Abstract
Objectives
The objective of this study was to describe the outcomes of patients in the University of Iowa (UI) Neuroendocrine Tumor (NET) Database treated with peptide receptor radionuclide therapy (PRRT).
Methods
135 patients from the UI NET Database who received PRRT were analyzed, their characteristics described and survival calculated.
Results
The median age at diagnosis was 51 years and 64% were men. The primary tumor was located in the small bowel (SBNET) in 37.8%, the pancreas (PNET) in 26.0%, the lung in13.3%, unknown primary in 9.6%, and other sites in 13.3 %. A radiographic response of any magnitude was observed in 65.8%, 11.1% had a mixed response, and 15.4% showed progression. The overall survival (OS) from first PRRT was 40 months and the median time to progression (TTP) was 23.9 months. Higher pre-treatment chromogranin A and pancreastatin levels predicted inferior OS.
Conclusions
PRRT resulted in a relatively long OS and TTP in heavily pretreated North American patients with advanced NETs. Elevated pre-treatment chromogranin A and pancreastatin predicted shorter OS after therapy. PRRT is a valuable treatment options in patients with advanced NETs, especially SBNETS.
Keywords: Peptide receptor radionuclide therapy, PRRT, neuroendocrine tumors, therapy, chromogranin A, pancreastatin
Introduction
Neuroendocrine tumors (NETs) encompass a spectrum of malignancies that arise from neuroendocrine cells in multiple organs of the body. NETs constitute a challenging clinical problems as these tumors frequently present with metastatic disease that require unique diagnostic and treatment approaches different from other commonly occurring cancers.1 The percentage of patients with metastatic disease at diagnosis varies greatly among studies but a recent large population-based study showed that 21% of NET patients present with metastatic disease and another 38% develop metastases after resection of the primary tumor.2 There is, therefore, a great need for effective systemic therapy for advanced NETs. The current treatment options for metastatic neuroendocrine tumors include surgical resection of both the primary tumor and metastatic lesions in those patients who have resectable tumors.3,4 For patients with liver-dominant disease, liver directed therapy such as microwave or radiofrequency ablation and hepatic artery embolization can be utilized.5 Options for systemic therapy include biotherapy with somatostatin analogues or interferons, targeted therapy with agents such as inhibitors of the mammalian target of rapamycin (mTOR) pathway and kinase inhibitors, cytotoxic chemotherapy, and peptide receptor radionuclide therapy (PRRT).6,7
Peptide receptor radionuclide therapy (PRRT) with radiolabeled somatostatin analogues relies on the expression of somatostatin receptors by neuroendocrine tumor cells, which allows delivery of a cytotoxic radiolabeled compound directly to the tumor.8 The cytotoxic effects are via the ß emission of the radioisotope.
Two different radiolabels are most frequently used: analogues labeled with 90Yttrium (90Y), or analogues labeled with 177Lutetium (177Lu).9 There are differences in the characteristic of the isotopes where 90Y has greater beta energy and tissue penetration than 177Lu. 90Y may therefore have greater activity against larger tumors but also possibly higher toxicity, especially nephrotoxicity. 90Y has been examined in at least 10 studies involving more than 440 patients with NETs of various origins, and these treatments resulted in complete tumor response occurring in 0–6 % of the patients, partial tumor regression in 7–37 %, and tumor stabilization (i.e., no tumor growth) in 42–86 %.10–13 Results of therapy with 177Lu have been reported in over 510 patients with various NETs (including 40 % with pancreatic NETs), with a complete response seen in 2 %, partial tumor regression in 28 %, minor tumor response in 16 %, and tumor stabilization in 35 %.14,15 A recent study of sequential therapy with, [90Y-DOTA]-TOC followed by [177Lu-DOTA]-TOC showed improved overall survival compared with [90Y-DOTA]-TOC alone in patients completing three or more cycles of treatment.16 In these trials, PRRT was well tolerated and had acceptable toxicity.
A randomized, multinational phase III trial comparing treatment with 177 Lu-DOTA-TYR-Octreotate to high-dose octreotide LAR (60 mg intramuscularly monthly) in patients with inoperable NETs (NETTER-1) has completed accrual and preliminary results were recently presented.17 This large phase III trial comparing PRRT to systemic therapy showed a substantial prolongation of progression-free survival (PFS) in patients receiving PRRT compared to patients receiving high-dose octreotide LAR. The PFS of patients on the high-dose octreotide LAR reference arm was 8.4 months while the PFS was not reached among the patients on the PRRT arm (p<0.0001).17
In this retrospective study, we report patient characteristics, treatment responses and survival outcomes as well analyze the predictive role of tumor markers in a University of Iowa cohort of PRRT-treated well differentiated NET patients.
Materials and Methods
A retrospective analysis of patients with metastatic neuroendocrine tumors undergoing PRRT between 2001 and 2011 was performed. Data was collected from the IRB approved University of Iowa Neuroendocrine Tumor Registry. The registry data included patient demographics and other patient data (such as age at diagnosis, sex, history of smoking, alcohol use and symptoms at the time of diagnosis), tumor specific data, and treatment related information. Tumor data included tumor site, tumor grade, stage, presence of tumor necrosis, number of mitoses and percentage of Ki-67 and MIB-1 positive cells (proliferative index). Treatment data included use of somatostatin analogs at the time of PRRT, location, isotope used and dose of isotope for each PRRT. Biomarker data (chromogranin A and pancreastatin) at the time of diagnosis, before and after the first PRRT, and after the second PRRT were also extracted. Radiographic findings prior to PRRT, radiographic response after PRRT, date of radiographic progression on imaging after PRRT, and status of disease on imaging at the last follow-up were also recorded. Overall survival (OS) was defined as the time from diagnosis to death of any cause. Time to progression (TTP) was defined as the time from the first PRRT until any radiographic progression. Radiographic treatment responses and progression were assessed with cross-sectional imaging with either computerized tomography (CT) or magnetic resonance imaging (MRI). Response was defined as any response of any magnitude. Radiographic progression was defined as any increase in lesion sizes and/or appearance of new metastatic lesions.
Statistical Analysis
Kaplan-Meier curves were constructed by tumor site for overall survival (OS) from diagnosis and from the first PRRT. Time to progression (TTP) was defined as the time from first PRRT until first evidence of any radiographic progression. Overall survival and progression rates were compared between primary tumor sites using the log-rank test. The association of pre-PRRT chromogranin A and pancreastatin levels with overall survival and time to progression was examined using Cox proportional hazard regression with age as a covariate. For this analysis, chromogranin A and pancreastatin levels were expressed as a proportion of the upper limit of the normal reference range (ULN), categorized into 3 intervals, <3 times normal, 3–10 times normal value, and >10 times normal value. Hazard ratio of death and hazard ratio of progression (with 95% confidence interval) are reported for the 2 upper levels relative to the lowest level computed from the fitted Cox model.
Results
Data from 135 patients from the University of Iowa NET Database were analyzed. The majority of the PRRT were performed at the University of Basel, Switzerland and the University of Iowa, USA. Patient characteristics are shown in Table 1. 64% of the patients were men and the median age at diagnosis was 51 years. The primary tumor was located in the small bowel in 38% of patients, the pancreas in 26%, lungs in 13% and other locations or unknown primary in the remainder of the patients. 90Y was used in 112 patients (83.2%) and 177Lu in 23 patients (16.8%). A second PRRT was given to 116 patients (86%). The median dose of radiation was 180 mCi (range: 7.6–360) for the first and 170 mCi (range: 7.5–360) for the second PRRT treatment.
Table 1.
Baseline patient characteristics of the 135 patients treated with PRRT.
| Patient characteristics | ||
|---|---|---|
|
| ||
| Age | Median Age | 51 years (range 18 – 70 years |
|
| ||
| Sex | Males | 64% |
| Females | 36% | |
|
| ||
| Primary Tumor Site | Small bowel (SBNET) | 37.8% |
| Pancreas (PNET) | 26.0% | |
| Lung | 13.3% | |
| Unknown Primary | 9.6% | |
| Other sites | 13.3% | |
|
| ||
| Type of PRRT (isotope) | 90Y | 83.2% |
| 177Lu | 15.3% | |
|
| ||
| Treatment Center | University of Basel | 68.8% |
| University of Iowa | 25.5% | |
| Other centers | 6% | |
Assessment of radiographic responses within the first 6 months after PRRT showed a response of any magnitude in 65.8% of patients, 11.1% had a mixed response defined as areas of both regression and progression, and 15.4% had progressive disease. The median overall survival (OS) from date of diagnosis for all patients was 122.7 months (interquartile range [IQR] 58.7–223.1 months), and the median OS from date of the first PRRT was 40.0 months (IQR 18.5–97.7 months). The median time to progression (TTP) after the first PRRT was 23.9 months (IQR 13.6–83.5 months). Median OS and TTP by tumor site are presented in Table 2. Overall survival from the date of diagnosis was significantly shorter for PNET (68.4 months) and unknown primary (64.5months) than SBNET (199.3 months) (Table 2). Similarly, overall survival after the first PRRT was 95.4 months, 37.3 months and 20 months for small bowel, pancreatic and unknown primary NETs respectively (p=0.009). Time to progression from the first PRRT was shorter for those with primary pulmonary NET (median 18.6 months) compared to SBNET and PNET, but was not statistically significant (p=0.093)
Table 2.
Median (25th – 75th percentile), overall survival (OS) from diagnosis, overall survival from the first PRRT and time to progression (TTP) by site (all survival values in months).
| Site | Overall survival from diagnosis date |
Overall survival from the first PRRT |
Time to progression from the first PRRT |
|---|---|---|---|
| All Sites | 122.7 (58.7 – 223.1) | 40.0 (18.5–97.7) | 23.9 (11.0–60.8) |
| SBNETs | 199.3 (117.2 – 265.9) | 95.4 (18.6–>118.8) | 27.2 (17.4–83.5) |
| PNETs | 68.4 (49.6 – 142.7) | 37.3 (18.1–48.0) | 37.0 (6.5–48.0) |
| Lung | 143.3 (52.1 – >191.2) | 32.4 (18.4–>137.6) | 18.6 (7.5–61.9) |
| Unknown primary | 64.5 (29.8–89.2) | 20.4 (17.2–29.2) | 20.0 (10.6–29.2) |
| Other | 85.5 (72.4–216.4) | 55.9 (40.0–88.9) | 13.7 (11.0–43.4) |
Results of pre-treatment chromogranin A (CgA) measurements were available for 81 patients (60%) and 80 patients (59%) had pre-treatment results for pancreastatin (PcSt). The tumor markers were expressed as proportion of the upper limit of the normal (ULN) reference range (Table 3) to account for differences in reference ranges for the markers over time and different laboratories utilized. The median relative CgA prior to PRRT was 3.0 times the ULN (IQR: 0.7–14.6). The median relative pre-PRRT PcSt level was 4.5 times the ULN (IQR: 1.4–24.5). The distribution of pre PRRT CgA and PcSt relative to the ULN of the reference value are presented in Table 3. A 50% or more decrease in CgA and PcSt was observed in 18% and 17% respectively.
Table 3.
Distribution of Pre PRRT CgA and PcSt relative to the upper limit of normal (ULN) of the reference range
| Ratio relative to normal | Pre PRRT CgA (n=81) Count (%) |
Pre PRRT PcSt (n=80) Count (%) |
|---|---|---|
| ≤1.0 | 26 (32%) | 16 (20%) |
| >1.0–<3.0 | 14 (17%) | 19 (24%) |
| 3.0–10.0 | 17 (21%) | 14 (17%) |
| >10.0 | 24 (30%) | 31 (39%) |
The association of pre-PRRT CgA and PcSt levels with OS and TTP after the first PRRT was examined using Cox proportional hazard regression with age as covariate. A significant association of elevated levels of pre-PRRT CgA (p=0.007) and pre-PRRT PcSt (p=0.033) with death (OS) was observed. Relative to those with CgA <3 times ULN, the hazard ratio (HR) of death, adjusted for age, was 2.81 (95% CI: 1.04 – 7.59; p=0.042) for those with CgA 3–10 times normal level, and 4.42 (95% CI: 1.72 – 11.34; p=0.002) for those with CgA >10 times normal. In regards to PcSt, the HR of death was 2.91 (95% CI: 1.20 – 7.08; p=0.018) for those with PcSt >10 times ULN relative to those with PcSt <3 times ULN. There was no significant difference in OS after the first PRRT between those with PcSt 3–10 times normal level and those with PcSt <3 times normal (HR: 0.97; 95% CI: 0.26 – 3.64; p=0.961). The Kaplan-Meier curves for OS after the first PRRT by level of CgA and PcSt are shown in Figures 1 and 2, respectively.
Fig 1.
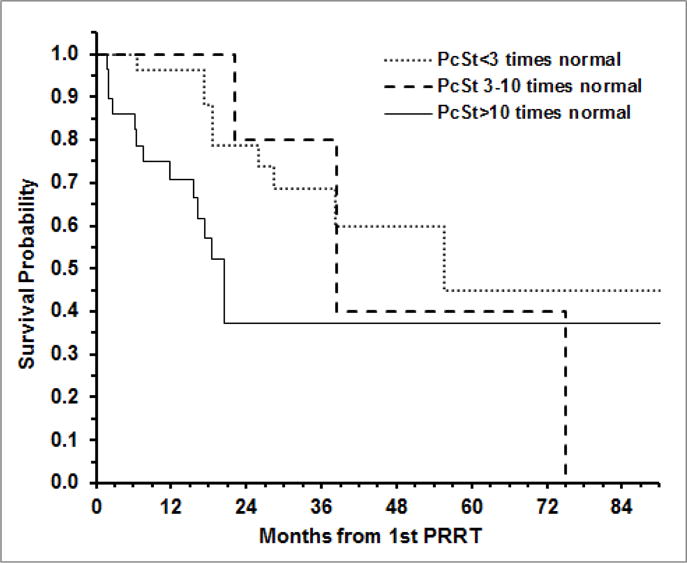
Overall survival after the first PRRT according to pancreastatin (PcSt) levels at the time of therapy.
Fig 2.
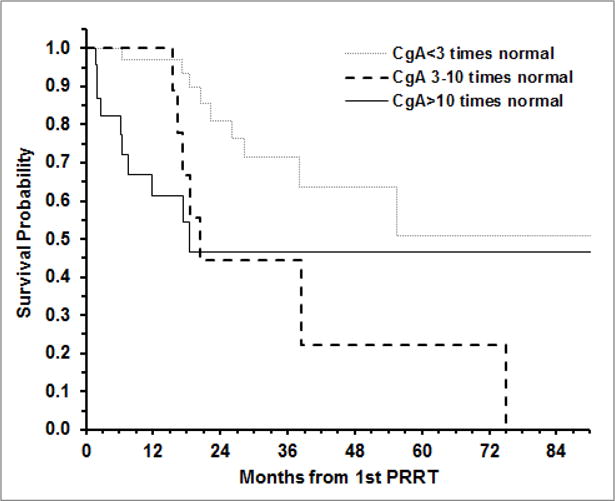
Overall survival after the first PRRT according to chromogranin A (CgA) levels at the time of therapy.
Cox hazard regression modeling for progression after the first PRRT showed no significant association with pre PRRT CgA levels (p=0.19) or pre PRRT PcSt levels (p=0.468).
Discussion
PRRT using radiolabeled somatostatin analogs is a promising option for patients with metastatic or inoperable somatostatin receptor-positive neuroendocrine tumors. Our results of PRRT in a cohort of North American patients suggest that this therapy is feasible and effective in this population with an encouraging survival, even in heavily pre-treated patients. Despite extensive use of PRRT in patients with advanced NETs in the past, the effect of PRRT on survival remains unclear.12 The heterogeneity of the published studies in terms of the radionuclide used and the patient populations studied, makes comparison across studies difficult. A wide range of survival following PRRT has been reported in the literature and the populations studied have differed in their characteristics such as the extent of previous therapy.10,14,16,18–24 Most of the reported studies have evaluated response rates but the association of radiographic response with OS and PFS remains uncertain following PRRT. One study reported a strong and significant association between radiographic response rates and overall survival with responders having a superior median OS (44.7 vs, 18.3 months).10 The preliminary results of the first randomized trial comparing PRRT with systemic therapy (high-dose octreotide LAR) were reported in late 2015 and the findings suggested a clinically significant improvement in progression-free survival.
The observed OS from date of diagnosis in both SBNETs and PNETs in our study was high but similar survival from the time of initial diagnosis has been reported by other investigators.14,25 The OS from diagnosis in patients with PNETs was substantially longer than has been reported in population-based studies.26 The long survival of patients with PNETs may be explained by factors such as wider range of treatment options in patients treated at large referral centers and also potential bias among patients referred to such centers.27 The prolonged OS from diagnosis may be a result of patients being extensively treated prior to PRRT in a multidisciplinary setting using a combination of systemic and locoregional therapy. The median overall survival from the first PRRT in our study was 40 months for all patients. Patients with SBNET had a longer OS after the first PRRT compared with patients with NETs of other primary sites, possibly owing to a more indolent nature of SBNETs. The reported OS after PRRT in other studies varies widely, likely in part as a reflection of the different populations studied and different treatments used prior to and after the PRRT. In our study, the time to progression after PRRT was comparable to the results of other published studies.
For example, a study of PRRT in patients with gastroenteropancreatic NETs, the PFS was 29 months the patients who had at least stable disease at the end of the treatment period.28 A retrospective study of 69 Danish NET patients treated with PRRT reported a progression-free survival (PFS) of 29 months.29 Contrary to our findings, patients with SBNETs in that study had a longer PFS than patients with PNETs. Patients with SBNETs had a very long OS from diagnosis or 199 months. This points towards a more indolent growth of this particular NET type and has been observed in other studies.
PRRT was used relatively late in the disease course in our cohort of patients and many were heavily pretreated. Prospective studies are needed to determine if early use of PRRT is beneficial in improving OS.
Despite the inferior OS of the patients with PNETs, the TTP for of PNETs patients after PRRT was better than the combined TTP of NETs of all other sites. Even though the TTP after PRRT in patients with PNETs was longer than in patients with SBNETs, the overall survival was substantially shorter. This may reflect a more indolent course of SBNETs when compared to PNETs.
Very limited data exists on the predictive role of circulating tumor markers in patients with NETs treated with PRRT. In our study, elevated chromogranin A prior to PRRT was associated with inferior OS from the first PRRT but no statistically significant association with TTP. Very elevated pancreastatin (>10 times ULN) was also associated with inferior OS. Two studies have reported on inferior OS in patients treated with PRRT who have elevated pre-treatment levels of neuron-specific enolase (NSE).18,19 Similar results have been seen in patients receiving systemic therapy but both CgA and NSE have been shown to have prognostic value in patient treated with everolimus where higher levels predict inferior survival.30,31 An association between tumor markers and outcome has also been seen after hepatic artery embolization where elevated pancreastatin predicts inferior outcome.32,33
There are limitations to this study, many of which are inherent to retrospective studies. The population studied as well as the treatment given prior to PRRT was heterogeneous. There is a potential for selection bias at several levels. The patients were referred to the University of Iowa which is a large tertiary medical center with extensive experience in the management of patients with NETs. The patients who were able to come for an evaluation may not accurately reflect the larger group of NET patients as younger and more fit patients may be more likely to travel for a second opinion. Indeed, studies have shown a better outcome for patients with PNETs treated at large referral centers compared with patients identified in registries.27 Furthermore, the majority of our patients was treated at the University of Basel in Switzerland. This could have potentiated the selection bias as the patients fit and able for international travel, may differ in several characteristics known to affect cancer treatment outcomes such as age, performance status and socioeconomic status. Another limitation of our study is the lack of formal assessment of radiographic responses according to commonly used response criteria such as RECIST. This can certainly be considered a limitation knowing that patients responding to PRRT seem to have an improved outcome.10 On the other hand, one can argue that radiographic responses are not an endpoint as clinically relevant as overall survival or time to progression, both primary endpoints in our study. Indeed, there is increasing literature on the limitations of objective response rates in cancer patients, especially those not treated with cytotoxic chemotherapy.34
Conclusion
North American patients with advanced NETs have a relatively long survival and PRRT was used late in the disease course. PRRT appears to be a valuable treatment option for advanced NETs regardless of the location of the primary tumor. Its role earlier in the disease course needs further investigation.
Elevated CgA at the time of diagnosis of NETs is prognostic as has been demonstrated in literature. There is a trend towards shorter TTP in patients with elevated pre-PRRT markers.
Prospective studies are ongoing to understand the role of PRRT in treatment of metastatic neuroendocrine tumors.
Acknowledgments
Funding Source: National Institute Health Neuroendocrine Special Program of Research Excellence Grant # P50 CA 174521
Footnotes
Disclosures: The authors declare no conflict of interest.
References
- 1.Oberg K. Neuroendocrine tumors of the digestive tract: impact of new classifications and new agents on therapeutic approaches. Curr Opin Oncol. 2012;24:433–440. doi: 10.1097/CCO.0b013e328353d7ba. [DOI] [PubMed] [Google Scholar]
- 2.Hallet J, Law CH, Cukier M, et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589–597. doi: 10.1002/cncr.29099. [DOI] [PubMed] [Google Scholar]
- 3.Knigge U, Hansen CP. Surgery for GEP-NETs. Best Pract Res Clin Gastroenterol. 2012;26:819–831. doi: 10.1016/j.bpg.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Pathak S, Dash I, Taylor MR, et al. The surgical management of neuroendocrine tumour hepatic metastases. Eur J Surg Oncol. 2013;39:224–228. doi: 10.1016/j.ejso.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Mayo SC, Herman JM, Cosgrove D, et al. Emerging approaches in the management of patients with neuroendocrine liver metastasis: role of liver-directed and systemic therapies. J Am Coll Surg. 2013;216:123–134. doi: 10.1016/j.jamcollsurg.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavel M, Kidd M, Modlin I. Systemic therapeutic options for carcinoid. Semin Oncol. 2013;40:84–99. doi: 10.1053/j.seminoncol.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Ito T, Igarashi H, Jensen RT. Therapy of metastatic pancreatic neuroendocrine tumors (pNETs): recent insights and advances. J Gastroenterol. 2012;47:941–960. doi: 10.1007/s00535-012-0642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Vliet EI, Teunissen JJ, Kam BL, et al. Treatment of gastroenteropancreatic neuroendocrine tumors with peptide receptor radionuclide therapy. Neuroendocrinology. 2013;97:74–85. doi: 10.1159/000335018. [DOI] [PubMed] [Google Scholar]
- 9.Bodei L, Mueller-Brand J, Baum RP, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800–816. doi: 10.1007/s00259-012-2330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29:2416–2423. doi: 10.1200/JCO.2010.33.7873. [DOI] [PubMed] [Google Scholar]
- 11.Bodei L, Cremonesi M, Kidd M, et al. Peptide Receptor Radionuclide Therapy for Advanced Neuroendocrine Tumors. Thorac Surg Clin. 2014;24:333–349. doi: 10.1016/j.thorsurg.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Gulenchyn KY, Yao X, Asa SL, et al. Radionuclide therapy in neuroendocrine tumours: a systematic review. Clin Oncol (R Coll Radiol) 2012;24:294–308. doi: 10.1016/j.clon.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 13.van Essen M, Krenning EP, Kam BL, et al. Peptide-receptor radionuclide therapy for endocrine tumors. Nat Rev Endocrinol. 2009;5:382–393. doi: 10.1038/nrendo.2009.105. [DOI] [PubMed] [Google Scholar]
- 14.Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 15.Forrer F, Valkema R, Kwekkeboom DJ, et al. Neuroendocrine tumors. Peptide receptor radionuclide therapy. Best Pract Res Clin Endocrinol Metab. 2007;21:111–129. doi: 10.1016/j.beem.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Villard L, Romer A, Marincek N, et al. Cohort study of somatostatin-based radiopeptide therapy with [(90)Y-DOTA]-TOC versus [(90)Y-DOTA]-TOC plus [(177)Lu-DOTA]-TOC in neuroendocrine cancers. J Clin Oncol. 2012;30:1100–1106. doi: 10.1200/JCO.2011.37.2151. [DOI] [PubMed] [Google Scholar]
- 17.Strosberg JR, Wolin E, Chasen B, et al. 177-Lu-Dotatate significantly improves progression-free survival in patients with midgut neuroendocrine tumours: Results of the phase III NETTER-1 trial (6LBA). Presented at the 2015 European Cancer Congress in Vienna; Austria. 2015. [Google Scholar]
- 18.Ezziddin S, Khalaf F, Vanezi M, et al. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2014;41:925–933. doi: 10.1007/s00259-013-2677-3. [DOI] [PubMed] [Google Scholar]
- 19.Ezziddin S, Attassi M, Yong-Hing CJ, et al. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 2014;55:183–190. doi: 10.2967/jnumed.113.125336. [DOI] [PubMed] [Google Scholar]
- 20.Romer A, Seiler D, Marincek N, et al. Somatostatin-based radiopeptide therapy with [177Lu-DOTA]-TOC versus [90Y-DOTA]-TOC in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2014;41:214–222. doi: 10.1007/s00259-013-2559-8. [DOI] [PubMed] [Google Scholar]
- 21.Kunikowska J, Krolicki L, Sowa-Staszczak A, et al. Polish experience in Peptide receptor radionuclide therapy. Recent Results Cancer Res. 2013;194:467–478. doi: 10.1007/978-3-642-27994-2_26. [DOI] [PubMed] [Google Scholar]
- 22.Cwikla JB, Sankowski A, Seklecka N, et al. Efficacy of radionuclide treatment DOTATATE Y-90 in patients with progressive metastatic gastroenteropancreatic neuroendocrine carcinomas (GEP-NETs): a phase II study. Ann Oncol. 2010;21:787–794. doi: 10.1093/annonc/mdp372. [DOI] [PubMed] [Google Scholar]
- 23.Bushnell DL, Jr, O’Dorisio TM, O’Dorisio MS, et al. 90Y-edotreotide for metastatic carcinoid refractory to octreotide. J Clin Oncol. 2010;28:1652–1659. doi: 10.1200/JCO.2009.22.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savelli G, Bertagna F, Franco F, et al. Final results of a phase 2A study for the treatment of metastatic neuroendocrine tumors with a fixed activity of 90Y-DOTA-D-Phe1-Tyr3 octreotide. Cancer. 2012;118:2915–2924. doi: 10.1002/cncr.26616. [DOI] [PubMed] [Google Scholar]
- 25.Horsch D, Ezziddin S, Haug A, et al. Peptide receptor radionuclide therapy for neuroendocrine tumors in Germany: first results of a multi-institutional cancer registry. Recent Results Cancer Res. 2013;194:457–465. doi: 10.1007/978-3-642-27994-2_25. [DOI] [PubMed] [Google Scholar]
- 26.Halfdanarson TR, Rabe KG, Rubin J, et al. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727–1733. doi: 10.1093/annonc/mdn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strosberg JR, Halfdanarson TR. Survival analyses of pancreatic neuroendocrine tumors: Contrasting institutional databases with population-based studies. Presented at the 2010 North American Neuroendocrine Tumor Society (NANETS) Meeting; Santa Fe, New Mexico. October 29–30, 2010. [Google Scholar]
- 28.Valkema R, Pauwels S, Kvols LK, et al. Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0,Tyr3]octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med. 2006;36:147–156. doi: 10.1053/j.semnuclmed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Pfeifer AK, Gregersen T, Gronbaek H, et al. Peptide receptor radionuclide therapy with Y-DOTATOC and (177)Lu-DOTATOC in advanced neuroendocrine tumors: results from a Danish cohort treated in Switzerland. Neuroendocrinology. 2011;93:189–196. doi: 10.1159/000324096. [DOI] [PubMed] [Google Scholar]
- 30.Yao JC, Pavel M, Phan AT, et al. Chromogranin A and neuron-specific enolase as prognostic markers in patients with advanced pNET treated with everolimus. J Clin Endocrinol Metab. 2011;96:3741–3749. doi: 10.1210/jc.2011-0666. [DOI] [PubMed] [Google Scholar]
- 31.Yao JC, Hainsworth JD, Wolin EM, et al. Multivariate analysis including biomarkers in the phase III RADIANT-2 study of octreotide LAR plus everolimus (E+O) or placebo (P+O) among patients with advanced neuroendocrine tumors (NET) J Clin Oncol 30, 2012 (suppl; abstr 4014) 2012 [Google Scholar]
- 32.Desai DC, O’Dorisio TM, Schirmer WJ, et al. Serum pancreastatin levels predict response to hepatic artery chemoembolization and somatostatin analogue therapy in metastatic neuroendocrine tumors. Regul Pept. 2001;96:113–117. doi: 10.1016/s0167-0115(00)00167-1. [DOI] [PubMed] [Google Scholar]
- 33.Bloomston M, Al-Saif O, Klemanski D, et al. Hepatic artery chemoembolization in 122 patients with metastatic carcinoid tumor: lessons learned. J Gastrointest Surg. 2007;11:264–271. doi: 10.1007/s11605-007-0089-z. [DOI] [PubMed] [Google Scholar]
- 34.Bradbury P, Seymour L. Tumor shrinkage and objective response rates: gold standard for oncology efficacy screening trials, or an outdated end point? Cancer J. 2009;15:354–360. doi: 10.1097/PPO.0b013e3181b9c506. [DOI] [PubMed] [Google Scholar]


