Abstract
Objective
To explore if creatine kinase (CK) levels correlate with survival in amyotrophic lateral sclerosis (ALS), and whether a correlation is independent of other well-studied predictors such as location of onset, gender, age, fat free mass, spasticity, cramps, and fasciculations.
Methods
We analyzed data from 80 ALS patients from a 48 week non-interventional longitudinal multicenter nutrition study with long term follow-up.
Results
The overall mean CK was 214 ± 191.8 U/L (range: 22-1,992 U/L). 45% of patients had at least one high CK value (>200 U/L), and about half maintained a high CK value, but there was no trend over the study period. Male gender and extremity onset were significantly associated with high CK. In univariate analysis, age, bioelectric impedance spectroscopy (BIS) fat free mass, spasticity, and fasciculations were not associated with CK level. There was an association between CK and muscle cramps (P<0.001). In survival analysis, low CK (≤200 U/L) was associated with a longer overall survival (P=0.02), when adjusting for location of onset, age, race, gender, BIS fat free mass, and study site.
Conclusions
CK may be a useful marker for ALS survival, which has implications for clinical care and the design of future clinical trials.
Keywords: CK, cramps, amyotrophic lateral sclerosis, biomarker, fasciculations
Introduction
Creatine kinase (CK) is a non-specific marker of muscle damage. A mild to moderately elevated CK level can be helpful in supporting the diagnosis of amyotrophic lateral sclerosis (ALS). CK elevation has been reported in 23-75% of ALS patients (1-9). Prior studies found no correlation between CK level and duration of symptoms, severity of weakness, or age of onset (1, 3, 6). Several studies show a significant elevation in male compared to female CK levels (3, 5, 10), but other studies do not (1). Additionally, a significant difference in CK levels in patients with limb onset compared to those with bulbar onset has been reported (1, 3).
The mechanism of CK elevation in ALS is unknown. It has been hypothesized that increased CK might reflect rate of atrophy or the extent of muscle involvement, as animal studies show muscle to be more permeable to CK in neurogenic atrophy than in normal or dystrophic muscle (11). It has also been hypothesized that ALS causes a state of disturbed muscle metabolism, resulting in increased endogenous adenosine triphosphatase activity in mitochondria, which leads to increased CK production (12). Additionally, others suggest that the elevation may be due to mild myopathic changes, as the degree of myopathic changes in ALS muscle biopsies has been correlated with CK values (13).
Beyond supporting the diagnosis, the clinical implication of an elevated CK in an ALS patient is unclear. It has been hypothesized that CK elevation is indicative of more rapid disease progression; however prior studies have not shown a correlation between ALS patient survival and CK level (1, 3, 6).
All prior studies are limited by size, duration, and follow-up evaluations. No studies have been published on the association of CK levels with body composition, spasticity, cramps, or fasciculations. We postulated that our study, which includes large number of patients with serial measurements over 48 weeks and over decade of survival data, could determine whether CK levels correlate with survival, and whether such a correlation was independent of other important factors such as location of onset, gender, age, fat free mass, spasticity, cramps, and fasciculations.
Materials and Methods
Longitudinal data from 80 patients enrolled in the University of Kentucky's multicenter nutrition study were reviewed (14). Patients with clinically definite, probable or laboratory supported probable, sporadic or familial ALS according to the revised El Escorial criteria (15) were enrolled in five multidisciplinary ALS treatment centers (Columbia University, Pennsylvania State University, University of Kentucky, University of Utah, and University of Vermont), from 2/2005 through 6/2007. Survival status was last updated in March 2014. Inclusion criteria at time of enrollment included; age of onset between 18-80 years, symptom onset within 5 years (although exceptions were granted up to 10 years), and a forced vital capacity (FVC) of greater than or equal to 50%. Patients received standard of care treatment during the length of the study based on AAN practice parameters, including initiation of non-invasive positive pressure ventilation (NIPPV) when FVC was less than 50% (16). Exclusion criteria included chronic obstructive pulmonary disease, thyroid abnormalities, inflammatory bowel disease, malabsorption syndrome, untreated or unstable medical condition, other neurodegenerative disease, and/or the inability to follow the study schedule. Participants were evaluated longitudinally, with visits at study baseline, 16, 32 and 48 weeks; although some laboratory tests were missed. Survival was ascertained by periodic follow-up with the study sites. Samples for CK were processed at site-based CLIA certified labs. The reported upper limit of normal CK from the site laboratories ranged from 145 U/L to 320 U/L, and the mode was 200 U/L.
Measurements including fat free mass by bioelectric impedance spectroscopy (BIS), modified Ashworth spasticity scale, fasciculation index, and cramp index were completed at each study visit. The modified Ashworth spasticity scale is a 0-16 point scale performed by the examiner to measure spasticity in each upper and lower extremity (16, 17). The fasciculation index is a 0-24 point scale used to quantify fasciculations throughout the body and rated during the examination with a combination of examiner and patient input (14). The cramp index is a 0-24 point scale completed by the patient to score the severity of cramps throughout the body since last study visit (14).
Statistical Analysis
Patients’ baseline characteristics were summarized as mean (± SD) for continuous variables and N (%) for binary or categorical variables. The CK measurements are displayed by a spaghetti plot that connected individuals’ CK values at baseline (week 0), 16 weeks, 32 weeks and 48 weeks. The primary endpoint was time to death, which was subject to right censoring. Survival probabilities (proportions of patients alive) were summarized using Kaplan-Meier plots with product limit estimates (18). Spasticity, cramps, and fasciculations were measured longitudinally, allowing for use of the generalized estimating equation (GEE) method to handle the repeated measurements by using the 1st order autoregressive (AR1) working correlation. The linear mixed effects model (LMEM) was used to assess the temporal trend of CK measured over time. We performed a Cox proportional hazards (PH) model to examine the effect of baseline CK on the survival outcome in which CK values were dichotomized at a threshold value of 200 U/L (19). Since a total of 12 subjects (15%) missed baseline CK measurements, a sensitivity analysis was performed to evaluate the impact of the missing data. A proportionality test was performed on the dichotomous CK data by adding the time and dichotomous CK interaction in the survival model. Statistical analyses were performed using SAS (SAS Inst. Inc., Cary, NC, USA) version 9.4 and Stata (StataCorp, College Station, TX, USA) version 13. All results with p<0.05 were considered as statistically significant (18).
Ethics and consent
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983. Formal approval for this clinical trial was by the local ethics committees.
Results
Patients Characteristics
Baseline characteristics for the 80 patients are shown in Table 1. Site enrollment was; Columbia University- 25%, Pennsylvania State University- 25%, University of Kentucky- 26. 3%, University of Utah- 11.3%, and University of Vermont- 12.5%.
Table 1.
Summary of patient characteristics, symptoms, and CK
| Variables | Categories | Mean (SD) | Range | N(%) |
|---|---|---|---|---|
| Age (years) | 59.5 (17.8) | 24.1 - 79.6 | ||
| Gender | Male | 52 (65.0) | ||
| Female | 28 (35.0) | |||
| Race | White | 72 (90.0) | ||
| Non-White | 8 (10.0) | |||
| Location of Onset | ||||
| Bulbar | 21 (26.3) | |||
| Generalized | 1 (1.3) | |||
| Lower Extremity | 16 (20.0) | |||
| Upper & Lower | 5 (6.3) | |||
| Upper Extremity | 37 (46.3) | |||
| Symptom Onset to Baseline (days) | 729.3 (585.6) | 140-3353 | ||
| CK (U/L) (baseline) (N = 68) | 221.9 (191.8) | 22-998 | ||
| CK (U/L) (16 weeks) (N = 52) | 222.9 (247.5) | 34-1489 | ||
| CK (U/L) (32 weeks) (N = 50) | 213.8 (306.1) | 39-1992 | ||
| CK (U/L) (48 weeks) (N = 48) | 192.3 (205.6) | 26-988 | ||
| CK (U/L) (overall) | 214 (191.8) | 22-1992 | ||
| BIS fat free mass (kg) (baseline) (N = 78) | 46.4 (11.5) | 20.7-84.0 | ||
| Ashworth (baseline) (N = 79) | 12.3 (3.8) | 0-16 | ||
| Ashworth (16 weeks) (N = 66) | 12.4 (3.7) | 0-16 | ||
| Ashworth (32 weeks) (N=54) | 11.9 (4.1) | 0-16 | ||
| Ashworth (48 weeks) (N = 47) | 12.1 (4.2) | 0-16 | ||
| Cramps (baseline) (N = 79) | 4.5 (4.6) | 0-18 | ||
| Cramps (16 weeks) (N = 66) | 3.9 (4.1) | 0-18 | ||
| Cramps (32 weeks) (N = 54) | 3.7 (3.2) | 0-12 | ||
| Cramps (48 weeks) (N = 46) | 4.0 (3.8) | 0-14 | ||
| Fasciculation (baseline) (N = 79) | 7.7 (5.3) | 0-21 | ||
| Fasciculation (16 weeks) (N = 64) | 7.4 (5.7) | 0-20 | ||
| Fasciculation (32 weeks) (N = 54) | 7.0 (5.6) | 0-18 | ||
| Fasciculation (48 weeks) (N = 48) | 7.0 (6.2) | 0-18 |
Creatine Kinase
The mean baseline CK was 221.9 ± 191.8 U/L. The CK value from all visits ranged from 22 to 1992 U/L. Figure 1 shows individual patient CK trajectory measurements across time via a spaghetti plot. Low (≤200) CK values were seen at all visits in 55% of patients, 24% had variable CKs with at least one high (>200) CK value recorded at one or more visits, and 21% of patients had high CK values at all visits. There was a slight increase in the mean CK from baseline to 16 weeks due to outliers, the median CK decreased (161 U/L at baseline, and 141 U/L at 16 weeks). Analysis of the temporal trend of CK over time using a LMEM revealed a negligible average decrease (P=0.27) in CK (0.0016 U/L/day) over the 48 week serial measurement study period. LMEM also revealed a statistically significant decreasing trend in average fat free mass over the 48 week serial measurement study period. On average, the fat free mass decreased by 0.011 kg/day (p<0.001).
Figure 1.
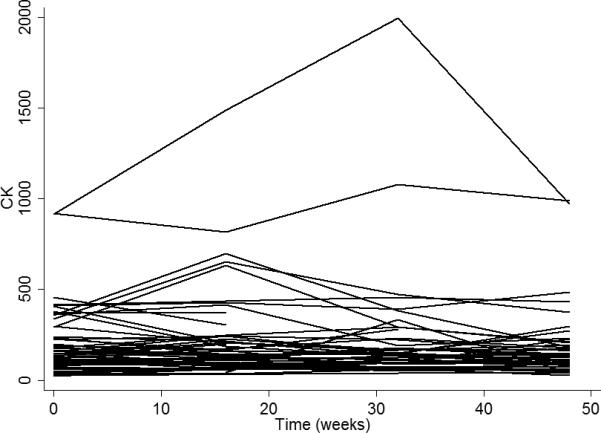
Spaghetti plot of CK versus time (weeks).
The average male CK for all time points was 257 U/L (above normal limits) and the average female CK was 135 U/L (within normal limits). Male CK values were significantly higher than female CK values (P=0.009). When adjusted for fat free mass, the difference in CK between male and female was no longer statistically significant (P=0.17).
The average baseline CK value was 247 U/L (SD=172) for upper extremity onset, 300 U/L (SD=308) for lower extremity onset, and 127 U/L (SD=95) for bulbar onset. Compared to bulbar-onset patients, the CK values in upper and lower extremity onset patients were both significantly elevated (P= 0.009 and 0.03, respectively). The difference in CK values in upper vs lower extremity onset was not significant (P=0.37).
There was a statistically significant association between CK and cramps (p<0.001). For one unit increase in cramp index (0-24 point scale), there is a 15.1 U/L increase in CK for all repeated measurements. No association between CK and spasticity or between CK and fasciculations was observed.
Association between Survival and Baseline Creatine Kinase
The primary goal was to investigate the correlation between baseline CK level and survival. The mean time from symptom onset to study entry for the low CK (CK≤200U/L) group was 777 days, not statistically significantly different from that for the high CK group, 579 days (p=0.18). Figure 2 presents survival functions for low and high baseline CK (≤200 versus >200) using the Kaplan-Meier product limit estimates. The median survival time was 4.21 years (95% CI: 3.04 – 5.88 years) for patients with low baseline CK and, 3.31 years (95% CI: 2.04 – 4.65 years) for patients with high baseline CK. A log rank test for comparing the survival functions of the two arms rejects the null hypothesis of equality of the two survival functions (P=0.02). Using median (160 U/L) and mean (222 U/L) CK of study patient as the dichotomous value, revealed significant results as well (p=0.002 and p=0.02 respectively).
Figure 2.
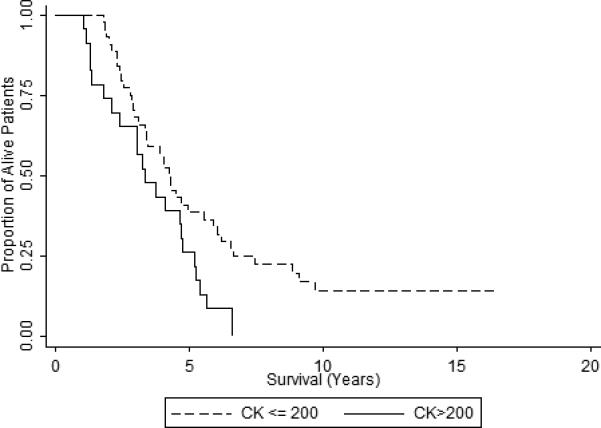
Kaplan-Meier Survival Curves for Low versus High CK.
We performed 10 Cox PH models to relate low/high CK to the primary end point, time to death, by adjusting for different sets of covariates (Table 2). In all models, baseline CK was statistically significantly associated with the overall survival. For example, in the unadjusted model (Model 1), the hazard ratio comparing high versus low CK (CK ≤ 200) is 1.88 (95% CI: 1.09-3.26, p=0.025). In the most controlled model (model 10), which was adjusted for age, gender, race, BIS fat free mass, location of onset, and study site, the hazard ratio between high and low baseline CK is 3.57 (95% CI: 1.84 - 7.27). In all models, an ALS patient with high baseline CK had a significantly higher hazard of death than the patient with low baseline CK when adjusted for all possible sets of variables.
Table 2.
Hazard ratios (HRs), standard error (SE), and 95% confidence intervals (CIs) for baseline CK.
| Model | HR | SE | P-value | 95% CI |
|---|---|---|---|---|
| Model 1 | 1.88 | 0.53 | 0.025 | (1.09, 3.26) |
| Model 2 | 1.90 | 0.54 | 0.023 | (1.09, 3.30) |
| Model 3 | 2.10 | 0.61 | 0.011 | (1.19, 3.71) |
| Model 4 | 2.26 | 0.67 | 0.005 | (1.27, 4.03) |
| Model 5 | 2.45 | 0.75 | 0.003 | (1.34, 4.67) |
| Model 6 | 2.66 | 0.86 | 0.002 | (1.41, 4.99) |
| Model 7 | 2.66 | 0.85 | 0.002 | (1.42, 4.97) |
| Model 8 | 3.20 | 1.09 | 0.001 | (1.63, 6.22) |
| Model 9 | 3.20 | 1.07 | 0.001 | (1.66, 6.15) |
| Model 10 | 3.57 | 1.28 | <0.001 | (1.84, 7.27) |
*Model 1: unadjusted; Model 2: adjusted for age; Model 3: adjusted for age, gender; model 4: adjusted for age, gender, race; Model 5: adjusted for age, gender, race, BIS fat free mass; Model 6: adjusted for age, gender, race, BIS fat free mass and study center; Model 7: adjusted for age, gender, race, location of onset; Model 8: adjusted for age, gender, race, location of onset and center; Model 9: adjusted for age, gender, race, BIS fat free mass and location of onset; Model 10: adjusted for age, gender, race, BIS fat free mass, location of onset and study center. The race in is coded as non-White (baseline) vs. White and the location of onset is categorized as bulbar, multiple location onset, lower extremity and upper extremity.
Finally, location of onset was significantly associated with ALS survival. The mortality hazard ratio comparing ALS with lower extremity onset versus bulbar onset is 0.25 (95% CI: 0.10 - 0.62, p=0.003) when controlled for age, gender, race, BIS fat free mass, baseline CK and study site.
Sensitivity analysis of the missing data showed that the missing values of baseline CK did not impact the significance of CKs effect on survival.
Discussion
This study reveals that low CK is significantly associated with longer ALS survival (P=0.02). A high CK was associated with an 88% higher hazard of death from ALS, when additional variables are controlled (age, gender, race, BIS fat free mass, location of onset, and study site), the hazard increases to 2.75. Prior studies, which have not shown a correlation between patient survival and CK level, were limited by their relatively short duration of study period (1, 3, 6), where we have over a decade of survival data.
The range of CK reported in this study (22-1992 U/L) is similar to those reported in other studies (1-9). Our study shows a significant (P=0.009) difference between male and female CK values (males 257 U/L; females 135 U/L), as shown in several previous studies (3, 5, 10). However, the difference in CK was no longer significant when adjusted for fat free mass, which might be why other studies did not find a difference (1). We also found a significant difference between CK values in the upper extremity (P=0.009) and lower extremity onset (P=0.03) when compared to bulbar onset, which has also been demonstrated in previous studies (1, 3). We did not find a significant difference in CK based on age, race or study site, consistent with the findings of prior studies.
This study uniquely characterizes CK with disease variables. No statistical difference was found for CK in relation to fasciculations or spasticity. However, we did find an association between CK and cramps (P<0.001), leading us to hypothesize that cramps may result in muscle breakdown. Based on animal studies it has been hypothesized that increased CK might reflect the rate of atrophy and the amount of muscle involvement (11), however CK did not correlate with change in BIS fat free mass, nor did it decline over time as did BIS fat free mass (−0.0107 kg/day). Because CK values had only a negligible average decrease (P=0.27, 0.0016 U/L/day) over the 48-week serial measurement study period, the mean time to the baseline visit (23.8 ± 19.5 months) is unlikely to be a major limitation.
The association of lower CK with survival has implications both for patient care and for future drug study design. In this study all ALS patients who survived beyond 8 years from symptom onset had a low CK (CK≤200). Although this is not intended to be applied to an individual ALS patient, these data may provide additional information to aide in the identification of very slowly progressive ALS patients in drug trials. It is unclear if treating cramps would have an effect on maintaining muscle mass and/or survival.
Supplementary Material
Acknowledgements
The University of Kentucky's multicenter nutrition study was funded by the National Institute of Neurological Disorders and Stroke grant 1 RO1 NS045087, the ALS Hope Foundation, and the Cynthia Shaw Crispen Endowment. Research reported in this publication was additionally supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR001066. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosure of Interests: none
References
- 1.Ilzecka J, Stelmasiak Z. Creatine kinase activity in amyotrophic lateral sclerosis patients. Neurol Sci. 2003;24:286–7. doi: 10.1007/s10072-003-0158-3. [DOI] [PubMed] [Google Scholar]
- 2.Chahin N, Sorenson EJ. Serum creatine kinase levels in spinobulbar muscular atrophy and amyotrophic lateral sclerosis. Muscle Nerve. 2009;40:126–9. doi: 10.1002/mus.21310. [DOI] [PubMed] [Google Scholar]
- 3.Felice KJ, North WA. Creatine kinase values in amyotrophic lateral sclerosis. J Neurol Sci. 1998;160(Suppl 1):S30–2. doi: 10.1016/s0022-510x(98)00195-6. [DOI] [PubMed] [Google Scholar]
- 4.Panitch HS, Franklin GM. Elevation of serum creatine phosphokinase in amyotrophic lateral sclerosis. Neurology. 1972;22:964–6. doi: 10.1212/wnl.22.9.964. [DOI] [PubMed] [Google Scholar]
- 5.Amrit AN, Anderson MS. Serum creatine phosphokinase in amyotrophic lateral sclerosis. Correlation with sex, duration, and skeletal muscle biopsy. Neurology. 1974;24:834–7. doi: 10.1212/wnl.24.9.834. [DOI] [PubMed] [Google Scholar]
- 6.Sinaki M, Mulder DW. Amyotrophic lateral sclerosis: relationship between serum creatine kinase level and patient survival. Arch Phys Med Rehabil. 1986;67:169–71. doi: 10.1016/0003-9993(86)90064-x. [DOI] [PubMed] [Google Scholar]
- 7.Lima AF, Evangelista T, de Carvalho M. Increased creatine kinase and spontaneous activity on electromyography, in amyotrophic lateral sclerosis. Electromyogr Clin Neurophysiol. 2003;43:189–92. [PubMed] [Google Scholar]
- 8.Harrington TM, Cohen MD, Bartleson JD, Ginsburg WW. Elevation of creatine kinase in amyotrophic lateral sclerosis. Potential confusion with polymyositis. Arthritis Rheum. 1983;26:201–5. doi: 10.1002/art.1780260212. [DOI] [PubMed] [Google Scholar]
- 9.Edmonds PJ, Ziegler DK. Diagnostic value of serum creatine phosphokinase in motor neuron disease. South Med J. 1975;68:1388–90. doi: 10.1097/00007611-197511000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Williams ER, Bruford A. Creatine phosphokinase in motor neurone disease. Clin Chim Acta. 1970;27:53–6. doi: 10.1016/0009-8981(70)90373-6. [DOI] [PubMed] [Google Scholar]
- 11.Dawson DM. Leakage of enzymes from denervated and dystrophic chicken muscle. Arch Neurol. 1966;14:321–5. doi: 10.1001/archneur.1966.00470090093013. [DOI] [PubMed] [Google Scholar]
- 12.Ernster L, Ikkos D, Luft R. Enzymic activities of human skeletal muscle mitochondria: a tool in clinical metabolic research. Nature. 1959;184:1851–4. doi: 10.1038/1841851a0. [DOI] [PubMed] [Google Scholar]
- 13.Achari AN, Anderson MS. Myopathic changes in amyotrophic lateral sclerosis. Pathologic analysis of muscle biopsy changes in 111 cases. Neurology. 1974;24:477–81. doi: 10.1212/wnl.24.5.477. [DOI] [PubMed] [Google Scholar]
- 14.Kasarskis EJ, Mendiondo MS, Wells S, Malguizo MS, Thompson M, Healey M, et al. The ALS Nutrition/NIPPV Study: design, feasibility, and initial results. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 2011;12:17–25. doi: 10.3109/17482968.2010.515225. [DOI] [PubMed] [Google Scholar]
- 15.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 16.Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73:1227–33. doi: 10.1212/WNL.0b013e3181bc01a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashworth B. Preliminary Trial of Carisoprodol in Multiple Sclerosis. The Practitioner. 1964;192:540–2. [PubMed] [Google Scholar]
- 18.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2nd edition ed. John Wiley & Sons; Hoboken, New Jersey: 2002. [Google Scholar]
- 19.Farinde A. [10/20/2014];Lab Values, Normal Adult. 2014 http://emedicine.medscape.com/article/2172316-overview: medscape [updated 5/20/2014]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


