Abstract
Objectives
Patients with Amyotrophic Lateral Sclerosis (ALS) have expressed desire to become living organ donors but are unable to do so with current organ donation policies. Our objective is to assess ALS patient's interest in organ donation, and perceived concerns of this practice by ALS neurologists.
Patients and Methods
An electronic survey was administered to ALS neurologists across the United States regarding living organ donation in ALS patients prior to respiratory failure.
Results
52 complete responses were received from 121 invites. 67% (35/52) of neurologists expressed no concerns about living organ donation in ALS patients, and 33% had concerns. The concerns related to respiratory failure, anesthesia exposure and renal dysfunction. With their concerns addressed, 71% of neurologists reported that they would endorse living organ donation.
49% of neurologists reported being asked by a patient for information regarding living organ donation. ALS neurologists felt that 22.8% of ALS patients (median 19%) would be interested in learning more about organ donation, while only 6% of neurologists broach this subject with their patients.
Conclusion
Our results indicate that 1 in every 4 ALS patients may be interested in exploring options for living organ donation, and this topic is not routinely addressed by ALS clinics. These results indicate an unexplored area of patient interest. To honor a patient's wishes to donate, the transplant community will have to accommodate living organ donation from terminally ill patients, and address neurologist concerns. Such a practice could benefit two groups of patients.
Search terms: Amyotrophic lateral sclerosis, living organ donation, kidney donation
Introduction
Organ transplantation is a widely practiced intervention in patients with terminal organ failure since the first successful organ transplant in humans performed in 19541. In 2015 alone, 30,974 organs (17,898 kidneys) were transplanted in the United States2. During the same period, a staggering 119,778 patients remained on the United Network for Organ Sharing (UNOS) waiting list, including 99,238 patients waiting for kidneys2,3. The vast majority of organs (88%) are recovered from deceased donors, while a lesser percentage are from living organ donors. Given the need for transplantable organs and the higher quality of organs with living donation, there have been recent campaigns to increase living or “good Samaritan” organ donation, which is strongly supported by public opinion4. The current UNOS policy for living organ donation dictates that potential donors be adults in good overall physical and mental health, and thus excludes all potential donors with chronic illness5.
Recent media reports have highlighted multiple patients diagnosed with a terminal disease who have exhibited a strong desire to participate in living organ donation6–9. Of note, all of these patients carry the diagnosis of Amyotrophic Lateral Sclerosis (ALS). ALS is a fatal neurodegenerative condition in which the overwhelming majority of clinical disease is motor10. A small subset of patients have, to varying degrees, a frontotemporal lobe pattern of cognitive dysfunction, but the majority retain decision making capacity10. These patients face an incurable disease and expected mortality due to respiratory failure within 3 years of symptom onset, prolonged only with tracheostomy, ventilation and aggressive life support10,11. Many such patients find solace in the idea of organ donation to cure others with terminal disease, prior to respiratory failure and end of life6–9. However, living organ donation by ALS patients is not supported by transplant centers and federal policies, and to the author's knowledge has not been carried out.
We designed and administered a survey to ALS neurologists to assess the level of interest amongst them and their patients regarding living organ donation, prior to respiratory failure and end of life. We also enquired about potential concerns from the ALS neurologist community towards this idea.
Materials and Methods
An electronic survey was designed which consisted mostly of binary (yes/no) and multiple-choice style questions with some options for free response. The survey questionnaire is available in the supplementary appendix of this manuscript (supplement 1). Research Electronic Data Capture (Redcap™) hosted at the University of Utah, was used to implement the survey12. REDCap™ is a secure, web-based application designed to support research, data collection and manipulation.
The authors compiled a list of ALS neurologists in the United States and a survey invite was sent electronically. Survey respondents were informed that “living organ donation is defined as donation of a single kidney, while the donor is alive, and that organ recovery surgery involves general anesthesia and typically lasts 1-2 hours.” Survey responses were managed, and statistical calculations done using REDCap™ and Microsoft Excel™.
Results
132 survey invites were sent and with 11 returned emails (due to incorrect contact information), the potential the total respondent list was 121. A total of 52 responses were received and included in the final analysis, with a response rate of 42.9%. Respondents were asked to provide a postal code of the location of their ALS practice and this geographic data is presented in Figure 1.
Figure 1. Geographical distribution of ALS neurologists surveyed in the United States.
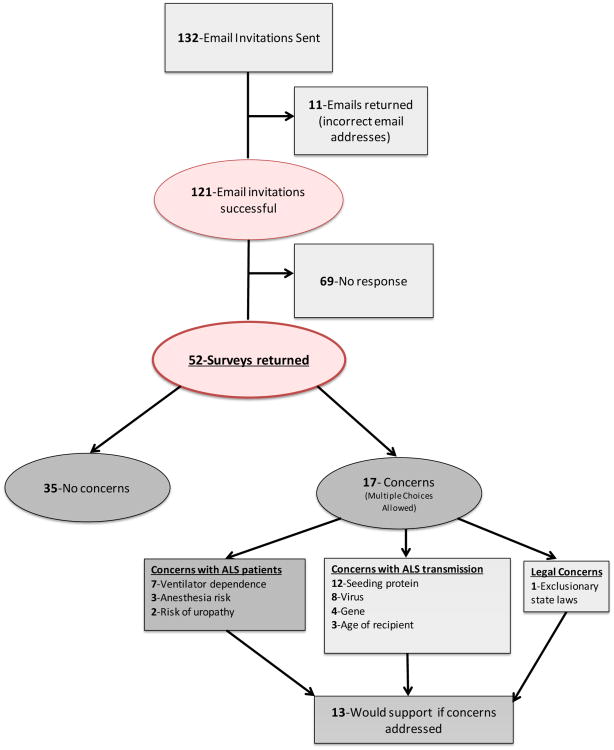
Graphical flowchart representation of the survey results is depicted in Figure 2. 5.9% (3 of 51) of ALS neurologists reported routinely discussing organ donation with their patients (deceased donation). 67.3% (35 of 52) reported no concerns with living organ donation in this patient population. The 32.7% (17 of 52) with concerns were asked to choose from different categories of concerns (more than one option could be chosen), as well as the ability to write in concerns. 9 of the 17 neurologists had concerns about the ALS patient/donor, 12 of the 17 had concerns related to the organ recipient, and 1 wrote a free response that stated that local state laws would exclude ALS patients from organ donation (the survey respondent was located in the state of Georgia).
Figure 2.
Flowchart depicting survey response rates and results.
Of the 9 neurologists (out of 17 with concerns) who reported safety concerns for the ALS donor patient, 7 opted to specify their concerns as: risks of prolonged ventilator dependence following anesthesia (7 of 7), exposure to anesthetic agents (3 of 7), and risk of uropathy or renal dysfunction with a single kidney (2 of 7).
Within the12 neurologists (out of 17) that reported concerns regarding the organ recipient, all focused on the possible transmission of disease to the organ recipient. Possible transmission vectors were: ALS-associated protein (12 of 12), ALS-associated virus (8 of 12), ALS-associated gene (4 of 12), and the age of the recipient for possible transmission (3 of 12). The 17 respondents with concerns were asked if they would support living organ donation if their concerns were addressed, and 76.5% (13 of 17) expressed support.
49% of ALS neurologists (25 of 51) reported being spontaneously asked by at least one patient, for information and options related to living organ donation. When neurologists were asked to estimate the percentage of ALS patients who would be interested in learning more about organ donation the mean percentage was 22.8% (range 0 – 76%, median 19%, standard deviation 17.3%, 49 respondents). ALS neurologists were queried on the average number of new ALS patients seen in their clinic annually, and the mean was 85.4 patients (range 5 – 400, median 72.5, standard deviation 72.5, 52 respondents).
Discussion
Living organ donation is the ultimate way for a person to consciously give the “gift of life.” Despite the tremendous need, this option is only available to healthy individuals and is currently denied to patients with terminal diseases, including ALS patients, despite their desire to participate. One such ALS patient in the United States said “there's nothing greater than for a family member to receive an organ so they can watch their family grow up………..I am not suicidal, I just know that it is a matter of time before I die and wish to do a good thing for those people who have a good life expectancy.”9 Such an altruistic desire has been expressed by many patients suffering from ALS, and has gone unfulfilled. Living organ donation is the most feasible option for these good Samaritans, as ALS does not lead to brain death, which provides maximum organ donation potential. The manner of death in ALS is respiratory failure and then circulatory arrest with subsequent poor organ perfusion. Organ donation in circulatory death is only possible after tracheostomy, ventilation and then withdrawal of life support to control the time of death. This is a high price to pay for most ALS patients, though it has happened at least once, where the patient said “I am glad that in spite of my disease, there is still something I can do to help others in a significant way. ALS is preventing me from accomplishing what I wanted to do in my life, but hopefully, my donation will give others a chance to live out their dreams.”13
Our results indicate that a small portion of ALS neurologists routinely discuss living organ donation with their patients (5.9%), almost half report being asked by their ALS patients about this option (49%), and the majority do not have concerns with living organ donation in ALS patients (67.3%). Furthermore the majority of concerns expressed by the neurologists can be readily addressed.
Neurologist respondents expressed concern with the ALS donors risk incurred during organ recovery surgery and beyond (Figure 2). The highest concern is the risk of post-operative respiratory failure (PRF), followed by exposure to anesthetic agents, and renal dysfunction/uropathy (due to single remaining kidney).
There are concerns that ALS patients without overt symptoms of respiratory failure may have subclinical diaphragmatic weakness, reduced respiratory reserve and thus be prone to PRF. However, this concern can be addressed by using the established guidelines for placement of percutaneous endoscopic gastrostomy (PEG) tubes in ALS patients, which are a FVC> 50% 14. Additionally, there are multiple validated predictive scores (e.g. the ARISCAT Score) for the general population15–17, which can be coupled with pulmonary function testing to provide an accurate risk assessment of PRF, enabling informed decisions by ALS patients.
A number of respondents expressed concern about worsening ALS symptoms after exposure to anesthetic agents and the systemic stress of surgery. These concerns are alleviated by abundant reports of anesthesia in a variety of surgeries in ALS patients, without evidence of decline. Surgeries have included feeding tube placement, diaphragmatic pacemaker implantation, spinal stem cell transplantation, and various other procedures18,19,20,21.
Concern for renal dysfunction or uropathy after kidney donation is an unlikely issue as there are multiple reports that living kidney donors have an extremely low peri-operative risk22, and no increased risk of end stage renal disease (ESRD) when compared to the general population 23–26. However, when compared to appropriate controls (i.e. those that fulfill kidney donation criteria) 2 studies show ESRD risk is slightly higher (0.27%) in living kidney donors27,28. The median time to develop ESRD is 8.6 years27, which is significantly longer than the median survival of ALS patients (3 years)10,11. Furthermore, individualized risk projection tools for ESRD in living donor candidates are available29. With the available ESRD risk data, delayed progression data and risk projection tools, potential ALS living organ donors will be able to make an informed decision regarding their donation options and inherent risks.
The other area of concern for this practice centers on the possibility of disease transmission through an undetermined vector. The highest source of concern identified in this study was with a misfolded protein vector, followed by viral and gene transmission, which reflects possible factors of disease etiology 30. In the unlikely event that a transmissible etiology is found for ALS, transplantation of a non-nervous system organ would be less likely to induce ALS in the recipient. There is ample experience with organ recovery and transplantation in patients with chronic hepatitis, and ALS donation could mirror this practice31. Transplants of ALS organs could also be limited to elderly patients (who may otherwise have a lower chance to be organ recipients), and significantly improve their quality of life.
It is evident from this survey that many ALS patients have spontaneously expressed interest and that there is a larger number that would likely consider living organ donation (1 in 4 patients). Yet, ALS centers are currently unable to honor such requests. There are no established mechanisms for screening, counseling or referral of these patients to transplant centers. With a reported mean of 85 new ALS patients per center per year, and 120 centers in the U.S., there is a potential of 2,550 ALS patients every year who wish to explore organ donation options 32. These options are best considered when ALS patients have independent respiratory function (!>50% predicted) and are not severely debilitated. Current practice does not expose the donor to any financial costs or liability for the organ recovery surgery or post-recovery care. Honoring these wishes will provide ALS patients with a sense of altruism, selflessness and philanthropy, which will be of significant psychological benefit and provide them comfort at the end of life.
The medical concerns for living organ donation in this population can be readily addressed to ensure the ALS donor has adequate information to make an informed decision. Patients with ALS are a vulnerable population, which poses ethical concerns regarding living organ donation. These concerns need to be addressed with protective mechanisms such as formal ethics review, consultation with multiple physicians and specialties, psychological evaluation and support, psychiatric evaluation and depression screening, as well as a mandatory time period to consider the decision in every ALS patient who volunteers. Once these ethical concerns are addressed, Good Samaritan donation by ALS patients will not only increase the number of life-saving kidney transplants, but also ensure better quality of life in the recipients as compared to kidneys from deceased donors33.
To enable living organ donation in terminally ill patients, federal agencies and organ donation governing bodies should consider a “donate life at the end of life” program. This would establish processes, benchmarks and awareness for organ donation in this population, and remove the penalties incurred by transplant centers and surgeons when terminally ill donors suffer clinical morbidity or mortality related to their primary disease and unrelated to organ recovery surgery6.
Our study has several limitations inherent to survey-based studies in general, such as small sample size, recall, belief and reporting bias. Survey respondents are also assumed to have different depths of knowledge regarding organ donation. This is a survey of ALS neurologists and not patients, and therefore, subject to neurologist's perceptions and biases. However, the strengths of this study are its high response rate, of 42.9% which is robust compared to the reported literature34. Our respondents were well distributed geographically across the United States of America (figure 1). Our questionnaire though comprehensive, was simple and easy to administer and revealed meaningful findings. This study is being followed with a suitably designed survey administered directly to ALS patients, to assess their interest and concerns with living organ donation and “donating life at the end of life”.
Supplementary Material
Highlights.
ALS patients are currently unable to become living organ donors due to current policies with donation.
Transplant centers are penalized if a living donor suffers morbidity even if due to terminal illness and not donation.
Our survey indicates that majority of neurologists (67%) do not have any concerns with ALS living organ donation.
Medical concerns expressed by ALS neurologists can be readily addressed in a systematic way.
Living organ donation in ALS patients needs to be explored to provide this valuable altruistic option to these patients.
Acknowledgments
We thank the Organ Donation and Transplantation Alliance, Leadership and Innovation Council for their support in conception and visualization of this project.
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR001066. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Study funding: This study was supported in part by the Center for Clinical and Translational Sciences grant support (8UL1TR000105 (formerly UL1RR025764) NCATS/NIH) and the National Center for Advancing Translational Sciences of the National Institutes of Health (TL1TR001066). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding agency had no role in the data collection or manuscript composition.
Footnotes
Author contribution: Dr. Ansari has made a substantive contribution to the design and conceptualization of the study, analysis and interpretation of the data and drafting and revising of the manuscript. Dr. Bromberg has made a substantive contribution to the design and conceptualization of the study, analysis and interpretation of the data and drafting and revising of the manuscript. Dr. Gibson has made a substantive contribution to the design and conceptualization of the study, interpretation of the data, and drafting and revising of the manuscript.
Author disclosures: Dr. Bromberg reports no disclosures. Dr. Gibson reports the following disclosures: Recursion Pharmaceuticals – Share Holder, and Dr. Ansari reports the following disclosure: Bard Medical – Consultant.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leeson S, Desai SP. Medical and ethical challenges during the first successful human kidney transplantation in 1954 at Peter Bent Brigham Hospital, Boston. Anesth Analg. 2015;120(1):239–45. doi: 10.1213/ANE.0000000000000521. [DOI] [PubMed] [Google Scholar]
- 2.Annual report | UNOS [Internet] [cited 2016 Dec 1];United Netw Organ Shar. Available from: https://www.unos.org/about/annual-report/
- 3.National Data - OPTN [Internet] [cited 2016 Dec 1];Organ Procure Transplant Netw. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/
- 4.Tong A, Chapman JR, Wong G, Josephson MA, Craig JC. Public awareness and attitudes to living organ donation: systematic review and integrative synthesis. Transplantation. 2013;96(5):429–37. doi: 10.1097/TP.0b013e31829282ac. [DOI] [PubMed] [Google Scholar]
- 5.Living donation | UNOS [Internet] [cited 2016 Apr 2];United Netw Organ Shar. Available from: https://www.unos.org/donation/living-donation/
- 6.Joseph Scalea JM. As They Lay Dying. [cited 2016 Apr 1];The Atlantic [Internet] 2015 Available from: http://www.theatlantic.com/magazine/archive/2015/04/as-they-lay-dying/386273/
- 7.Cherokee man with ALS wants to die by donating organs [Internet] [cited 2016 Apr 1];Atlanta J -Const. 2010 Available from: http://www.ajc.com/news/news/local/cherokee-man-with-als-wants-to-die-by-donating-org/nQhtj/
- 8.Wahlberg, David Imminent death organ donation could help others, stir distrust[Internet] [cited 2016 Apr 1];Wis State J. 2015 Available from: http://host.madison.com/wsj/news/local/health-med-fit/imminent-death-organ-donation-could-help-others-stir-distrust/article_6c5d178a-48bc-5eee-9ba6-6c5c72fce62d.html.
- 9.CNN Wire staff. Man with Lou Gehrig's makes extraordinary offer of his organs [Internet] [cited 2016 Apr 1];CNN - Cable News Netw. :2010. Available from: http://www.cnn.com/2010/HEALTH/07/29/georgia.right.to.die/
- 10.Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet Lond Engl. 2011;377(9769):942–55. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 11.Connolly S, Galvin M, Hardiman O. End-of-life management in patients with amyotrophic lateral sclerosis. Lancet Neurol. 2015;14(4):435–42. doi: 10.1016/S1474-4422(14)70221-2. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toossi S, Lomen-Hoerth C, Josephson SA, et al. Organ donation after cardiac death in amyotrophic lateral sclerosis - Case report. Ann Neurol. 2012;71(2):154–6. doi: 10.1002/ana.22525. [DOI] [PubMed] [Google Scholar]
- 14.Miller RG, Jackson CE, Kasarskis EJ, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73(15):1227–33. doi: 10.1212/WNL.0b013e3181bc01a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta H, Gupta PK, Fang X, et al. Development and validation of a risk calculator predicting postoperative respiratory failure. Chest. 2011;140(5):1207–15. doi: 10.1378/chest.11-0466. [DOI] [PubMed] [Google Scholar]
- 16.Canet J, Sabaté S, Mazo V, et al. Development and validation of a score to predict postoperative respiratory failure in a multicentre European cohort: A prospective, observational study. Eur J Anaesthesiol. 2015;32(7):458–70. doi: 10.1097/EJA.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 17.Mazo V, Sabaté S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications - ARISCAT Score. Anesthesiology. 2014;121(2):219–31. doi: 10.1097/ALN.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 18.Onders RP, Carlin AM, Elmo M, Sivashankaran S, Katirji B, Schilz R. Amyotrophic lateral sclerosis: the Midwestern surgical experience with the diaphragm pacing stimulation system shows that general anesthesia can be safely performed. Am J Surg. 2009;197(3):386–90. doi: 10.1016/j.amjsurg.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Pinto S, Swash M, de Carvalho M. Does surgery accelerate progression of amyotrophic lateral sclerosis? J Neurol Neurosurg Psychiatry. 2014;85(6):643–6. doi: 10.1136/jnnp-2013-305770. [DOI] [PubMed] [Google Scholar]
- 20.Lee D, Lee KC, Kim JY, Park YS, Chang YJ. Total intravenous anesthesia without muscle relaxant in a patient with amyotrophic lateral sclerosis. J Anesth. 2008;22(4):443–5. doi: 10.1007/s00540-008-0655-x. [DOI] [PubMed] [Google Scholar]
- 21.Olivieri C, Castioni CA, Livigni S, et al. Non-invasive ventilation after surgery in amyotrophic lateral sclerosis. Acta Neurol Scand. 2014;129(4):e16–19. doi: 10.1111/ane.12187. [DOI] [PubMed] [Google Scholar]
- 22.Segev DL, Muzaale AD, Caffo BS, et al. PErioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303(10):959–66. doi: 10.1001/jama.2010.237. [DOI] [PubMed] [Google Scholar]
- 23.Cherikh WS, Young CJ, Kramer BF, Taranto SE, Randall HB, Fan PY. Ethnic and gender related differences in the risk of end-stage renal disease after living kidney donation. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2011;11(8):1650–5. doi: 10.1111/j.1600-6143.2011.03609.x. [DOI] [PubMed] [Google Scholar]
- 24.Wafa EW, Refaie AF, Abbas TM, et al. End-stage renal disease among living-kidney donors: single-center experience. Exp Clin Transplant Off J Middle East Soc Organ Transplant. 2011;9(1):14–9. [PubMed] [Google Scholar]
- 25.Ibrahim HN, Foley R, Tan L, et al. Long-Term Consequences of Kidney Donation. N Engl J Med. 2009;360(5):459–69. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fehrman-Ekholm I, Nordén G, Lennerling A, et al. Incidence of end-stage renal disease among live kidney donors. Transplantation. 2006;82(12):1646–8. doi: 10.1097/01.tp.0000250728.73268.e3. [DOI] [PubMed] [Google Scholar]
- 27.Muzaale AD, Massie AB, Wang M, et al. RIsk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579–86. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mjøen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int. 2014;86(1):162–7. doi: 10.1038/ki.2013.460. [DOI] [PubMed] [Google Scholar]
- 29.Grams ME, Sang Y, Levey AS, et al. Kidney-Failure Risk Projection for the Living Kidney-Donor Candidate. N Engl J Med. 2016;374(5):411–21. doi: 10.1056/NEJMoa1510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grad LI, Fernando SM, Cashman NR. From molecule to molecule and cell to cell: prion-like mechanisms in amyotrophic lateral sclerosis. Neurobiol Dis. 2015;77:257–65. doi: 10.1016/j.nbd.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Huprikar S, Danziger-Isakov L, Ahn J, et al. Solid organ transplantation from hepatitis B virus-positive donors: consensus guidelines for recipient management. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2015;15(5):1162–72. doi: 10.1111/ajt.13187. [DOI] [PubMed] [Google Scholar]
- 32.The ALS Association Certified Centers and Clinics [Internet] [cited 2016 Aug 5];ALSA org. Available from: http://www.alsa.org/community/centers-clinics/
- 33.Cohen DJ, St Martin L, Christensen LL, Bloom RD, Sung RS. Kidney and Pancreas Transplantation in the United States, 1995–2004. Am J Transplant. 2006;6(5p2):1153–69. doi: 10.1111/j.1600-6143.2006.01272.x. [DOI] [PubMed] [Google Scholar]
- 34.Edwards PJ, Roberts I, Clarke MJ, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009 Jul;8(3) doi: 10.1002/14651858.MR000008.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.