Abstract
Chemokines are involved in many aspects of oncogenesis, including regulation of cancer cell growth, dissemination and host-tumor response. However, the potential of the chemokine receptors, CXCR4 and CXCR7, in serving as biomarkers in extramammary Paget's disease (EMPD) has been rarely examined. Expressions of CXCR4 and CXCR7 were evaluated in 92 EMPD specimens by immunohistochemistry. High expression of CXCR4 and CXCR7 were both correlated with regional lymph node metastasis and presence of lymphovascular invasion. High expression of CXCR7 also correlated with the depth of invasion. The prognostic value of these two chemokines were also investigated in progression-free survival (PFS) and cancer-specific survival (CSS). Both high expression of CXCR4 and CXCR7 were indicative of shorter PFS and CSS. In the combined prognostic model, concomitant high expression of CXCR4 and CXCR7 were suggestive of poor prognosis compared with the other two groups. In the multivariate analysis, depth of invasion, combined prognostic model and regional lymph node metastasis at diagnosis were the independent prognostic factors for EMPD patients for PFS, and the former two factors independently impacted CSS. Our results demonstrated that CXCR4 and CXCR7 can be used as prognostic biomarkers and prediction of aggressiveness of EMPD. Therapy targeting CXCR4 and CXCR7 may helpful to prevent EMPD progression and improve the prognosis of EMPD.
Keywords: EMPD, CXCR7, CXCR4, invasive, prognosis.
Introduction
Extramammary Paget's disease (EMPD) is a rare cutaneous malignant neoplasm of unknown histogenesis. It mostly arises in areas rich in apocrine sweat glands, e.g. the anogenital areas, the perineal area and axilla.1, 2 Early lesions generally present as red or brown plaques and the lesions become erosive, infiltrated in the late stage. Patients classified as regional intraepithelial invasion experience good prognosis regardless of tumor diameter.4-6 However, in some instances, EMPD tumor cells invade deeply into the dermis and develop nodules and ulcerations. Tumors then rapidly metastasize to regional lymph nodes and distant organs, leading to poor outcomes.4-7 Treatment for advanced EMPD with systemic metastasis often ends up with a disappointing result, as no highly effective therapy has been established. Identification of reliable prognostic molecular markers for EMPD could provide novel therapeutic targets, which are critically important for developing molecular profile-directed therapies for patients with EMPD.
Chemokines belong to a superfamily of chemo-attracting proteins that bind to chemokine receptors. They are involved in many aspects of oncogenesis, including regulation of cancer cell growth, dissemination and host-tumor response.8, 9 Alterations of chemokine expression levels are significantly associated with more aggressive disease and worse prognosis in diverse malignancies.10, 11 CXCR7 and CXCR4 shared the same chemokine ligands, CXCL12,12 with demonstrated critical roles in tumor growth, angiogenesis, and tumor cell homing in lymph nodes and distant metastases in a variety of solid human malignancies, such as lung cancer,13 breast cancer,13 prostate cancer,14 rhabdomyosarcomas15 and human glioma,16 among others. In different tumor cell types, depending on the differentiation status and environment, they may function uniquely or even in combination. Based on these findings, we speculated whether CXCR4 and CXCR7 participate in the progression of EMPD and could serve as potential prognostic markers for the disease.
In this study, we investigated the expression of intratumoral CXCR4 and CXCR7 in a large cohort of EMPD patients. We evaluated the correlation between expression of these proteins and patient clinicopathological characteristics and assessed the prognostic value of these factors in progression-free survival (PFS) and cancer-specific survival (CSS).
Materials and Methods
Patients and methods
Patients with EMPD who underwent surgical excision of primary skin lesions at our institution between June 2003 and March 2013 were screened. All patients underwent a thorough physical examination and diagnostic tests, including cystoscopy, colonoscopy, chest X-ray, abdominal and pelvic CT before surgery. Patients who presented with synchronous or metachronous internal noncutaneous malignancies were excluded. In total, 92 patients who met these criteria and with available specimens were included in this study. Patients' clinical characteristics, such as age, sex, time delayed in diagnosis, lesion location, primary treatment, and adjuvant therapy, were recorded. Histopathological parameters, including longest diameter of lesion, surgical margin status, depth of invasion and lymphovascular invasion, were examined by two experienced pathologists. We stratified the patients into three groups according to depth of invasion: (i) intraepitheliar; (ii) upper dermis; and (iii) lower dermis. The pathological stage was determined according to the 7th edition of the American Joint Committee on Cancer TNM classification of skin appendageal carcinoma.17 Both inguinal and pelvic lymph nodes were deemed as regional nodes for the specific location of the lesion. PFS and CSS were assessed to determine the time to disease recurrence and cancer-related death, respectively. The end point for PFS was the date of local or regional recurrence or the date of distant metastasis. The end point for CSS was the date of cancer-related death. For CSS, patients who died of a cause unrelated to EMPD were considered as censored observations at the date of death. Hematoxylin and eosin sections were reviewed by pathologists to select representative areas of the original samples. The study was carried out in accordance with the ethical standards of the Helsinki Declaration II and approved by the Institution Review Board of Fudan University Shanghai Cancer Center. Informed consent was obtained from each patient and the study was approved by our Institutional Ethics Committee.
Immunohistochemistry staining
Immunostainings of CXCR4 and CXCR7 were performed using antibodies (CXCR4, Abcam, Cambridge, MA; dilution 1:50; CXCR7, Abgent, R&D Systems, Abingdon, UK; dilution 1:200) and the Envision detection kit (Dako, Carpinteria, CA, USA). EMPD tissue sections (4 µm) from archived formalin-fixed, paraffin-embedded tissue blocks were deparaffinized in a series of xylene and rehydrated in graded ethanol solutions. Endogenous peroxidase was quenched by incubation in 0.3 % H2O2 for 15 min at 37°C and nonspecific binding was blocked with 10% normal goat serum for 60 min at room temperature. For antigen retrieval, sections in 0.01 M sodium citrate buffer, pH 6.0, were heated in a pressure cooker (20 psi for 10 min). Incubation with primary antibodies was conducted overnight at 4°C. Chromogenic detection was carried out using a peroxidase-conjugated secondary antibody (60 min) and DAB reagents (1 min) provided in the Envision detection kit. Tissue sections were then counterstained with Meyer's Hematoxylin (Thermo Fisher Scientific, Waltham, MA, USA). The positive controls for CXCR7, and CXCR4 were conducted by using normal human tonsil and spleen tissues. The negative control was prepared using phosphate-buffered saline with omission of the primary antibody.
All slides were examined and scored by two experienced pathologists, who were blinded to the outcome and clinical data, in an open discussion. The CXCR4 and CXCR7 immunostaining was measured based on the intensity of staining (intensity score) and the quantity of immunoreactive cells (quantity score), as previously reported.18 The intensity of immune staining was scored as 0, negative; 1, weak; 2, moderate; and 3, intense. The quantity of immunoreactive cells was scored as 0%, none; 1, 1%-30%; 2, 31%-60%; 3, >60%.19 The product of intensity score and quantity score was used as the total score, in which 0-3 indicates low expression, and 4-9 indicates high expression.20
Statistical analysis
To evaluate the relationship between the immunostaining and clinicopathological parameters, the Chi-square test and Fisher's exact test were used. PFS and CSS were calculated by the Kaplan-Meier method with the log-rank test to assess differences between groups based on the gene expression status. Univariate and multivariate analysis of prognostic factors was performed using the Cox proportional hazard model. All statistical tests were performed using SPSS, version 20 (SPSS Inc., Chicago, IL). P < 0.05 was considered to indicate statistical significance.
Results
Patient characteristics
Among the total 92 patients recruited in the study, 87 were males and 5 were females. After a median (range) follow-up of 34 months (range 6-130 months), 25.0% (23/92) of the patients experienced clinical recurrence and 15.2% (14/92) patients died of EMPD. Non-regional lymph nodes (10.9%, 10/92) and bone (7.6%, 7/92) were the most common sites of distant metastasis. The median PFS was 28 months (range 3~116 months) and the median CSS was 30 months (range 5~130 months). The detailed clinicopathological characteristics of the patients are summarized in Table 1.
Table 1.
Clinicopathological characteristics in relation to CXCR4 and CXCR7 expression status
| Variable | Entire group (n=92) | CXCR4 | P value | CXCR7 | P value | ||
|---|---|---|---|---|---|---|---|
| Low | High | Low | High | ||||
| Age, years | 68 (35~87) | 0.799 | 0.983 | ||||
| <70 | 48(52.2) | 36 | 12 | 37 | 11 | ||
| ≥70 | 44(47.8) | 34 | 10 | 34 | 10 | ||
| Delay in diagnosis, months | 36 (2~244) | 0.575 | 0.071 | ||||
| <24 | 29(31.5) | 21 | 8 | 19 | 10 | ||
| ≥24 | 63(68.5) | 49 | 14 | 52 | 11 | ||
| Longest diameter of lesion, cm | 5 (1~12) | 0.815 | 0.634 | ||||
| <5 | 44(47.8) | 33 | 11 | 33 | 11 | ||
| ≥5 | 48(52.2) | 37 | 11 | 38 | 10 | ||
| Surgical margin status | 0.851 | 0.946 | |||||
| Positive | 18(19.6) | 14 | 4 | 14 | 4 | ||
| Negative | 74(80.4) | 56 | 18 | 57 | 17 | ||
| Depth of invasion | 0.318 | 0.010 | |||||
| Intraepithelial | 45(48.9) | 36 | 9 | 40 | 5 | ||
| Upper dermis | 21(22.8) | 17 | 4 | 16 | 5 | ||
| Lower dermis | 26(28.3) | 17 | 9 | 15 | 11 | ||
| Lymphovascular invasion | 0.007 | 0.000 | |||||
| Yes | 16(17.4) | 8 | 8 | 7 | 9 | ||
| No | 76(82.6) | 62 | 14 | 64 | 12 | ||
| Regional lymph node metastasis at diagnosis | 0.037 | 0.025 | |||||
| Yes | 19(20.7) | 11 | 8 | 11 | 8 | ||
| No | 73(79.3) | 59 | 14 | 60 | 13 | ||
CXCR4 and CXCR7 expression in EMPD
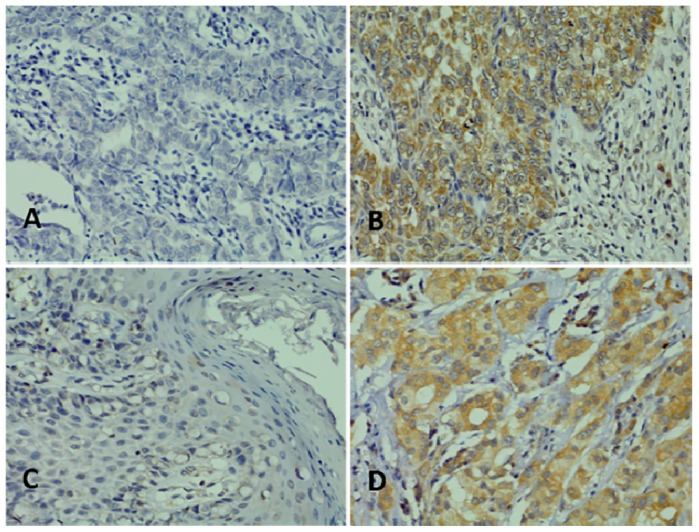
Immunohistochemical staining showed that both CXCR4 and CXCR7 were expressed in the cytoplasm in EMPD specimens. Among the 92 EMPD patients, 22 (23.9%) and 21 (22.9%) had high expression of CXCR4 and CXCR7, respectively. Representative examples of high and low CXCR4 and CXCR7 EMPD specimens are shown in Figure 1.
Figure 1.
Representative images of immunohistochemical staining. (A) Low expression of CXCR4; (B) High expression of CXCR4; (C) Low expression of CXCR7; (D) High expression of CXCR7. Magnification, ×400.
Association of CXCR4 and CXCR7 expression with patient characteristics
The clinicopathological characteristics of patients grouped by CXCR4 and CXCR7 expression level are listed in Table 1. High expression of CXCR4 was correlated with lymphovascular invasion (P=0.007) and regional lymph node metastasis at diagnosis (P=0.037). In addition to strong indication of lymphovascular invasion (P=0.000) and regional lymph node metastasis at diagnosis (P=0.025), high expression of CXCR7 was indicative of deeper dermis invasion (P=0.010). We found no significant association of CXCR4 and CXCR7 expression with other clinicopathological features including age, time delayed in diagnosis, longest diameter of lesion and surgical margin status.
High expressions of CXCR4 and CXCR7 were associated with adverse prognosis
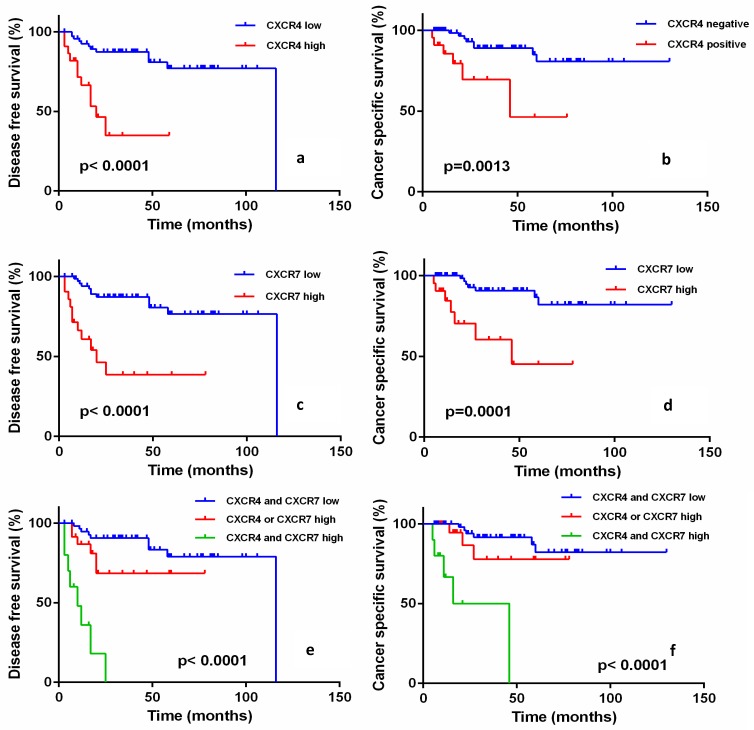
In the survival analysis, high expression of CXCR4 was significantly associated with worse prognosis of EMPD in both PFS (P<0.0001, Fig. 2a) and CSS (P=0.0013, Fig. 2b). The same pattern of survival analysis was also observed with CXCR7 and PFS and CSS (P<0.0001, P<0.0001, respectively; Fig. 2c, Fig. 2d). The combined prognostic model was established based on the expression of CXCR4 and CXCR7. Patients with concomitant high expression of both CXCR4 and CXCR7 showed the worst prognosis in PFS and CSS, and patients with both low expression of CXCR4 and CXCR7 showed better PFS and CSS (P<0.0001, P<0.0001, respectively; Fig. 2e, Fig. 2f).
Figure 2.
Progression-free survival (PFS) and cancer-specific survival (CSS) of EMPD patients according to the expression of CXCR4 and CXCR7. CXCR4 high expression patients versus CXCR4 low expression patients for PFS (a) and CSS (b) (log-rank P<0.0001 and P=0.0013, respectively). CXCR7 high expression patients versus CXCR7 low expression patients for PFS (c) and CSS (d) (log-rank P<0.0001 and P=0.0001). There was a significant difference among groups stratified according to CXCR4 and CXCR7 expression in PFS (e) and CSS (f) (log-rank P<0.0001 and P<0.0001), respectively. Patients with both high CXCR4 and CXCR7 expression had the worst prognosis.
Univariate and multivariate analyses were performed to assess the independent prognostic value of clinicopathological and immunohistochemical parameters for PFS and CSS. The results revealed that four parameters, including depth of invasion, lymphovascular invasion, regional lymph node metastasis at diagnosis, high expression of CXCR4 and CXCR7 and the combined prognostic model were associated with shorter PFS and CSS (P<0.05) in the univariate analysis (Table 2). Subsequent multivariate analysis suggested that depth of invasion, regional lymph node metastasis at diagnosis and the combined prognostic model were the independent prognostic factors of shorter PFS (P<0.05). Only depth of invasion and combined prognostic model impacted the CSS independently (P<0.05) (Table 3).
Table 2.
Univariate analysis of clinicopathological parameters affecting disease-free survival and cancer-specific survival
| Variables | DFS | CSS | ||
|---|---|---|---|---|
| HR(95%CI) | P value | HR(95%CI) | P value | |
| Age (<70 vs ≥70 years) | 0.711(0.304~1.664) | 0.431 | 0.723(0.250~2.091) | 0.549 |
| Delay in diagnosis (<24 vs ≥24 months) | 1.047(0.426~2.573) | 0.920 | 1.732(0.482~6.221) | 0.400 |
| Longest diameter of lesion (<5 vs ≥5 cm) | 0.691(0.298~1.600) | 0.388 | 0.573(0.198~1.658) | 0.304 |
| Surgical margin status (positive vs negative) | 1.377(0.538~3.532) | 0.505 | 2.010(0.673~6.010) | 0.211 |
| Depth of invasion (IE vs UD vs LD) | 4.705(2.465~8.982) | 0.000 | 13.209(3.460~50.435) | 0.000 |
| Lymphovascular invasion (Yes vs No) | 20.040(7.353~54.618) | 0.000 | 51.210(10.767~243.564) | 0.000 |
| Regional lymph node metastasis at diagnosis (Yes vs No) | 22.132(8.158~60.042) | 0.000 | 13.841(4.471~42.847) | 0.000 |
| CXCR4 (High vs Low) | 5.549(2.320~13.275) | 0.000 | 5.098(1.706~15.235) | 0.004 |
| CXCR7 (High vs Low) | 5.637(2.409~13.188) | 0.000 | 6.218(2.152~17.963) | 0.001 |
| combined prognostic model (both high vs either high vs both low) | 4.389(2.472~7.795) | 0.000 | 4.445(2.171~9.100) | 0.000 |
IE: Intraepithelial; UD: Upper dermis; LD: Lower dermis; both high: CXCR4 and CXCR7 high; either high: CXCR4 or CXCR7 high; both low: CXCR4 and CXCR7 low
Table 3.
Multivariate analysis of clinicopathological parameters affecting disease-free survival and cancer-specific survival
| Variables | DFS | CSS | ||
|---|---|---|---|---|
| HR(95%CI) | P value | HR(95%CI) | P value | |
| Depth of invasion (IE vs UD vs LD) | 3.874(1.595~9.412) | 0.003 | 8.226(1.910~35.420) | 0.005 |
| Lymphovascular invasion(Yes vs No) | 0.128(0.016~1.062) | 0.057 | 0.843(0.062~11.394) | 0.898 |
| Regional lymph node metastasis at diagnosis(Yes vs No) | 34.166(5.909~197.544) | 0.000 | 7.748(0.972~61.782) | 0.053 |
| Combined prognostic model (both high vs either high vs both low) | 3.203(1.611~6.367) | 0.001 | 2.662(1.097~6.462) | 0.030 |
IE: Intraepithelial; UD: Upper dermis; LD: Lower dermis; both high: CXCR4 and CXCR7 high; either high: CXCR4 or CXCR7 high; both low: CXCR4 and CXCR7 low
Discussion
In the present study, we evaluated the expression of two chemokine receptors, CXCR4 and CXCR7, in a series of EMPD patients and correlated their expression patterns with clinicopathological characteristics and patient outcomes. We found that either high expression of CXCR4 or CXCR7 was indicative of lymphovascular invasion, regional lymph node metastasis at diagnosis and an adverse prognosis. In addition, high expression of CXCR7 was also correlated with the depth of invasion. Impressively, a combined prognostic model based on the expression of CXCR4 and CXCR7 could stratify the whole group into three categories, and showed that patients overexpressing both CXCR4 and CXCR7 experienced the worst prognosis. Our findings suggest that CXCR4 and CXCR7 could be highly relevant molecular markers of malignant potential and therapeutic targets for patients with EMPD.
Our findings showing that patients with high CXCR4 and CXCR7 expression exhibited the worst prognosis were consistent with similar findings in other tumors. In Florian's study, the expression status of CXCR4 and CXCR7 was examined in 249 pancreatic adenocarcinoma patients and 47 patients that were CXCR7-positive also showed CXCR4 expression, which underlines the functional affiliation between these two molecules.21 In Heinrich's study, immunohistochemical staining of 51 human pancreatic cancer specimens demonstrated high frequency of CXCR4 and CXCR7 co-expression: 37 showed double staining and 5 showed single staining, while 9 had no staining.19 D'Alterio et al. demonstrated that concomitant high expression of CXCR4 and CXCR7 (P=0.0235) was an independent prognostic factor for renal cell carcinoma.22
Under pathological conditions, CXCR4 and CXCR7 signaling mediates several cellular effects affecting leukocyte recruitment, neovascularization and tumor progression. Activation of MAP and Akt kinase pathways promotes cell survival and proliferation, and transcriptional regulation of multiple genes affects angiogenesis, invasion and adhesion of cells. When co-expressed, CXCR4 and CXCR7 may form homo- and heterodimers, and heterodimerization seems to play an important role in the modulation of downstream signaling.23-25 CXCR4 and CXCR7 co-expression on the same cells resulted in stronger calcium flux and more robust phosphorylation of MAPKp42/44 in response to SDF-1 stimulation compared with cells that express only CXCR4.26 This suggests that the heterodimeric receptor potentially might activate a broader panel of intracellular pathways than activation of only one receptor.27, 28 This may explain why the patients high expressing both receptors showed worse prognosis compared with the other groups.
For EMPD patients, identification of invasive disease is of great value in clinical practice. Consistent with previous published studies, our study also suggested that depth of invasion was an important prognosticator in EMPD. Patients diagnosed as invasive disease tend to experience rapid progression and adverse prognosis.29-33 However, it is difficult to distinguish invasive disease with noninvasive disease before surgery. Thus, the identification of reliable biomarkers to distinguish invasive and noninvasive disease at the time of biopsy is desperately needed. A retrospective study including 44 specimens from 38 primary EMPD cases found that invasive lesions and metastatic lymph nodes tended to express significantly higher MUC5AC levels than in situ lesions (P < 0.01).34 Other studies reported that Stat5a, E-cadherin and even FDG PET/CT may play some role in the invasion of EMPD.35, 36 Aoyagi et al. reported that combined high expression of Ki-67 and cyclin D1 were useful for the early detection of micro-invasive EMPD. 3 However, several reasons have prevented the use of these biomarkers. First, due to the rarity of EMPD, most of these cohorts consisted of relatively small sample sizes, especially patients with invasive disease. Furthermore, the short follow-up also restricted the validation and application of these markers. In our study, we evaluated the expression of CXCR7 in 92 specimens, among which 47 were invasive cases. Over the median follow-up of 34 months (range 6-130 months), one quarter of the patients experienced clinical recurrence and over 15% patients died of EMPD. We found that high expression of CXCR7 closely correlated with invasive disease. This suggests that patients with high expression of CXCR7 at preoperative biopsy may be linked with invasive disease. Aggressive treatment might be advisable for these patients, including wider and deeper excision of primary lesion with or without adjuvant radiation therapy, and intensive follow-up. To decrease the possibility of local recurrence and distant metastasis, complementary resection may also be recommended.
Although the clinical significance of CXCR4 and CXCR7 in EMPD has been revealed, several limitations of this study warrant further discussion. First, this study was conducted in a single center. An independent external cohort is necessaryto confirm our findings. Second, our results were based on a retrospective analysis, which may have resulted in selection bias because of the various therapeutic strategies that were performed. Therefore, prospective studies are also needed to validate our findings. Finally, functional studies should be conducted to uncover the biological mechanisms of CXCR4 and CXCR7 in EMPD.
Acknowledgments
This study was supported in part by a grant from the Natural Science Foundation of Shanghai Municipality (No. 16ZR1406500), a grant from the Outstanding Young Talent Training Plan of Shanghai Municipal Commission of Health and Family Planning (No. XYQ2013102), and a grant from Shanghai Cancer Research Charity Center.
Author Contributions
KC and GXL designed the study, analyzed and interpreted the clinical data, and wrote the manuscript. KC and ZWJ conducted the experiments. YYQ and YW helped with patient follow-up. YYK, BD and DWY supervised the project and revised the manuscript. All authors approved the final version and agreed to publish the manuscript.
References
- 1.Lloyd J, Evans DJ, Flanagan AM. Extension of extramammary Paget disease of the vulva to the cervix. J Clin Pathol. 1999;52:538–540. doi: 10.1136/jcp.52.7.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohara K, Fujisawa Y, Yoshino K. et al. A proposal for a TNM staging system for extramammary Paget disease: Retrospective analysis of 301 patients with invasive primary tumors. J Dermatol Sci. 2016;83:234–239. doi: 10.1016/j.jdermsci.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Aoyagi S, Akiyama M, Shimizu H. et al. High expression of Ki-67 and cyclin D1 in invasive extramammary Paget's disease. J Dermatol Sci. 2008;50:177–184. doi: 10.1016/j.jdermsci.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka R, Sasajima Y, Tsuda H. et al. Human epidermal growth factor receptor 2 protein overexpression and gene amplification in extramammary Paget disease. Br J Dermatol. 2013;168:1259–1266. doi: 10.1111/bjd.12249. [DOI] [PubMed] [Google Scholar]
- 5.Shiomi T, Noguchi T, Nakayama H. et al. Clinicopathological study of invasive extramammary Paget's disease: Subgroup comparison according to invasion depth. J Eur Acad Dermatol Venereol. 2013;27:589–592. doi: 10.1111/j.1468-3083.2012.04489.x. [DOI] [PubMed] [Google Scholar]
- 6.Hatta N, Yamada M, Hirano T. et al. Extramammary Paget's disease: Treatment, prognostic factors and outcome in 76 patients. Br J Dermatol. 2008;158:313–318. doi: 10.1111/j.1365-2133.2007.08314.x. [DOI] [PubMed] [Google Scholar]
- 7.Dai B, Kong YY, Chang K. et al. Primary invasive carcinoma associated with penoscrotal extramammary Paget's disease: A clinicopathological analysis of 56 cases. Bju Int. 2015;115:153–160. doi: 10.1111/bju.12776. [DOI] [PubMed] [Google Scholar]
- 8.Craig MJ, Loberg RD. CCL2 (Monocyte Chemoattractant Protein-1) in cancer bone metastases. Can Met Rev. 2006;25:611–619. doi: 10.1007/s10555-006-9027-x. [DOI] [PubMed] [Google Scholar]
- 9.Homey B, Muller A, Zlotnik A. et al. Chemokines: Agents for the immunotherapy of cancer? Nat Rev Immunol. 2002;2:175–184. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- 10.Zlotnik A. Chemokines in neoplastic progression. Semin Can Biol. 2004;14:181–185. doi: 10.1016/j.semcancer.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Strieter RM, Belperio JA, Phillips RJ. et al. CXC chemokines in angiogenesis of cancer. Semin Can Biol. 2004;14:195–200. doi: 10.1016/j.semcancer.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Balabanian K, Lagane B, Infantino S. et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 13.Miao Z, Luker KE, Summers BC. et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci USA. 2007;104:15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Shiozawa Y, Wang J. et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283:4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 15.Tarnowski M, Grymula K, Reca R. et al. Regulation of expression of stromal-derived factor-1 receptors: CXCR4 and CXCR7 in human rhabdomyosarcomas. Mol Can Res. 2010;8:1–14. doi: 10.1158/1541-7786.MCR-09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hattermann K, Held-Feindt J, Lucius R. et al. The chemokine receptor CXCR7 is highly expressed in human glioma cells and mediates antiapoptotic effects. Can Res. 2010;70:3299–3308. doi: 10.1158/0008-5472.CAN-09-3642. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 18.Kononen J, Bubendorf L, Kallioniemi A. et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 19.Heinrich EL, Lee W, Lu J. et al. Chemokine CXCL12 activates dual CXCR4 and CXCR7-mediated signaling pathways in pancreatic cancer cells. J Transl Med. 2012;10:68. doi: 10.1186/1479-5876-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo JC, Li J, Zhou L. et al. CXCL12-CXCR7 axis contributes to the invasive phenotype of pancreatic cancer. Oncotarget. 2016;7:62006–62018. doi: 10.18632/oncotarget.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebauer F, Tachezy M, Effenberger K. et al. Prognostic impact of CXCR4 and CXCR7 expression in pancreatic adenocarcinoma. J Surg Oncol. 2011;104:140–145. doi: 10.1002/jso.21957. [DOI] [PubMed] [Google Scholar]
- 22.D'Alterio C, Consales C, Polimeno M. et al. Concomitant CXCR4 and CXCR7 expression predicts poor prognosis in renal cancer. Curr Cancer Drug Targets. 2010;10:772–781. doi: 10.2174/156800910793605839. [DOI] [PubMed] [Google Scholar]
- 23.Decaillot FM, Kazmi MA, Lin Y. et al. CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. J Biol Chem. 2011;286:32188–32197. doi: 10.1074/jbc.M111.277038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levoye A, Balabanian K, Baleux F. et al. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113:6085–6093. doi: 10.1182/blood-2008-12-196618. [DOI] [PubMed] [Google Scholar]
- 25.Luker KE, Steele JM, Mihalko LA. et al. Constitutive and chemokine-dependent internalization and recycling of CXCR7 in breast cancer cells to degrade chemokine ligands. Oncogene. 2010;29:4599–4610. doi: 10.1038/onc.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sierro F, Biben C, Martinez-Munoz L. et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci USA. 2007;104:14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mellado M, Rodriguez-Frade JM, Vila-Coro AJ. et al. Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. Embo J. 2001;20:2497–2507. doi: 10.1093/emboj/20.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maksym RB, Tarnowski M, Grymula K. et al. The role of stromal-derived factor-1-CXCR7 axis in development and cancer. Eur J Pharmacol. 2009;625:31–40. doi: 10.1016/j.ejphar.2009.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam C, Funaro D. Extramammary Paget's disease: Summary of current knowledge. Dermatol Clin. 2010;28:807–826. doi: 10.1016/j.det.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Ito Y, Igawa S, Ohishi Y. et al. Prognostic indicators in 35 patients with extramammary Paget's disease. Dermatol Surg. 2012;38:1938–1944. doi: 10.1111/j.1524-4725.2012.02584.x. [DOI] [PubMed] [Google Scholar]
- 31.Hatta N, Yamada M, Hirano T. et al. Extramammary Paget's disease: Treatment, prognostic factors and outcome in 76 patients. Br J Dermatol. 2008;158:313–318. doi: 10.1111/j.1365-2133.2007.08314.x. [DOI] [PubMed] [Google Scholar]
- 32.Karam A, Dorigo O. Treatment outcomes in a large cohort of patients with invasive Extramammary Paget's disease. Gynecol Oncol. 2012;125:346–351. doi: 10.1016/j.ygyno.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 33.Crawford D, Nimmo M, Clement PB. et al. Prognostic factors in Paget's disease of the vulva: A study of 21 cases. Int J Gynecol Pathol. 1999;18:351–359. doi: 10.1097/00004347-199910000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Hata H, Abe R, Hoshina D. et al. MUC5AC expression correlates with invasiveness and progression of extramammary Paget's disease. J Eur Acad Dermatol Venereol. 2014;28:727–732. doi: 10.1111/jdv.12156. [DOI] [PubMed] [Google Scholar]
- 35.Liu H, Urabe K, Uchi H. et al. Expression and prognostic significance of Stat5a and E-cadherin in extramammary Paget's disease. J Cutan Pathol. 2007;34:33–38. doi: 10.1111/j.1600-0560.2006.00568.x. [DOI] [PubMed] [Google Scholar]
- 36.Li ZG, Qin XJ. Extensive invasive extramammary Paget disease evaluated by F-18 FDG PET/CT: A case report. Medicine (Baltimore) 2015;94:e371. doi: 10.1097/MD.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]