Abstract
Defects in intestinal barrier function are associated with diseases of the gastrointestinal (GI) tract. There is growing evidence that increases in intestinal permeability plays a pathogenic role in diseases, such as inflammatory bowel disease (IBD) and celiac disease, and functional bowel disorders, such as irritable bowel syndrome (IBS). This review takes a unique translational approach to discuss the physiological and pathophysiological mechanisms involved in the regulation of intestinal barrier function in IBS. The review summarizes the components of the intestinal barrier including the tight junction complex within the epithelium, and the methods used to assess gut permeability both in vitro and in vivo. Throughout the review, the authors have attempted to critically review the latest research from both experimental animal models and human studies to appraise whether intestinal barrier dysfunction is a primary cause of functional GI disorders, such as IBS....
Keywords: glutamine, intestinal barrier, irritable bowel syndrome, microRNA, tight junction proteins
INTRODUCTION
The ability of the intestinal epithelium to function as a barrier between the external environment and the closely regulated internal milieu is essential for human health. Increased gut permeability is associated with several different human diseases, including inflammatory bowel disease (IBD), celiac disease, and irritable bowel syndrome (IBS). However, whether this increase in gut permeability is an epiphenomenon, an early manifestation of disease, or a critical step in disease pathogenesis remains unknown and has been the subject of much debate. The overall objective of this review is to provide a critical analysis on intestinal barrier function and its role in IBS.
Intestinal permeability: basic physiology
Several components form the multilayered intestinal barrier.1 In the lumen, there is degradation of bacteria and antigens by gastric acid and pancreatic juice; in addition, commensal bacteria inhibit the colonization of pathogens by production of antimicrobial substances. Next, the microclimate close to the epithelium consists of the unstirred water layer, glycocalyx, and mucus layer that prevent bacterial adhesion and contains antimicrobial products secreted by Paneth cells and secretory IgA from the enterocytes.2–6 Below the unstirred water layer, glycocalyx, and mucus layer, there are epithelial cells separated by junctions that represent homo- and heterotypic binding of extra-cellular domains of tight junction proteins.
Several types of proteins contribute to the development of tight junctions (Fig. 1)
Figure 1.
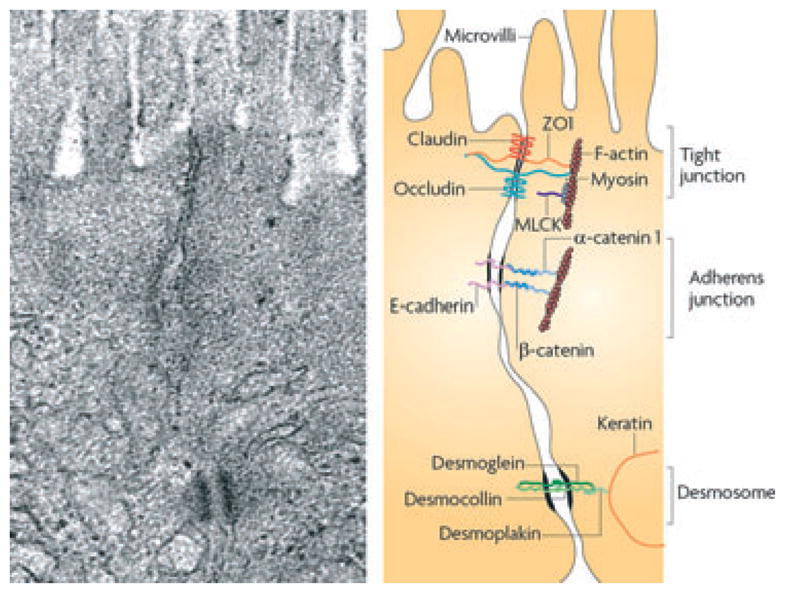
Plasma membranes of adjacent cells fuse at tight junctions (reproduced from Turner).11
Integral membrane proteins include the claudin family of proteins that form the actual paracellular pore within the tight junction and are associated with other transmembrane proteins called occludins.
Junctional complex proteins: zonula occludens (ZO)-1 and other cytoplasmic proteins, such as ZO-2 and ZO-3 attach to this complex.
Cell cytoskeleton structures: microtubules, intermediate- and microfilaments.
Occludin and ZO-1 interact directly with actin.7,8 Claudin-1, 3, 4, 5, and 8 strengthen the barrier, whereas claudin-2, 7, 10, and 12 weaken it. Claudin-2 is a prototype channel forming, tight junction protein responsible for specific paracellular transfer of solutes across epithelium, selective for small cations, but nearly impermeable to anions and uncharged solutes of any size. Interestingly, claudin-2 is not expressed in a human colon carcinoma cell line (Caco-2). This cell line, when cultured under specific conditions, resembles the enterocytes lining the small intestine and has been used as a model system to study intestinal epithelial permeability.9 Claudin-5, a tight junction protein found in all endothelia and in some epithelia (e.g. kidney tubule), is a barrier builder. Claudin-8 is another barrier builder that is relevant to the permeability in the colon and is regulated by Na+ uptake in surface epithelial cells of the human colon.10 Also relevant to the barrier properties are adherens junctions (or zonula adherens, intermediate junction, or ‘belt desmosome’) that are defined as a cell junction where the cytoplasmic face is linked to the actin cytoskeleton.11 At the basal pole of the intercellular space, desmosomes are formed by interactions between desmoglein, desmocollin, desmoplakin, and keratin filaments.11 Passive permeability (relevant for the passage of larger hydrophilic compounds) is only one of several passage routes across the epithelium. Other routes are: transcellular route (lipophilic and small hydrophilic compounds); transcellular route via aqueous pores (small hydrophilic compounds); active carrier-mediated absorption (nutrients, electrolytes, and some exogenous substances, such as peptidomimetic antiobiotics); and endocytosis, followed by transcytosis and exocytosis (larger peptides, proteins, and particles).1 Thus, solute and particulate matter moves across the intestinal epithelium in a regulated manner either between epithelial cells via the tight junction region or across the apical membrane of epithelial cells. Far from being a static region, tight junctions are continually being monitored and regulated by both intra- and extracellular signals. Signaling molecules that control the assembly and disassembly of tight junctions through phosphorylation and dephosphorylation reactions include myosin light chain kinase, Rho GTPases, protein kinase C, and mitogen-activated protein kinases. For example, zonulin is known to be an important regulator of tight junction permeability being released by luminal factors including food and bacterial toxins to act on apical receptors to increase permeability and facilitate absorption.12
Relative permeability of the GI tract
Studies of transepithelial resistance in the rat show that the colon is less permeable than the small bowel; in fact the colon has strong expression of the barrier claudins-1, 3, 4, 5, and 8. In the colon, the claudins mediating permeability are claudins-2, 7, and 12.13 Within the small intestinal mucosa, there are different permeation characteristics in the villi and crypts. Arrieta et al. reported that the pore size at the villus tip is 4–5 Å, at the villus base 10–15 Å, and at the crypt base >20 Å.14 These pore sizes would allow passage of mannitol (~ 3 Å) at the villus tip, and solvent drag may occur in this part of the villus given the distribution of the sodium-glucose transporter (SGLT1) in the top half of the villi. Moreover, permeability probe molecules, lactulose (~6 Å), and 51Cr-EDTA (5.3 Å) are able to pass through the barrier at the base of the villi where solvent drag does not occur as there is no SGLT1. Finally, the probe molecule, inulin (18 Å), can be absorbed at the base of crypts.
Measuring intestinal permeability
Permeability and the factors controlling it can be assessed in vitro in several ways15–19, and was reviewed recently by Shen and colleagues20 including: (i) measurements of transepithelial resistance and or assessment of macromolecular flux across isolated segments of GI tissue or colonic biopsies in Ussing chambers, (ii) assessing the effects of human biopsy extracts or fecal supernatants on permeability to fluorescein isothiocyanate (FITC)-dextran in confluent monolayers of Caco-2 cells, (iii) morphological measurements of the tight junction components, such as myosin light chain kinase and ZO-1 proteins in mucosal biopsies, (iv) measurement of dilution potentials, and (v) polyethylene glycol (PEG) profiling to assess pore pathways (high capacity size and charge selective route) vs leak pathways (low capacity paracellular route).
In patients, the typical measurement of intestinal permeability involves oral ingestion of probe molecules, which are not metabolized, but excreted in urine where they can be readily measured. Thus, the factors that determine the excretion of the probe molecules are the size and charge of the molecule and gut metabolic and renal factors. The molecular size of probe molecules for in vivo measurements has typically ranged from around 150–3350 Da, with most of the probes around 150–500 Da.21 Although molecular size is a major determinant of intestinal permeability, molecular structure and diameter are also important. For example, PEG 400 (a polydisperse of molecules ranging from ~290 to 520 Da) has a higher absorption profile than mannitol whose molecular weight is 182 Da.22 Typically, sucrose is used as a probe for gastric permeability, mannitol as a marker of small bowel permeability (proportional to surface area), lactulose as a marker of damaged small bowel permeability, and sucralose as a marker of colonic permeability.14,23 The gut factors that influence the measurement of permeability are: concentration gradient across the barrier, the barrier function or permeability, contact time, location of the probes (and therefore the transit profile of the probe molecules), the surface area of the small and large intestine, and the potential for degradation of the molecules by digestion or bacterial degradation. Bacterial degradation of sugars by colonic bacteria was investigated in vitro.23 In these experiments, aliquots containing all probes were incubated overnight with an aliquot of colonic contents. Sucralose does not undergo bacterial degradation, whereas mannitol and lactulose undergo approximately the same amount (average ~75%) of bacterial degradation overnight, leading to the use of sucralose as a marker of colonic permeability. Typically, studies using a nutrient test meal have assumed that the L: M excretion ratio from the 0 to 6 h urine collection reflects permeability of the small intestine. In other studies, urine collections during 0–3, 3–5, and 5–24 h were chosen to reflect the mucosal permeability of the proximal small intestine, distal small intestine, and large intestine, respectively. Recent studies that administered sugars orally without additional nutrient found that the optimal time for urine collections that combine residence of the probe molecules in the small bowel is 0–2 h for small bowel and 8–24 h for colonic permeability.24 Rao and coworkers have shown that individual sugar excretion profiles reflect the greater small bowel compared with colonic permeability; in contrast, the L: M ratios were higher in the 8–24 h collections attributed to colonic permeability compared with 0–2 and 2–4 h urine collections (Table 1).24
Table 1.
Examples of the variations in urine collecting periods in studies using excretion of orally administered probes to evaluate intestinal permeability in patients with IBS
| Reference | IBS group | Probe | In vivo/in vitro | Urine collection timing (h) | Permeability |
|---|---|---|---|---|---|
| Spiller et al.47 | Post infectious (PI-IBS) | Lactulose, mannitol | In vivo | 0–6 | Increased |
| Marshall et al.48 | IBS | Sucrose, lactulose, mannitol | In vivo | Overnight | Increased |
| Dunlop et al.54 | IBS-D (PI and non-PI), IBS-C | 51Cr-EDTA | In vivo | 0–3, 3–5, 5–24 | Increased |
| Shulman et al.81 | Children with IBS, and functional abdominal pain | Sucrose, lactulose, mannitol, sucralose | In vivo | 0–3 | Increased (sucrose/lactulose; sucralose/lactulose only) |
| Zhou et al.70 | IBS-D | Lactulose, mannitol | In vivo | 0–24 | Increased in 39% of patients |
| Zhou et al.17 | IBS-D | Lactulose, mannitol | In vivo | 0–5, 6–24 | Increased in 42% of patients |
IBS, irritable bowel syndrome.
Potential neuroimmune modulation of intestinal mucosal barrier function
Extrinsic vagal and/or sympathetic efferents or enteric nerves influence the mucosal barrier through direct effects via acetylcholine or vasoactive intestinal polypeptide on epithelial cells, tight junction protein expression, or through interaction with immune (e.g. mast or plasma) cells. During stress and inflammation mast cell mediators, such as TNF-α, tryptase [via protease-activated receptor type 2 (PAR-2)], nerve growth factor (NGF), and interleukins may affect paracellular permeability (by altering expression of claudins in the tight junctions) or the transcellular uptake route (by increasing macropinocytosis), thereby disrupting the barrier to antigens and bacteria.14,25–27 The release of serine proteases from mast cells results in the activation of PAR-2 on epithelial cells;28 further, activation of PAR-2 has been linked with tight junction disassembly and increased permeability.29
Gut permeability and microflora
In a strain and dose-dependent manner, microbes have been shown to directly alter tight junction protein expression and/or localization in both in vivo and in vitro models.30–32 Gut permeability can be modulated directly by microbes through the release of soluble peptides or toxins,30,33 by cellular structural components34 or by metabolites.35–37 The short chain fatty acids, acetate36 and butyrate37 have been shown to have a direct role in the enhancement of intestinal epithelial barrier function and subsequent protection against pathogens. Microbes can also alter epithelial permeability indirectly through effects on host immune cells and the release of cytokines, which can both reduce (i.e. TNFα, IFNγ) or enhance (i.e. TGFβ, IL-10) barrier function.14 Manipulation of the gut microflora with probiotics, antibiotics, or microbial products results in both an attenuation of disease and a restoration of normal gut permeability; thus it is challenging to differentiate whether a restoration of gut permeability occurs as a result of an improvement of disease, or if a direct effect on gut permeability results in attenuation of disease.30,38,39
Intestinal permeability in animal models
In an attempt to answer the question of whether small intestinal permeability can be an initiating factor in colonic disease, studies were carried out using an antagonist to zonulin in the IL-10−/− mouse.40 Zonulin receptors only exist in the small intestine; thus, effects of the antagonist can only be a result of events occurring in the small intestine. In these studies, small intestinal permeability was initially reduced in IL-10−/− mice by the zonulin antagonist and development of inflammation was subsequently attenuated, indicating that therapy aimed at reducing small intestinal permeability can have effects on disease in distal organs possibly via an altered immune response.40 Animal models have shown that alterations in intestinal permeability can occur owing to a number of factors as shown in Table 2. Overall, the use of animal models to investigate the role of increased gut permeability in disease processes has clearly demonstrated that in many situations, an increase in small intestinal permeability precedes the development of colonic disease, and that treatment of the permeability defect can prevent, or attenuate, disease. However, studies have also demonstrated that abnormal small intestinal permeability is not necessarily sufficient to induce disease and that other defects in either intestinal homeostasis or immune function must be present.41
Table 2.
Mechanisms of altered intestinal permeability
| Mechanism | References |
|---|---|
| Alterations in tight junction protein expression or localization | Chen et al.82 |
| Abnormal regulation of tight junction function | Su et al.41 |
| Dysbiosis in microbial flora resulting in the lack of signals to maintain barrier function | Bansal et al.,36 Fukuda et al.,37 Hamer et al.37 |
| Dysbiosis resulting in an increase in signals that break the barrier | Fasano et al.33 |
| Presence of active inflammation and increased presence of pro-inflammatory cytokines and oxidative species | Arrieta et al.14 |
| Increased density of epithelial gaps caused by increased cell shedding | Liu et al.43 |
Another potential mechanism of barrier dysfunction is an increase in epithelial cell shedding. Epithelial cell shedding occurs along the entire villus in the small intestine; increases in cell shedding may contribute to increased gut permeability.42 In the IL-10−/− mouse, there is a 2-fold increase in the density of epithelial gaps compared with wild-type mice, and this correlates with the increased intestinal permeability of IL-10−/− mice.43 Furthermore, in several mouse models, TNFα was shown to increase epithelial cell shedding, along with enhancing gut permeability.44 Host-microbial interactions can involve toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD), and nucleotide leucine-rich repeat (NLR) containing proteins. Toll-like receptors detect numerous microbe-associated molecular patterns (MAMPs), including lipopolysaccharide (LPS) by TLR4; lipoproteins and lipoteichoic acids by TLR2; flagellin by TLR5; CpG DNA by TLR9; dsRNA by TLR3; and ssRNA by TLR7. Activation of TLR2 helps maintain gut barrier function through effects on ZO-1 and signaling between PI3Kinase-AkT and PKC isoforms via MyD88.39 In addition, activation of the PI3K-Akt pathway also results in a reduction in pro-inflammatory signaling through the MAPK-NF-κB pathway.34 In contrast to the protective effects of TLR2 signaling, activation of TLR4 can directly increase paracellular permeability, whereas TLR2 activation can protect against a TLR-4 induced increase in epithelial permeability.45
Intestinal permeability in functional GI disorders
There are several lines of evidence that support a link between abnormal intestinal permeability and functional GI disorders, such as irritable bowel syndrome (IBS)
A. Infection
Infectious gastroenteritis increases gut permeability46 so it was no surprise that serial measurements of small intestinal permeability using the urinary L: M excretion ratio showed substantially increased values 6 and 12 weeks after Campylobacter jejuni gastroenteritis. However, the fact that it remained elevated in individuals with post infective IBS (PI-IBS) 4 months to 4 years after their initial infection was unexpected.47 This same study also showed that the acute increases in inflammatory cells, particularly T lymphocytes and macrophages persisted in a subgroup of patients whose symptoms continued 12 months after the initial infection. The persistent increase in small intestinal permeability after gastro-enteritis was confirmed when the water supply of Walkerton, Ontario, Canada was contaminated with Escherichia coli 0147 and C. jejuni. Over 2300 residents developed acute bacterial gastroenteritis, and 24 months after the infection, 35% of those with PI-IBS had urine L: M excretion ratios >0.02 compared with 13% of those who were infected, but did not develop IBS.48 These and other studies suggested the hypothesis that delayed recovery from the inflammatory response was a factor in predisposing to PI-IBS.
B. Genetic predisposition
This hypothesis was pursued using a candidate gene approach examining 51 genes regulating the response to bacteria, inflammation, and permeability. They identified four single nucleotide polymorphisms (SNPs) in three genes, which increased the risk of PI-IBS, two in toll-like receptor (TLR)-9, and one each in interleukin (IL)-6 and cadherin 1 (CDH1).49 TLR-9 is a pattern recognition receptor, which is expressed intracellularly and recognizes unmethylated CpG sequences in bacterial and viral DNA, initiating a pro-inflammatory response producing cytokines, such as IL-1 and IL-12. IL-6 is a pro-inflammatory cytokine, activated by infection and stress, whereas CDH-1 is a cell-cell adhesion glycoprotein that may also control tight junction formation and hence influence permeability. Two further SNPs (rs12597188 and rs10431923), identified in the fine mapping of CDH1 locus, have also been associated with Crohn’s disease and abnormal permeability.50 These findings support the concept that a genetically determined over exuberant production of inflammatory mediators or abnormal tight junctions might contribute to the persistent increase in gut permeability seen in PI-IBS. More recently, an over-expression of TNFSF15 mRNA has been identified after C. jejuni infection and in IBS with diarrhea (IBS)-D.51 Zucchelli and colleagues in 201152 independently identified TNFSF15 as linked to IBS. In both studies, a SNP linked to an increased risk of Crohn’s disease and resulting in over-expression of TNFSF15 was more common in IBS than controls. However, the relationship of this risk allele to intestinal permeability has not yet been demonstrated.
C. Stress
Although infection is the most obvious cause of increased permeability, there are numerous animal studies showing that stress may also be a factor. Stress appears to act via mast cells, whose products stimulate T lymphocytes to produce inflammatory cytokines including interferon-γ that leads, after a delay of about 48 h, to an increase in colonic permeability.53
Evidence for increased permeability in IBS patients
Several studies have shown increased gut permeability in IBS. Studies using the urine excretion of 50Cr-EDTA taken with a 200 kcal nutrient test meal showed elevations in both 0–6 h and 6–24 h urinary collections, which approximate to small and large bowel permeability in both PI-IBS and IBS with constipation (IBS-C).54 Another study using the ratio of PEG 3500–400 urine excretions showed increases in both IBS-D and IBS-C.55 Using intestinal biopsies placed directly in mini-Ussing chambers, Piche and colleagues15 demonstrated that the paracellular permeability to FITC sulfonic acid was significantly increased regardless of IBS subtype in a small sample (12 IBS patients). They also showed a reduction in a tight junction protein ZO-1 mRNA, but no difference in occludin. Caco-2 monolayers provide a more convenient model for studying the effect of supernatant from IBS biopsies. This technique was used in 39 subjects to show that, in most patients with IBS, supernatant obtained from incubated biopsies (regardless of IBS clinical subtype) increases the permeability of the Caco-2 monolayer. Interestingly, the effect on permeability in this model correlated positively with pain scores.15 The increase in gut permeability caused by the IBS supernatant was neither blocked by histamine receptor antagonists nor mimicked by histamine. The role of other mast cell mediators, such as proteases and TNF, was not defined. Other studies to investigate novel approaches to treat barrier dysfunction in IBS have shown that probiotics can enhance barrier function56,57 and the anti-inflammatory agent, mesalazine, showed decreased mast cell numbers in IBS in a small pilot study.58
More recently, the role of proteasome degradation of tight junctions proteins has been examined in IBS. There was increased trypsin-like proteasome activity and this was associated with increased expression of 20S proteasome subunits, both constitutive and inducible (β1, β1i, β2, and β2i). Furthermore, the degradation of occludin was increased in IBS.59 A subsequent study from the same group showed decreased ZO-1 and occludin protein levels in IBS as a whole. The occludin decrease was only seen in IBS-D but not IBS-C.60 Of particular interest was the disruption of the normal apical expression of claudin-1, occludin, and ZO-1 that was irregularly distributed in the IBS patients. Multivariate analysis showed only occludin expression was negatively correlated with severity of abdominal pain.60 The mechanism underlying increased occludin degradation remains unclear, but increased proteasome activity is a feature of human mucosal immune activation61 which many other studies suggest plays a role in IBS.
Following several animal studies showing that acute stress increases mast cell numbers and gut permeability, studies in humans have demonstrated that acute pain stress, induced by immersing the hand in ice cold water, activates mast cells increasing the release of histamine, tryptase, and prostaglandin D2 into jejunal perfusates.62 Chronic psychological stress might well account for the increased mast cells recorded in the colon of patients with IBS who are often anxious or depressed.63 A recent study showed that mast cell numbers in the colon of IBS patients correlated (r = 0.64) with fatigue, a common non-colonic feature of IBS.64 Increased mast cells have also been found in the terminal ileum of patients with PI-IBS65, whereas others have found increased mast cell numbers in duodenal biopsies.66,67 Human mast cells express corticotrophin releasing factor (CRF) receptors subtype 1 and 2, and working with mini-Ussing chambers, it has been shown that human colonic mucosal permeability to horseradish peroxidase was increased by CRF, an effect blocked by a mast cell stabilizer.68 The potential implication of this increase in permeability is to allow access of bacterial antigens to TLR that activate the innate immune system. This hypothesis is supported by the finding of increased colonic mucosal expression of human defensin 2 (HD2) and increased levels of HD2 in the stool of patients with IBS.69 Recent studies suggest that increased permeability as measured by L: M ratio is associated with increased severity of pain.70 Possible mediators include inflammatory cytokines, mast cell tryptase or serotonin, but defining their precise role requires further studies with specific antagonists. In summary, both GI infection and chronic psychological stress in susceptible individual appears to be able to decrease epithelial barrier function. The associated immune activation may well contribute to the elevated cytokines, fatigue, and non-GI symptoms that are characteristic of IBS. Mast cell activation releases tryptase, and may act via PAR-2 receptors on enteric nerves to contribute to abdominal pain.
Glutamine and intestinal permeability
Previous studies have established that deficiencies in glutamine may lead to increased epithelial permeability. Glutamine is a major energy source for rapidly dividing mucosal cells of the GI tract. The major utilization of glutamine is in the human GI tract and depletion of intestinal glutamine results in epithelial atrophy of intestinal cells and a subsequent increase in permeability of the intestinal barrier. On the other hand, supplementation with glutamine can restore intestinal membrane permeability71,72, and decrease bacterial translocation and intestinal permeability after intestinal injury.73 In IBD and in patients with advanced esophageal cancer undergoing radiochemotherapy, glutamine supplementation has been shown to decrease intestinal permeability and improve GI function.74,75 The administration of glutamine may also provide clinical benefit to critically ill patients who develop increased intestinal permeability that leads to septicemia of enteric origin.72
Glutamine synthetase (GS) is a key intestinal enzyme that catalyzes the conversion of glutamate and ammonia to glutamine. It plays a major role in ammonia detoxification, cell signaling, inter-organ nitrogen flux, and acid-base homeostasis. As a result of the multiple functions and importance of glutamine synthetase in cellular metabolism, both synthesis and catalytic activities are highly regulated. Glutamine synthetase is an important enzyme that has been shown to be important for cell proliferation in rat intestinal crypt cells. Inhibition of glutamine synthetase has been shown to decrease proliferation of cultured rat intestinal cells.76 Mucosal epithelial cells lining the GI tract have a relatively small free glutamine pool and rely on glutamine synthetase to maintain adequate levels of intestinal glutamine. Decreased levels of intestinal glutamine synthetase present in certain conditions may lead to decreased levels of glutamine resulting in a shortage of the energy supply for intestinal epithelial cells, resulting in increased intestinal permeability. Thus, in conditions in which there are decreased levels of intestinal glutamine synthetase present, this may also lead to decreased glutamine and increased intestinal permeability.
Interestingly, decreased glutamine synthetase has recently been demonstrated in IBS patients with increased intestinal permeability associated with altered microRNA (miRNA) expression.16,70 miRNAs are small, endogenous, approximately 21–23 nucleotide long, non-coding RNAs that were first identified in 1993, and have the capacity for gene regulation.77,78 miRNAs are now recognized as regulators of biological function processes, such as differentiation, proliferation, cellular development, apoptosis, and metabolism. Unique miRNAs are cleaved from 70- to 100-nucleotide hairpin pre-miRNA precursors which then bind to the three prime untranslated region (3′-UTR) of target mRNAs through partial sequence complementarity blocking translation and also causing some degree of mRNA degradation. Thus, miRNAs have specific cellular functions that occur through altered pairing with target mRNAs of protein coding genes.77,78
miRNAs have been shown to impact a number of chronic diseases and may be involved in regulating disease activity in specific GI disorders. One study evaluated patients with both active and inactive ulcerative colitis.79 They found differential expression of specific miRNAs present in active ulcerative colitis. A study evaluated miR-510 in IBS-D patients and found an unique miRNA that targeted the serotonin receptor gene, HTR3E, in IBS patients.80 In an extensive study profiling all available miRNAs to identify all possible miRNAs that might have potential functional regulation properties in IBS patient’s17 intestinal biopsies from a subset of IBS-D, who had increased intestinal permeability revealed an up-regulation of miR-29a supporting a potential role for miR-29a in regulating intestinal permeability in IBS-D patients. The results also confirmed that intestinal glutamine synthetase is a target of miR-29a.17 Thus, it was concluded that altered miR-29a expression may regulate intestinal permeability in IBS patients through glutamine dependent mechanisms based on the functional interaction between miR29a and the GLUL gene.17 In vitro cell culture data also confirmed that miR-29a modulates colon and small intestinal cell permeability. In addition, miR-29a is also predicted to target claudin-1, an important mucosal tight junction protein that regulates paracellular permeability to pathogens and ions. As miRNAs have multiple targets, miR-29a may be involved in the regulation of intestinal permeability in some IBS patients through multiple target genes. The above study evaluated IBS patients with increased intestinal permeability also had reduced levels of intestinal glutamine synthetase activity that was functionally associated with increased miR-29a expression.17 Therefore, miR-29a appears to play a key role in directly modulating intestinal permeability through glutamine dependent signaling pathways that down regulate glutamine synthetase expression and lead to increased intestinal permeability.
SUMMARY AND CONCLUSION
In summary, our understanding of intestinal permeability and the mechanisms that regulate barrier function have increased significantly over the past few years. However, future exploration in experimental models and translational research in human tissue samples are required to enhance our understanding of the relationship between the immune system, inflammation, intestinal microbial flora, and the intestinal barrier. An important finding in experimental models is that increases in gut permeability occur before the development of colonic disease. Therefore, if specific agent(s) that prevent intestinal barrier dysfunction and reduce intestinal permeability are identified, they may serve as novel future therapeutic approaches to treat IBS. It is our belief that future research should focus on ways of enhancing gut barrier function that might well include probiotics and/or gut-selective anti-inflammatory agents.
Acknowledgments
FUNDING
No funding declared.
Abbreviations
- ACh
acetylcholine
- CDH-1
cadherin-1
- Cr-EDTA
chromium ethylenediaminetetracetic acid
- CRF
corticotrophin releasing factor
- ENaC
epithelial sodium channel
- FITC
fluorescein isothiocyanate
- GI
gastrointestinal
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- LR
leucine rich
- MAMP
microbe-associated molecular patterns
- miRNA
microribonucleic acid
- NGF
nerve growth factor
- NOD
nucleotide-binding oligomerization domain
- PAR
protease activated receptor
- PEG
polyethylene glycol
- PI-IBS
post infectious irritable bowel syndrome
- SEM
standard error of the mean
- SGLT-1
sodium glucose transporter
- SNP
single nucleotide polymorphism
- TLR
toll-like receptors
- TNF
tumor necrosis factor
- UTR
untranslated region
- VIP
vasoactive intestinal polypeptide
- ZO
zonula occludens
Footnotes
COMPETING INTERESTS
The authors have no competing interests.
AUTHOR CONTRIBUTION
MC, RS, KM, GNV, & BG-VM wrote the study.
References
- 1.Keita AV, Söderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil. 2010;22:718–33. doi: 10.1111/j.1365-2982.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–30. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley MA, Wertz JE. Bacteriocins: evolution, ecology, and application. Annu Rev Microbiol. 2002;56:117–37. doi: 10.1146/annurev.micro.56.012302.161024. [DOI] [PubMed] [Google Scholar]
- 4.Bevins CL, Salzmann NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–68. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 5.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol. 2001;280:G922–9. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 6.Korjamo T, Heikkinen AT, Mönkkönen J. Analysis of unstirred water layer in vitro permeability experiments. J Pharm Sci. 2009;98:4469–79. doi: 10.1002/jps.21762. [DOI] [PubMed] [Google Scholar]
- 7.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–91. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- 8.Visser J, Rozing J, Sapone A, Lammers K, Fasano A. Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms. Ann NY Acad Sci. 2009;1165:95–205. doi: 10.1111/j.1749-6632.2009.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hildalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96:736–49. [PubMed] [Google Scholar]
- 10.Amasheh M, Andres S, Amasheh S, Fromm M, Schulzke JD. Barrier effects of nutritional factors. Ann NY Acad Sci. 2009;1165:267–73. doi: 10.1111/j.1749-6632.2009.04063.x. [DOI] [PubMed] [Google Scholar]
- 11.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 12.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151–75. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 13.Veshnyakova A, Fromm M, Amasheh M, Amasheh S. Segmental expression of claudin proteins correlates with tight junction barrier properties in rat intestine. J Comp Physiol B. 2010;180:591–8. doi: 10.1007/s00360-009-0440-7. [DOI] [PubMed] [Google Scholar]
- 14.Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512–20. doi: 10.1136/gut.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of irritable bowel syndrome patients: involvement of soluble mediators. Gut. 2009;58:196–201. doi: 10.1136/gut.2007.140806. [DOI] [PubMed] [Google Scholar]
- 16.Lee JW, Park JH, Park DI, et al. Subjects with diarrhea-predominant IBS have increased rectal permeability responsive to tryptase. Dig Dis Sci. 2010;55:2922–8. doi: 10.1007/s10620-009-1094-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010;59:775–84. doi: 10.1136/gut.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Itallie CM, Anderson JM. Measuring size-dependent permeability of the tight junction using PEG profiling. Methods Mol Biol. 2011;762:1–11. doi: 10.1007/978-1-61779-185-7_1. [DOI] [PubMed] [Google Scholar]
- 19.Yu AS. Electrophysiological characterization of claudin ion permeability using stably transfected epithelial cell lines. Methods Mol Biol. 2011;762:27–41. doi: 10.1007/978-1-61779-185-7_3. [DOI] [PubMed] [Google Scholar]
- 20.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Ann Rev Physiol. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camilleri M, Gorman H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol Motil. 2007;19:545–52. doi: 10.1111/j.1365-2982.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- 22.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–81. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 23.Meddings JB, Gibbons I. Discrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology. 1998;114:83–92. doi: 10.1016/s0016-5085(98)70636-5. [DOI] [PubMed] [Google Scholar]
- 24.Rao AS, Camilleri M, Eckert DJ, et al. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol. 2011 Aug 11;301:G919–28. doi: 10.1152/ajpgi.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neunlist M, Toumi F, Oreschkova T, et al. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-association protein Z)-1 via VIPergic pathways. Am J Physiol Gastrointestin Liver Physiol. 2003;285:G1028–36. doi: 10.1152/ajpgi.00066.2003. [DOI] [PubMed] [Google Scholar]
- 26.Jacob C, Yang PC, Darmoul D, et al. Mast cell tryptase controls paracellular permeability of the intestine Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem. 2005;280:31936–48. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 27.Bueno L, Fioramonti J. Protease-activated receptor 2 and gut permeability: a review. Neurogastroenterol Motil. 2008;20:580–7. doi: 10.1111/j.1365-2982.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- 28.Hollenberg MD. Proteinase-mediated signaling: proteinase-activated receptors (PARs) and much more. Life Sci. 2003;74:237–46. doi: 10.1016/j.lfs.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Goldblum SE, Rai U, Tripathi A, et al. The active Zot domain (aa 288–293) increases ZO-1 and myosin 1C serine/threonine phosphorylation, alters interaction betnween ZO-1 and its binding partners, and induces tight junction disassembly through proteinase activated receptor 2 activation. FASEB J. 2011;25:144–58. doi: 10.1096/fj.10-158972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ewaschuk J, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol. 2008;295:G1025–34. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- 31.Ukena S, Singh A, Dringenberg U, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS Biol. 2007;2:e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson RC, Cookson AL, McNabb WC, et al. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010;10:316. doi: 10.1186/1471-2180-10-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fasano A, Fiorentini C, Donelli G, et al. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J Clin Invest. 1995;96:710–20. doi: 10.1172/JCI118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–74. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 35.Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci USA. 2010;107:228–33. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukuda S, Toh H, Hase K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543–7. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 37.Hamer HM, Jonkers D, Venema K, et al. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–19. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 38.Pagnini C, Saeed R, Bamias G, Arseneau KO, Pizarro TT, Cominelli F. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci USA. 2010;107:454–9. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Resta-Lenert SC, Barrett KE. Modulation of intestinal barrier properties by probiotics: role in reversing colitis. Ann NY Acad Sci. 2009;1165:175–82. doi: 10.1111/j.1749-6632.2009.04042.x. [DOI] [PubMed] [Google Scholar]
- 40.Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut. 2009;58:41–8. doi: 10.1136/gut.2008.150888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su L, Shen L, Clayburgh DR, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551–63. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson AJ, Chu S, Sieck L, et al. Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterology. 2005;129:902–12. doi: 10.1053/j.gastro.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 43.Liu JJRJ, Mah SJ, Thiesen AL, et al. Epithelial gaps in a rodent model of inflammatory bowel disease: a quantitative validation study. Clin Transl Gastroenterol. 2011;2:e3. doi: 10.1038/ctg.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiesslich R, Goetz M, Angus EM, et al. Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology. 2007;133:1769–78. doi: 10.1053/j.gastro.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Hanson PJ, Moran AP, Butler K. Paracellular permeability is increased by basal lipopolysaccharide in a primary culture of colonic epithelial cells; an effect prevented by an activator of Toll-like receptor-2. Innate Immun. 2011;17:269–82. doi: 10.1177/1753425910367813. [DOI] [PubMed] [Google Scholar]
- 46.Zuckerman MJ, Watts MT, Bhatt BD, Ho H. Intestinal permeability to [51Cr]EDTA in infectious diarrhea. Dig Dis Sci. 1993;38:1651–7. doi: 10.1007/BF01303174. [DOI] [PubMed] [Google Scholar]
- 47.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–11. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marshall JK, Thabane M, Garg AX, et al. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004;20:1317–22. doi: 10.1111/j.1365-2036.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 49.Villani AC, Lemire M, Thabane M, et al. Genetic risk factors for post-infectious irritable bowel syndrome following a waterborne outbreak of gastroenteritis. Gastroenterology. 2010;138:1502–13. doi: 10.1053/j.gastro.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 50.Imielinski M, Baldassano RN, Griffiths A, et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat Genet. 2009;41:1335–40. doi: 10.1038/ng.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spiller R, Swan C, Campbell E, et al. Identifying and testing candidate genes underlying the inflammatory basis of irritable bowel syndrome. Gut. 2011;60:A164. [Google Scholar]
- 52.Zucchelli M, Camilleri M, Nixon AA, et al. Association of TNFSF15 polymorphism with irritable bowel syndrome. Gut. 2011;41:134–40. doi: 10.1136/gut.2011.241877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demaude J, Salvador-Cartier C, Fioramonti J, et al. Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut. 2006;55:655–61. doi: 10.1136/gut.2005.078675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunlop SP, Hebden J, Campbell E, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288–94. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 55.Park JH, Park DI, Kim HJ, et al. The relationship between small-intestinal bacterial overgrowth and intestinal permeability in patients with irritable bowel syndrome. Gut. 2009;3:174–9. doi: 10.5009/gnl.2009.3.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Z, Qin H, Yang Z, et al. Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery – a double-blind study. Aliment Pharmacol Ther. 2011;33:50–63. doi: 10.1111/j.1365-2036.2010.04492.x. [DOI] [PubMed] [Google Scholar]
- 57.Zeng J, Li YQ, Zuo XL, et al. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:994–1002. doi: 10.1111/j.1365-2036.2008.03818.x. [DOI] [PubMed] [Google Scholar]
- 58.Corinaldesi R, Stanghellini V, Cremon C, et al. Effect of mesalazine on mucosal immune biomarkers in irritable bowel syndrome: a randomized controlled proof of concept study. Aliment Pharmacol Ther. 2009;30:245–52. doi: 10.1111/j.1365-2036.2009.04041.x. [DOI] [PubMed] [Google Scholar]
- 59.Coeffier M, Gloro R, Boukhettala N, et al. Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. Am J Gastroenterol. 2010;105:1181–8. doi: 10.1038/ajg.2009.700. [DOI] [PubMed] [Google Scholar]
- 60.Bertiaux-Vandaele N, Youmba SB, Belmonte L, et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol. 2011;106:2165–73. doi: 10.1038/ajg.2011.257. [DOI] [PubMed] [Google Scholar]
- 61.Visekruna A, Slavova N, Dullat S, et al. Expression of catalytic proteasome subunits in the gut of patients with Crohn’s disease. Int J Colorectal Dis. 2009;24:1133–9. doi: 10.1007/s00384-009-0679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santos J, Saperas E, Nogueiras C, et al. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology. 1998;114:640–8. doi: 10.1016/s0016-5085(98)70577-3. [DOI] [PubMed] [Google Scholar]
- 63.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 64.Piche T, Saint-Paul MC, Dainese R, et al. Mast cells and cellularity of the colonic mucosa correlated with fatigue and depression in irritable bowel syndrome. Gut. 2008;57:468–73. doi: 10.1136/gut.2007.127068. [DOI] [PubMed] [Google Scholar]
- 65.Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096–101. doi: 10.1136/gut.2003.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guilarte M, Santos J, deTorres I, et al. Diarrhoea-predominant IBS patients show mast cell activation and hyper-plasia in the jejunum. Gut. 2007;56:203–9. doi: 10.1136/gut.2006.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foley S, Garsed K, Singh G, et al. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation. Gastroenterology. 2011;140:1434–43. doi: 10.1053/j.gastro.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 68.Wallon C, Yang PC, Keita AV, et al. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2008;57:50–8. doi: 10.1136/gut.2006.117549. [DOI] [PubMed] [Google Scholar]
- 69.Langhorst J, Junge A, Rueffer A, et al. Elevated human beta-defensin-2 levels indicate an activation of the innate immune system in patients with irritable bowel syndrome. Am J Gastroenterol. 2009;104:404–10. doi: 10.1038/ajg.2008.86. [DOI] [PubMed] [Google Scholar]
- 70.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41–6. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klimberg VA, Souba WW. The importance of intestinal glutamine metabolism in maintaining a healthy gastrointestinal tract and supporting the body’s response to injury and illness. Surg Annu. 1990;22:61–76. [PubMed] [Google Scholar]
- 72.Labow BI, Souba WW. Glutamine. World J Surg. 2000;24:1503–13. doi: 10.1007/s002680010269. [DOI] [PubMed] [Google Scholar]
- 73.Souba WW, Klimberg VS, Plumley DA, et al. The role of glutamine in maintaning a healthy gut and supporting the metabolic response to injury and infection. J Surg Res. 1990;48:383–91. doi: 10.1016/0022-4804(90)90080-l. [DOI] [PubMed] [Google Scholar]
- 74.Yoshida S, Matsui M, Shirouzu Y, et al. Effects of glutamine supplements and radiochemotherapy on the systemic immune and gut barrier function in patients with advanced esophageal cancer. Ann Surg. 1998;227:485–91. doi: 10.1097/00000658-199804000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sido B, Seel C, Hochlehnert A, et al. Low intestinal glutamine level and low glutaminase activity in Crohn’s Disease: a rational for glutamine supplementation? Dig Dis Sci. 2006;51:2170–9. doi: 10.1007/s10620-006-9473-x. [DOI] [PubMed] [Google Scholar]
- 76.DeMarco V, Dyess K, Strauss D, et al. Inhibition of glutamine synthetase decreases proliferation of cultured rat intestinal epithelial cells. J Nutr. 1999;129:57–62. doi: 10.1093/jn/129.1.57. [DOI] [PubMed] [Google Scholar]
- 77.Kim J, Krichevsky A, Grad Y, et al. Identification of many microRNAs that copurify with polyribomes in mammalian neurons. Proc Natl Acad Sci USA. 2004;101:360–5. doi: 10.1073/pnas.2333854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farh KK, Grimson A, Jan C, et al. The widespread impact of mammalian microRNA on mRNA repression and evolution. Science. 2005;310:1817–21. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- 79.Wu F, Zikusoka M, Trindade A, Dassopoulus T, et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2α. Gastroenterology. 2008;135:1624–35. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 80.Kapeller J, Houghton LA, Monnikes H, et al. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17:2967–77. doi: 10.1093/hmg/ddn195. [DOI] [PubMed] [Google Scholar]
- 81.Schulman RJ, Eakin MN, Czyzewski DJ, et al. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J Pediatr. 2008;153:646–50. doi: 10.1016/j.jpeds.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen HQ, Yang J, Zhang M, et al. Lactobacillus plantarum ameliorates colonic epithelial barrier dysfunction by modulating the apical junctional complex and PepT1 in IL-10 knockout mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1287–97. doi: 10.1152/ajpgi.00196.2010. [DOI] [PubMed] [Google Scholar]


