Abstract
Objective
Perceived discrimination has been associated with psychosocial distress and adverse health outcomes. We examined associations of perceived discrimination measures with changes in kidney function in a prospective cohort study, the Healthy Aging in Neighborhoods of Diversity Across the LifeSpan.
Methods
Our study included 1,620 participants with preserved baseline kidney function (estimated glomerular filtration rate (eGFR) ≥60 ml/min/1.73m2) (662 Whites and 958 African-Americans (AA), aged 30–64 years). Self-reported perceived racial discrimination (PRD) and perceived gender discrimination (PGD) and a general measure of experience of discrimination (EOD) [“Medium vs. low”, “High vs. low”] were examined in relation to baseline, follow-up and annual rate of change in eGFR using multiple mixed-effects regression (γbase, γrate) and OLS models (γfollow).
Results
Perceived gender discrimination “High vs. Low PGD” was associated with a lower baseline eGFR in all models (γbase=−3.51(1.34), p=0.009 for total sample). Among White women, High EOD was associated with lower baseline eGFR, an effect that was strengthened in the full model (γbase=−5.86(2.52), p=0.020). Overall, “High vs. Low” PGD was associated with lower follow-up eGFR (γfollow=−3.03(1.45), p=0.036). Among AA women, both PRD and PGD were linked to lower follow-up kidney function, an effect that was attenuated with covariate adjustment, indicating mediation through health-related, psychosocial and lifestyle factors. In contrast, EOD was not linked to follow-up eGFR in any of the sex by race groups.
Conclusions
Perceived racial and gender discrimination are associated with poor kidney function assessed by glomerular filtration rate and the strength of associations differ by sex and race groups. Perceived discrimination deserves further investigation in psychsocial risk factors for kidney disease.
Keywords: Perceived discrimination, race, gender, urban adults, kidney function
INTRODUCTION
Chronic kidney disease (CKD) is a public health problem affecting 13% of US adults. (1) Clinical factors, such as hypertension and diabetes, and genetic factors (2) do not fully explain CKD burden. Therefore, attention has been recently paid to other social, economic, and psychosocial factors which may underlie kidney function decline.(3, 4) Among psychosocial factors, perceived discrimination (general experience of discrimination (EOD), race/ethnicity-related (PRD) or gender-related discrimination (PGD)) has been linked to adverse health outcomes, possibly through stress-related pathways, including hypertension, cardiovascular disease, poor general health status, and mental illness.(5) Stress is a condition whereby environmental factors tax or exceed the adaptive capacity of individuals to a point where psychological and physiological responses may place them at risk for disease.(6) Studies of stressors and their relation to pathophysiology have revealed alterations in blood pressure, heart rate and vascular reactivity in response to acute stress.(7–10)
These links suggest that adverse health outcomes are influenced by perceived racial discrimination (11–29) and in other instances by perceived gender discrimination (PGD). (13, 15, 16, 20–23, 30) Nevertheless, in one earlier study, reporting no or low discrimination had an unexpected positive relationship with worse health outcomes, such as hypertension, specifically among African-American women. (13) Thus, the direction of the association between perceived discrimination is still debated, particularly within different socio-demographic strata, such as sex and race.
To our knowledge, there have been no empirical studies of the relation of perceived discrimination and kidney function. Therefore, we examined the associations of PRD, PGD and EOD with longitudinal kidney function change in a bi-racial socioeconomically diverse sample from Baltimore City, Maryland, and tested differential associations by sex and race.
METHODS
Study Design
Initiated in 2004, The Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study is an ongoing prospective cohort study focused on the cardiovascular and cognitive health of a socioeconomically diverse sample of African Americans and Whites (baseline age: 30–64y), residing in Baltimore, Maryland. Race was self-reported in answer to the question: Please look at this card and tell me which category best describes you. Are you:
White;
Black/African American;
American Indian or Alaska Native;
Asian;
Native Hawaiian or other Pacific Islander;
Some other race?
Only those with self-described race of white or African American were eligible for the HANDLS study. Briefly, thirteen neighborhoods were selected using an area probability sampling methodology as detailed elsewhere.(31) Phase 1 consisted of screening, recruitment, and household interviews, while phase 2 collected more extensive data in a mobile Medical Research Vehicle (MRV). The present study uses baseline visit 1 (2004–2009) and the first follow-up visit 2 (2009–2013), with mean follow-up time of ~5y.
All participants provided written informed consent, after accessing a protocol booklet in layman’s terms and a video detailing all procedures and future re-contacts. HANDLS study was ethically approved by National Institute on Environmental Health Sciences, National Institutes of Health, Institutional Review Board.
Participants
Of 3720 total baseline HANDLS participants initially selected with complete Phase 1 of visit 1 data (i.e. home visit), 2,743 had complete data on estimated glomerular filtration rate (eGFR) at either visit measured on the MRV (Phase 2, visit 1), while 1,993 had complete eGFR data at both baseline and follow-up (N=750 at baseline only). We further excluded participants with missing data on PRD/PGD/EOD (n=63) or with baseline eGFR<60 ml/min/1.73m2 (N=84). Of the remaining participants (N=1,846) with preserved kidney function, those with missing data on any of the covariates entered into the model were excluded (i.e. complete case analysis; N=68 missing on hypertension or diabetes, an additional N=125 missing on smoking/drug use, an additional N=35 missing on elevated depressive symptoms status at baseline and an additional N=2 missing on education) yielding a final sample size of N=1,616. Compared to the HANDLS cohort that was not selected, our selected sample included a higher proportion who did not live in poverty and more females (P<0.05); though no age or race differences were detected. This sample selectivity was accounted for in the analysis through a 2-stage Heckman selection model as discussed in the Statistical Analysis section.
Perceived Racial Discrimination
Baseline PRD was measured using an adapted 9-item Discrimination Scale of the Experience of Discrimination (EOD) questionnaire, (13) and two global PRD items(32) (Supplemental Digital Content 1), eliciting perceived discrimination because of race, ethnicity/culture on a 4-point Likert scale (‘not at all’ to ‘a lot’). The five PRD binary (yes/no) situations from the EOD were racial discrimination at school, getting a job, at work, getting housing, and getting medical care. The sum of the five situational items (range: 5–10), and that of the two global items (range:2–8), were entered as two measured variables in a factor analysis with one common factor being extracted and predicted using the regression method. The predicted factor (z-score) was then grouped into the following categories: “Low PRD” (factor score<0), “Medium PRD” (factor score: 0–1) and “High PRD” (factor score >1).
Perceived Gender Discrimination
Similarly, PGD included one global item measured on a 4-point Likert scale (‘not at all’ to ‘a lot’) and 5 binary “yes/no” items, namely: “Have you ever experienced discrimination, or has anyone stopped you from doing something, hassled you, or made you feel inferior because of your gender?” in five distinctive situations: at school, getting a job, at work, at home, or when getting medical care?, with a total score range of 5–10.(13, 33) Using a similar approach, a factor analysis was conducted to combine the global measure with the situational measures that were also summed. The common factor was predicted and categorized as: “low PGD” (<0), “Medium PGD” (0–1) and “High PGD” (>1). The correlation between the sum of global PGD items and the sum of situational PGD items was 0.49, while that of global vs. situational PRD items was 0.54. The factor score for PGD were highly correlated with each sum of items (r=0.87 (factor score vs. global), r=0.86 (factor score vs. situational). Those correlations were both 0.88 for PRD. Cronbach’s α, assuming we are summing up items for each scale, were 0.79 and 0.67, for PRD and PGD, respectively. In addition to using the final factor score in the main analysis, summation of the items of PRD and PGD was also used as a continuous outcome in a small portion of the analysis.
Experience of discrimination (EOD)
Perceived discrimination was also measured using the EOD.(34, 35) The 9-item EOD measures the everyday experiences of unfair treatment and is by far the most commonly used scale in previous studies. This measure asks respondents ‘how often in your day-to-day life have the following things happened to you?’ (e.g. “You are treated with less courtesy”; “You are treated with less respect”; “You get worse service at restaurants and stores”; “People act as if you are not smart”; “People act as if they are afraid of you”; “People act as if they think you are dishonest”) on a Likert response scale (1 (never), 2 (less than once a year), 3 (a few times a year), 4 (a few times a month), 5 (at least once a week) and 6 (almost every day). Items were reverse coded so higher scores reflect more everyday discrimination (Cronbach’s α = .84 and item-total correlations ranging from 0.54–0.77).
A similar factor analytic approach was carried out whereby each of the 9 items were entered as measured variables and one factor was extracted. This common factor was then predicted and categorized in a similar fashion as for PRD and PGD (“Low EOD” (factor score<0), “Medium EOD” (factor score: 0–1) and “High EOD” (factor score>1). In addition to using the final factor score in the main analysis, summation of the items of EOD was also used as a continuous outcome in a small portion of the analysis.
Kidney Function
Our primary outcomes were baseline, annual rate of change and follow-up estimated glomerular filtration rate (eGFR). Using participant fasting venous blood specimens, baseline serum creatinine was measured at the National Institute on Aging, Clinical Research Branch Core Laboratory, using a modified kinetic Jaffe method (CREA method, Dade Dimension X-Pand Clinical Chemistry System, Siemens Healthcare Diagnostics Inc., Newark, DE) for a small group of participants (n=88); while the majority of participants (n=1,528) had baseline serum creatinine analyzed at Quest Diagnostics, Inc. by isotope dilution mass spectrometry (IDMS) (Olympus America Inc., Melville, NY) and standardized to the reference laboratory, Cleveland Clinic. While inter-assay coefficients of variation (CV) for this sample could not be calculated due to the use of only one or the other measurement of creatinine at baseline, only intra-assay CVs (mean/SD) could be estimated and those were 0.192 and 0.187 for the CREA and the IDMS methods, respectively. All follow-up serum creatinine concentrations were measured using IDMS at Quest Diagnostics, Inc.
For participants having spot urine data, micro-albumin concentration was measured at Quest Diagnostics, Inc. using an immunoturbimetric assay (Kamiya Biomedical Co., Seattle, WA). Estimated GFR was calculated using the CKD Epidemiology Collaboration equation(36), truncating values at 150 mL/min/1.73 m2 (37). Urine albumin-to-creatinine ratio (ACR) was estimated and included in a sensitivity analysis, due to its appreciable missingness from the selected sample (>10%).
Covariates
Age, sex, race (White or AA), completed years of education, poverty status (household income less than 125% of 2004 Department of Health and Human Services guideline) (38), marital status, current cigarette smoking, illicit drug use and self-rated health were self-reported at baseline. Baseline diabetes mellitus status combined fasting serum glucose concentration ≥126 mg/dL, self-reported diabetes, and/or prescription diabetic medication. Using two sitting blood pressure measurements, with brachial artery auscultation and an inflatable cuff,(39) hypertension was defined as the average of two systolic or diastolic blood pressures ≥ 140 mm Hg or ≥ 90 mm Hg, respectively, or self-reported hypertension, or anti-hypertensive medication prescription. Body mass index (BMI) was calculated as weight over height-squared (kg/m2). Elevated depressive symptoms (EDS) were defined as ≥16 score on the 20-item Center for Epidemiologic Studies-Depression (CES-D) scale.(40, 41)
Statistical Analysis
Bivariate associations of PRD and PGD with each of the baseline covariates were tested using one-way ANOVA from a bivariate ordinary-least-square (OLS) regression model for continuous variables and χ2 tests of independence for categorical variables. Similarly, we compared means of baseline, follow-up and annual rates of change in eGFR across PRD and PGD, stratifying by sex×race.
We used mixed-effects linear regression models to examine associations of baseline PRD and PGD (high vs. low) with eGFR (baseline and annual rate of change), controlling for key confounders. To account for non-random participant selection by age, sex, race and poverty status, in each mixed-effect regression model, we conducted a 2-stage Heckman selection process, as described elsewhere.(42, 43) In the basic model, we estimated the alternative associations of PRD and PGD with baseline and annual rate of change in eGFR, adjusting slopes and intercepts for age, sex and race (Model 1). Moving forward, we adjusted for factors that were considered modifiable socio-economic, lifestyle and health-related. While some can be considered potential confounders, others such as health-related factors are often the result of lifestyle and socio-economic factors as well as psychosocial factors, and thus may be mediating the effect of perceived discrimination on kidney function outcomes. Therefore, a stepwise adjustment was used in order to examine the potential omnibus effect of adding several groups of variables into the models in a cumulative manner. In Model 2, we further adjusted Model 1 for poverty status, education and marital status (i.e. in addition to age, sex, and race); in Model 3, we adjusted Model 2 for current smoking and illicit drug use, self-rated health, BMI and EDS; with Model 4 controlling Model 3 further for diabetes and hypertensive status. We added interaction terms and stratified by sex and race, because AAs report greater PRD(44) and reactions to psychological stressors differ by gender(45). Predictive margins of eGFR from stratified mixed-effects regression models were selectively plotted across time to illustrate key findings. Finally, we conducted OLS regression models, evaluating PRD and PGD’s independent associations with follow-up eGFR. Thus, two types of longitudinal analyses were conducted. While the first method investigates whether discrimination has a potential effect on the rate of change in kidney function, the second method investigates the effect of baseline discrimination on the level of kidney function 5 years later. A type I error of 0.05 was considered in all analyses which were conducted using Stata version 13 (StataCorp, College Station, TX). A sensitivity analysis is presented and discussed in Supplemental Digital Content 2 whereby ACR was included in Model 5, after excluding all participants with missing data on ACR. In a second sensitivity analysis (data not reported), the method/laboratory used for creatinine measurement was added as an additional covariate in all models and results of the full models were compared. In a third sensitivity analysis (data not reported), the 1,846 individuals with complete data on eGFR at both visits were selected, by including a category for missing (e.g. missing=“9”). Depressive symptoms were categorized as (0:<16, 1:≥16, 9:missing).
RESULTS
Baseline Study Characteristics by EOD groups
Overall, participants’ mean age was 48 years; 59% were AA; 41% were male. High PRD was reported by 13.7%, High PGD by 11.3% and High EOD by 15.2%. Both PRD and PGD factor scores (See factor analysis in methods section) had a positive and linear association with EOD tertiles. A larger proportion of AA men was found among participants with High EOD as opposed to low EOD (35.1% vs. 21.6%). High EOD was also associated with a higher proportion below poverty, poor/fair self-rated health, current smoking, current illicit drug use and elevated depressive symptoms. Overall, there was only a marginally significant higher mean baseline eGFR in the “High EOD” as opposed to the “low EOD” group. No linear trend was detected between EOD and the prevalence rates of hypertension and diabetes, the distribution in educational level and marital status or in mean BMI (Table 1)
Table 1.
Study participant baseline characteristics, overall and by EOD group, HANDLS study
| Overall (n=1,616) | Low EOD (n=934) | Medium EOD (n =437) | High EOD (n =245) | P-trend | |
|---|---|---|---|---|---|
| % | 100 | 57.8 | 27.0 | 15.2 | |
| Sex×Race | |||||
| White women | 23.6 | 24.6 | 23.8 | 19.2 | <0.001 |
| AA women | 35.0 | 36.5 | 34.3 | 30.0 | |
| White men | 17.2 | 17.2 | 18.1 | 15.5 | |
| AA men | 24.1 | 21.6 | 23.8 | 35.1 | |
| Baseline Age, Mean (SE) | 48.3(0.2) | 49.3(0.3) | 47.1(0.4) | 46.5(0.5) | 0.001 |
| Married, % | 33.4 | 33.7 | 32.7 | 33.4 | 0.94 |
| Educational level, % | |||||
| <HS | 6.5 | 6.4 | 6.4 | 6.9 | 0.84 |
| HS | 58.0 | 57.8 | 60.0 | 60.8 | |
| >HS | 35.4 | 35.8 | 36.6 | 32.2 | |
| Poverty status, % | 0.035 | ||||
| <125% PIR | 38.0 | 36.3 | 37.8 | 45.3 | |
| ≥125% PIR | 61.9 | 63.7 | 62.2 | 54.7 | |
| Self-rated health, % | 0.001 | ||||
| Poor/fair | 24.0 | 21.7 | 24.5 | 31.8 | |
| Good | 40.7 | 40.0 | 45.1 | 35.5 | |
| Very good/Excellent | 35.4 | 38.4 | 30.4 | 32.7 | |
| Current smoking status, % | 45.2 | 42.7 | 44.2 | 56.3 | 0.001 |
| Current illicit drug use, % | 17.3 | 14.3 | 18.8 | 25.7 | <0.001 |
| BMI, Mean (SE) | 30.1 (0.2) | 29.9 (0.2) | 30.7 (0.4) | 29.6(0.5) | 0.88 |
| Elevated depressive symptoms, CES-D total score ≥16, % | 40.1 | 32.2 | 49.0 | 54.3 | <0.001 |
| Hypertension, % yes | 44.7 | 45.6 | 44.2 | 42.4 | 0.65 |
| Diabetes, % yes | 9.7 | 9.6 | 10.8 | 8.2 | 0.54 |
| Baseline eGFR, Mean (SE) | 101.6(0.5) | 101.0(0.6) | 102.0(0.9) | 103.3(1.3) | 0.09 |
| PRD factor score, Mean (SE) | −0.003(0.018) | −0.21(0.02) | +0.18(0.04) | +0.47(0.06) | <0.001 |
| Low | 59.4 | 71.2 | 47.4 | 35.9 | <0.001 |
| Medium | 26.8 | 22.8 | 33.9 | 29.4 | |
| High | 13.7 | 6.0 | 18.8 | 34.7 | |
| PGD factor score, Mean (SE) | −0.004(0.017) | −0.22(0.02) | +0.21(0.04) | +0.46(0.05) | <0.001 |
| Low | 58.8 | 80.7 | 35.1 | 10.3 | <0.001 |
| Medium | 30.0 | 17.4 | 55.9 | 33.2 | |
| High | 11.3 | 1.9 | 9.0 | 56.3 | |
| EOD factor score, Mean (SE) | +0.007(0.023) | −0.62(0.013) | +0.42(0.01) | +1.68(0.03) | <0.001 |
Note: Selected study participants had preserved kidney function. Values are percent or Mean (SE). P-value for trend was based on a one-way ANOVA when row variable is continuous and χ2 when row variable is categorical.
Abbreviations: BMI = body mass index; eGFR CKD-EPI = estimate glomerular filtration rate Chronic Kidney Disease Epidemiology Collaboration; EOD= 9-item EveryDay Discrimination Scale; PIR=poverty income ratio.
Baseline, follow-up and annual rate of change in eGFR by PRD, PGD and EOD groups
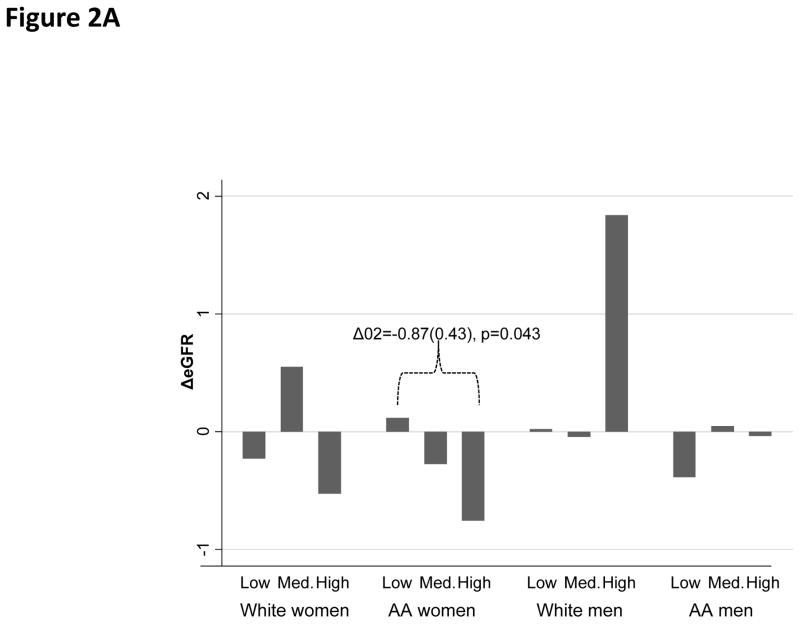
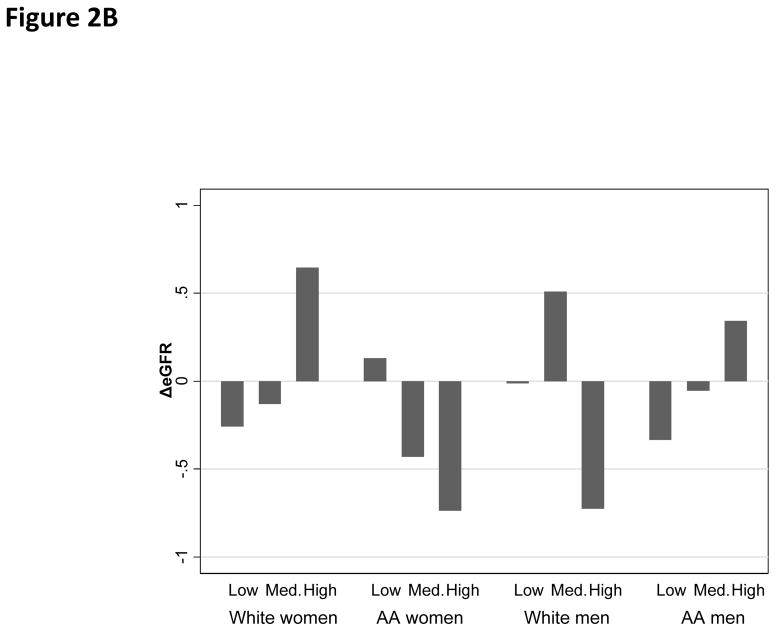
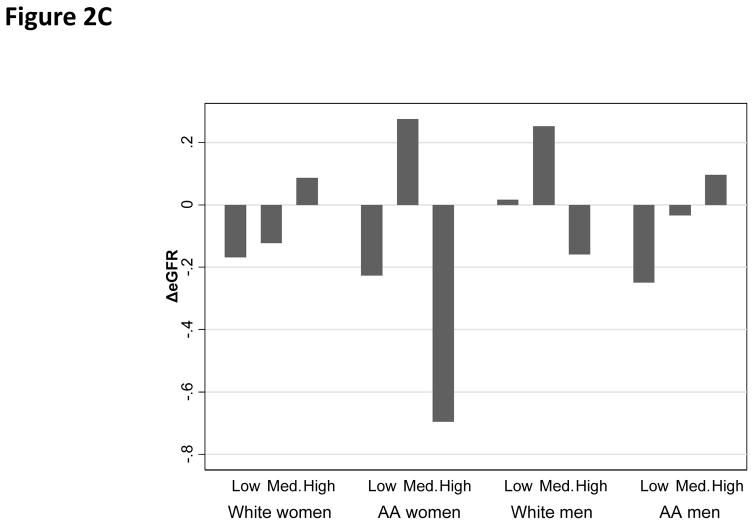
Overall, the mean annual rate of change in eGFR was estimated at −0.10 units/year, with a standard deviation of 3.35 (range: −18.7;+17.1). “High PRD” was associated with a faster rate of decline in eGFR among AA women as compared with “Low PRD”. (Figure 2A) In contrast, PGD and EOD were not associated with the rate of change in eGFR in any of the sex by race groups (Figure 2C–2D).
Figure 2.
Figure 2A. Annual rate of change in eGFR by PRD category
Figure 2B. Annual rate of change in eGFR by PGD category
Figure 2C. Annual rate of change in eGFR by EOD category
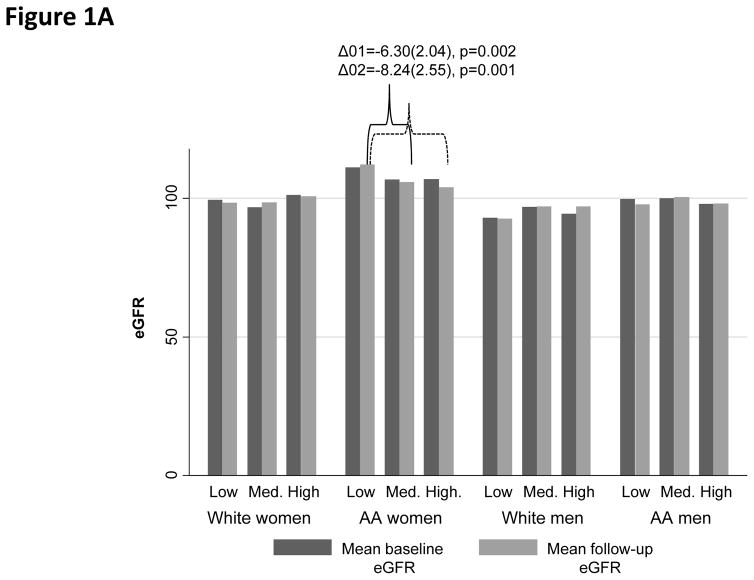
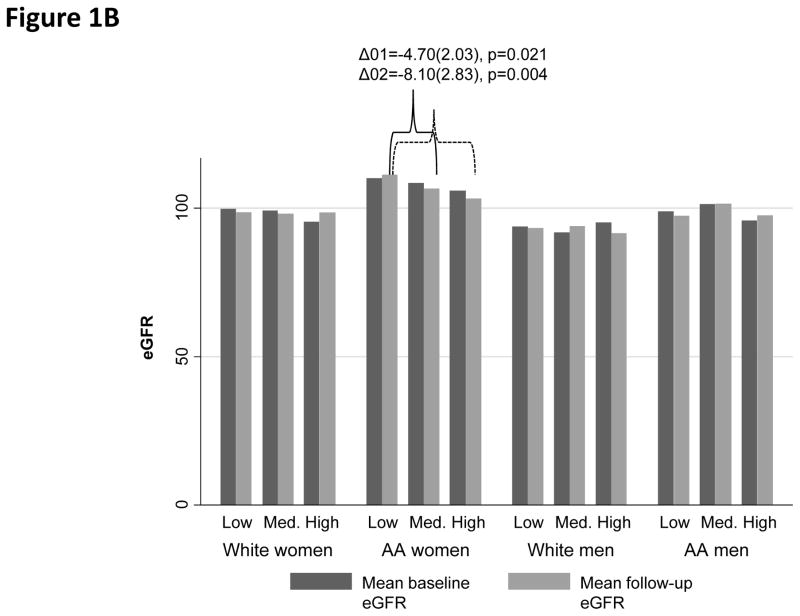
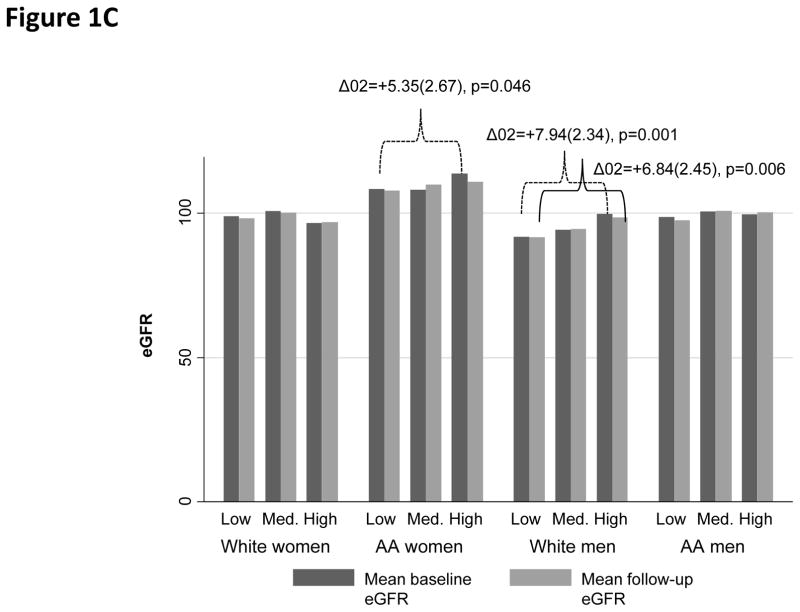
When examining baseline and follow-up eGFR (overall means±SD: 101.64±19.11 and 101.32±20.16, respectively), among AA women, High PRD (vs. Low PRD) and High PGD (vs. Low PGD) were both associated with lower follow-up eGFR (Figure 1A–1B). In contrast, “High EOD” was linked to higher eGFR among both AA women (baseline) and White men (baseline and follow-up), when compared with “Low EOD” (Figure 1C).
Figure 1.
Figure 1A. Baseline and follow-up mean eGFR by PRD category
Figure 1B. Baseline and follow-up mean eGFR by PGD category
Figure 1C. Baseline and follow-up mean eGFR by EOD category
Unadjusted association between PRD/PGD/EOD summation scores and key outcomes
Table S1 shows the unadjusted correlations between outcome measures and key exposures, overall and stratifying simultaneously by sex and race. Although most correlation coefficients were weak (<0.3), statistical significance was observed for AA women, whereby the PRD summation score was inversely related to baseline, follow-up and annual rate of change in eGFR. PGD among AA women was also inversely related to two of three outcomes, namely baseline and follow-up eGFR. This is in stark contrast with the EOD summation score which showed a positive association with baseline and follow-up eGFR, overall and among White men. Finally, the EOD summation score was also positively associated with baseline eGFR among AA women.
Net associations between PRD/PGD and EOD with baseline and annual rate of change in eGFR
In mixed effects regression models examining the net effect of PRD and PGD on eGFR (baseline and annual rate of change), In the total sample, “High vs. Low PGD” was associated with a lower baseline eGFR in all models (full model: PGD effect: −3.51(1.34), p=0.009), an effect restricted to Whites. (Table 2)
Table 2.
Baseline and annual rate of change in eGFR by perceived racial/gender discrimination (PRD, PGD), overall and by sex×race: Mixed-effects linear regression models.
| Models | N | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| β(SE) | P | β(SE) | P | β(SE) | P | β(SE) | P | ||
| Overall | 1616 | ||||||||
| Model A: PRD | |||||||||
| PRD10 | −1.72(1.00) | 0.085 | −1.63(1.00) | 0.10 | −1.59(1.00) | 0.11 | −1.60(1.00) | 0.11 | |
| PRD10×Time | +0.05(0.19) | 0.80 | +0.02(0.19) | 0.91 | +0.02(0.20) | 0.91 | +0.03(0.19) | 0.88 | |
| PRD20 | −1.37(1.31) | 0.30 | −1.11(1.31) | 0.40 | −1.35(1.32) | 0.31 | −1.31(1.32) | 0.32 | |
| PRD20×Time | −0.15(0.25) | 0.55 | −0.23(0.25) | 0.37 | −0.18(0.25) | 0.49 | −0.20(0.25) | 0.42 | |
| Model B: PGD | |||||||||
| PGD10 | −0.36(0.92) | 0.70 | −0.32(0.92) | 0.72 | −0.40(0.92) | 0.66 | −0.41(0.92) | 0.66 | |
| PGD10×Time | −0.12(0.18) | 0.49 | − −0.14(0.18) | 0.43 | −0.12(0.18) | 0.50 | −0.14(0.18) | 0.43 | |
| PGD20 | −3.41(1.33) | 0.010 | −3.28(1.37) | 0.014 | −3.53(1.34) | 0.009 | −3.51(1.34) | 0.009 | |
| PGD20×Time | +0.13(0.26) | 0.61 | +0.09(0.26) | 0.73 | +0.14(0.26) | 0.60 | +0.11(0.26) | 0.68 | |
| White women | 381 | ||||||||
| Model A: PRD | |||||||||
| PRD10 | −2.81(2.20) | 0.20 | −3.25(2.19) | 0.14 | −3.35(2.18) | 0.13 | −3.55(2.19) | 0.11 | |
| PRD10×Time | +0.53(0.46) | 0.25 | +0.62(0.46) | 0.17 | +0.70(0.45) | 0.12 | +0.66(0.45) | 0.14 | |
| PRD20 | +0.83(4.86) | 0.86 | −0.37 (4.85) | 0.94 | +0.23 (4.87) | 0.96 | +0.33(4.86) | 0.95 | |
| PRD20×Time | +0.25(1.00) | 0.81 | +0.54(0.99) | 0.58 | +0.34(0.98) | 0.73 | +0.32(0.98) | 0.74 | |
| Model B: PGD | |||||||||
| PGD10 | +0.27(1.73) | 0.88 | −0.18(1.75) | 0.92 | −0.18(1.75) | 0.92 | −0.06(1.76) | 0.97 | |
| PGD10×Time | −0.02(0.37) | 0.96 | +0.01(0.37) | 0.98 | −0.01(0.37) | 0.98 | −0.10(0.37) | 0.79 | |
| PGD20 | −4.50(2.55) | 0.078 | −4.73(2.55) | 0.064 | −4.73(2.55) | 0.065 | −4.88(2.57) | 0.058 | |
| PGD20×Time | +0.99(0.54) | 0.067 | +1.02(0.54) | 0.058 | +1.02(0.54) | 0.058 | +0.93(0.53) | 0.077 | |
| White men | 278 | ||||||||
| Model A: PRD | |||||||||
| PRD10 | +3.57(2.03) | 0.079 | +3.82(1.97) | 0.052 | +3.37(1.97) | 0.088 | +3.28(1.98) | 0.098 | |
| PRD10×Time | +0.15(0.40) | 0.72 | +0.09(0.40) | 0.81 | +0.30(0.40) | 0.47 | +0.27(0.40) | 0.50 | |
| PRD20 | −1.84(4.53) | 0.68 | −2.25(4.41) | 0.61 | −3.46(4.37) | 0.43 | −3.66(4.39) | 0.40 | |
| PRD20×Time | −0.25(0.89) | 0.78 | −0.22(0.88) | 0.80 | +0.35(0.89) | 0.70 | +0.31(0.88) | 0.73 | |
| Model B: PGD | |||||||||
| PGD10 | −2.77(1.92) | 0.15 | −2.48(1.87) | 0.19 | −3.45(1.84) | 0.062 | −3.36(1.85) | 0.069 | |
| PGD10×Time | +0.55(0.38) | 0.15 | +0.54(0.38) | 0.16 | +0.63(0.37) | 0.093 | +0.63(0.38) | 0.094 | |
| PGD20 | −1.67(5.36) | 0.76 | −2.80(5.20) | 0.59 | −4.45(5.22) | 0.39 | −4.50(5.22) | 0.39 | |
| PGD20×Time | −0.54(0.92) | 0.56 | −0.46(0.92) | 0.62 | +0.05(0.94) | 0.96 | +0.05(0.93) | 0.96 | |
| AA women | 565 | ||||||||
| Model A: PRD | |||||||||
| PRD10 | −2.44(1.77) | 0.16 | −2.70(1.79) | 0.13 | −2.44(1.81) | 0.18 | −2.34(1.81) | 0.20 | |
| PRD10×Time | −0.33(0.32) | 0.31 | −0.33(0.33) | 0.32 | −0.33(0.33) | 0.31 | −0.33(0.33) | 0.32 | |
| PRD20 | −0.51(2.23) | 0.82 | −1.00(2.27) | 0.66 | −0.88(2.31) | 0.70 | −0.72(2.32) | 0.76 | |
| PRD20×Time | −0.73(0.41) | 0.078 | −0.62(0.42) | 0.14 | −0.57(0.43) | 0.18 | −0.57(0.43) | 0.18 | |
| Model B: PGD | |||||||||
| PGD10 | −0.70(1.76) | 0.69 | −1.08(1.77) | 0.54 | −0.93(1.78) | 0.60 | −0.92(1.78) | 0.61 | |
| PGD10×Time | −0.65(0.33) | 0.044 | −0.60(0.33) | 0.066 | −0.57(0.33) | 0.081 | −0.59(0.32) | 0.074 | |
| PGD20 | −1.25(2.45) | 0.61 | −1.81(2.48) | 0.47 | −1.67(2.52) | 0.51 | −1.59(2.52) | 0.53 | |
| PGD20×Time | −0.72(0.45) | 0.11 | −0.61(0.45) | 0.18 | −0.57(0.46) | 0.21 | −0.57(0.46) | 0.21 | |
| AA men | 392 | ||||||||
| Model A: PRD | |||||||||
| PRD10 | −1.14(2.00) | 0.57 | −1.16(2.01) | 0.57 | −1.06(1.98) | 0.59 | −1.46(1.98) | 0.46 | |
| PRD10×Time | +0.52(0.39) | 0.18 | +0.40(0.39) | 0.30 | +0.40(0.38) | 0.29 | +0.48(0.38) | 0.21 | |
| PRD20 | −1.20(2.17) | 0.58 | −0.78(2.19) | 0.72 | −1.31(2.16) | 0.54 | −1.22(2.15) | 0.57 | |
| PRD20×Time | +0.43(0.42) | 0.30 | +0.27(0.42) | 0.72 | +0.23(0.41) | 0.57 | +0.22(0.41) | 0.59 | |
| Model B: PGD | |||||||||
| PGD10 | +1.82(1.84) | 0.32 | +2.09(1.85) | 0.26 | +1.86(1.81) | 0.31 | +1.79(1.81) | 0.32 | |
| PGD10×Time | +0.34(0.35) | 0.34 | +0.24(0.36) | 0.50 | +0.24(0.35) | 0.50 | +0.25(0.35) | 0.47 | |
| PGD20 | −3.03(2.43) | 0.21 | −2.90(2.43) | 0.23 | −3.90(2.39) | 0.10 | −3.84(2.38) | 0.11 | |
| PGD20×Time | +0.67(0.47) | 0.15 | +0.59(0.46) | 0.20 | +0.63(0.46) | 0.17 | +0.59(0.45) | 0.11 | |
Note: Selected participants with preserved kidney function. PRD and PGD are coded as 2=High, 1=Medium, 0=Low, were entered separately in models A or B. For instance PGD10 refers to Medium PGD contrasted with Low PGD. Model 1: adjusted for inverse mills ratio, age, sex and race; Model 2: further adjusted for poverty status, marital status and educational level; Model 3: further adjusted for current smoking status and illicit drug use, BMI, self-rated health and elevated depressive symptoms; Model 4: further adjusted for diabetes and hypertension.
Other key findings emerged in the sex and race-stratified mixed-effects regression models with EOD (Table 3). Specifically, among White women, High EOD was associated with lower baseline eGFR, an effect that was strengthened in the full model (full model: EOD effect −5.86(2.52), p=0.020). Among White men, high EOD was linked to a marginally significant faster decline in eGFR in Model 1, which was fully attenuated by socio-economic factors in Model 2.
Table 3.
Baseline and annual rate of change in eGFR by everyday discrimination (EOD), overall and by sex×race: Mixed-effects linear regression models
| Models | N | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| β(SE) | P | β(SE) | P | β(SE) | P | β(SE) | P | ||
| Overall | 1616 | ||||||||
| EOD10 | −0.57(0.95) | 0.54 | −0.55(0.95) | 0.56 | −0.55(0.96) | 0.57 | −0.57(0.96) | 0.56 | |
| EOD 10×Time | +0.20(0.19) | 0.30 | +0.19(0.19) | 0.75 | +0.18(1.20) | 0.99 | +0.19(0.19) | 0.31 | |
| EOD 20 | +0.35(1.18) | 0.77b | +0.38(1.18) | 0.75b | +0.02(1.20) | 0.99b | +0.03(1.20) | 0.98b | |
| EOD 20×Time | −0.12(0.23) | 0.61 | −0.12(0.23) | 0.61 | −0.04(0.23) | 0.86 | −0.05(0.23) | 0.84 | |
| White women | 381 | ||||||||
| EOD10 | +0.19(1.78) | 0.92 | +0.12(1.78) | 0.95 | −0.07(1.83) | 0.97 | −0.05(1.83) | 0.98 | |
| EOD10×Time | −0.08(0.38) | 0.84 | −0.06(0.38) | 0.87 | +0.12(0.39) | 0.76 | +0.10(0.39) | 0.80 | |
| EOD20 | −4.28(2.43) | 0.078 | −4.81(2.43) | 0.048 | −5.55(2.51) | 0.027 | −5.86(2.52) | 0.020 | |
| EOD20×Time | +0.12(0.52) | 0.82 | +0.25(0.52) | 0.64 | +0.72(0.53) | 0.18 | +0.65(0.53) | 0.22 | |
| White men | 278 | ||||||||
| EOD10 | +0.91(1.64) | 0.56 | +0.99(1.61) | 0.54 | +0.01(1.64) | 1.00 | −0.03(1.64) | 0.99 | |
| EOD10×Time | −0.28(0.33) | 0.40 | −0.31(0.33) | 0.34 | −0.24(0.34) | 1.00 | −0.25(0.33) | 0.46 | |
| EOD20 | +4.98(2.19) | 0.023 | +3.49(2.16) | 0.11 | +2.83(2.19) | 0.20 | +2.77(2.21) | 0.21 | |
| EOD20×Time | −0.75(0.44) | 0.088 | −0.60(0.44) | 0.17 | −0.52(0.45) | 0.25 | −0.55(0.45) | 0.22 | |
| AA women | 565 | ||||||||
| EOD10 | −1.66(1.83) | 0.37 | −1.13(1.84) | 0.54 | −1.04(1.86) | 0.58 | −1.08(1.86) | 0.56 | |
| EOD10×Time | +0.40(0.34) | 0.23 | +0.35(0.34) | 0.30 | +0.40(0.34) | 0.37 | +0.40(0.34) | 0.24 | |
| EOD20 | +3.49(2.39) | 0.14 | +3.67(2.39) | 0.13 | +3.67(2.43) | 0.13 | +3.67(2.43) | 0.13 | |
| EOD20×Time | −0.58(0.45) | 0.19 | −0.53(0.45) | 0.24 | −0.41(0.46) | 0.37 | −0.41(0.46) | 0.37 | |
| AA men | 392 | ||||||||
| EOD10 | −0.03(2.02) | 0.99 | +0.00(2.02) | 1.00 | +0.22(2.02) | 0.91 | +0.11(2.01) | 0.96 | |
| EOD10×Time | +0.33(0.39) | 0.40 | +0.29(0.39) | 0.31 | +0.09(0.39) | 0.82 | +0.12(0.38) | 0.76 | |
| EOD20 | −0.79(2.16) | 0.72 | −0.50(2.16) | 0.82 | −0.65(2.12) | 0.76 | −0.48(2.12) | 0.82 | |
| EOD20×Time | +0.42(0.41) | 0.31 | +0.42(0.41) | 0.31 | +0.32(0.41) | 0.43 | +0.27(0.40) | 0.50 | |
Note: Selected participants with preserved kidney function. EOD is coded as 2=High, 1=Medium, 0=Low. For instance EOD10 refers to Medium EOD contrasted with Low EOD. Model 1: adjusted for inverse mills ratio, age, sex and race; Model 2: further adjusted for poverty status, marital status and educational level; Model 3: further adjusted for current smoking status and illicit drug use, BMI, self-rated health and elevated depressive symptoms; Model 4: further adjusted for diabetes and hypertension.
In a separate model with sex×race (0=White women vs. each of the other categories), sex×race×Time, sex×race×EODk0, sex×race× EODk0×Time, (in addition to the other covariates in each model), p<0.05 for the null hypothesis that the term sex×race× EODk0×Time =0.
In a separate model with sex×race (0=White women vs. each of the other categories), sex×race×Time, sex×race×EODk0, sex×race× EODk0×Time, (in addition to the other covariates in each model), p<0.05 for the null hypothesis that the term sex×race×EODk0=0.
PRD/PGD and EOD and their adjusted associations with follow-up eGFR
Our sequential OLS models with alternative predictors PRD and PGD (Supplemental Digital Content 2, Table S2) indicated that, overall, “High vs. Low” PGD was associated with lower follow-up eGFR (full model: PGD effect: −3.03(1.45), p=0.036). “Medium vs. Low PRD” was specifically positively associated with eGFR, indicating better kidney function, among White men in Model 3 (PRD effect: +4.33(2.07), p=0.037), an effect attenuated with adjustment for hypertension and diabetes status (PRD effect: +4.09(2.08), p=0.050). Among AA women, both PRD and PGD were linked to lower kidney function at follow-up, an effect that was attenuated systematically between Models 2 and 4, indicating an effect of health-related (e.g. self-rated health, BMI, hypertension and diabetes), psychosocial (depressive symptoms) and lifestyle factors (smoking and drug use). In contrast, EOD was not linked to follow-up eGFR in any of the sex by race groups (Table S3). A few marked changes were observed in the sensitivity analysis in the sample with complete ACR, mostly due to a reduced overall sample size (n=1,158), (Tables S4–S7), Supplemental Digital Content 2). In a second sensitivity analysis (data not reported), the method/laboratory used for creatinine measurement was restricted to Quest Diagnostics, the most commonly used laboratory at both waves, and the only one used in the follow-up wave (n=1,528 of 1,616). The results were not altered, as was the case for a third sensitivity analysis of N=1,846 individuals with complete baseline and follow-up visit eGFR (data not reported).
DISCUSSION
Within a biracial urban sample of adults in Baltimore City, Maryland, High PRD was reported by 13.7%, High PGD by 11.3% and High EOD by 15.2%. Associations between perceived discrimination and kidney function varied by race and sex groups. Among Whites, High PGD was associated with a lower baseline eGFR. Among White women, High EOD was associated with lower baseline eGFR. Overall, High PGD was associated with lower follow-up eGFR. Notably, among AA women, both PRD and PGD were linked to lower kidney function at follow-up, an effect which appeared mediated by health-related, psychosocial and lifestyle factors. In contrast, EOD was not linked to follow-up eGFR in any of the sex by race groups.
Our findings of variation of assocations between perceived discrimination and kidney function change across gender and race groups are consistent with nuanced findings of several other studies, underscoring the complex effects of discrimination on health outcomes. (18, 21, 23–25) For example, in a large sample of Asian-American adults, perceived discrimination was associated with adverse health outcomes among both men and women, with the strongest association being with women’s mental health. The threshold for an association of discrimination with adverse health outcomes was also lower among women as compared to men.(17) Based on the CARDIA study, the experience of 1 or 2 episodes of discrimination were only associated with higher levels of inflammation (as measured by C-reactive protein) among AA women. There were no such associations observed among men or White women.(19)
The findings of our study could have implications for the well-established race and gender differences in kidney disease outcomes. For example, Whites have equal or greater overall prevalence of reduced kidney function when compared to African Americans,(46) however African Americans experience faster declines in kidney function,(47) and bear a greater burden of advanced and end-stage renal disease (ESRD).(48) While few studies have examined the intersectionality of race and gender in kidney disease, White women have been documented to have greater overall prevalence of reduced kidney function,(49) as compared to women of other race/ethnic groups, however, African American men(50) have the highest incident rate for ESRD. Our study argues for closer examination of psychosocial stressors for their impact on these differences.
Biologically speaking, chronic psychosocial stress may induce changes in neuroendocrine, autonomic and immune systems(51), and perceived discrimination has been linked with increased levels of oxidative stress,(52) a pathway through which allostatic load (53)) may be transduced into chronic diseases (54). In fact, stress-induced allostatic load was hypothesized to cause an epigenetically induced pro-inflammatory state, leading to an increased risk for cardiovascular disease.(55) Moreover, both racial and gender differences in coping with psychosocial stress, including discrimination, are important to consider, as they were detected in various non-CKD samples, and coping strategies have been noted to vary among men and women with CKD, with women showing a broader range of strategies that can buffer the effects of stress. (56)
Perceived discrimination can lead to hopelessness and low self-efficacy,(57) affecting the ability to self-manage one’s health, perhaps differentially by gender and race.(58) For instance, among hypertensive AAs, PRD was linked to lower medication adherence.(59) Another study suggested lifetime discrimination was associated with medical care delays and nonadherence, (60) a possible contributor to racial disparities in health, in general, and CKD progression in particular. Similarly, education-related discrimination was linked to poorer glycemic control among type 2 diabetes patients(61), while gender discrimination among women was linked to non-adherence to mammography services.(30) Among CKD patients, lifetime discrimination was associated with lower odds of desiring a kidney transplant, suggesting that patients with significant prior exposure to discrimination do not want to risk new treatment situations, such as transplantation, because they have a lower expectation of successful outcomes.(62) Using longitudinal data obtained from the Study of Women’s Health Across the Nation SWAN (n = 2063; mean age at baseline = 46.0), Upchurch et al. found that race and SES’s total effect on womnen’s allostatic load was at least partially mediated by psychosocial factors such as perceived discrimination, perceived stress and hostility.(29) Another recent study exploring the association between perceived racism and ambulatory blood pressure among Hispanics, reported that lower perceived racism was associated with ambulatory blood pressure non-dipping, a cardiovascular risk factor, only among Black Hispanics. This reveals a coping mechanism among this group that differs from White Hispanics.(63) In a third recent study examining heart rate variability (HRV) across three racial groups (Black, brown and White) found a gradient (Black>brown>Whites) in HRV that clearly mediated by perceived discrimination.(28) Examining sleep quality outcomes, another recent study reported that perceived discrimination mediated racial differences in most sleep quality measures, with nonWhite consistently showing poor sleep outcomes compared to Whites.(27) Finally, a study of mutliple ethnic groups reported that perceived ethnic discimination was positively associated with the metabolic syndrome (MetS) that ethnic differences in MetS were partially explained by this discrimination measure. (26)
Our study had limitations, including residual confounding, specifically by time-dependent blood pressure, urinary albumin excretion and apolipoprotein L1 risk variants among AAs. Third, perceived discrimination may have a different effect on kidney function decline from personally mediated or internalized forms of racism or sexism, which we did not examine. Fourth, kidney function decline was estimated only from two measures, while baseline ACR data was incomplete. Fifth, significant declines in eGFR was a relatively rare event, with 45 (2.8%) participants declining to an (e.g. eGFR<60 ml/min/1.73m2 at follow-up and 150 (9.3%) declining to an eGFR between 60 and 90 ml/min/1.73m2,) which precluded examining the association of perceived discrimination with the development of significantly reduced kidney function.. Related to this limitation, our sample had limited kidney function decline in relation to the two main exposures, yielding our finding of potentially limited clinical significance. Moreover, no valid data was available on whether participants had received a diagnosis of or treatment for CKD. Finally, given the sampling methodology and the large percentage of missing data between initial screening, baseline and follow-up examinations, our study findings are generalizable only to urban US adults. Thus, future studies should include geographically diverse samples, ideally with multiple eGFR and ACR assessments and longer follow-up.
The limitations of our study are balanced by its longitudinal design and the elucidation of a novel risk factor for kidney function decline. If validated in other studies, our findings emphasize the role of psychosocial stressors as potentially modifiable risk factors for adverse kidney outcomes. Further intervention studies addressing psychosocial stressors and CKD are likely warranted and future studies should also examine potential biomarkers that may mediate the relationship between perceived discrimination and kidney function decline.
In conclusion, in this sample of urban adults, perceived gender discrimination was associated with modestly lower kidney function among White women and AA men. Consistent findings were observed among AA women with respect to perceived racial discrimination and lower kidney function. Perceived discrimination, a psychosocial stressor, deserves further investigation for its potential contribution to kidney outcomes.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health (NIH). D.C.C. was supported by grant K23DK097184 from the National Institute of Diabetes and Digestive and Kidney Diseases, NIH.
ABBREVIATIONS
- AA
African-American
- ACR
Albumin:creatinine ratio
- CKD
Chronic Kidney Disease
- BMI
Body Mass Index
- CES-D
Center for Epidemiologic Studies-Depression
- EDS
Elevated Depressive Symptoms
- eGFR
Estimated Glomerular Filtration Rate
- EOD
Experience of Discrimination
- HANDLS
Health Aging in Neighborhoods of Diversity Across the LifeSpan
- OLS
Ordinary Least Square
- PRD
Perceived Racial Discrimination
- PGD
Perceived Gender Discrimination
Footnotes
Disclosure statement: The authors declare no conflict of interest.
Author contributions:
MAB: Study concept, literature search and review, plan of analysis, data management, statistical analysis, write-up and revision of the manuscript.
APB: Plan of analysis, data management, literature search and review, write-up and revision of the manuscript.
ABZ: Data acquisition, plan of analysis, write-up of parts of the manuscript, revision of the manuscript.
OSR: Plan of analysis, write-up of parts of the manuscript, revision of the manuscript.
MKE: Data acquisition, revision of the manuscript.
DCC: Plan of analysis, literature search and review, write-up of parts of the manuscript, revision of the manuscript.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. Jama. 2007;298:2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT, Jr, Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ, Investigators AS, Investigators CS. APOL1 risk variants, race, and progression of chronic kidney disease. The New England journal of medicine. 2013;369:2183–96. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crews DC, Pfaff T, Powe NR. Socioeconomic factors and racial disparities in kidney disease outcomes. Seminars in nephrology. 2013;33:468–75. doi: 10.1016/j.semnephrol.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Bruce MA, Griffith DM, Thorpe RJ., Jr Stress and the kidney. Advances in chronic kidney disease. 2015;22:46–53. doi: 10.1053/j.ackd.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: findings from community studies. American journal of public health. 2003;93:200–8. doi: 10.2105/ajph.93.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen SKRC, Gordon LU. Measuring Stress. New York, NY: Oxford University Press; 1995. Personality characteristics as moderators of the relationship between stress and disorder. [Google Scholar]
- 7.Kovach JA, Nearing BD, Verrier RL. Angerlike behavioral state potentiates myocardial ischemia-induced T-wave alternans in canines. Journal of the American College of Cardiology. 2001;37:1719–25. doi: 10.1016/s0735-1097(01)01196-2. [DOI] [PubMed] [Google Scholar]
- 8.Lind L, Johansson K, Hall J. The effects of mental stress and the cold pressure test on flow-mediated vasodilation. Blood pressure. 2002;11:22–7. doi: 10.1080/080370502753543927. [DOI] [PubMed] [Google Scholar]
- 9.Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30:1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Williams JE, Nieto FJ, Sanford CP, Couper DJ, Tyroler HA. The association between trait anger and incident stroke risk: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke; a journal of cerebral circulation. 2002;33:13–9. doi: 10.1161/hs0102.101625. [DOI] [PubMed] [Google Scholar]
- 11.Bratter JL, Gorman BK. Is discrimination an equal opportunity risk?: racial experiences, socioeconomic status, and health status among black and white adults. J Health Soc Behavior. doi: 10.1177/0022146511405336. [DOI] [PubMed] [Google Scholar]
- 12.Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: findings from community studies. American journal of public health. 2003;93:200–8. doi: 10.2105/ajph.93.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krieger N. Racial and gender discrimination: risk factors for high blood pressure? Social science & medicine. 1990;30:1273–81. doi: 10.1016/0277-9536(90)90307-e. [DOI] [PubMed] [Google Scholar]
- 14.Ro AE, Choi KH. Social status correlates of reporting gender discrimination and racial discrimination among racially diverse women. Women & health. 2009;49:1–15. doi: 10.1080/03630240802694756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shelton RC, Puleo E, Bennett GG, McNeill LH, Sorensen G, Emmons KM. The association between racial and gender discrimination and body mass index among residents living in lower-income housing. Ethnicity & disease. 2009;19:251–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Borrell C, Muntaner C, Gil-Gonzalez D, Artazcoz L, Rodriguez-Sanz M, Rohlfs I, Perez K, Garcia-Calvente M, Villegas R, Alvarez-Dardet C. Perceived discrimination and health by gender, social class, and country of birth in a Southern European country. Preventive medicine. 2010;50:86–92. doi: 10.1016/j.ypmed.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Hahm HC, Ozonoff A, Gaumond J, Sue S. Perceived discrimination and health outcomes a gender comparison among Asian-Americans nationwide. Women’s health issues : official publication of the Jacobs Institute of Women’s Health. 2010;20:350–8. doi: 10.1016/j.whi.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brodish AB, Cogburn CD, Fuller-Rowell TE, Peck S, Malanchuk O, Eccles JS. Perceived Racial Discrimination as a Predictor of Health Behaviors: the Moderating Role of Gender. Race and social problems. 2011;3:160–9. doi: 10.1007/s12552-011-9050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham TJ, Seeman TE, Kawachi I, Gortmaker SL, Jacobs DR, Kiefe CI, Berkman LF. Racial/ethnic and gender differences in the association between self-reported experiences of racial/ethnic discrimination and inflammation in the CARDIA cohort of 4 US communities. Social science & medicine. 2012;75:922–31. doi: 10.1016/j.socscimed.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry BL, Harp KL, Oser CB. Racial and Gender Discrimination in the Stress Process: Implications for African American Women’s Health and Well-Being. Sociological perspectives : SP : official publication of the Pacific Sociological Association. 2013;56:25–48. [PMC free article] [PubMed] [Google Scholar]
- 21.Kim IH, Noh S. Ethnic and gender differences in the association between discrimination and depressive symptoms among five immigrant groups. Journal of immigrant and minority health/Center for Minority Public Health. 2014;16:1167–75. doi: 10.1007/s10903-013-9969-3. [DOI] [PubMed] [Google Scholar]
- 22.McDonald JA, Terry MB, Tehranifar P. Racial and gender discrimination, early life factors, and chronic physical health conditions in midlife. Women’s health issues : official publication of the Jacobs Institute of Women’s Health. 2014;24:e53–9. doi: 10.1016/j.whi.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otiniano Verissimo AD, Gee GC, Ford CL, Iguchi MY. Racial discrimination, gender discrimination, and substance abuse among Latina/os nationwide. Cultural diversity & ethnic minority psychology. 2014;20:43–51. doi: 10.1037/a0034674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otiniano Verissimo AD, Grella CE, Amaro H, Gee GC. Discrimination and substance use disorders among Latinos: the role of gender, nativity, and ethnicity. American journal of public health. 2014;104:1421–8. doi: 10.2105/AJPH.2014.302011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brondolo E, Monge A, Agosta J, Tobin JN, Cassells A, Stanton C, Schwartz J. Perceived ethnic discrimination and cigarette smoking: examining the moderating effects of race/ethnicity and gender in a sample of Black and Latino urban adults. Journal of behavioral medicine. 2015;38:689–700. doi: 10.1007/s10865-015-9645-2. [DOI] [PubMed] [Google Scholar]
- 26.Ikram UZ, Snijder MB, Agyemang C, Schene AH, Peters RJ, Stronks K, Kunst AE. Perceived Ethnic Discrimination and the Metabolic Syndrome in Ethnic Minority Groups: The Healthy Life in an Urban Setting Study. Psychosom Med. 2017;79:101–11. doi: 10.1097/PSY.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 27.Owens SL, Hunte HE, Sterkel A, Johnson DA, Johnson-Lawrence V. Association Between Discrimination and Objective and Subjective Sleep Measures in the Midlife in the United States Study Adult Sample. Psychosom Med. 2017 doi: 10.1097/PSY.0000000000000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kemp AH, Koenig J, Thayer JF, Bittencourt MS, Pereira AC, Santos IS, Dantas EM, Mill JG, Chor D, Ribeiro AL, Bensenor IM, Lotufo PA. Race and Resting-State Heart Rate Variability in Brazilian Civil Servants and the Mediating Effects of Discrimination: An ELSA-Brasil Cohort Study. Psychosom Med. 2016;78:950–8. doi: 10.1097/PSY.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 29.Upchurch DM, Stein J, Greendale GA, Chyu L, Tseng CH, Huang MH, Lewis TT, Kravitz HM, Seeman T. A Longitudinal Investigation of Race, Socioeconomic Status, and Psychosocial Mediators of Allostatic Load in Midlife Women: Findings From the Study of Women’s Health Across the Nation. Psychosom Med. 2015;77:402–12. doi: 10.1097/PSY.0000000000000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dailey AB, Kasl SV, Jones BA. Does gender discrimination impact regular mammography screening? Findings from the race differences in screening mammography study. Journal of women’s health. 2008;17:195–206. doi: 10.1089/jwh.2006.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis. 2010;20:267–75. [PMC free article] [PubMed] [Google Scholar]
- 32.Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Social science & medicine. 2005;61:1576–96. doi: 10.1016/j.socscimed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Williams DR. Measuring Discrimination Resource. 2012 http://scholar.harvard.edu/files/davidrwilliams/files/measuring_discrimination_resource_feb_2012_0_0.pdf.
- 34.Essed P. Understanding everyday racism. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 35.Williams DR, Yan Y, Jackson JS, Anderson NB. Racial Differences in Physical and Mental Health: Socio-economic Status, Stress and Discrimination. Journal of health psychology. 1997;2:335–51. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- 36.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peralta CA, Vittinghoff E, Bansal N, Jacobs D, Jr, Muntner P, Kestenbaum B, Lewis C, Siscovick D, Kramer H, Shlipak M, Bibbins-Domingo K. Trajectories of kidney function decline in young black and white adults with preserved GFR: results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Kidney Dis. 2013;62:261–6. doi: 10.1053/j.ajkd.2013.01.012. Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy aging in neighborhoods of diversity across the life span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethnicity & disease. 2010;20:267–75. [PMC free article] [PubMed] [Google Scholar]
- 39.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart Lung and Blood Institute, Joint National Committee on Prevention Detection Evaluation and Treatment of High Blood Pressure, National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA : the journal of the American Medical Association. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 40.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977:1. [Google Scholar]
- 41.Beydoun MA, Fanelli Kuczmarski MT, Beydoun HA, Hibbeln JR, Evans MK, Zonderman AB. omega-3 fatty acid intakes are inversely related to elevated depressive symptoms among United States women. The Journal of nutrition. 2013;143:1743–52. doi: 10.3945/jn.113.179119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beydoun MA, Beydoun HA, Kitner-Triolo MH, Kaufman JS, Evans MK, Zonderman AB. Thyroid hormones are associated with cognitive function: moderation by sex, race, and depressive symptoms. The Journal of clinical endocrinology and metabolism. 2013;98:3470–81. doi: 10.1210/jc.2013-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heckman JJ. Sample selection bias as a specification error. Econometrica. 1979;47:153–61. [Google Scholar]
- 44.Landrine H, Klonoff EA. African American Acculturation: Deconstructing Race and Reviving Culture. Thousand Oaks, CA: Sage; 1996. [Google Scholar]
- 45.Guidi J, Offidani E, Rafanelli C, Roncuzzi R, Sonino N, Fava GA. The Assessment of Allostatic Overload in Patients with Congestive Heart Failure by Clinimetric Criteria. Stress and health : journal of the International Society for the Investigation of Stress. 2014 doi: 10.1002/smi.2579. [DOI] [PubMed] [Google Scholar]
- 46.McClellan WM, Newsome BB, McClure LA, Howard G, Volkova N, Audhya P, Warnock DG. Poverty and racial disparities in kidney disease: the REGARDS study. American journal of nephrology. 2010;32:38–46. doi: 10.1159/000313883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peralta CA, Katz R, DeBoer I, Ix J, Sarnak M, Kramer H, Siscovick D, Shea S, Szklo M, Shlipak M. Racial and ethnic differences in kidney function decline among persons without chronic kidney disease. Journal of the American Society of Nephrology : JASN. 2011;22:1327–34. doi: 10.1681/ASN.2010090960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.United States Renal Data System: USRDS. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda: 2013. [Google Scholar]
- 49.Kramer H, Palmas W, Kestenbaum B, Cushman M, Allison M, Astor B, Shlipak M. Chronic kidney disease prevalence estimates among racial/ethnic groups: the Multi-Ethnic Study of Atherosclerosis. Clinical journal of the American Society of Nephrology : CJASN. 2008;3:1391–7. doi: 10.2215/CJN.04160907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3–5 in the United States. Am J Kidney Dis. 2013;62:245–52. doi: 10.1053/j.ajkd.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of psychosomatic research [Review] 2002;53:865–71. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 52.Szanton SL, Rifkind JM, Mohanty JG, Miller ER, 3rd, Thorpe RJ, Nagababu E, Epel ES, Zonderman AB, Evans MK. Racial discrimination is associated with a measure of red blood cell oxidative stress: a potential pathway for racial health disparities. International journal of behavioral medicine. 2012;19:489–95. doi: 10.1007/s12529-011-9188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 54.Kooman JP, Kotanko P, Schols AM, Shiels PG, Stenvinkel P. Chronic kidney disease and premature ageing. Nature reviews Nephrology. 2014;10:732–42. doi: 10.1038/nrneph.2014.185. [DOI] [PubMed] [Google Scholar]
- 55.Saban KL, Mathews HL, DeVon HA, Janusek LW. Epigenetics and social context: implications for disparity in cardiovascular disease. Aging and disease [Review] 2014;5:346–55. doi: 10.14336/AD.2014.0500346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gemmell LA, Terhorst L, Jhamb M, Unruh M, Myaskovsky L, Kester L, Steel J. Gender and Racial Differences in Stress, Coping, and Health-Related Quality of Life in Chronic Kidney Disease. Journal of pain and symptom management. 2016 doi: 10.1016/j.jpainsymman.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanders-Phillips K, Settles-Reaves B, Walker D, Brownlow J. Social inequality and racial discrimination: risk factors for health disparities in children of color. Pediatrics. 2009;124(Suppl 3):S176–86. doi: 10.1542/peds.2009-1100E. [DOI] [PubMed] [Google Scholar]
- 58.Ahmed AT, Mohammed SA, Williams DR. Racial discrimination & health: pathways & evidence. The Indian journal of medical research. 2007;126:318–27. [PubMed] [Google Scholar]
- 59.Cuffee YL, Hargraves JL, Rosal M, Briesacher BA, Schoenthaler A, Person S, Hullett S, Allison J. Reported racial discrimination, trust in physicians, and medication adherence among inner-city African Americans with hypertension. American journal of public health. 2013;103:e55–62. doi: 10.2105/AJPH.2013.301554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Casagrande SS, Gary TL, LaVeist TA, Gaskin DJ, Cooper LA. Perceived discrimination and adherence to medical care in a racially integrated community. Journal of general internal medicine. 2007;22:389–95. doi: 10.1007/s11606-006-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynolds DB, Walker RJ, Campbell JA, Egede LE. Differential effect of race, education, gender, and language discrimination on glycemic control in adults with type 2 diabetes. Diabetes technology & therapeutics. 2015;17:243–7. doi: 10.1089/dia.2014.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klassen AC, Hall Ag, Saksvig B, Curbow B, Klassen DK. Relationship between patients’ perceptions of disadvantage and discrimination and listing for kidney transplantation. American journal of public health. 2002;92:811–7. doi: 10.2105/ajph.92.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodriguez CJ, Gwathmey TM, Jin Z, Schwartz J, Beech BM, Sacco RL, Di Tullio MR, Homma S. Perceived Discrimination and Nocturnal Blood Pressure Dipping Among Hispanics: The Influence of Social Support and Race. Psychosom Med. 2016;78:841–50. doi: 10.1097/PSY.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.