Abstract
Radiotherapy is one of the most important treatment options for localized early-stage or advanced-stage prostate cancer (CaP). Radioresistance (relapse after radiotherapy) is a major challenge for the current radiotherapy. There is great interest in investigating mechanisms of radioresistance and developing novel treatment strategies to overcome radioresistance. MicroRNAs (miRNAs) are small, non-coding RNAs that regulate gene expression at the post-transcriptional level, participating in numerous physiological and pathological processes including cancer invasion, progression, metastasis and therapeutic resistance. Emerging evidence indicates that miRNAs play a critical role in the modulation of key cellular pathways that mediate response to radiation, influencing the radiosensitivity of the cancer cells through interplaying with other biological processes such as cell cycle checkpoints, apoptosis, autophagy, epithelial-mesenchymal transition and cancer stem cells. Here, we summarize several important miRNAs in CaP radiation response and then discuss the regulation of the major signalling pathways and biological processes by miRNAs in CaP radiotherapy. Finally, we emphasize on microRNAs as potential predictive biomarkers and/or therapeutic targets to improve CaP radiosensitivity.
Keywords: Prostate cancer, MicroRNAs, Radiotherapy, Radioresistance, Signalling pathway.
Introduction
Prostate cancer (CaP) remains a significant medical problem in western countries and accounts for estimated 161,360 new cases and 26,730 deaths in the USA in 2017 1. Around 70% of CaP patients present with organ-confined disease, with the majority presenting with low- or intermediate-risk CaP 2. At the early stage, patients can be treated effectively with surgery with or without radiotherapy (RT). RT is an important treatment option for CaP patients with early-stage or advanced-stage disease.
RT, through ionizing radiation (IR), damages cancer cells by producing intermediate ions and free radicals at various levels, particularly but not only on the DNA, causing DNA single-strand breaks (SSBs) or double-strand breaks (DSBs), triggering a number of signalling pathways mediating the DNA damage response (DDR). DDR is the determining factor of the therapeutic effect, during which the most relevant processes are DSB repair pathways including homologous recombination (HR) and non-homologous end-joining (NHEJ) pathways 3.
In CaP clinical settings, RT is an effective therapeutic modality for clinically localized CaP. Common RT modalities include external beam radiation therapy (EBRT) and radioactive isotopes as used in brachytherapy. Over the several past decades, there has been a rapid advancement in the treatment of CaP with EBRT. The previous standard RT was 3-dimensional (3D) conformal radiation therapy (3D-CRT), where multiple shaped radiation beams were used to limit dose to adjacent tissues rather than the prostate 4. Later, intensity modulated radiation therapy (IMRT) for CaP was introduced in the mid-1990s, which creates dynamic fields that vary in intensity across their cross section, providing a more close conformity to a specific tumor region 5. In addition, proton beam radiation and carbon ion RT offer superior dose conformity in the treatment of deep-seated malignant tumors compared with conventional X-ray therapy 6, 7. The long-term results of proton beam radiation yield comparable disease-free survival to alternative treatments for localized CaP with minimal morbidity 8. Moreover, excellent outcomes for biochemical relapse-free survival were obtained from carbon ion RT in high-risk CaP compared with the photon or proton radiation 9.
Several phase III trials for localized CaP patients have shown the benefit of dose escalation with the high-dose conformal RT with conventional 2 Gy daily fractions to a total dose of 74 Gy 10, 11. However, it was found that the high-dose RT (74-80 Gy) is associated with an increased risk of late grade 2 or higher gastrointestinal toxicity compared with lower doses of RT (64-70.2 Gy) 12. Hypofractionated RT is the delivery of fewer and larger fractions of radiation. The recent randomised, phase III CHHiP trial recommended hypofractionated RT as a new standard therapy of EBRT for localized CaP, which may improve the therapeutic ratio, resource usage and patient convenience 13.
Despite advances in the approaches of delivering radiation to the prostate gland of CaP patients, failure to control a tumor with a curative dosage is a major problem in RT which eventually occurs in almost one-third of CaP patients 14. The relapse after RT can be attributed to many factors such as geographic miss, changes in the target volume, disseminated tumor cells (occult metastases) and micro-metastases at the time of diagnosis, and most importantly, radioresistance of tumor cells 15.
Radioresistance may arise from many biological processes including mutations of oncogenes and/or tumor suppressors, deregulated signalling pathways, abnormal DDR, as well as disordered cell death and cell cycle checkpoint 16. During the past decades, considerable efforts have been put into the investigation of these contributing factors. However, direct manipulation of coding genes and essential signalling pathways in vivo are difficult due to technical issues on stable gene delivery and expression, as well as diverse crosstalk among different signalling pathways, and altering coding genes often causes substantial toxicity and side effects.
MicroRNAs (miRNAs) are a class of endogenous, small non-coding RNAs composed of ~22 nucleotides. They play an important role in the regulation of gene expression at the post-transcriptional level by targeting mRNAs, whereby modulating various processes including cell growth, differentiation, cell cycle and cell death 17. As inherent gene regulators, miRNAs could offer new predictive and therapeutic possibilities. It was not until recent years have miRNAs been recognized as an important regulator of radiation response. Accumulating evidence has shown that there is a close link between miRNA expression and radioresistance in cancers 18, 19, 20, 21, 22, 23. Nevertheless, the number of studies on the regulatory mechanisms of miRNA in CaP radioresistance is very limited and the understanding of the functional role exerted by individual miRNAs in CaP radioresistance is largely in their infancy and requires a great number of further studies. In this review, we summarize the roles of miRNA in CaP, elaborate on miRNAs in CaP radiation response, discuss the putative roles of miRNA-related signalling pathways and biological processes in various cancers including CaP, and finally emphasize its potentials as predictive biomarkers and therapeutic targets in CaP RT.
miRNA in CaP
It becomes increasingly evident that miRNAs function as a negative regulator of gene expressions by binding to complementary mRNAs, resulting in translational repression and mRNA degradation. As such, dysfunction of miRNAs is known to disrupt the expression of mRNAs and proteins, and has been found to be involved in various human cancers, including CaP 24. It was found that about 26% (12 out of 47) long-range epigenetic silencing contained miRNA genes in 4 CaP cell lines (LNCaP, DU145, PC3 and MDA-2A) 25. As there are more than 2000 unique human miRNAs in the miRNAnome (miRbase.org) with hundreds of predicted targets individually, their role in CaP carcinogenesis and therapeutic resistance is widespread and remains elusive. A recent review has demonstrated that there are 30 miRNA studies associated with CaP diagnosis and another over 30 miRNA studies related with CaP prognosis 26. In one study, Volinia et al. identified 39 up-regulated miRNAs and 6 down-regulated miRNAs out of 45 human CaP primary tumor samples compared with normal prostate tissues from a miRnome analysis 27. In another study, Porkka et al. detected differential expression of 51 individual miRNAs between benign prostatic hyperplasia (BPH) and CaP tissues, 37 of them showing down-regulation and 14 up-regulation in CaP samples 28. Sun et al. demonstrated that up-regulation of miR-221 reprogrammed androgen receptor (AR) signalling and promoted the transition to castration-resistant CaP (CRPC) 29. More recently, the tumor-suppressive miR-221/222 cluster was identified in one study demonstrating the down-regulation of miR-221/222 cluster enhanced migration and invasion in CaP cells which led to CRPC 30.
The differences from various studies imply that the regulatory role of miRNAs in CaP carcinogenesis and progression is still inconclusive, with the explanation being the complexity of miRNAnome and diversity of mRNA targets for each miRNA. Also, the genetic and microenvironmental tumor heterogeneity may confound the identification of miRNA expression patterns 23. Despite the conflicting results, miRNAs have been proved as a biomarker for prognosis of CaP in several studies. Guan et al. found the up-regulation of miR-21 as an independent predictor of progression-free survival (PFS) in patients with advanced CaP 31. Spahn et al. demonstrated that down-regulation of miR-221 was associated with clinicopathological parameters, including the Gleason score and the clinical recurrence during follow-up in 92 CaP patients 32. Introduction of ectopic miR-145 in 22Rv1 CaP cells generated an inhibitory effect on the AR at both transcript and protein levels as well as its activity and downstream targets PSA, hence reduce the transformation to the deadly CRPC 33. In addition, it was reported that CaP patients with post-surgery biochemical relapse displayed a distinct expression profile of 16 miRNAs in formalin-fixed paraffin-embedded prostatectomy specimens, as compared with non-relapsed patients 34, indicating that aberrant miRNA expression features may reflect a tendency for early disease relapse. Similarly, miR-145 expression was found to be significantly increased in patients demonstrating good response (PSA<2.0ng/mL/year) to neoadjuvant RT, while the expression of miR-145-regulated γ-H2AX gene was significantly decreased 35. Moreover, up-regulated miR-135b, miR-194, miR-222 and miR-125b were observed to predict the risk of early recurrence in CaP 34, 36. Several different studies in evaluating the expression of specific miRNAs in CaP tissue samples indicated that the expression levels of miRNAs including miR-205 37, 38, miR-224 39, 40, miR-21 41, miR-126 42, miR-34b 43 and miR-15/16 44 are progressively deregulated in lymph node or bone metastases and correlated with clinical outcome, thus supporting a possible role of these miRNAs as prognostic biomarkers and novel therapeutic targets 45.
Taken together, altered miRNA expression is highly prevalent in CaP and miRNA expression profiles have been found to be closely correlated with unique pathophysiological features in CaP, which still requires rigorous biological and statistical validation in addition to a clear definition of applicable target populations. Elucidation of differentially expressed miRNAs presents an attractive avenue toward the development of novel CaP prognostic indicators and therapeutic targets.
miRNA in CaP radiation response
One of the approaches in defining the changes of miRNAs in response to IR is to determine which one exhibits significant changes after IR compared with controls 20, 46, 47. Accumulating evidence demonstrates that exposure to IR can significantly alter miRNA expression levels in cancer cells including CaP 48, 49. Leung et al. exposed PC-3 CaP cells to different dose levels of radiation and identified 6 increased miRNAs (miR-9, miR-22, miR-25, miR-30a, miR-550a and miR-548h) and 16 decreased miRNAs (let-7c/d/e, miR-15a, miR-17, miR-30d, miR-92a, miR-125a, miR-197, miR-221, miR-320b, miR-342, miR-361, miR-374a, miR-501 and miR-671) compared with the parental cells 50.
MiR-106b is one of the 3 highly conserved miRNAs within the miR-106b-25 cluster located in a 515-bp region at chromosome 7q22, in intron 13 of the host gene MCM7 51. The members of the cluster are co-transcribed in the context of the primary transcript of MCM7, the over-expression of which has been associated with worse prognosis in CaP 52. Expression of miR-106b was found to be decreased by 3.3-fold 24 hours post-radiation in LNCaP CaP cells compared with untreated parental cells 53, indicating that miR-106b expression is modulated by IR. MiR-449a is the best-characterized member of the miR-449 family (miR-449a, miR-449b and miR-449c), deregulated in various types of cancers including CaP 54, 55. Mao et al. found that miR-449a was markedly up-regulated in LNCaP cells following IR and its expression reached the maximum level 24 hours post-IR with 4 Gy X-ray, and overexpression of miR-449a enhanced radiosensitivity by down-regulating c-Myc in LNCaP CaP cells 56, suggesting its important role in CaP radioresistance. MiR-521 was also found to be significantly altered in response to radiation treatment in CaP 57. It was demonstrated that overexpression of miR-521 significantly sensitized LNCaP and C4-2 CaP cells to RT while ectopic inhibition of miR-521 resulted in radioresistance 57. Further study indicated that miR-521 modulates radiosensitivity through Cockayne syndrome protein A (CSA) (a DNA repair protein) and the levels of miR-521 are correlated inversely with the levels of CSA 57.
let-7 was initially found as the second miRNA after lin-4 in Caenorhabditis elegans 58. The human let-7 family consists of let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7g, let-7i and miR-98. This family is commonly viewed as a tumor suppressor consistent with down-regulation of oncogenes such as Ras 59 and c-Myc 60 by binding to 3'-UTR of these target mRNAs. Moreover, decreased let-7 expression was found in many cancers, including CaP 61. John-Aryankalayil et al. compared miRNA expression in LNCaP, PC-3 and DU145 CaP cells treated with the single-dose radiation in the same CaP cells treated with fractionated radiation by microarray, and found that fractionated radiation significantly altered more miRNAs as compared to single-dose radiation 49. Moreover, tumor suppressor miR-34a and let-7 were found to be up-regulated by fractionated radiation in radiosensitive LNCaP (p53-positive) and PC-3 (p53-null) cells, indicating that radiation-induced miRNA expression may not be regulated by one factor (p53) alone, but potentially by multiple pathways, which will be discussed in detail next. Using a global analysis and a miRNA PCR array data analysis, our group found that the expressions of miR-200 family (miR-200a/b/c, miR-141 and miR-429), miR-4474, miR-7641 and miR-4521 are repressed in CaP-radioresistant (RR) cell lines compared to CaP-radiosensitive cell lines (unpublished data), suggesting these miRNAs are involved in CaP radioresistance. The miRNA expressions altered by IR in CaP are summarized in Table 1.
Table 1.
miRNAs modulated by IR in CaP
| miRNA expression | IR dosage | Model | Reference |
|---|---|---|---|
| 116 miRNAs aberrant expression | 5 Gy and 10 Gy, single dose, and 0.5 Gy × 10 and 1 Gy × 10, fractionated | PC-3, DU145, LNCaP cell lines | John-Aryankalayil et al., 2012 [49] |
| miR-521↑, miR-196a↑, miR-133b↑, miR-487↑, miR-122a↑, miR-145↑, miR-100↑, miR-107↑, miR-187↑, miR-UL22A-1↑; miR-34c↓, miR-372↓, miR-520c↓, miR-520f↓, miR-449↓ | 6 Gy, single dose | LNCaP cell line | Josson et al., 2008 [57] |
| miR-521↑, miR-196a↑, miR-133b↑, miR-143↑, miR-135b↑, miR-218↑; miR-34c↓, miR-383↓, miR-154*↓, miR-488↓, miR-379↓ | 6 Gy, single dose | C4-2 cell line | Josson et al., 2008 [57] |
| miR-106b↓, miR199a↓, miR-29b↑, miR-191↑, miR-22↑, miR-200c↑, miR-141↑, miR-24↑, miR-30a-5p↑, miR-9-1↑ | 6 Gy, single dose | LNCaP cell line | Li et al., 2011 [53] |
| miR-9↑, miR-22↑, miR-25↑, miR-30a↑, miR-550a↑, miR-548h↑; let-7c/d/e↓, miR-15a↓, miR-17↓, miR-30d↓, miR-92a↓, miR-125a↓, miR-197↓, miR-221↓, miR-181a, -1207-5p, -20b, -19b, -3156, -3175, -92a-1*, -503 and -224 , miR-342↓, miR-361↓, miR-374a↓, miR-501↓, miR-671↓ | 10 Gy, single dose | PC-3 cell line | Leung et al., 2014 [50] |
| miR-449a, b and c↑ | 4 Gy, single dose | LNCaP cell line | Mao et al., 2014 [56] |
| miR-1207-5p↓, miR-20b↓, miR-19b↓, miR-3156↓, miR-3175↓, miR-92a-1*↓, miR-503↓, miR-224↓ | 5 Gy and 10 Gy, single dose | LNCaP cell line | Morii et al., 2013 [62] |
| miR-200-5p↓, miR-4474-3p↓, miR-7704↓, miR-7641↓, miR-4521↓, miR-6746-5p↑ | 2 Gy ×5, fractionated | PC-3RR, DU145RR, LNCaPRR and 22Rv1RR cell lines versus PC-3, DU145, LNCaP and 22Rv1 cell lines | unpublished data |
Notes: ↑, increased; ↓, decreased; RR, radioresistant.
It is worth mentioning that, in many cases, miRNAs changes reported in one study were not highlighted in another one, indicating the changes are not consistent or common. This observation suggests that other potential targets may exist and other conflicting factors need to be further elucidated. To the best of our knowledge, the changes of miRNA profile induced by IR in CaP are only limited in in vitro cell line studies. Therefore, the study of miRNA changes after IR from animal models or human samples are needed to deeply investigate its roles in CaP radiation response or radioresistance in the future.
Putative roles of miRNAs as a regulator of signalling pathways in cancer radioresistance
PI3K/Akt/mTOR pathway
The PI3K/Akt/mTOR signalling pathway is implicated in a diverse array of cellular functions including survival, growth, proliferation, differentiation, stem cell-like properties, metabolism and angiogenesis 63. This pathway is closely associated with 3 major radiation resistance mechanisms including intrinsic radiosensitivity, tumor cell proliferation ability and hypoxia microenvironment 64 and is regulated by several miRNAs at different levels. A key downstream molecule in this pathway, Akt, is directly mediated by miRNA regulation. Evidence showed that miR-302a sensitized radioresistant breast cancer cells to radiation therapy in vitro and in vivo and reduced the expression of Akt1 65, providing a rationale for investigating the role of this miRNA in CaP cell survival and cell signalling after RT. The suppression of radiation-induced Akt activation was also observed in U373 malignant glioma cells as a consequence of miR-21 silencing by synthetic inhibitors 66.
The PI3K/Akt/mTOR signalling pathway was mediated by phosphatase and tensin homolog (PTEN), a negative regulator of Akt expression. It was reported increased miR-21 was induced after IR, facilitating the repression of PTEN and the activation of Akt to promote the DNA damage repair and cell survival in U251 glioblastoma cell line 67, and several articles have demonstrated sensitisation to IR by antagonising miR-21, ultimately inhibited the PI3K/Akt pathway in malignant glioma cell lines 66 and esophageal cancer cell lines 68, suggesting it is worthwhile testing miR-21 in CaP-RR cells. Similarly, miR-205 and miR-221/222 were found to regulate radiosensitivity via direct modulation of PTEN in gastric 69 and nasopharyngeal 70 cancers. In CaP, it was found that siRNA-mediated knockdown of SGPP1 ugregulated phospho-Akt levels in PC-3 CaP cells and conferred increased radiation resistance in PC-3 and DU145 CaP cells, phenocopying that seen in miR-95-overexpressing PC-3 and DU145 CaP cell lines 71. However, the direct link between miRNAs and the PI3K/Akt signalling pathway in CaP radioresistance has not been reported so far. It will be of great interest to investigate the role of miRNAs in the regulation of the PI3K/Akt pathway in the future study. Targeting miRNAs to repress the PI3K/Akt signalling pathway may become a valid approach to treating CaP and overcoming radioresistance.
MAPK/ERK pathway
The MAPK pathway was demonstrated to be associated with growth factor-mediated regulation of cellular events such as proliferation, differentiation and apoptosis 72. Particularly, the MAPK/ERK pathway, also known as the 'classical' MAPK pathway, is closely related with IR. After stimulating EGFR family receptors by radiation, the MAPK pathway activates the downstream protein Ras, leading to translocation of the RAF proteins to the plasma membrane. The active form of RAF, RAF-1, phosphorylates MAPK kinase 1/2 (MEK1/2), which subsequently phosphorylates extracellular signal-regulated kinase 1/2 (ERK1/2), altering transcription factor function and the apoptotic threshold of cells 73. The RAF/MEK/ERK pathway is critical for efficient DNA damage repair 74.
Similar to the PI3K/Akt pathway, the MAPK pathway has a number of miRNA regulators. Members of the let-7 family were reported to down-regulate the expression of K-Ras (a protein in Ras family) and the overexpression of let-7a decreased expression of K-Ras and radiosensitized A549 lung carcinoma cells 75. Another study also demonstrated that overexpression of let-7 family increased the cellular radiation response, whereas decreasing their levels causes radioresistance, partly through control of the proto-oncogene homologue let-60/Ras and genes in the DNA damage response pathway in A549 lung cancer cells in vitro and in a Caenorhabditis el egans model in vivo 48. These two studies indicate that let-7 plays an important role in the Ras protein and radiation response in lung cancer cells.
MiR-17-5p is usually overexpressed and acts as an oncomiR in tumor to effectively activate the p38 MAPK pathway and elevate the phosphorylation level of heat shock protein 27 (hsp27) 64. The p38-MAPK pathway was found to play a role in the phosphorylation of hsp27 induced by miR-17-5p, promoting tumor invasion and metastasis in Huh-7 and HepG2 hepatocellular carcinoma cells in vitro and in vivo 76. Hence, regulating the expression of miR-17-5p and interfering its interaction with the MAPK/ERK signalling pathway may have potential to exploit the possible mechanisms of radioresistance and improve CaP radiation response.
Putative roles of miRNAs as a regulator of different cellular biological events in CaP radioresistance
DDR
DDR is an essential process during IR, which can maintain genome integrity in cells by reversing the exogenous and endogenous DNA damage and is indispensable in cell survival during DNA replication 77. Cells have evolved high conservative yet diverse DDR pathways, which contain three major components: sensors, signal transducers and effectors.
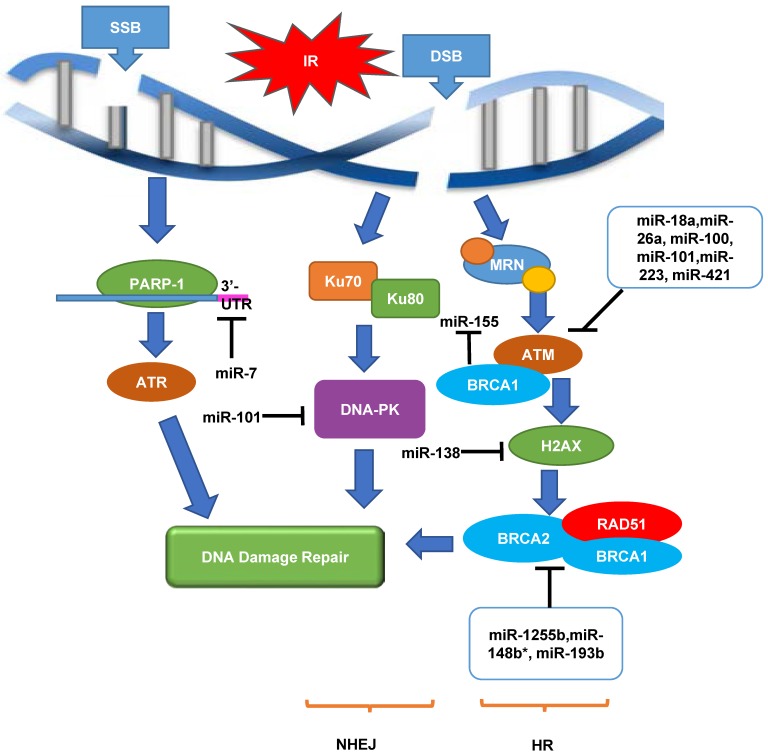
Initial step after damage by IR includes DNA damage sensing and cell cycle checkpoint activation. SSB sensing is often performed by poly [ADP-ribose] polymerase-1 (PARP-1) while the sensing of DSB, the more common form of IR-induced damage, is conducted by MRN complex (MRE11/RAD50/NBS1). Up to date, there are no validated miRNAs that target PARP-1 or the MRN complex.
At the level of transducers, ATM and ATR (ATM-Rad3-related) are proximal kinases in the central of the entire DDR and can be used to detect various forms of damaged DNA and trigger the downstream DDR cascades 78. ATR generally responds to SSBs while ATM is the main activator of the DDR after DSBs. As DSB is the more common form of IR-induced damage, the miRNA regulation of ATM will be discussed in detail. ATM is directly regulated by several miRNAs in tumor cells, including miR-18a in colorectal cancer 79, miR-26a in glioblastoma 80, miR-100 in glioma 81, miR-101 in lung cancer and glioblastoma 82, miR-223 in glioblastoma 83 and miR-421 in neuroblastoma 84. In particular, Hu et al. discovered that miR-421 suppressed ATM expression by targeting the 3'-UTR of ATM transcripts in LA-N-1 and LA-N-5 neuroblastoma cells in vitro 84. Ectopic expression of miR-421 resulted in S-phase cell cycle checkpoint changes and sensitised LA-N-1 and LA-N-5 neuroblastoma cells to IR, and blocking the interaction between miR-421 and ATM 3'-UTR rescued the defective phenotype caused by miR-421 overexpression, indicating that ATM mediates the effect of miR-421 on cell cycle checkpoint and radiosensitivity 84. The findings from Hu et al.'s study were supported by another study in which miR-421 was found to be able to induce clinical-manifested radiosensitivity in SKX head and neck squamous cell carcinoma in vitro 85, further confirming the putative role of miR-421 in the regulation of ATM and radiation response. ATM is responsible for the phosphorylation of several downstream effectors, including histone H2AX, a mediator of DSB repair that recruits DNA repair proteins to the site of damage. MiR-138 was found to directly target the histone H2AX 3'-UTR, reduce histone H2AX expression, and induce chromosomal instability after DNA damage in U2OS osteosarcoma cell line 86. Overexpression of miR-138 in U2OS osteosarcoma cell line inhibited HR pathway and enhanced cellular sensitivity to IR, causing increased chromosomal instability after DNA damage 86.
DNA-dependent protein kinase (DNA-PK) is another important effector in the DDR (NHEJ pathway). Yan et al. demonstrated that miR-101 could target the 3′ -UTR regions of DNA-PK and ATM mRNA, and up-regulating miR-101 efficiently reduced the protein levels of DNA-PKcs and ATM in U87MGD malignant glioma cells and 95C lung cancer cells, sensitizing the tumor cells to IR in vitro and in vivo 82.
Apart from ATM and DNA-PK, BRCA1 and BRCA2 are also important tumor suppressor genes. A recent genomic analysis revealed that inactivating mutations in BRCA1 or BRCA2 or other genes involved in the HR pathway of DNA repair collectively occur in as much as 20-25% of advanced CaP 87, indicating that BRCA1 and BRCA2 play an essential role in DNA damage repair pathways, specifically the HR pathway. A synthetic lethal therapeutic approach using PARP inhibitor has been developed for BRCA mutant- and HR deficient-related cancers including metastatic CRPC and is being investigated in multiple Phase I and Phase II clinical trials (NCT01972217, NCT02861573 and NCT01682772). Chang et al. found that BRCA1 epigenetically represses miR-155 expression via its association with HDAC2, which deacetylates histones H2A and H3 on the miR-155 promoter 88, and overexpression of miR-155 accelerated but the knockdown of miR-155 attenuated the growth of MDA-MB-468 breast cancer cells in vivo 88, suggesting that miR-155 may have the potential to treat BRCA1-associated CaP by regulating DDR and increase radiosensitivity. It was also found that the transcripts of HR factors including BRCA1, BRCA2 and RAD51 can be targeted by miR-1255b, miR-148b* and miR-193b*, and inhibiting these miRNAs increased expression of BRCA1/BRCA2/RAD51, specifically in the G1-phase, which blocked NHEJ impairing DSB repair in MDA-MB-231 breast cancer cell line in vitro 89. A recent study showed that two potent IR sensitizing miRNAs (miR-890 and miR-744-3p) significantly delayed IR-induced DNA damage repair in CaP. In this study, miR-890 directly targeted MAD2L2, as well as WEE1 and XPC, whereas miR-744-3p directly targeted RAD23B 90.
The putative regulatory roles of miRNAs in DDR in CaP radioresistance are shown in Figure 1. Taken together, it is tempting to say that investigation of regulatory mechanisms between miRNA and DDR genes will possibly lead to the discovery of novel and promising targets to overcome CaP radioresistance.
Figure 1.
An overview of roles of miRNAs as a regulator of DDR in response to radiation in cancers. When SSB or DSB occurs, DNA damage repair pathway is initiated. This pathway contains a cascade of components including sensors (MRN and PAPR-1), transducers (ATM and ATR) and effectors (DNA-PK, RAD51 and BRCA1/2), in order to achieve DNA damage repair through NHEJ and HR pathways. Multiple miRNAs play important roles in up-regulating or down-regulating these biological processes in cancer radiation response. ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia and Rad3-related protein; DNA-PK, DNA-dependent protein kinase; DSB, double-strand breaks; HR, homologous recombination; IR, irradiation; MRN, MRE11/RAD50/NBS1; NHEJ, non-homologous end joining; PARP-1, Poly [ADP-ribose] polymerase 1; SSB, single-strand breaks. '⊣' indicates down-regulation and '⟶' indicates up-regulation.
Cell cycle checkpoint activation
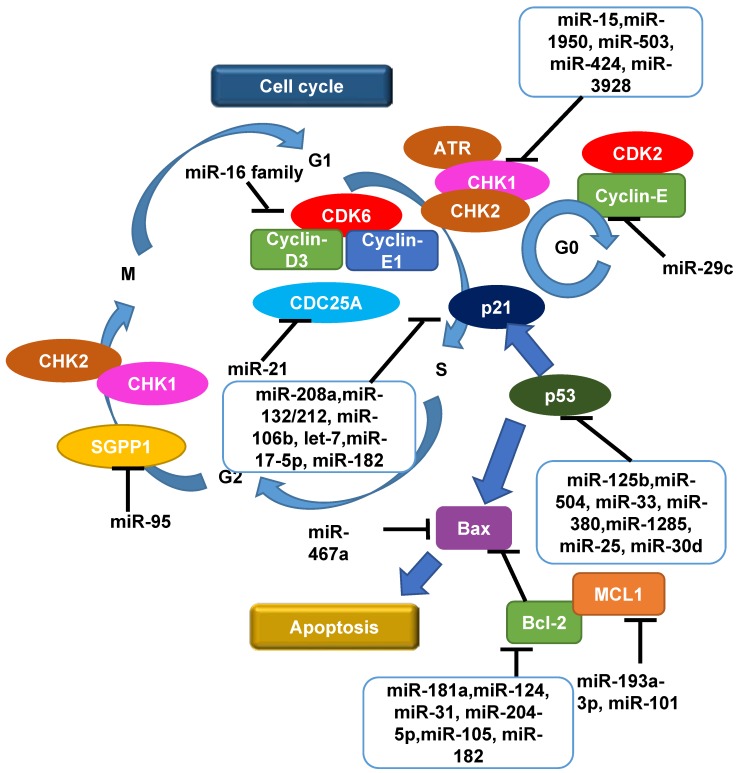
The eukaryotic cell cycle consists of four phases and transition from one to the other is regulated by Cyclins and Cyclin-dependent kinases (CDKs). Following the induction of DNA damage, cell cycle checkpoints are triggered to interrupt cell cycle progression to allow time for DNA damage repair by targeting the Cyclin/CDKs complexes that normally promote cell cycle progression. Once the damage is repaired, cells will re-enter the cell cycle; if the damage is too broad or severe, cells will go on to apoptosis or senescence 91. Cell cycle checkpoint activation is pivotal for safeguarding genomic stability. Otherwise, there will be an increasing rate of genomic instability, resulting in tumorigenesis. The cell has two main checkpoints to maintain genomic stability throughout the cell cycle; one at the G1/S interphase and the other at the G2/M interphase, which is thought to be able to prevent cells from replication or undergoing mitosis, respectively, in the presence of DNA damage 92. Interfering the cell cycle process contributes to the tumor radiation response 93.
At the G1/S checkpoint, multiple miRNAs regulate Cyclins and CDKs. It was reported that miR-29c was found to be frequently down-regulated in 5 esophageal squamous cell carcinoma cell lines (KYSE150, KYSE410, KYSE450, KYSE510 and EC9706) in vitro and 60 advanced human esophageal squamous cell carcinoma tissue samples 94. Overexpression of miR-29c suppressed EC9706 and KYSE150 esophageal squamous cell carcinoma cell growth in vitro by inducing cell cycle G1/G0 arrest mainly through modulating Cyclin-E expression 94. Another report showed that the miR-16 family triggers an accumulation of A549 lung carcinoma cells in G1 arrest by silencing multiple cell cycle genes including Cyclin-D3, Cyclin-E1 and CDK6 simultaneously 95. These studies support a key role of miRNAs in regulating the cell cycle.
CHK1 and CHK2 are the two most common cell cycle checkpoint proteins and both of them were found to be activated in CaP-RR cells 96. Recent results suggested that a post-transcriptional regulation mechanism, involving miR-195 and miR-503, was inversely correlated with expression of CHK1 in RR AGS and MKN45 gastric cancer cells 97. Another report also showed that expression of the miR-15 family contributes to increased radiosensitivity of MCF-7 and MDA-MB-231 breast cancer cells by targeting 3'-UTR of CHK1 98. MiR-3928 was shown to bind directly to the 3'-UTR of Dicer mRNA, which consequently induced DNA damage, activated ATR, and phosphorylated CHK1 accompanied by G1 arrest in response to IR in HeLa cervical carcinoma cells and 786-O renal cell carcinoma cells 99. Xu et al. reported that enforced expression of miR-424 inhibited the growth of SiHa and CaSki cervical cancer cells by both enhancing apoptosis and blocking G1/S transition, as well as suppressing the expression of CHK1 and phosphorylated CHK1 by targeting the 3'-UTR of CHK1, which implies that miR-424 functions as a tumor suppressor and cell cycle checkpoint regulator in cancer progression 100.
CDC25A is a phosphatase serving as another cell cycle checkpoint effector that plays an important role in checkpoints by activating CDKs to lift the checkpoint arrest. CDC25A is a direct target of miR-21 in colon cancer 101, let-7 in lung cancer 102 and miR-449a/b in osteosarcoma 103 in DDR. In particular, Wang et al. identified that, in RKO and DLD1 colon cancer cell lines, miR-21 suppressed CDC25A expression through a defined sequence in its 3'-UTR, and miR-21 was induced by DNA damage, negatively regulates G1/S transition and participates in DNA damage-induced G2/M checkpoint through down-regulation of CDC25A 101. Li et al. found that miR-21 expression was up-regulated in response to IR in U251 glioblastoma cells, suggesting that miR-21 could be involved in the response of glioblastoma cancer cells to radiation and used as a biomarker to predict RT response 67.
While the cell cycle arrest driven by CDC25A is transient, the more long-term arrest effect is achieved by p53. The p53-dependent G1/S checkpoint not only functions to permanently arrest damaged cells but also to temporarily prolong the time for repair 92. p53 is a well-studied tumor suppressor and closely related to radiosensitivity in various cancers including CaP 104, 105, which is also regulated by miRNAs 106. Several recent publications demonstrated that miRNAs contribute to the tight control of p53 by directly interacting with the 3ʹ-UTR of TP53 mRNA, including miR-125b, miR-504, miR-33, miR-380, miR-1285, miR-25 and miR-30d 106. Le et al. found that IR can repress expression of miR-125b in zebrafish embryos and that this repression contributes to the radiation-induced expression of p53, leading to stress-induced apoptosis 107. In return, it was shown that p53 also mediates the processing and functions of miRNAs on different levels including transcription induction, processing by Drosha and Dicer, and mRNA target selection via interaction with RNA-binding-motif protein 38 in U2OS osteosarcoma and MCF-7 breast cancer cells in vitro 106, 108. p21, one key component in the downstream of p53, is also regulated by miRNAs in cancer radioresistance, including miR-208a and miR-132/212 cluster in lung cancer 109, 110, miR-106b in colon and CaP 53, 111, let-7 in cervical cancer 112, miR-17-5p in oral squamous cell carcinoma 113. Interestingly, miR-106b was shown to be able to override radiation-induced cell cycle arrest mediated by p21, and can be used as a potential therapeutic target in LNCaP and MDA-PCa-2b CaP cells whose radioresistance is likely due to a consistently elevated level of miR-106b 53.
Moreover, at G2/M checkpoint, it was reported that miR-95 overexpression recapitulated an aggressive phenotype including deregulated G2/M checkpoint following IR and increased invasive potential in PC-3 CaP and MDA-MB-231 breast cancer cell lines in vitro and s.c mouse models in vivo, by targeting sphingolipid phosphatase SGPP1, an antagonist of sphingosine-1-phosphate signalling 71, indicating that G2 to M phase transition is also subject to miRNA regulation. The roles of miRNAs as a regulator of cell cycle in cancer radiation treatment including CaP are shown in Figure 2.
Figure 2.
An overview of roles of miRNAs as a regulator of cell cycle and apoptosis in radiation response in cancers including CaP. Following the induction of DNA damage by radiation, the reaction that initiates the G1/S or G2/M cell cycle checkpoints is phosphorylation of CHK1 and CHK2 by ATM or ATR. Cell cycle progression is then interrupted to allow time for DNA damage repair by targeting the Cyclin/CDKs complexes whereby affect other components in cell cycle including CDC25, p53 and p21, in order to achieve cell cycle arrest and apoptosis. Multiple miRNAs play important roles in up-regulating or down-regulating these biological processes. CDK, cyclin-dependent kinase; CHK, checkpoint kinase; MCL1, myeloid cell leukaemia 1; SGPP1, sphingosine-1-phosphate phosphatase 1; '⊣' indicates down-regulation and '⟶' indicates up-regulation.
Apoptosis and autophagy
Failure to repair radiation-induced DNA damage triggers cell death directly or indirectly by apoptosis, mitotic catastrophe or senescence. While it is not considered as a major mode of cell death in carcinomas, apoptosis is a major defence mechanism against malignant transformation. In fact, failures in normal apoptotic pathways contribute to carcinogenesis and tumor cells use a variety of molecular mechanisms to evade apoptosis. Thus, an agent that can selectively enhance apoptotic cell death in malignant cells without affecting normal cells would be an ideal selection for cancer therapy. Not surprisingly, miRNA plays a regulatory role in apoptotic cell death. Two core pathways (the extrinsic-death receptor pathway and intrinsic-mitochondrial pathway) exist to induce cancer cell apoptosis 114. The extrinsic pathway is triggered mainly by binding of Fas plasma-membrane death receptor with its extracellular ligand-Fas-L. The complex of Fas/FasL then activates its pro-caspase-8, which proceeds to trigger pro-caspase-3 for execution of the apoptotic process 115. Gao et al. suggest that miR-467a is oncogenic in radiation-induced thymic lymphoma cells and overexpression of miR-467a may confer a growth advantage on thymic lymphoma tumor cells via ectopic expression of Fas and Bax 116. MiR-23a/b were found to be up-regulated in radiation-induced thymic lymphoma tissue samples, and cell death and apoptosis were increased by miR-23a/b inhibitor (antisense oligos) and decreased by miR-23a/b mimics in EL4 lymphoma cells in vitro 117. Knockdown of miR-155 using anti-miR155 miRCURY LNA™ probes in OCI-AML3 acute myeloid leukaemia cells resulted in an increased expression of cleaved caspase-3, leading to an activation of apoptosis cascade 118. In addition to miR-155, let-7a overexpression confers radioresistance to A431 human squamous carcinoma and HepG2 hepatocellular carcinoma cells in vitro 119.
The intrinsic pathway also leads to apoptosis but under the control of mitochondrial pro-enzymes. One of the most important regulators of this pathway is the Bcl-2 family of proteins including myeloid cell leukemia1 (MCL1) and Bcl-2 itself 120. MCL1 is a direct target of miR-193a-3p in U251 glioblastoma and HeLa cervical cancer cells 121 and miR-101 in HepG2 hepatocellular carcinoma cells 122. Bcl-2 is a target of miR-181a which modulates radiosensitivity in human U87MG malignant glioma cells 123. It was also demonstrated that miR-124 was a negative regulator of MYC and Bcl-2 expression in OCI-Ly1 B-cell lymphoma cell line 124. Körner et al. identified protein kinase C epsilon as a novel direct target of miR-31 which was associated with increased radiosensitivity in MDA-MB-231 breast cancer cells via down-regulation of Bcl-2 125. In CaP, miR-204-5p directly targeted the 3'-UTR of Bcl-2 mRNA leading to increased apoptosis in PC-3, 22Rv1 and LNCaP cell lines 126. In addition, both Bcl-2 and p21 were identified to be potential target genes of miR-182 in PC-3 CaP cells using TargetScan and PicTar in vitro 127. MiR-205 was found to promote apoptosis in PC-3 CaP cells in response to DNA damage by targeting Bcl-2 128. Taken together, various miRNAs play a functional role in modulating apoptosis in different kinds of human cancers including CaP. The regulatory roles of miRNAs in apoptosis in CaP radiation response are summarized in Figure 2.
Autophagy is a regulated and catabolic process which plays an important part in response to IR 129. When uncovering relationships between autophagy and cancer, a common challenge is to determine whether autophagy protects cell survival or contributes to cell death, which remains largely unknown. Similarly, contribution of autophagy to radiation response remains elusive because of some contradicting reports. In BxPC3 and PANC-1 pancreatic cancer cells, reduced levels of the miR-23b were found to increase levels of autophagy to promote radioresistance 130. In line with this finding, Liao et al. found that overexpressed miR-32 reduced IR-induced cell apoptosis by enhancing autophagy in DU145 and PC-3 CaP cells 131, and Wang et al. found that through downregulating NDRG2, miR-301a and miR-301b could decrease autophagy of CaP cells in hypoxia and promote radioresistance 132. On the contrary, overexpression of miR-199a-5p was demonstrated to enhance IR-induced autophagy and sensitised cells to IR in highly invasive MDA-MB-231 breast cancer cells 133. Using flux analysis, Schaaf et al. assessed autophagy activity changes after IR in 8 cell lines including DU145 CaP cell line, and concluded that canonical autophagy does not contribute to cellular radioresistance 134. The discrepancies of the relationships among miRNAs, autophagy and tumor radioresistance may be attributed to the interaction between the promoter regions of the genes or the cell-line specific manner. Collectively, miRNAs play a double-faceted role in regulating autophagy in RT, and further comprehensive investigations are warranted.
Hypoxia
Areas of hypoxia within prostate tumors are known to be involved in radiation response 135. Hypoxia, in addition to a low pH and lack of nutrients, provides a favourable environment for tumor progression and the development of metastases 136. One of the most important hypoxia-associated proteins, hypoxia-inducible factor 1-α (HIF-1α), is a critical transcription factor involved in the adaptation of a number of different types of cancer cells, including prostate, to hypoxic conditions 137. The precise roles of HIF-1α in the regulation of radiosensitivity are highly complicated: there is a close interplay between HIF-1α and radiosensitivity, but the degrees of impact vary by tumor types. In non-small cell lung cancer cells, HIF-1α was up-regulated by miR-21 in RR cells, resulting in promotion of the key enzymes of glycolysis. Inhibition of HIF-1α suppressed glycolysis and re-sensitized the cancer cells to radiation, whereas the recovery of HIF-1α in RR cells resulted in recovery of radioresistance 138. It was recently reported that hypoxia down-regulates Drosha and Dicer in several ovarian and breast cancer cell lines and leads to dysregulation of miRNA biogenesis and increased tumor progression 139. Using the ovarian cancer data from the Cancer Genome Atlas project, it was also observed that higher levels of hypoxia and lower levels of Drosha and Dicer are significantly correlated with worse survival rates in ovarian cancer patients 139. These two observations suggest hypoxia-induced miRNA changes are highly involved in ovarian cancer progression. It is also known the down-regulation of miR-210 by antisense lentiviral vectors in hypoxic human hepatocarcinoma led to increased radiosensitivity, both in SMMC-7721, HepG2 and HuH7 cell lines in vitro 140 and SMMC-7721 mouse model in vivo 141. Further understanding of the regulatory mechanisms of miRNA in tumor hypoxia could lead to enhanced CaP radiosensitivity.
Epithelial-mesenchymal transition (EMT)
EMT is characterized by the loss of adhesive features, enhanced cell motility as well as the acquisition of mesenchymal characteristics, such as invasive motility 142. The role of miRNA in EMT is double-sided. Recently, it was demonstrated that overexpression of miR-144 conferred significant radioresistance on MDA-MB-231 and SKBR-3 breast cancer cell lines via enhancing EMT by inhibiting E-cadherin expression and promoting Snail expression 143. Yoo et al. found that miR-5003-3p functioned as a metastasis activator by promoting EMT through dual regulation of Snail stability and E-cadherin 144. It was demonstrated that hypoxia led to enhanced EMT phenotypes associated with increased expression of miR-21 in PC-3 and LNCaP CaP cell lines 145. Conversely, studies from other groups gave the opposite answer. Ectopic overexpression of miR-449a in LM9 and Huh-7 hepatocellular carcinoma cell lines promoted EMT by attenuating the accumulation of Snail 146. Similarly, several other miRNAs, including miR-143, miR-506 and miR-156a were also found to be a negative regulator of EMT by targeting a diverse range of sites in esophageal, nasopharyngeal and cervical cancers, respectively 147, 148, 149. Our preliminary data showed that forced expression of either miR-200a-5p or miR-4474-3p using miRNA mimics could reverse EMT in PC-3RR CaP cell line (unpublished data). Collectively, whether miRNA promotes or represses EMT remains elusive and is largely in a miRNA- and cell type-specific fashion and further investigation is needed to facilitate the development of novel miRNA-based anti-tumor strategies for personalised medicine.
Cancer stem cell (CSC)
In the last decade, CSC theory has offered an explanation for tumor relapse and therapeutic resistance, and CSCs are closely associated with radiosensitivity in CaP via multiple mechanisms, which has recently been reviewed 150. However, to date, there is no review that systemically discusses the regulatory role of miRNA in CSC-related radioresistance. Recently, the basis of radioresistance was attributed to CSCs which contain lower reactive oxygen species (ROS) and enhanced ROS defences therefore suffer from less DNA damage 151. A limited number of experiments showed that miRNAs are involved in modulating radioresistance via CSCs. For example, CSC marker genes such as Nanog, Oct4 and EZH2 were found to be increased concurrently with the expression of miR-21 in PC-3 and LNCaP CaP cells under hypoxia 145. Rane et al. demonstrated that low expression of miR-99a and miR-100 is present in prostate cancer stem cell (PCSC) populations which are relatively radiation insensitive 152. Strikingly, Rane's group examined 11 randomly selected miRNAs in a CSC phenotype CD133+α2β1hi subpopulation enriched from CaP tissue, and discovered that miRNA expression profile is predominantly dependent on the differentiation stage and can be possibly used to stratify CaP-stem-like cells and CRPC-stem-like cells. The regulation of miRNAs with CSCs in CaP radioresistance is a very interesting research area, and needs to be thoroughly investigated for developing innovative therapies to overcome CaP radioresistance.
The miRNAs involved in regulating radiosensitivity in CaP are summarized in Table 2. The discoveries identified a key regulatory role of miRNAs in various biological processes of CaP cells in response to IR, highlighting the opportunity to develop therapeutic targets aimed at these processes to treat CaP.
Table 2.
miRNAs regulating radiosensitivity in CaP
| miRNA | Model | Target | Effect | Reference |
|---|---|---|---|---|
| miR-32 | DU145 and PC-3 CaP cell lines and normal prostate cell line RWPE-1 | DAB2IP | autophagy ↑ IR-induced apoptosis ↓ | Liao et al., 2015 [131] |
| miR-890 | LNCaP, C4-2, PC-3 and DU145 CaP cell lines | MAD2L2, WEE1 and XPC | IR-induced DNA damage repair ↓ | Hatano et al., 2015 [90] |
| miR-744-3p | LNCaP, C4-2, PC-3 and DU145 cell lines | RAD23B | IR-induced DNA damage repair ↓ | Hatano et al., 2015 [90] |
| miR-145 | LNCaP and PC-3 CaP cell lines; subcutaneous PC3 tumors in mice | n/a | repair of IR-induced DSB ↓ IR-induced tumor growth delay ↑ | Gong et al., 2015 [35] |
| miR-521 | LNCaP and C4-2 cell lines |
CSA | sensitized CaP cells to IR | Josson et al., 2008 [57] |
| miR-145 | PC-3 cell line | DNMT3b | sensitized CaP cells to X-ray radiation | Xue et al., 2015 [153] |
| miR-106b | LNCaP cell line | p21 | overrode IR-induced cell cycle arrest; cell growth ↑ | Li et al., 2011 [53] |
| miR-95 | PC-3 and DU145 cell lines; subcutaneous PC-3 tumors in mice | SGPP1 | proliferation ↑ deregulated G2-M checkpoint and invasive potential in vitro ↑; tumor growth in vivo ↑; resistance to IR in vivo ↑ | Huang et al., 2013 [71] |
| miR-200a-5p, miR-4474-3p | PC-3 radioresistant cells | EMT and CSC | re-sensitized radioresistant cells | unpublished data |
| miR-99a and miR-100 | 22Rv1, LNCaP, PC-3, and DU145 cell lines | SMARCA5 and SMARCD1 | sensitized CaP cells to IR | Rane et al., 2016 [152] |
Abbreviations: CSA, Cockayne syndrome protein A; CSC, cancer stem cell; DAB2IP, DAB2 interacting protein; DNMT3B, DNA methyltransferase 3 beta; EMT, epithelial-mesenchymal transition; MAD2L2, mitotic arrest deficient-like 2; RAD23B, RAD23 homolog B; SGPP1, sphingosine-1-phosphate phosphatase 1; SMARCA5, SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 5; SMARCD1, SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily d, member 1.
miRNAs as biomarkers or therapeutic targets for CaP RT
miRNAs as biomarkers for CaP RT
It is clear that miRNAs are actively involved in tumorigenesis and treatment responses, and mounting data suggest that they may serve as promising diagnostic and prognostic biomarkers in cancer treatment 154, including CaP 155.
Recently, Montani et al. performed a large-scale and comprehensive validation study of the miR-Test based on serum miRNAs in high-risk individuals (n=1115) enrolled in the Continuous Observation of Smoking Subjects (COSMOS) lung cancer screening program. The overall accuracy, sensitivity, and specificity of the miR-Test are 74.9% (95% confidence interval [CI] = 72.2% to 77.6%), 77.8% (95% CI = 64.2% to 91.4%), and 74.8% (95% CI = 72.1% to 77.5%), respectively 156. These striking results support that the miR-based test might be a useful tool for cancer screening in high-risk individuals. Another interesting study conducted by Hur et al. measured the miRNA profile of colorectal cancer patients in primary tumor tissues compared to the matched liver metastases tissues, and identified 23 differentially-expressed miRNAs 157. Four miRNAs that were down-regulated in liver metastases (let-7i, miR-10b, miR-221, and miR-320a) were further validated in primary colorectal tissues and it was found that let-7i expression was statistically significantly reduced, and miR-10b increased in the primary colorectal cancer patients who suffered synchronous distant metastases compared with patients who did not, while miR-221 and miR-320a were non-statistically correlated 157. These unique miRNAs may be clinically applicable to predict prognosis and distant metastasis in colorectal cancer.
In CaP, more than 40 studies performed in investigating miRNAs in whole-blood samples including serum, plasma, peripheral mononuclear cells, exosomes and urine have been published, which was recently reviewed by Fendler et al. 158. From the analysis, it was found the heterogeneity of the disease, differences in population size and study design and disparity in the statistical analysis contribute to the controversial results among the studies. Also, most of the reports identified various miRNA signatures as biomarkers for CaP diagnosis, positive biopsy results and prognosis, with none as biomarkers for the RT response in CaP treatment. The investigation of feasibility and efficacy of miRNAs in clinics for the treatment of CaP is still largely in its infancy.
With the controversial PSA-based screening, overdiagnosis and overtreatment have become a major problem in the management of CaP 159. To alleviate these negative effects, active surveillance (AS) was recently advocated to delay any kind of curative local therapy until the natural history and cancer threat can be more accurately characterized. The selection of AS candidates combines PSA level, Gleason score, clinical stage, life expectancy and percent biopsy involvement 160. However, even when these criteria are strictly used, misclassification rates are still around 20% or higher, remaining as a limitation to the accurate identification of low-risk CaP patients 161. Wang et al. showed that pre-surgical serum levels of miR-19, miR-345 and miR-519c-5p could help identify adverse pathology in CaP patients eligible for AS, independent of their pre-surgical age, PSA, stage, or percent biopsy involvement 162, suggesting that miRNAs can be used as a new way to better select AS candidates in CaP patients.
Preliminary data from Gonzales et al. demonstrated a strong correlation between miR-141 levels and chemotherapy response in CaP patients; thus, it could be potentially used as a useful tool for evaluating prognosis or treatment efficacy of CaP. Perhaps, the full utility of miR-141 could be in a combinatorial, multivariate panel with other validated markers such as PSA, circulating tumor cells (CTC) and lactate dehydrogenase (LDH) 163. There is also one active clinical trial (clinicaltrial.gov identifier: NCT02366494) aiming to identify exosomal miRNAs that predict response to androgen deprivation therapy (ADT) from peripheral blood of CaP patients with systemic disease using next generation sequencing.
In terms of CaP RT, the lack of effective and accurate biomarkers that predict the presence of, or the potential leading to radioresistance in patients, remains a barrier in optimizing treatment strategies. The miRNA profiles identified in patients' body fluids hold promise for prediction of disease stage and treatment response to achieve proper stratification of patients. Gade et al. fused patient tissue-based mRNA and miRNA expression data into one prediction model and improved clinical outcome prediction in 98 CaP patients 164. Santos et al. found that patients diagnosed with high-Gleason score tumors and higher expression levels of miR-221 have an early CRPC compared to patients with lower miR-221 expression levels (10 vs. 46 months, log-rank test P = 0.012) and observed a significantly lower overall survival in patients with higher peripheral whole-blood expression levels of miR-7 (28 vs. 116 months, log-rank test P = 0.001) 165. For the first time, Cheng et al. raised the possibility that serum miR-210 levels could be used to identify a biologically distinct, subset of metastatic CRPC patients for whom the development of alternative therapeutic approaches could be considered 166. It would be of great value to develop a miRNA signature which is capable of predicting radiation treatment response, to achieve personalised therapy.
In radiation research, several miRNAs have been used as a biomarker to minimize the acute and latent radiation-related toxicity. MiR-150, abundant in lymphocytes, exhibited a dose- and time-dependent decrease in serum, which was proposed as a sensitive marker indicative of lymphocyte depletion and bone marrow damage during and after RT 167. Someya et al. identified a good correlation between the post-RT rectal bleeding and the combination of low Ku80 expression and high miR-99a expression in peripheral blood lymphocytes for CaP patients, suggesting that miRNAs, combined with other biomarkers, could form a promising panel for predicting rectal bleeding after RT in CaP 168. Fendler et al. developed a classifier based on two serum miRNAs (miR-30a and miR-126) that can reproducibly predict radiation-induced mortality in radiation-exposed macaques 169. Hamama et al. also reported the overexpression of miR-210 in intestinal samples obtained from patients with radiation enteropathy and fibrotic cultured cells 170, suggesting that miRNAs could serve as an indicator of radiation injury. In the perspective study, detecting miRNA profiles from human bio-fluids such as blood or urine could be useful for predicting radiation response pre-RT and monitoring cancer recurrence after RT in CaP patients.
miRNAs as potential therapeutic targets for CaP RT
Numerous preliminary studies have shown a promising future of miRNA-based application in cancer treatment. As miRNAs play an oncogenic or tumor suppressive role in cancer progression and therapeutic resistance, multiple strategies have been employed to target miRNAs in current cancer treatment. These modern treatment approaches include the use of small molecule inhibitors, miRNA vectors, miRNA mimics, miRNA sponges, the clustered regularly interspaced short palindromic repeat (CRISPR)-Cas9 technology, antisense oligonucleotides and miR-mask oligonucleotides 26. Forced expression of miRNA can be achieved with delivery of miRNA into cells using viral based vectors such as retroviruses, lentiviruses, and adenoviruses or adeno-associated viruses 171. Alternatively, non-viral delivery systems, such as physical and chemical approaches, have also been utilized in delivering miRNA 171, 172. The advantages of non-viral systems are less toxicity, less immunogenicity, and no limitation on the size of the transferred miRNA. The disadvantages are lower transfection efficiency, worse biodegradability and shorter half-lives, largely limiting the use in in vivo studies 173, 174.
MRX34 is a liposomal miR-34a mimic, and recent results from the first in-human, phase I study which assessed the maximum tolerated dose (MTD), safety, pharmacokinetics, and clinical activity of MRX34 in patients with advanced solid tumors showed that MRX34 treatment (by intravenous infusion twice a week for 3 weeks followed by 1 week of rest) with dexamethasone premedication was associated with acceptable safety and showed evidence of antitumor activity in a subset of patients with refractory advanced solid tumors 175. It was also found that intratumoral delivery of miR-890 mimetics prior to IR therapy significantly enhanced IR (6Gy, single dose) therapeutic efficacy in a DU145 subcutaneous (s.c) CaP mouse model in vivo 90.
Aptamers, as chemical antibodies that bind to targets with high affinity and specificity, are a novel agent for both cancer diagnostic and therapeutic applications 176. After conjugated with an aptamer, the miRNA can be specifically delivered to its target that expresses proteins recognized by the aptamer. Esposito et al. engineered a multifunctional aptamer-miRNA conjugate by linking a tumor suppressor let-7g to an aptamer that specifically antagonizes the oncogenic receptor tyrosine kinase Axl, and this conjugate demonstrated tumor-growth inhibiting capacity in A549 lung cancer cells in vitro and in a s.c mouse model in vivo 177. Similarly, intratumoral injection of miRNA mimics may prove beneficial in controlling tumor growth, for example, reconstitution of miR-15a and miR-16-1 expression results in growth arrest, apoptosis and marked regression of LNCaP CaP orthotopic xenografts in vivo 178. On the other hand, miRNAs down-regulation or inhibition can be achieved by using miRNA sponges which equipped with multiple miRNA binding sites targeting endogenous miRNAs, decreasing the expression level of targeted miRNA 179. Alternatively, the latest CRISPR-Cas9 technology could modify miRNA genes, thereby up-regulating or down-regulating the transcription of miRNAs 180. Therefore, once the potential miRNAs associated with CaP radiosensitivity are identified, all above-mentioned miRNA-targeting techniques available could be exploited for developing targeted therapy or combination therapy to sensitize the tumor cells to radiation in order to reduce the radiation dosage and radiation-related toxicity, leading to an improvement of the overall effect of RT.
Although the miRNA-based therapies have made great progress in cancer preclinical studies, the implementation of these techniques in reality is facing with many challenges. First, as mentioned before, there are great variations in the expression of miRNAs among different human cancer types, even among studies of the same tumor type from different research groups. Second, tumor microenvironment plays an essential role in regulating tumor biological behaviour and may confound the identification of miRNA expression. Third, tumor progression during treatment is a dynamic process accompanied with dynamic miRNA expression. Therefore, further technical and scientific development and thorough clinical investigation are warranted to overcome these challenges.
Conclusions
The regulatory roles and mechanisms of miRNAs in radioresistance have been a hot topic in the cancer research for the past decade. The expression of miRNAs is regulated by radiation, and in turn, altered miRNAs play an important role in changing radiation sensitivity via multiple cellular signalling pathways as well as a diverse range of biological processes. Currently, most data on the regulation of miRNAs in CaP radiation research are limited to in vitro cell line study. Their actual role in modulating radiosensitivity in CaP needs to be properly and further defined in in vivo models such as patient-derived xenografts (PDX) as well as in human RR CaP tissues such as patient-derived explant (ex-vivo) model. Additional data validating the diagnostic, prognostic and therapeutic values of miRNA in human samples from CaP patients with RT are much needed over the next few years, which will further strengthen the enthusiasm for this new class of anticancer agents. miRNAs are important biomarkers for predicting CaP radiation response and need to be investigated in human RR CaP samples. Targeting miRNAs using current miRNA-based techniques combined with RT holds promise for overcoming CaP radioresistance. A deeper understanding of the role of miRNAs in cancer radioresistance will not only provide new insights for radiation oncology but also achieve personalized cancer treatment and bring new hope to the CaP patients.
Acknowledgments
Our prostate cancer radiation research project is partly supported by the St George Cancer Care Cancer Research Trust Fund and the Prostate and Breast Cancer Foundation.
Abbreviations
- 3D
three-dimensional
- 3D-CRT
three-dimensional conformal radiation therapy
- ADT
androgen deprivation therapy
- AR
androgen receptor
- AS
active surveillance
- ATM
ataxia telangiectasia mutated
- ATR
ataxia telangiectasia and Rad3-related protein
- BPH
benign prostatic hyperplasia
- CaP
prostate cancer
- CDK
Cyclin-dependent kinases
- CHK
checkpoint kinase
- COSMOS
continuous observation of smoking subjects
- CRISPR
clustered regularly interspaced short palindromic repeat
- CRPC
castration-resistant prostate cancer
- CSA
Cockayne syndrome protein A
- CSC
cancer stem cell
- CTC
circulating tumor cells
- DDR
DNA damage response
- DNA-PK
DNA-dependent protein kinase
- DSB
double-strand break
- EBRT
external beam radiation therapy
- EMT
epithelial-mesenchymal transition
- ERK
extracellular signal-regulated kinase
- HIF-1α
hypoxia-inducible factor 1-α
- HR
homologous recombination
- IMRT
intensity modulated radiation therapy
- IR
ionizing radiation
- LDH
lactate dehydrogenase
- MCL1
myeloid cell leukemia1
- miRNA
microRNA
- MTD
maximum tolerated dose
- NHEJ
non-homologous end-joining
- PARP-1
poly [ADP-ribose] polymerase-1
- PCSC
prostate cancer stem cell
- PDX
patient-derived xenografts
- PFS
progress-free survival
- PTEN
phosphatase and tensin homolog
- RR
radioresistant
- RT
radiotherapy
- s.c
subcutaneous
- SSB
single-strand break.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Story M, Legerski RJ. Cellular responses to ionizing radiation damage. Int J Radiat Oncol Biol Phys. 2001;49:1157–62. doi: 10.1016/s0360-3016(00)01524-8. [DOI] [PubMed] [Google Scholar]
- 4.Soffen EM, Hanks GE, Hunt MA, Epstein BE. Conformal static field radiation therapy treatment of early prostate cancer versus non-conformal techniques: a reduction in acute morbidity. Int J Radiat Oncol Biol Phys. 1992;24:485–8. doi: 10.1016/0360-3016(92)91063-s. [DOI] [PubMed] [Google Scholar]
- 5.Ling CC, Burman C, Chui CS, Kutcher GJ, Leibel SA, LoSasso T. et al. Conformal radiation treatment of prostate cancer using inversely-planned intensity-modulated photon beams produced with dynamic multileaf collimation. Int J Radiat Oncol Biol Phys. 1996;35:721–30. doi: 10.1016/0360-3016(96)00174-5. [DOI] [PubMed] [Google Scholar]
- 6.Ohno T. Particle radiotherapy with carbon ion beams. Epma j. 2013;4:9. doi: 10.1186/1878-5085-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efstathiou JA, Trofimov AV, Zietman AL. Life, liberty, and the pursuit of protons: an evidence-based review of the role of particle therapy in the treatment of prostate cancer. Cancer J. 2009;15:312–8. doi: 10.1097/PPO.0b013e3181b14ec0. [DOI] [PubMed] [Google Scholar]
- 8.Pugh TJ, Lee AK. Proton beam therapy for the treatment of prostate cancer. Cancer J. 2014;20:415–20. doi: 10.1097/PPO.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 9.Shioyama Y, Tsuji H, Suefuji H, Sinoto M, Matsunobu A, Toyama S. et al. Particle radiotherapy for prostate cancer. Int J Urol. 2015;22:33–9. doi: 10.1111/iju.12640. [DOI] [PubMed] [Google Scholar]
- 10.Zaorsky NG, Ohri N, Showalter TN, Dicker AP, Den RB. Systematic review of hypofractionated radiation therapy for prostate cancer. Cancer Treat Rev. 2013;39:728–36. doi: 10.1016/j.ctrv.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dearnaley DP, Jovic G, Syndikus I, Khoo V, Cowan RA, Graham JD. et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464–73. doi: 10.1016/S1470-2045(14)70040-3. [DOI] [PubMed] [Google Scholar]
- 12.Hou Z, Li G, Bai S. High dose versus conventional dose in external beam radiotherapy of prostate cancer: a meta-analysis of long-term follow-up. J Cancer Res Clin Oncol. 2015;141:1063–71. doi: 10.1007/s00432-014-1813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D. et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–60. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam JS, Belldegrun AS. Salvage cryosurgery of the prostate after radiation failure. Rev Urol. 2004;6(Suppl 4):S27–36. [PMC free article] [PubMed] [Google Scholar]
- 15.Baumann M, Krause M, Overgaard J, Debus J, Bentzen SM, Daartz J. et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016;16:234–49. doi: 10.1038/nrc.2016.18. [DOI] [PubMed] [Google Scholar]
- 16.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11:239–53. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 17.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 18.Hu H, Gatti RA. MicroRNAs: new players in the DNA damage response. J Mol Cell Biol. 2011;3:151–8. doi: 10.1093/jmcb/mjq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czochor JR, Glazer PM. microRNAs in cancer cell response to ionizing radiation. Antioxid Redox Signal. 2014;21:293–312. doi: 10.1089/ars.2013.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metheetrairut C, Slack FJ. MicroRNAs in the ionizing radiation response and in radiotherapy. Curr Opin Genet Dev. 2013;23:12–9. doi: 10.1016/j.gde.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cellini F, Morganti AG, Genovesi D, Silvestris N, Valentini V. Role of microRNA in response to ionizing radiations: evidences and potential impact on clinical practice for radiotherapy. Molecules. 2014;19:5379–401. doi: 10.3390/molecules19045379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaudhry MA. Radiation-induced microRNA: discovery, functional analysis, and cancer radiotherapy. J Cell Biochem. 2014;115:436–49. doi: 10.1002/jcb.24694. [DOI] [PubMed] [Google Scholar]
- 23.Korpela E, Vesprini D, Liu SK. MicroRNA in radiotherapy: miRage or miRador? Br J Cancer. 2015;112:777–82. doi: 10.1038/bjc.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanmi K, Ignacimuthu S, Paulraj MG. MicroRNA in prostate cancer. Clin Chim Acta. 2015;451:154–60. doi: 10.1016/j.cca.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 25.Coolen MW, Stirzaker C, Song JZ, Statham AL, Kassir Z, Moreno CS. et al. Consolidation of the cancer genome into domains of repressive chromatin by long-range epigenetic silencing (LRES) reduces transcriptional plasticity. Nat Cell Biol. 2010;12:235–46. doi: 10.1038/ncb2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matin F, Jeet V, Clements JA, Yousef GM, Batra J. MicroRNA Theranostics in Prostate Cancer Precision Medicine. Clin Chem; 2016. [DOI] [PubMed] [Google Scholar]
- 27.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F. et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–61. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TL, Visakorpi T. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007;67:6130–5. doi: 10.1158/0008-5472.CAN-07-0533. [DOI] [PubMed] [Google Scholar]
- 29.Sun T, Wang X, He HH, Sweeney CJ, Liu SX, Brown M. et al. MiR-221 promotes the development of androgen independence in prostate cancer cells via downregulation of HECTD2 and RAB1A. Oncogene. 2014;33:2790–800. doi: 10.1038/onc.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goto Y, Kojima S, Nishikawa R, Kurozumi A, Kato M, Enokida H. et al. MicroRNA expression signature of castration-resistant prostate cancer: the microRNA-221/222 cluster functions as a tumour suppressor and disease progression marker. Br J Cancer. 2015;113:1055–65. doi: 10.1038/bjc.2015.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guan Y, Wu Y, Liu Y, Ni J, Nong S. Association of microRNA-21 expression with clinicopathological characteristics and the risk of progression in advanced prostate cancer patients receiving androgen deprivation therapy. Prostate. 2016;76:986–93. doi: 10.1002/pros.23187. [DOI] [PubMed] [Google Scholar]
- 32.Spahn M, Kneitz S, Scholz CJ, Stenger N, Rudiger T, Strobel P. et al. Expression of microRNA-221 is progressively reduced in aggressive prostate cancer and metastasis and predicts clinical recurrence. Int J Cancer. 2010;127:394–403. doi: 10.1002/ijc.24715. [DOI] [PubMed] [Google Scholar]
- 33.Larne O, Hagman Z, Lilja H, Bjartell A, Edsjo A, Ceder Y. miR-145 suppress the androgen receptor in prostate cancer cells and correlates to prostate cancer prognosis. Carcinogenesis. 2015;36:858–66. doi: 10.1093/carcin/bgv063. [DOI] [PubMed] [Google Scholar]
- 34.Tong AW, Fulgham P, Jay C, Chen P, Khalil I, Liu S. et al. MicroRNA profile analysis of human prostate cancers. Cancer Gene Ther. 2009;16:206–16. doi: 10.1038/cgt.2008.77. [DOI] [PubMed] [Google Scholar]
- 35.Gong P, Zhang T, He D, Hsieh JT. MicroRNA-145 Modulates Tumor Sensitivity to Radiation in Prostate Cancer. Radiat Res. 2015;184:630–8. doi: 10.1667/RR14185.1. [DOI] [PubMed] [Google Scholar]
- 36.Singh PK, Preus L, Hu Q, Yan L, Long MD, Morrison CD. et al. Serum microRNA expression patterns that predict early treatment failure in prostate cancer patients. Oncotarget. 2014;5:824–40. doi: 10.18632/oncotarget.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagman Z, Haflidadottir BS, Ceder JA, Larne O, Bjartell A, Lilja H. et al. miR-205 negatively regulates the androgen receptor and is associated with adverse outcome of prostate cancer patients. Br J Cancer. 2013;108:1668–76. doi: 10.1038/bjc.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalogirou C, Spahn M, Krebs M, Joniau S, Lerut E, Burger M. et al. MiR-205 is progressively down-regulated in lymph node metastasis but fails as a prognostic biomarker in high-risk prostate cancer. Int J Mol Sci. 2013;14:21414–34. doi: 10.3390/ijms141121414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mavridis K, Stravodimos K, Scorilas A. Downregulation and prognostic performance of microRNA 224 expression in prostate cancer. Clin Chem. 2013;59:261–9. doi: 10.1373/clinchem.2012.191502. [DOI] [PubMed] [Google Scholar]
- 40.Lin ZY, Huang YQ, Zhang YQ, Han ZD, He HC, Ling XH. et al. MicroRNA-224 inhibits progression of human prostate cancer by downregulating TRIB1. Int J Cancer. 2014;135:541–50. doi: 10.1002/ijc.28707. [DOI] [PubMed] [Google Scholar]
- 41.Li T, Li RS, Li YH, Zhong S, Chen YY, Zhang CM. et al. miR-21 as an independent biochemical recurrence predictor and potential therapeutic target for prostate cancer. J Urol. 2012;187:1466–72. doi: 10.1016/j.juro.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 42.Sun X, Liu Z, Yang Z, Xiao L, Wang F, He Y. et al. Association of microRNA-126 expression with clinicopathological features and the risk of biochemical recurrence in prostate cancer patients undergoing radical prostatectomy. Diagn Pathol. 2013;8:208. doi: 10.1186/1746-1596-8-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forno I, Ferrero S, Russo MV, Gazzano G, Giangiobbe S, Montanari E. et al. Deregulation of MiR-34b/Sox2 Predicts Prostate Cancer Progression. PLoS One. 2015;10:e0130060. doi: 10.1371/journal.pone.0130060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonci D, Coppola V, Patrizii M, Addario A, Cannistraci A, Francescangeli F. et al. A microRNA code for prostate cancer metastasis. Oncogene. 2016;35:1180–92. doi: 10.1038/onc.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doldi V, Pennati M, Forte B, Gandellini P, Zaffaroni N. Dissecting the role of microRNAs in prostate cancer metastasis: implications for the design of novel therapeutic approaches. Cell Mol Life Sci. 2016;73:2531–42. doi: 10.1007/s00018-016-2176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Templin T, Paul S, Amundson SA, Young EF, Barker CA, Wolden SL. et al. Radiation-induced micro-RNA expression changes in peripheral blood cells of radiotherapy patients. Int J Radiat Oncol Biol Phys. 2011;80:549–57. doi: 10.1016/j.ijrobp.2010.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simone NL, Soule BP, Ly D, Saleh AD, Savage JE, Degraff W. et al. Ionizing radiation-induced oxidative stress alters miRNA expression. PLoS One. 2009;4:e6377. doi: 10.1371/journal.pone.0006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weidhaas JB, Babar I, Nallur SM, Trang P, Roush S, Boehm M. et al. MicroRNAs as potential agents to alter resistance to cytotoxic anticancer therapy. Cancer Res. 2007;67:11111–6. doi: 10.1158/0008-5472.CAN-07-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.John-Aryankalayil M, Palayoor ST, Makinde AY, Cerna D, Simone CB 2nd, Falduto MT. et al. Fractionated radiation alters oncomir and tumor suppressor miRNAs in human prostate cancer cells. Radiat Res. 2012;178:105–17. doi: 10.1667/rr2703.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leung CM, Li SC, Chen TW, Ho MR, Hu LY, Liu WS. et al. Comprehensive microRNA profiling of prostate cancer cells after ionizing radiation treatment. Oncol Rep. 2014;31:1067–78. doi: 10.3892/or.2014.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petrocca F, Vecchione A, Croce CM. Emerging role of miR-106b-25/miR-17-92 clusters in the control of transforming growth factor beta signaling. Cancer Res. 2008;68:8191–4. doi: 10.1158/0008-5472.CAN-08-1768. [DOI] [PubMed] [Google Scholar]
- 52.Zhao ZN, Bai JX, Zhou Q, Yan B, Qin WW, Jia LT. et al. TSA suppresses miR-106b-93-25 cluster expression through downregulation of MYC and inhibits proliferation and induces apoptosis in human EMC. PLoS One. 2012;7:e45133. doi: 10.1371/journal.pone.0045133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li B, Shi XB, Nori D, Chao CK, Chen AM, Valicenti R. et al. Down-regulation of microRNA 106b is involved in p21-mediated cell cycle arrest in response to radiation in prostate cancer cells. Prostate. 2011;71:567–74. doi: 10.1002/pros.21272. [DOI] [PubMed] [Google Scholar]
- 54.Noonan EJ, Place RF, Pookot D, Basak S, Whitson JM, Hirata H. et al. miR-449a targets HDAC-1 and induces growth arrest in prostate cancer. Oncogene. 2009;28:1714–24. doi: 10.1038/onc.2009.19. [DOI] [PubMed] [Google Scholar]
- 55.Kraemer A, Anastasov N, Angermeier M, Winkler K, Atkinson MJ, Moertl S. MicroRNA-mediated processes are essential for the cellular radiation response. Radiat Res. 2011;176:575–86. doi: 10.1667/rr2638.1. [DOI] [PubMed] [Google Scholar]
- 56.Mao A, Zhao Q, Zhou X, Sun C, Si J, Zhou R. et al. MicroRNA-449a enhances radiosensitivity by downregulation of c-Myc in prostate cancer cells. Sci Rep. 2016;6:27346. doi: 10.1038/srep27346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Josson S, Sung SY, Lao K, Chung LW, Johnstone PA. Radiation modulation of microRNA in prostate cancer cell lines. Prostate. 2008;68:1599–606. doi: 10.1002/pros.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE. et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–6. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 59.Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA. et al. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc Natl Acad Sci U S A. 2008;105:3903–8. doi: 10.1073/pnas.0712321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim HH, Kuwano Y, Srikantan S, Lee EK, Martindale JL, Gorospe M. HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 2009;23:1743–8. doi: 10.1101/gad.1812509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barh D, Malhotra R, Ravi B, Sindhurani P. MicroRNA let-7: an emerging next-generation cancer therapeutic. Curr Oncol. 2010;17:70–80. doi: 10.3747/co.v17i1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morii A, Ogawa R, Watanabe A, Cui ZG, Takasaki I, Doi N. et al. Utilization of microRNAs with decreased expression levels in response to X-ray irradiation for fine-tuning radiation-controlled gene regulation. Int J Mol Med. 2013;32:9–16. doi: 10.3892/ijmm.2013.1360. [DOI] [PubMed] [Google Scholar]
- 63.Chang L, Graham PH, Ni J, Hao J, Bucci J, Cozzi PJ. et al. Targeting PI3K/Akt/mTOR signaling pathway in the treatment of prostate cancer radioresistance. Crit Rev Oncol Hematol. 2015;96:507–17. doi: 10.1016/j.critrevonc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Zhao L, Lu X, Cao Y. MicroRNA and signal transduction pathways in tumor radiation response. Cell Signal. 2013;25:1625–34. doi: 10.1016/j.cellsig.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liang Z, Ahn J, Guo D, Votaw JR, Shim H. MicroRNA-302 replacement therapy sensitizes breast cancer cells to ionizing radiation. Pharm Res. 2013;30:1008–16. doi: 10.1007/s11095-012-0936-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Gwak HS, Kim TH, Jo GH, Kim YJ, Kwak HJ, Kim JH. et al. Silencing of microRNA-21 confers radio-sensitivity through inhibition of the PI3K/AKT pathway and enhancing autophagy in malignant glioma cell lines. PLoS One. 2012;7:e47449. doi: 10.1371/journal.pone.0047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y, Zhao S, Zhen Y, Li Q, Teng L, Asai A. et al. A miR-21 inhibitor enhances apoptosis and reduces G(2)-M accumulation induced by ionizing radiation in human glioblastoma U251 cells. Brain Tumor Pathol. 2011;28:209–14. doi: 10.1007/s10014-011-0037-1. [DOI] [PubMed] [Google Scholar]
- 68.Huang S, Li XQ, Chen X, Che SM, Chen W, Zhang XZ. Inhibition of microRNA-21 increases radiosensitivity of esophageal cancer cells through phosphatase and tensin homolog deleted on chromosome 10 activation. Dis Esophagus. 2013;26:823–31. doi: 10.1111/j.1442-2050.2012.01389.x. [DOI] [PubMed] [Google Scholar]
- 69.Chun-Zhi Z, Lei H, An-Ling Z, Yan-Chao F, Xiao Y, Guang-Xiu W. et al. MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell proliferation and radioresistance by targeting PTEN. BMC Cancer. 2010;10:367. doi: 10.1186/1471-2407-10-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qu C, Liang Z, Huang J, Zhao R, Su C, Wang S. et al. MiR-205 determines the radioresistance of human nasopharyngeal carcinoma by directly targeting PTEN. Cell Cycle. 2012;11:785–96. doi: 10.4161/cc.11.4.19228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang X, Taeb S, Jahangiri S, Emmenegger U, Tran E, Bruce J. et al. miRNA-95 mediates radioresistance in tumors by targeting the sphingolipid phosphatase SGPP1. Cancer Res. 2013;73:6972–86. doi: 10.1158/0008-5472.CAN-13-1657. [DOI] [PubMed] [Google Scholar]
- 72.Lyng FM, Maguire P, McClean B, Seymour C, Mothersill C. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiat Res. 2006;165:400–9. doi: 10.1667/rr3527.1. [DOI] [PubMed] [Google Scholar]
- 73.Valerie K, Yacoub A, Hagan MP, Curiel DT, Fisher PB, Grant S. et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- 74.Golding SE, Rosenberg E, Neill S, Dent P, Povirk LF, Valerie K. Extracellular signal-related kinase positively regulates ataxia telangiectasia mutated, homologous recombination repair, and the DNA damage response. Cancer Res. 2007;67:1046–53. doi: 10.1158/0008-5472.CAN-06-2371. [DOI] [PubMed] [Google Scholar]
- 75.Oh JS, Kim JJ, Byun JY, Kim IA. Lin28-let7 modulates radiosensitivity of human cancer cells with activation of K-Ras. Int J Radiat Oncol Biol Phys. 2010;76:5–8. doi: 10.1016/j.ijrobp.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 76.Yang F, Yin Y, Wang F, Wang Y, Zhang L, Tang Y. et al. miR-17-5p Promotes migration of human hepatocellular carcinoma cells through the p38 mitogen-activated protein kinase-heat shock protein 27 pathway. Hepatology. 2010;51:1614–23. doi: 10.1002/hep.23566. [DOI] [PubMed] [Google Scholar]
- 77.Gonfloni S. Targeting DNA damage response: threshold, chromatin landscape and beyond. Pharmacol Ther. 2013;138:46–52. doi: 10.1016/j.pharmthera.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 78.Lopez-Contreras AJ, Fernandez-Capetillo O. The ATR barrier to replication-born DNA damage. DNA Repair (Amst) 2010;9:1249–55. doi: 10.1016/j.dnarep.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu CW, Dong YJ, Liang QY, He XQ, Ng SS, Chan FK. et al. MicroRNA-18a attenuates DNA damage repair through suppressing the expression of ataxia telangiectasia mutated in colorectal cancer. PLoS One. 2013;8:e57036. doi: 10.1371/journal.pone.0057036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo P, Lan J, Ge J, Nie Q, Guo L, Qiu Y. et al. MiR-26a enhances the radiosensitivity of glioblastoma multiforme cells through targeting of ataxia-telangiectasia mutated. Exp Cell Res. 2014;320:200–8. doi: 10.1016/j.yexcr.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 81.Ng WL, Yan D, Zhang X, Mo YY, Wang Y. Over-expression of miR-100 is responsible for the low-expression of ATM in the human glioma cell line: M059J. DNA Repair (Amst) 2010;9:1170–5. doi: 10.1016/j.dnarep.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 82.Yan D, Ng WL, Zhang X, Wang P, Zhang Z, Mo YY. et al. Targeting DNA-PKcs and ATM with miR-101 sensitizes tumors to radiation. PLoS One. 2010;5:e11397. doi: 10.1371/journal.pone.0011397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liang L, Zhu J, Zaorsky NG, Deng Y, Wu X, Liu Y. et al. MicroRNA-223 enhances radiation sensitivity of U87MG cells in vitro and in vivo by targeting ataxia telangiectasia mutated. Int J Radiat Oncol Biol Phys. 2014;88:955–60. doi: 10.1016/j.ijrobp.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 84.Hu H, Du L, Nagabayashi G, Seeger RC, Gatti RA. ATM is down-regulated by N-Myc-regulated microRNA-421. Proc Natl Acad Sci U S A. 2010;107:1506–11. doi: 10.1073/pnas.0907763107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mansour WY, Bogdanova NV, Kasten-Pisula U, Rieckmann T, Kocher S, Borgmann K. et al. Aberrant overexpression of miR-421 downregulates ATM and leads to a pronounced DSB repair defect and clinical hypersensitivity in SKX squamous cell carcinoma. Radiother Oncol. 2013;106:147–54. doi: 10.1016/j.radonc.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y, Huang JW, Li M, Cavenee WK, Mitchell PS, Zhou X. et al. MicroRNA-138 modulates DNA damage response by repressing histone H2AX expression. Mol Cancer Res. 2011;9:1100–11. doi: 10.1158/1541-7786.MCR-11-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dhawan M, Ryan CJ, Ashworth A. DNA Repair Deficiency Is Common in Advanced Prostate Cancer: New Therapeutic Opportunities. Oncologist. 2016;21:940–5. doi: 10.1634/theoncologist.2016-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang S, Wang RH, Akagi K, Kim KA, Martin BK, Cavallone L. et al. Tumor suppressor BRCA1 epigenetically controls oncogenic microRNA-155. Nat Med. 2011;17:1275–82. doi: 10.1038/nm.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choi YE, Pan Y, Park E, Konstantinopoulos P, De S, D'Andrea A. et al. MicroRNAs down-regulate homologous recombination in the G1 phase of cycling cells to maintain genomic stability. Elife. 2014;3:e02445. doi: 10.7554/eLife.02445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hatano K, Kumar B, Zhang Y, Coulter JB, Hedayati M, Mears B. et al. A functional screen identifies miRNAs that inhibit DNA repair and sensitize prostate cancer cells to ionizing radiation. Nucleic Acids Res. 2015;43:4075–86. doi: 10.1093/nar/gkv273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Medema RH, Macurek L. Checkpoint control and cancer. Oncogene. 2012;31:2601–13. doi: 10.1038/onc.2011.451. [DOI] [PubMed] [Google Scholar]
- 92.Deckbar D, Jeggo PA, Lobrich M. Understanding the limitations of radiation-induced cell cycle checkpoints. Crit Rev Biochem Mol Biol. 2011;46:271–83. doi: 10.3109/10409238.2011.575764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gogineni VR, Nalla AK, Gupta R, Dinh DH, Klopfenstein JD, Rao JS. Chk2-mediated G2/M cell cycle arrest maintains radiation resistance in malignant meningioma cells. Cancer Lett. 2011;313:64–75. doi: 10.1016/j.canlet.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ding DP, Chen ZL, Zhao XH, Wang JW, Sun J, Wang Z. et al. miR-29c induces cell cycle arrest in esophageal squamous cell carcinoma by modulating cyclin E expression. Carcinogenesis. 2011;32:1025–32. doi: 10.1093/carcin/bgr078. [DOI] [PubMed] [Google Scholar]
- 95.Liu Q, Fu H, Sun F, Zhang H, Tie Y, Zhu J. et al. miR-16 family induces cell cycle arrest by regulating multiple cell cycle genes. Nucleic Acids Res. 2008;36:5391–404. doi: 10.1093/nar/gkn522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ. et al. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013;4:e875. doi: 10.1038/cddis.2013.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bargiela-Iparraguirre J, Prado-Marchal L, Fernandez-Fuente M, Gutierrez-Gonzalez A, Moreno-Rubio J, Munoz-Fernandez M. et al. CHK1 expression in Gastric Cancer is modulated by p53 and RB1/E2F1: implications in chemo/radiotherapy response. Sci Rep. 2016;6:21519. doi: 10.1038/srep21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mei Z, Su T, Ye J, Yang C, Zhang S, Xie C. The miR-15 family enhances the radiosensitivity of breast cancer cells by targeting G2 checkpoints. Radiat Res. 2015;183:196–207. doi: 10.1667/RR13784.1. [DOI] [PubMed] [Google Scholar]
- 99.Chang L, Hu W, Ye C, Yao B, Song L, Wu X. et al. miR-3928 activates ATR pathway by targeting Dicer. RNA Biol. 2012;9:1247–54. doi: 10.4161/rna.21821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu J, Li Y, Wang F, Wang X, Cheng B, Ye F. et al. Suppressed miR-424 expression via upregulation of target gene Chk1 contributes to the progression of cervical cancer. Oncogene. 2013;32:976–87. doi: 10.1038/onc.2012.121. [DOI] [PubMed] [Google Scholar]
- 101.Wang P, Zou F, Zhang X, Li H, Dulak A, Tomko RJ Jr. et al. microRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells. Cancer Res. 2009;69:8157–65. doi: 10.1158/0008-5472.CAN-09-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D. et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–22. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 103.Yang X, Feng M, Jiang X, Wu Z, Li Z, Aau M. et al. miR-449a and miR-449b are direct transcriptional targets of E2F1 and negatively regulate pRb-E2F1 activity through a feedback loop by targeting CDK6 and CDC25A. Genes Dev. 2009;23:2388–93. doi: 10.1101/gad.1819009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Suetens A, Konings K, Moreels M, Quintens R, Verslegers M, Soors E. et al. Higher Initial DNA Damage and Persistent Cell Cycle Arrest after Carbon Ion Irradiation Compared to X-irradiation in Prostate and Colon Cancer Cells. Front Oncol. 2016;6:87. doi: 10.3389/fonc.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Feng FY, Zhang Y, Kothari V, Evans JR, Jackson WC, Chen W. et al. MDM2 Inhibition Sensitizes Prostate Cancer Cells to Androgen Ablation and Radiotherapy in a p53-Dependent Manner. Neoplasia. 2016;18:213–22. doi: 10.1016/j.neo.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hermeking H. MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat Rev Cancer. 2012;12:613–26. doi: 10.1038/nrc3318. [DOI] [PubMed] [Google Scholar]
- 107.Le MT, Teh C, Shyh-Chang N, Xie H, Zhou B, Korzh V. et al. MicroRNA-125b is a novel negative regulator of p53. Genes Dev. 2009;23:862–76. doi: 10.1101/gad.1767609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leveille N, Elkon R, Davalos V, Manoharan V, Hollingworth D, Oude Vrielink J. et al. Selective inhibition of microRNA accessibility by RBM38 is required for p53 activity. Nat Commun. 2011;2:513. doi: 10.1038/ncomms1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang Y, Cui Y, Li Z, Jiao Z, Zhang Y, He Y. et al. Radiation-induced miR-208a increases the proliferation and radioresistance by targeting p21 in human lung cancer cells. J Exp Clin Cancer Res. 2016;35:7. doi: 10.1186/s13046-016-0285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luo J, Meng C, Tang Y, Zhang S, Wan M, Bi Y. et al. miR-132/212 cluster inhibits the growth of lung cancer xenografts in nude mice. Int J Clin Exp Med. 2014;7:4115–22. [PMC free article] [PubMed] [Google Scholar]
- 111.Zheng L, Zhang Y, Liu Y, Zhou M, Lu Y, Yuan L. et al. MiR-106b induces cell radioresistance via the PTEN/PI3K/AKT pathways and p21 in colorectal cancer. J Transl Med. 2015;13:252. doi: 10.1186/s12967-015-0592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu B, Liu M, Wang J, Zhang X, Wang X, Wang P. et al. DICER-dependent biogenesis of let-7 miRNAs affects human cell response to DNA damage via targeting p21/p27. Nucleic Acids Res. 2015;43:1626–36. doi: 10.1093/nar/gku1368. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.Wu SY, Lin KC, Chiou JF, Jeng SC, Cheng WH, Chang CI. et al. MicroRNA-17-5p post-transcriptionally regulates p21 expression in irradiated betel quid chewing-related oral squamous cell carcinoma cells. Strahlenther Onkol. 2013;189:675–83. doi: 10.1007/s00066-013-0347-9. [DOI] [PubMed] [Google Scholar]
- 114.Eum KH, Lee M. Crosstalk between autophagy and apoptosis in the regulation of paclitaxel-induced cell death in v-Ha-ras-transformed fibroblasts. Mol Cell Biochem. 2011;348:61–8. doi: 10.1007/s11010-010-0638-8. [DOI] [PubMed] [Google Scholar]
- 115.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gao F, Chen S, Sun M, Mitchel RE, Li B, Chu Z. et al. MiR-467a is upregulated in radiation-induced mouse thymic lymphomas and regulates apoptosis by targeting Fas and Bax. Int J Biol Sci. 2015;11:109–21. doi: 10.7150/ijbs.10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li B, Sun M, Gao F, Liu W, Yang Y, Liu H. et al. Up-regulated expression of miR-23a/b targeted the pro-apoptotic Fas in radiation-induced thymic lymphoma. Cell Physiol Biochem. 2013;32:1729–40. doi: 10.1159/000356607. [DOI] [PubMed] [Google Scholar]
- 118.Palma CA, Al Sheikha D, Lim TK, Bryant A, Vu TT, Jayaswal V. et al. MicroRNA-155 as an inducer of apoptosis and cell differentiation in Acute Myeloid Leukaemia. Mol Cancer. 2014;13:79. doi: 10.1186/1476-4598-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsang WP, Kwok TT. Let-7a microRNA suppresses therapeutics-induced cancer cell death by targeting caspase-3. Apoptosis. 2008;13:1215–22. doi: 10.1007/s10495-008-0256-z. [DOI] [PubMed] [Google Scholar]
- 120.Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin. 2005;55:178–94. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 121.Kwon JE, Kim BY, Kwak SY, Bae IH, Han YH. Ionizing radiation-inducible microRNA miR-193a-3p induces apoptosis by directly targeting Mcl-1. Apoptosis. 2013;18:896–909. doi: 10.1007/s10495-013-0841-7. [DOI] [PubMed] [Google Scholar]
- 122.Su H, Yang JR, Xu T, Huang J, Xu L, Yuan Y. et al. MicroRNA-101, down-regulated in hepatocellular carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer Res. 2009;69:1135–42. doi: 10.1158/0008-5472.CAN-08-2886. [DOI] [PubMed] [Google Scholar]
- 123.Chen G, Zhu W, Shi D, Lv L, Zhang C, Liu P. et al. MicroRNA-181a sensitizes human malignant glioma U87MG cells to radiation by targeting Bcl-2. Oncol Rep. 2010;23:997–1003. doi: 10.3892/or_00000725. [DOI] [PubMed] [Google Scholar]
- 124.Jeong D, Kim J, Nam J, Sun H, Lee YH, Lee TJ. et al. MicroRNA-124 links p53 to the NF-kappaB pathway in B-cell lymphomas. Leukemia. 2015;29:1868–74. doi: 10.1038/leu.2015.101. [DOI] [PubMed] [Google Scholar]
- 125.Korner C, Keklikoglou I, Bender C, Worner A, Munstermann E, Wiemann S. MicroRNA-31 sensitizes human breast cells to apoptosis by direct targeting of protein kinase C epsilon (PKCepsilon) J Biol Chem. 2013;288:8750–61. doi: 10.1074/jbc.M112.414128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lin YC, Lin JF, Tsai TF, Chou KY, Chen HE, Hwang TI. Tumor suppressor miRNA-204-5p promotes apoptosis by targeting BCL2 in prostate cancer cells. Asian J Surg; 2016. [DOI] [PubMed] [Google Scholar]
- 127.Peng X, Li W, Yuan L, Mehta RG, Kopelovich L, McCormick DL. Inhibition of proliferation and induction of autophagy by atorvastatin in PC3 prostate cancer cells correlate with downregulation of Bcl2 and upregulation of miR-182 and p21. PLoS One. 2013;8:e70442. doi: 10.1371/journal.pone.0070442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Verdoodt B, Neid M, Vogt M, Kuhn V, Liffers ST, Palisaar RJ. et al. MicroRNA-205, a novel regulator of the anti-apoptotic protein Bcl2, is downregulated in prostate cancer. Int J Oncol. 2013;43:307–14. doi: 10.3892/ijo.2013.1915. [DOI] [PubMed] [Google Scholar]
- 129.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E. et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–44. [PubMed] [Google Scholar]
- 130.Wang P, Zhang J, Zhang L, Zhu Z, Fan J, Chen L. et al. MicroRNA 23b regulates autophagy associated with radioresistance of pancreatic cancer cells. Gastroenterology. 2013;145:1133–43.e12. doi: 10.1053/j.gastro.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 131.Liao H, Xiao Y, Hu Y, Xiao Y, Yin Z, Liu L. microRNA-32 induces radioresistance by targeting DAB2IP and regulating autophagy in prostate cancer cells. Oncol Lett. 2015;10:2055–62. doi: 10.3892/ol.2015.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang W, Liu M, Guan Y, Wu Q. Hypoxia-Responsive Mir-301a and Mir-301b Promote Radioresistance of Prostate Cancer Cells via Downregulating NDRG2. Med Sci Monit. 2016;22:2126–32. doi: 10.12659/MSM.896832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yi H, Liang B, Jia J, Liang N, Xu H, Ju G. et al. Differential roles of miR-199a-5p in radiation-induced autophagy in breast cancer cells. FEBS Lett. 2013;587:436–43. doi: 10.1016/j.febslet.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 134.Schaaf MB, Jutten B, Keulers TG, Savelkouls KG, Peeters HJ, van den Beucken T. et al. Canonical autophagy does not contribute to cellular radioresistance. Radiother Oncol. 2015;114:406–12. doi: 10.1016/j.radonc.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 135.Musiyenko A, Bitko V, Barik S. Ectopic expression of miR-126*, an intronic product of the vascular endothelial EGF-like 7 gene, regulates prostein translation and invasiveness of prostate cancer LNCaP cells. J Mol Med (Berl) 2008;86:313–22. doi: 10.1007/s00109-007-0296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Osinsky S, Zavelevich M, Vaupel P. Tumor hypoxia and malignant progression. Exp Oncol. 2009;31:80–6. [PubMed] [Google Scholar]
- 137.Marignol L, Coffey M, Lawler M, Hollywood D. Hypoxia in prostate cancer: a powerful shield against tumour destruction? Cancer Treat Rev. 2008;34:313–27. doi: 10.1016/j.ctrv.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 138.Jiang S, Wang R, Yan H, Jin L, Dou X, Chen D. MicroRNA-21 modulates radiation resistance through upregulation of hypoxia-inducible factor-1alpha-promoted glycolysis in non-small cell lung cancer cells. Mol Med Rep. 2016;13:4101–7. doi: 10.3892/mmr.2016.5010. [DOI] [PubMed] [Google Scholar]
- 139.Rupaimoole R, Wu SY, Pradeep S, Ivan C, Pecot CV, Gharpure KM. et al. Hypoxia-mediated downregulation of miRNA biogenesis promotes tumour progression. Nat Commun. 2014;5:5202. doi: 10.1038/ncomms6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang W, Sun T, Cao J, Liu F, Tian Y, Zhu W. Downregulation of miR-210 expression inhibits proliferation, induces apoptosis and enhances radiosensitivity in hypoxic human hepatoma cells in vitro. Exp Cell Res. 2012;318:944–54. doi: 10.1016/j.yexcr.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 141.Yang W, Wei J, Sun T, Liu F. Effects of knockdown of miR-210 in combination with ionizing radiation on human hepatoma xenograft in nude mice. Radiat Oncol. 2013;8:102. doi: 10.1186/1748-717X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233:706–20. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- 143.Yu L, Yang Y, Hou J, Zhai C, Song Y, Zhang Z. et al. MicroRNA-144 affects radiotherapy sensitivity by promoting proliferation, migration and invasion of breast cancer cells. Oncol Rep. 2015;34:1845–52. doi: 10.3892/or.2015.4173. [DOI] [PubMed] [Google Scholar]
- 144.Kwak SY, Yoo JO, An HJ, Bae IH, Park MJ, Kim J, miR-5003-3p promotes epithelial-mesenchymal transition in breast cancer cells through Snail stabilization and direct targeting of E-cadherin. J Mol Cell Biol; 2016. [DOI] [PubMed] [Google Scholar]
- 145.Bao B, Ahmad A, Kong D, Ali S, Azmi AS, Li Y. et al. Hypoxia induced aggressiveness of prostate cancer cells is linked with deregulated expression of VEGF, IL-6 and miRNAs that are attenuated by CDF. PLoS One. 2012;7:e43726. doi: 10.1371/journal.pone.0043726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chen SP, Liu BX, Xu J, Pei XF, Liao YJ, Yuan F. et al. MiR-449a suppresses the epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma by multiple targets. BMC Cancer. 2015;15:706. doi: 10.1186/s12885-015-1738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu J, Mao Y, Zhang D, Hao S, Zhang Z, Li Z. et al. MiR-143 inhibits tumor cell proliferation and invasion by targeting STAT3 in esophageal squamous cell carcinoma. Cancer Lett. 2016;373:97–108. doi: 10.1016/j.canlet.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 148.Zhang M, Xu Q, Yan S, Li Z, Yan W, Jia X. Suppression of forkhead box Q1 by microRNA-506 represses the proliferation and epithelial-mesenchymal transition of cervical cancer cells. Oncol Rep. 2016;35:3106–14. doi: 10.3892/or.2016.4651. [DOI] [PubMed] [Google Scholar]
- 149.Tian Y, Cai L, Tian Y, Tu Y, Qiu H, Xie G. et al. miR156a Mimic Represses the Epithelial-Mesenchymal Transition of Human Nasopharyngeal Cancer Cells by Targeting Junctional Adhesion Molecule A. PLoS One. 2016;11:e0157686. doi: 10.1371/journal.pone.0157686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chang L, Graham P, Hao J, Ni J, Deng J, Bucci J. et al. Cancer stem cells and signaling pathways in radioresistance. Oncotarget. 2016;7:11002–17. doi: 10.18632/oncotarget.6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN. et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–3. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rane JK, Erb HH, Nappo G, Mann VM, Simms MS, Collins AT, Inhibition of the glucocorticoid receptor results in an enhanced miR-99a/100-mediated radiation response in stem-like cells from human prostate cancers. Oncotarget; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xue G, Ren Z, Chen Y, Zhu J, Du Y, Pan D. et al. A feedback regulation between miR-145 and DNA methyltransferase 3b in prostate cancer cell and their responses to irradiation. Cancer Lett. 2015;361:121–7. doi: 10.1016/j.canlet.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 154.Pichler M, Calin GA. MicroRNAs in cancer: from developmental genes in worms to their clinical application in patients. Br J Cancer. 2015;113:569–73. doi: 10.1038/bjc.2015.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fabris L, Ceder Y, Chinnaiyan AM, Jenster GW, Sorensen KD, Tomlins S. et al. The Potential of MicroRNAs as Prostate Cancer Biomarkers. Eur Urol. 2016;70:312–22. doi: 10.1016/j.eururo.2015.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Montani F, Marzi MJ, Dezi F, Dama E, Carletti RM, Bonizzi G. et al. miR-Test: a blood test for lung cancer early detection. J Natl Cancer Inst. 2015;107:djv063. doi: 10.1093/jnci/djv063. [DOI] [PubMed] [Google Scholar]
- 157.Hur K, Toiyama Y, Schetter AJ, Okugawa Y, Harris CC, Boland CR, Identification of a metastasis-specific MicroRNA signature in human colorectal cancer. J Natl Cancer Inst; 2015. p. 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fendler A, Stephan C, Yousef GM, Kristiansen G, Jung K. The translational potential of microRNAs as biofluid markers of urological tumours. Nat Rev Urol. 2016;13:734–52. doi: 10.1038/nrurol.2016.193. [DOI] [PubMed] [Google Scholar]
- 159.Filella X, Foj L. miRNAs as novel biomarkers in the management of prostate cancer. Clin Chem Lab Med. 2017;55:715–36. doi: 10.1515/cclm-2015-1073. [DOI] [PubMed] [Google Scholar]
- 160.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–31. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 161.Palisaar JR, Noldus J, Loppenberg B, von Bodman C, Sommerer F, Eggert T. Comprehensive report on prostate cancer misclassification by 16 currently used low-risk and active surveillance criteria. BJU Int. 2012;110:E172–81. doi: 10.1111/j.1464-410X.2012.10935.x. [DOI] [PubMed] [Google Scholar]
- 162.Wang SY, Shiboski S, Belair CD, Cooperberg MR, Simko JP, Stoppler H. et al. miR-19, miR-345, miR-519c-5p serum levels predict adverse pathology in prostate cancer patients eligible for active surveillance. PLoS One. 2014;9:e98597. doi: 10.1371/journal.pone.0098597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gonzales JC, Fink LM, Goodman OB Jr, Symanowski JT, Vogelzang NJ, Ward DC. Comparison of circulating MicroRNA 141 to circulating tumor cells, lactate dehydrogenase, and prostate-specific antigen for determining treatment response in patients with metastatic prostate cancer. Clin Genitourin Cancer. 2011;9:39–45. doi: 10.1016/j.clgc.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 164.Gade S, Porzelius C, Falth M, Brase JC, Wuttig D, Kuner R. et al. Graph based fusion of miRNA and mRNA expression data improves clinical outcome prediction in prostate cancer. BMC Bioinformatics. 2011;12:488. doi: 10.1186/1471-2105-12-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Santos JI, Teixeira AL, Dias F, Mauricio J, Lobo F, Morais A. et al. Influence of peripheral whole-blood microRNA-7 and microRNA-221 high expression levels on the acquisition of castration-resistant prostate cancer: evidences from in vitro and in vivo studies. Tumour Biol. 2014;35:7105–13. doi: 10.1007/s13277-014-1918-9. [DOI] [PubMed] [Google Scholar]
- 166.Cheng HH, Mitchell PS, Kroh EM, Dowell AE, Chery L, Siddiqui J. et al. Circulating microRNA profiling identifies a subset of metastatic prostate cancer patients with evidence of cancer-associated hypoxia. PLoS One. 2013;8:e69239. doi: 10.1371/journal.pone.0069239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jacob NK, Cooley JV, Yee TN, Jacob J, Alder H, Wickramasinghe P. et al. Identification of sensitive serum microRNA biomarkers for radiation biodosimetry. PLoS One. 2013;8:e57603. doi: 10.1371/journal.pone.0057603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Someya M, Yamamoto H, Nojima M, Hori M, Tateoka K, Nakata K. et al. Relation between Ku80 and microRNA-99a expression and late rectal bleeding after radiotherapy for prostate cancer. Radiother Oncol. 2015;115:235–9. doi: 10.1016/j.radonc.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 169.Fendler W, Malachowska B, Meghani K, Konstantinopoulos PA, Guha C, Singh VK, Evolutionarily conserved serum microRNAs predict radiation-induced fatality in nonhuman primates. Sci Transl Med; 2017. p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hamama S, Noman MZ, Gervaz P, Delanian S, Vozenin MC. MiR-210: A potential therapeutic target against radiation-induced enteropathy. Radiother Oncol. 2014;111:219–21. doi: 10.1016/j.radonc.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 171.Nayerossadat N, Maedeh T, Ali PA. Viral and nonviral delivery systems for gene delivery. Adv Biomed Res. 2012;1:27. doi: 10.4103/2277-9175.98152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang H, Jiang Y, Peng H, Chen Y, Zhu P, Huang Y. Recent progress in microRNA delivery for cancer therapy by non-viral synthetic vectors. Adv Drug Deliv Rev. 2015;81:142–60. doi: 10.1016/j.addr.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 173.Yang N. An overview of viral and nonviral delivery systems for microRNA. Int J Pharm Investig. 2015;5:179–81. doi: 10.4103/2230-973X.167646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ibrahim AF, Weirauch U, Thomas M, Grunweller A, Hartmann RK, Aigner A. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res. 2011;71:5214–24. doi: 10.1158/0008-5472.CAN-10-4645. [DOI] [PubMed] [Google Scholar]
- 175.Beg MS, Brenner AJ, Sachdev J, Borad M, Kang YK, Stoudemire J, Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xiang D, Shigdar S, Qiao G, Wang T, Kouzani AZ, Zhou SF. et al. Nucleic acid aptamer-guided cancer therapeutics and diagnostics: the next generation of cancer medicine. Theranostics. 2015;5:23–42. doi: 10.7150/thno.10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Esposito CL, Cerchia L, Catuogno S, De Vita G, Dassie JP, Santamaria G. et al. Multifunctional aptamer-miRNA conjugates for targeted cancer therapy. Mol Ther. 2014;22:1151–63. doi: 10.1038/mt.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bonci D, Coppola V, Musumeci M, Addario A, Giuffrida R, Memeo L. et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med. 2008;14:1271–7. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- 179.Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4:721–6. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347–55. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]