml) or combined with artemether (300 μmol/L) for 48 h. Then, the mRNA expression of B7-H3 was measured by real-time PCR and results showed that doxorubicin decreased the expression of B7-H3 in SH-SY5Y (Figure 3A), SK-N-SH (Figure 3C), and SK-N-SH cells (Figure 3E). Particularly, co-treatment with artemether further inhibited the B7-H3 mRNA levels in the above-mentioned neuroblastoma cell lines. In addition, the protein expression of B7-H3 was suppressed in the presence of doxorubicin, and further reduced in combined treatment with artemether and doxorubicin (Figure 3B, 3D, 3F). Taken together, these data suggest that artemether incubation reduces the expression of B7-H3 in neuroblastoma cells.
Figure 3.
Down-regulation of B7-H3 by artemether in SH-SY5Y cells. Neuroblastoma cell lines SH-SY5Y, SK-N-SH, and SK-N-BE2 were incubated with artemether (300 μmol/L) or doxorubicin (0.5 μg/ml) alone or in combination. Real-time PCR was used to detect the mRNA expression of B7-H3 (A, C, E). The protein level of B7-H3 was measured by Western blot (B, D, F). * p<0.05, ** p<0.01, compared with control; # p<0.05, ## p<0.01, compared with doxorubicin (Dox)-treated cells.
Effects of B7-H3 overexpression on drug resistance in neuroblastoma cells
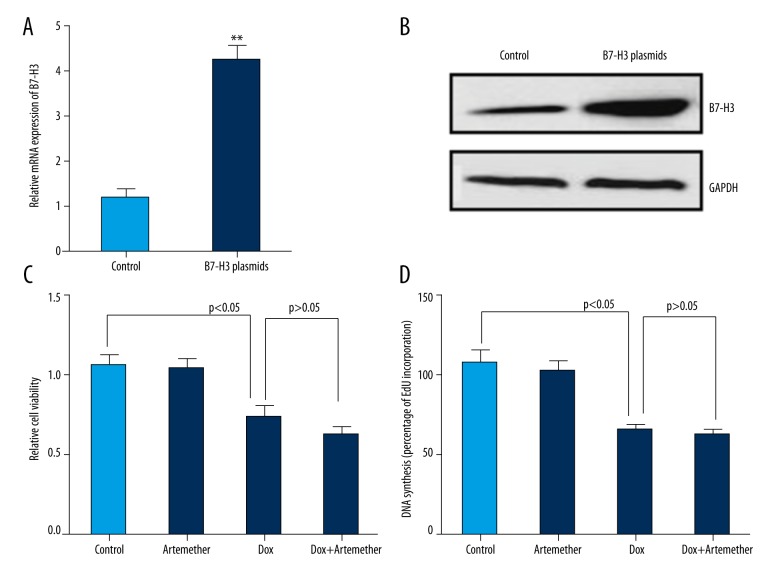
To confirm the regulatory role of B7-H3 in drug resistance, SH-SY5Y cells were transiently transfected with pcDNA(−)3.1-B7-H3 or empty control vector. At 48 h after transfection, the mRNA and protein expression of B7-H3 were determined by real-time PCR and Western blot analysis to evaluate the transfection efficiency. Results indicated a high transfection efficiency of B7-H3 plasmids, which resulted in obviously elevated (more than 4-fold) transcriptional and protein levels of B7-H3 in neuroblastoma cells (Figure 4A, B). Consequently, we observed no significant differences in cell viability (Figure 4C) and DNA synthesis (Figure 4D) in SH-SY5Y cells treated with doxorubicin alone (0.5 μg/ml) or combined with artemether (300 μmol/L). These results suggest that artemether failed to sensitize the SY-5Y cell to doxorubicin when B7-H3 was overexpressed in neuroblastoma cells.
Figure 4.
Effects of B7-H3 overexpression on drug resistance in neuroblastoma cells. Ectopic expression of B7-H3 in SH-SY5Y cells was confirmed by real-time PCR (A) and Western blot (B). Cell viability (C) and DNA synthesis (D) were measured by CCK-8 assay and EdU incorporation assay, respectively. ** p<0.01, compared with control.
Discussion
Artemether, a derivative extracted from a Chinese traditional herb, has been widely prescribed in patients diagnosed with malaria [17]. In recent years, emerging evidence demonstrated that artemether has potential therapeutic effects on several malignancies, such as gliomas, liver cancer, and breast cancer [8–11]. In the present study, our results indicated that artemether increased the doxorubicin cytotoxicity, probably through regulation of B7-H3 in neuroblastoma cells.
Neuroblastoma is the third most frequent cancer in childhood, only preceded by acute lymphocytic leukemia and central nervous system-related cancers [18]. However, the etiology of neuroblastoma tumorigenesis remains poorly understood. Despite the remarkable achievements in neuroblastoma treatment, their therapeutic efficacies are greatly compromised because of the development of drug resistance to the chemotherapeutic agents [19,20]. Accumulating evidence shows that artemisinin and its derivatives have potential antitumor activities through regulation of cancer cell behaviors. For example, dihydroartemisinin, a semi-synthetic derivative of artemisinin, results in significant inhibition of HCC both in vitro and in vivo via induction of mitochondria-dependent apoptosis [21]. In addition, another anti-malarial drug artemether has been shown to inhibit the proliferation, migration, and invasion of gastric cancer and brain gliomas [8–11]. We found that artemether (>300 μmol/L) significantly reduced the proliferation of neuroblastoma cell lines SH-SY5Y, SK-N-SH, and SK-N-BE2. Moreover, the cell viability and DNA synthesis in tumor cells were remarkably lower in the presence of artemether and doxorubicin than in doxorubicin-treated cells alone. Taken together, these data suggest that artemether increases the sensitivity of doxorubicin in neuroblastoma cells.
Several studies have reported the aberrant expression of B7-H3 in a number of solid tumors, such as prostate, lung, liver, colorectal, renal cell cancer, and neuroblastoma [22–26]. Functional investigations have shown the critical roles of B7-H3 in tumor behaviors [27]. For example, B7-H3 was identified as an independent predictor of poor prognosis in patients with non-small cell lung cancer [29]. Chen et al. found that B7-H3 knockdown reduced cell migration, adhesion, and invasion of breast cancer cells [29]. Liu et al. demonstrated that B7-H3-silenced cells become sensitive to chemotherapeutics via inhibition of Jak2/Stat3 phosphorylation [30]. These studies suggest that B7-H3 is a potential therapeutic target for tumors. We found that doxorubicin alone could inhibit B7-H3 expression in SH-SY5Y, SK-N-SH, and SK-N-BE2 cells, which was further suppressed in the presence of artemether. Moreover, enforced expression of B7-H3 did not completely abolish the doxorubicin cytotoxicity in SY-5Y cell; thus, we speculate that doxorubicin exerts its cytotoxicity by regulating multiple targets; therefore, manipulation of a single molecule was not sufficient to counteracted its cytotoxicity. In addition, we found that artemether failed to sensitize SY-5Y cells to doxorubicin in neuroblastoma cells overexpressing B7-H3, suggesting that B7-H3 may serve as a novel target for neuroblastoma therapy.
Conclusions
In conclusion, the present study demonstrates that artemether increased the doxorubicin sensitivity through suppression of B7-H3 in neuroblastoma cells. Our study provides a potential novel therapy for neuroblastoma.
Footnotes
Source of support: Departmental sources
References
- 1.Newman EA, Nuchtern JG. Recent biologic and genetic advances in neuroblastoma: Implications for diagnostic, risk stratification, and treatment strategies. Semin Pediatr Surg. 2016;25:257–65. doi: 10.1053/j.sempedsurg.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Domingo-Fernandez R, Watters K, Piskareva O, et al. The role of genetic and epigenetic alterations in neuroblastoma disease pathogenesis. Pediatr Surg Int. 2013;29:101–19. doi: 10.1007/s00383-012-3239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung NK, Dyer MA. Neuroblastoma: Developmental biology, cancer genomics and immunotherapy. Nat Rev Cancer. 2013;13:397–411. doi: 10.1038/nrc3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gómez S, Castellano G, Mayol G, Suñol M. DNA methylation fingerprint of neuroblastoma reveals new biological and clinical insights. Epigenomics. 2015;7:1137–46. doi: 10.2217/epi.15.49. [DOI] [PubMed] [Google Scholar]
- 5.Kreissman SG, Seeger RC, Matthay KK, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol. 2013;14:999–1008. doi: 10.1016/S1470-2045(13)70309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakase I, Lai H, Singh NP, Sasaki T. Anticancer properties of artemisinin derivatives and their targeted delivery by transferrin conjugation. Int J Pharm. 2008;354:28–33. doi: 10.1016/j.ijpharm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Suberu JO, Romero-Canelon I, Sullivan N, et al. Comparative cytotoxicity of artemisinin and cisplatin and their interactions with chlorogenic acids in MCF7 breast cancer cells. Chem Med Chem. 2014;9:2791–97. doi: 10.1002/cmdc.201402285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alcantara DD, Ribeiro HF, Cardoso PC, et al. In vitro evaluation of the cytotoxic and genotoxic effects of artemether, an antimalarial drug, in a gastric cancer cell line (PG100) J Appl Toxicol. 2013;33:151–56. doi: 10.1002/jat.1734. [DOI] [PubMed] [Google Scholar]
- 9.Farsam V, Hassan ZM, Zavaran-Hosseini A, et al. Antitumor and immunomodulatory properties of artemether and its ability to reduce CD4+ CD25+ FoxP3+ T reg cells in vivo. Int Immunopharmacol. 2011;11:1802–8. doi: 10.1016/j.intimp.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Hou J, Wang D, Zhang R, Wang H. Experimental therapy of hepatoma with artemisinin and its derivatives: In vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin Cancer Res. 2008;14:5519–30. doi: 10.1158/1078-0432.CCR-08-0197. [DOI] [PubMed] [Google Scholar]
- 11.Wang YB, Hu Y, Li Z, et al. Artemether combined with shRNA interference of vascular cell adhesion molecule-1 significantly inhibited the malignant biological behavior of human glioma cells. PLoS One. 2013;8:834–43. doi: 10.1371/journal.pone.0060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapoval AI, Ni J, Lau JS, et al. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–74. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 13.Collins M, Ling V, Carreno BM. The B7 family of immune-regulatory ligands. Genome Biol. 2005;6:223. doi: 10.1186/gb-2005-6-6-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo L, Chapoval AI, Flies DB, et al. B7-H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+ cytolytic T cells. J Immunol. 2004;173:5445–50. doi: 10.4049/jimmunol.173.9.5445. [DOI] [PubMed] [Google Scholar]
- 15.Castriconi R, Dondero A, Augugliaro R, et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci USA. 2004;101:12640–45. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, Cheung IY, Guo HF, Cheung NK. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: Potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69:6275–81. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esu E, Effa EE, Opie ON, et al. Artemether for severe malaria. Cochrane Database Syst Rev. 2014;(9):CD010678. doi: 10.1002/14651858.CD010678.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics, 2014. Cancer J Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 19.Harvey H, Piskareva O, Creevey L, et al. Modulation of chemotherapeutic drug resistance in neuroblastoma SK-N-AS cells by the neural apoptosis inhibitory protein and miR-520f. Int J Cancer. 2015;136:1579–88. doi: 10.1002/ijc.29144. [DOI] [PubMed] [Google Scholar]
- 20.Keshelava N, Seeger RC, Groshen S, Reynolds CP. Drug resistance patterns of human neuroblastoma cell lines derived from patients at different phases of therapy. Cancer Res. 1998;58:5396–405. [PubMed] [Google Scholar]
- 21.Zhang CZ, Zhang H, Yun J, et al. Dihydroartemisinin exhibits antitumor activity toward hepatocellular carcinoma in vitro and in vivo. Biochem Pharmacol. 2012;83:1278–89. doi: 10.1016/j.bcp.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Xu H, Cheung IY, Guo HF, Cheung NK. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: Potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69:6275–81. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun TW, Gao Q, Qiu SJ, et al. B7-H3 is expressed in human hepatocellular carcinoma and is associated with tumor aggressiveness and postoperative recurrence. Cancer Immunol Immunother. 2012;61:2171–82. doi: 10.1007/s00262-012-1278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arigami T, Narita N, Mizuno R, et al. B7-h3 ligand expression by primary breast cancer and associated with regional nodal metastasis. Ann Surg. 2010;252:1044–51. doi: 10.1097/SLA.0b013e3181f1939d. [DOI] [PubMed] [Google Scholar]
- 25.Castriconi R, Dondero A, Augugliaro R, et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci USA. 2004;101:12640–45. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crispen PL, Sheinin Y, Roth TJ, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14:5150–57. doi: 10.1158/1078-0432.CCR-08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nygren MK, Tekle C, Ingebrigtsen VA, Fodstad O. B7-H3 and its relevance in cancer; immunological and non-immunological perspectives. Front Biosci (Elite Ed) 2011;3:989–93. doi: 10.2741/e304. [DOI] [PubMed] [Google Scholar]
- 28.Mao Y, Li W, Chen K, et al. B7-H1 and B7-H3 are independent predictors of poor prognosis in patients with non-small cell lung cancer. Oncotarget. 2015;6:3452–61. doi: 10.18632/oncotarget.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen YW, Tekle C, Fodstad O. The immunoregulatory protein human B7H3 is a tumor-associated antigen that regulates tumor cell migration and invasion. Curr Cancer Drug Targets. 2008;8:404–3. doi: 10.2174/156800908785133141. [DOI] [PubMed] [Google Scholar]
- 30.Kang FB, Wang L, Jia HC, et al. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015;15:45. doi: 10.1186/s12935-015-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]