Abstract
Objective
Obesity and hypertension often coexist and represent risk factors for atrial fibrillation. This study hypothesized that their single and joint effects on atrial remodeling would be reflected in the PR interval and P-wave durations on electrocardiogram (ECG).
Methods
This cross-sectional analysis of a community-based study included 11,308 men and women age 45–64. Atrial indices were obtained from digital standard 12-lead resting ECG. Analyses were adjusted for traditional cardiovascular risk factors.
Results
Both ECG indices displayed a progressive increase across anthropometric indices. Each 5-unit increment in body mass index (BMI) increased P-wave duration by 1.9 ms (95% CI 1.5–2.2) and PR interval by 2.4 ms (95% CI 1.9–3.0), with similar trends for central obesity, even among those without obesity by BMI. Both ECG indices displayed graded increases across levels of blood pressure control, including prehypertension. A joint effect of overweight and hypertension on both ECG indices was detected. P-wave duration or PR interval among people with obesity was not additionally increased by hypertension.
Conclusions
P-wave indices increase in general and central obesity. Hypertension exerts an incremental effect in people with overweight but not in people with obesity. The study furthered the understanding of atrial remodeling in the setting of major atrial fibrillation risk factors.
Introduction
Despite increased awareness to its clinical consequences and public health burden, the prevalence of obesity among U.S. adults remains high (36.5%), with no clear signs of decrease (1). Similarly, the prevalence of hypertension (HTN) among adults remained unchanged during recent years (30.8%) (2). At the same time, atrial fibrillation (AF) represents an increasing clinical and public health problem (3). The prevalence of AF has been estimated at 2.7 to 6.1 million in the U.S. (4,5) and at 6.5 to 12.3 million in the EU (6). These figures are forecasted to double during the coming decades (7), due to population aging and increasing prevalence of predisposing chronic conditions. AF often remains silent and undetected (8).
Large epidemiological studies have estimated that about half of the AF risk at population level can be explained by traditional cardiovascular disease (CVD) risk factors (9), among which, obesity and HTN are the major contributors (10,11). Chronic exposure to these risk factors induces an insidious process of atrial electrophysiological remodeling through chronic volume and/or pressure overload. Prolongations of the PR interval and of several P-wave indices derived from the 12-lead resting electrocardiogram (ECG) have been proposed as intermediate phenotypes to mark this atrial remodeling process (10,11). Several studies have found that these ECG measures are associated with an increased risk of new-onset AF (12–15) and are also independently associated with obesity (11,16), metabolic syndrome (11), and HTN status (11,17,18). As obesity and HTN have a high prevalence and often coexist, we hypothesized that their single and joint effects on atrial remodeling would be reflected in the PR interval and P-wave durations.
We conducted this analysis in order to explore the cross-sectional clinical and electrocardiographic correlates of P-wave duration and PR interval in a large, community-based study. Second, we aimed to examine the single and joint effects of overweight/obesity and HTN on the P-wave and PR interval durations.
Methods
The Polish-Norwegian Study (PONS) is a prospective community-based investigation of risk factors for chronic diseases, conducted in the Kielce region of southeast Poland (19). The study enrolled 13,172 men and women, age 45 to 64 years, in 2010 to 2011. The study was approved by the ethics committee within the Cancer Center and Institute of Oncology in Warsaw, Poland. Trained nurses collected information on participant demographics, lifestyle factors, and medical history and conducted physical examinations, anthropometric, ECG, and laboratory measurements. Standardized questionnaires collected information on education, alcohol, tobacco use (19), and leisure-time physical activity (20). Blood pressure was measured twice in a seated position and the average was used for this analysis. HTN was defined as a systolic blood pressure (SBP) ≥140 mm Hg, or diastolic blood pressure (DBP) ≥90 mm Hg, or self-reported HTN diagnosis or use of anti-hypertensive medications. Diabetes was defined as fasting glucose ≥126 mg/dL, or self-reported diagnosis of diabetes, or use of antidia-betic medication. History of CVD was defined by any self-reported diagnosis of coronary heart disease, heart failure, or stroke. A diagnosis of HTN was not included in this definition. Medication use during the 30-day period before the clinic visit was self-reported, inspected, classified, and recorded by a trained nurse at the time of the clinic visit.
The present analysis is based on the cross-sectional, baseline data of PONS, from which we excluded participants with the following conditions: prevalent AF or flutter (n = 46), lacking ECG (n = 118), lacking information on covariates (n = 418), pre-excitation (Wolff-Parkinson-White) (n = 18), paced rhythms (n = 15), third degree atrioventricular blocks or conduction abnormalities with QRS >120 ms (n = 375), PR intervals ≤80 ms or ≥320 ms (n = 744), cardiac glycosides, or antiarrhythmic drugs (n = 130).
Anthropometric measurements and definition of the metabolic syndrome
Body mass index (BMI) was calculated as weight (kg) divided by the square of the height (m2). The cutoffs for BMI, waist circumference, and waist-to-hip ratio (WHR) were based on WHO definitions and obesity was defined as a BMI ≥30 kg/m2. An increased waist circumference was defined as ≥88 cm for women and ≥102 cm for men. Metabolic syndrome was defined according to the harmonized definition proposed by the International Diabetes Federation, American Heart Association, and the National Heart, Lung, and Blood Institute (21).
ECG measurements
Study participants underwent a standard 12-lead resting digital ECG in the supine position using an AsCARD Mr.Grey v.201 portable electrocardiograph (Aspel Inc., Zabierzow, Poland). This machine is commonly used in primary care. This electrocardiograph’s software provides automatic analysis, interpretation, and storage of several ECG indices. Measures of P-wave duration, PR, RR, and QT intervals, and QRS duration were digitally obtained by automated ECG software. Trained nurses visually inspected all ECGs for quality and legibility at acquisition. Study cardiologists reviewed all ECG recordings and made a judgment whether the ECG was “normal” or presented pathological findings such as AF, pathological Q waves, T-wave inversions, and intraventricular conduction defects. Left ventricular hypertrophy (LVH) was defined based on Cornell criteria (22). P-wave duration and PR interval were not manually measured; instead, they were obtained only by automatic measurements.
Statistical analysis
Continuous variables were expressed as means and standard deviations (SD), while categorical variables were expressed as percentages. Data were checked for normality using quantilequantile plots. To describe distributions and to enable comparisons with other studies, we applied standard cut points of 200 ms for the PR interval and of 110 ms and 120 ms for P-wave duration (23). To explore the relation with other ECG measures, P-wave duration and PR interval were treated as quartiles and/or as continuous variables. Trends across categorical variables were assessed by the χ2 test for trend. Pearson’s correlation coefficient was used to assess correlations between continuous variables. The main analysis consisted in estimating the means of the P-wave duration and PR intervals across categories of BMI, waist circumference, and blood pressure, using generalized linear models. We adjusted for age, sex, and other covariates in hierarchical models, adding lifestyle variables (smoking, alcohol, physical activity, education), CVD risk factors and comorbidities (prevalent CVD, diabetes, SBP and DBP, heart rate, cholesterol, antilipidemics, antihypertensive drugs), and LVH, sequentially. We tested for interactions by sex by including a multiplicative term in the models. We explored nonlinear relations between P-wave duration and PR interval with BMI, waist circumference, and blood pressure by creating cubic splines with knots at the 10th, 25th, 50th, 75th, and 90th percentiles. All P values refer to two-sided tests and values <0.05 were considered statistically significant. We used the Scheff’e correction to adjust for multiple statistical comparisons. Analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Study population
The study population for this analysis included 11,308 participants and their demographic, clinical, and ECG characteristics are presented in Table 1. The prevalence of obesity was 30.6%. More than one third of the participants had HTN (38.8%) and/or metabolic syndrome (38.8%). P-wave duration was ≥110 ms in 39.8% participants and ≥120 ms in 20% participants. PR interval was ≥200 ms in 524 (4.6%) participants. These percentages were higher among men than in women.
TABLE 1.
Demographic and clinical characteristics of the PONS participants
| N=11,308 | |
|---|---|
| Age (years) | 55.6 (5.4) |
| Men, N (%) | 3,663 (32.4) |
| Smoking, current, N (%) | 2,216 (19.6) |
| Systolic blood pressure (mm Hg) | 137.6 (19.2) |
| Diastolic blood pressure (mm Hg) | 82.2 (10.6) |
| Total cholesterol (mg/dL) | 210.1 (38.5) |
| LDL cholesterol (mg/dL) | 127.2 (33.9) |
| HDL cholesterol (mg/dL) | 59.1 (14.6) |
| Metabolic syndrome, N (%) | 4,382 (38.8) |
| Hypertension, N (%) | 4,390 (38.8) |
| Diabetes, N (%) | 687 (6.1) |
| Total cardiovascular diseases, N (%) | 1,528 (13.5) |
| Obesity measures | |
| BMI (kg/m2) | 28.2 (4.7) |
| BMI ≤24.9 | 2,916 (25.8) |
| BMI 25–29.9 | 4,935 (43.6) |
| BMI ≥30 | 3,457 (30.6) |
| Waist circumference (cm) | 91.8 (12.5) |
| Increased waist circumference, N (%) | 5,147 (45.5) |
| Waist-hip ratio | 0.89 (0.1) |
| ECG measures | |
| P-wave duration (ms) | 106.7 (16.3) |
| PR interval duration (ms) | 156.3 (24.3) |
| Heart rate (bpm) | 67 (10) |
| QRS duration (ms) | 92.7 (9.4) |
| QT interval duration (ms) | 418.7 (30.8) |
| Left axis deviation, N (%) | 695 (6.1) |
| LVH, N (%) | 379 (3.4) |
| Intraventricular conduction defects, N (%) | |
| QRS ≥120 ms | 88 (0.8) |
| Right bundle branch block (complete and incomplete) | 1,590 (14.1) |
| Left bundle branch block | 10 (0.1) |
| Pathological Q wave, N (%) | 772 (6.8) |
| T-wave inversion, N (%) | 572 (5.1) |
Values are means (SD) except as noted.
LDL, low-density lipoprotein; HDL, high-density lipoprotein; BMI, body mass index; ECG, electrocardiogram; LVH, left ventricular hypertrophy.
ECG correlates
P-wave duration and PR interval were moderately correlated (r = 0.62, P <0.0001) with each other. The prevalence of LVH increased significantly across quartiles of P-wave duration (5.6%, 5.6%, 6.2%, and 7.1%, P for trend <0.001). The prevalence of left axis deviation increased significantly across quartiles of PR interval (4.9%, 6.0%, 6.6%, 7.1%, P for trend <0.001). Detailed data are presented in Supporting Information Tables S1 and S2.
Association with anthropometric indices
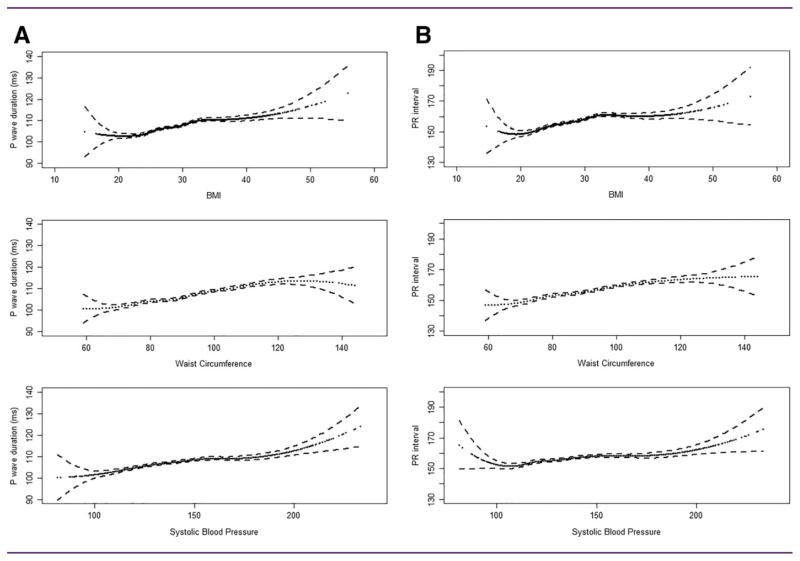
Both P-wave duration and PR interval exhibited progressive increases with BMI and waist circumference, starting at BMI >20 kg/m2 and extending beyond BMI values of 30 kg/m2 (Figure 1). The associations persisted after adjustment for age, sex, smoking, alcohol, physical activity, education, prevalent CVD, diabetes, antilipidemics, antihypertensive drugs, SBP and DBP, total and HDL cholesterol, heart rate, and LVH. For each 5-unit increase in BMI, P-wave duration increased by 1.9 ms (95% CI 1.5–2.2) and the PR interval increased by 2.4 ms (95% CI 1.9–3.0). Each 5-cm increase in waist circumference was associated with an increase in P-wave duration of 0.8 ms (95% CI 0.7–1.0) and an increase of 1.1 ms (95% CI 0.9–1.4) in the PR interval (Table 2). The association remained significant, with a magnitude of 2 to 3 ms, for both ECG indices, even after additionally adjusting for BMI and even among people without obesity as defined by BMI. The relations to WHR showed similar patterns. Detailed data are presented in Supporting Information Tables S3 and S4.
Figure 1.
Association of (A) P-wave duration and (B) PR interval with BMI, waist circumference, and systolic blood pressure in PONS. Cubic restricted splines showing mean durations and 95% confidence intervals (dotted lines).
TABLE 2.
Association of P-wave duration and PR interval with obesity and blood pressure: multivariable adjusted models
| Clinical characteristic | P-wave duration, ms, mean (95% CI) | PR interval, ms, mean (95% CI) |
|---|---|---|
| Association with general obesity (BMI) | ||
| ≤24.9 kg/m2 | 104.55 (103.93–105.18) | 153.60 (152.68–154.53) |
| 25–29.99 kg/m2 | 106.39 (105.94–106.84) | 155.72 (155.05–156.39) |
| ≥30 kg/m2 | 109.01 (108.44–109.57)** | 159.23 (158.39–160.08)** |
| Per 5-unit increment | +1.86 (1.50–2.22) | +2.44 (1.90–2.98) |
| Association with visceral obesity (waist circumference) | ||
| ≤93.9 cm (M), ≤79.9 cm (W) | 104.90 (104.28–105.53) | 153.88 (152.95–154.81) |
| 94–101.9 cm (M), 80–87.9 cm (W) | 106.36 (105.81–106.91) | 155.64 (154.81–156.46) |
| ≥102 cm (M), ≥88 cm (W) | 107.96 (107.50–108.42)** | 157.97 (157.28–158.66)** |
| Per 5-cm increment | +0.82 (0.67–0.97) | +1.13 (0.90–1.35) |
| Association with visceral obesity (WHR) | ||
| WHR <0.90 (M), <0.85 (W) | 105.58 (105.08–106.08) | 154.70 (153.96–155.45) |
| WHR ≥0.90 (M), ≥0.85 (W) | 107.46 (107.07–107.86)* | 157.26 (156.67–157.86)* |
| Association with blood pressure/HTN status | ||
| <120/80 mm Hg | 105.10 (104.33–105.89) | 155.30 (154.13–156.47) |
| 120–139/80–89 mm Hg | 106.67 (106.29–107.05) | 156.15 (155.58–156.72) |
| ≥140/90 mm Hg | 107.76 (107.17–108.36)* | 157.02 (156.14–157.91)** |
| Systolic blood pressure per 10–unit increment | 0.48 (0.32–0.65) | 0.41 (0.16–0.66) |
| Diastolic blood pressure per 10-unit increment | 0.67 (0.37–0.97) | 0.52 (0.08–0.97) |
| Association with obesity and HTN, single and joint effects | ||
| Normal weight, normotensive | 103.65 (102.96–104.33) | 152.46 (151.44–153.49) |
| Normal weight, hypertensive | 105.47 (104.16–106.78) | 154.83 (152.88–156.79) |
| Overweight, normotensive | 105.51 (104.94–106.08) | 154.33 (153.48–155.17) |
| Overweight, hypertensive | 107.80 (107.05–108.55) | 157.97 (156.84–159.09) |
| Obesity, normotensive | 109.15 (108.32–109.98) | 159.27(158.03–160.51) |
| Obesity, hypertensive | 109.81 (109.07–110.55)** | 160.35 (159.24–161.45)** |
| Association with metabolic syndrome (no. components) | ||
| 0 | 102.79 (101.91–103.66) | 152.13 (150.82–153.43) |
| 1 | 106.05 (105.44–106.66) | 154.90 (15.96–155.77) |
| 2 | 106.98 (106.38–107.57) | 156.68 (155.80–157.57) |
| 3 | 107.40 (106.71–108.09) | 157.17 (156.14–158.20) |
| 4 | 108.85 (108.03–109.67) | 158.53 (157.31–159.76) |
| 5 | 109.05 (107.91–110.20)** | 159.80 (158.10–161.51)** |
Generalized linear models with P-wave indices as dependent variables were used to obtain adjusted least square means for P-wave indices. Linear models were used for estimating increment per unit. P values for trend were calculated across the levels of categorical variables.
P for trend <0.05;
P for trend <0.0001.
BMI, body mass index; WHR, waist-to-hip ratio; HTN, hypertension.
Association with blood pressure/HTN
Both P-wave duration and PR interval exhibited progressive increases with SBP (Figure 1). There was no significant relation with DBP. The linear trend was also present across categorized blood pressure values and the association persisted, even after adjusting for BMI and other factors (age, sex, smoking, alcohol intake, physical activity, education, prevalent CVD, diabetes antilipemics, antihypertensives, total and HDL cholesterol, heart rate, and LVH) (Table 2).
Joint effect of obesity and HTN
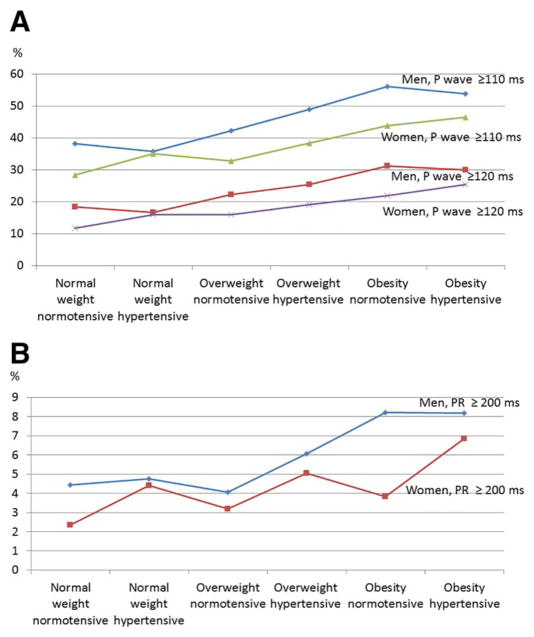
We further investigated P-wave duration and PR interval across categories defined by the single and joint presence of HTN and increased BMI (Table 2). HTN was associated with statistically significant increases in the P-wave duration and PR interval among normal-weight or overweight individuals, but not among those with obesity. This finding was confirmed in multivariable regression models, in which the standardized coefficients for BMI were twice the coefficients for SBP. Additional adjustment for LVH had minimal effect on the mean estimates. We further explored the prevalence of extreme P-wave duration which displayed a monotonically increasing relation with the body weight-HTN status, in both sexes (Figure 2).
Figure 2.
Sex-specific prevalence of increased (A) P-wave duration and (B) PR interval, by body weight-HTN status in PONS. [Color figure can be viewed at wileyonli-nelibrary.com.]
We also found increasing trends in both indices with increasing number of metabolic syndrome components. The association was only mildly attenuated after adjusting for age, sex, smoking, alcohol, physical activity, and education. Each additional metabolic syndrome component was associated with an increase of 1.2 ms (95% CI 1.0–1.4) in P-wave duration and of 1.3 ms (95% CI 1.0–1.7) in the PR interval. However, these relations were mainly driven by the overweight/obesity and HTN components of the metabolic syndrome. Neither P-wave duration nor PR interval were associated with diabetes or with fasting glucose levels, after taking anthropometric measures into account.
Subgroup and sensitivity analyses
We repeated the previous analyses in the subgroup of individuals with ECGs judged as “normal” by the study cardiologists (n = 6,564, i.e., 58% of the total sample). The same patterns and similar magnitude of statistically significant associations across BMI, waist circumference, and blood pressure categories emerged, for both P-wave duration and PR interval. Detailed data are presented in the Supporting Information Table S5. We found similar associations between P-wave duration or PR interval with anthropometric measures, blood pressure, and metabolic syndrome, in a sensitivity analysis conducted after excluding those with prevalent CVD (n = 1,528).
Discussion
In this large, community-based study, we found that BMI, waist circumference, and WHR had a monotonically increasing association with longer P-wave duration and PR interval, even after adjusting for multiple relevant covariates. Both ECG indices were associated with SBP and with the level of blood pressure control. While the joint effect of overweight and HTN was statistically significant, HTN did not additionally increase P-wave duration or PR interval among individuals with obesity.
Our findings on the relations between P-wave indices and BMI are consistent with those identified in the ARIC (11) and MESA studies (16). We further showed that central obesity, as measured by waist circumference, increases the two ECG indices, even among those who do not have obesity based on BMI alone and who might be at increased AF risk.
Previous studies have shown a relation of AF risk with levels of blood pressure (Framingham Heart Study (24), a study in Norwegian men (25) and MESA study (26)). Our study extends these findings by showing a graded relation between P-wave indices across blood pressure categories, including pre-HTN. These findings may be relevant in light of data suggesting that a more aggressive blood pressure control reduces the risk of AF (27).
Similar to the ARIC study (11), we found a relation with the number of metabolic syndrome components, a relation mainly driven by obesity and HTN. We expanded these findings by investigating the single and joint effects of overweight/obesity and HTN and showed an incremental effect of HTN in those with overweight but not those with obesity. Consistent with this finding, obesity but not HTN, was associated with the 10-year change in left atrial (LA) enlargement, measured echocardiographically in the MONICA/KORA study (28). Whether obesity is a stronger risk factor for atrial remodeling than HTN, potentially acting through different pathways, remains a question to be addressed by future studies.
The high prevalence of increased P-wave duration (≥110 ms or ≥120 ms) among individuals who have HTN, obesity, or overweight has clinical significance. An increased P-wave duration was recently found to increase the risk of AF by 22% if between 112 and 119 ms and by 50% if between 120 and 139 ms (15) or by 55% when >120 ms versus ≤120 ms (29). Similarly, a PR interval >200 ms was associated with a 46% increased risk of incident heart failure (12). The role of PR interval in predicting AF is currently unclear and the evidence is conflicting. In risk prediction scores incorporating various other clinical characteristics, a PR interval prolongation predicted AF in Framingham (14) and Copenhagen (30) studies but not in Health ABC study (31) and the Cohorts for Heart and Aging Research in Genomic Epidemiology-AF Consortium (32). In the ARIC study, PR prolongation did not have incremental predictive value in the presence of P-wave duration prolongation (33). The clinical value of P-wave duration and PR interval needs to be interpreted in the light of these indices being intermediate phenotypes between various risk factors and AF (10,11).
Strengths of our study include large sample size, extensive information on covariates, availability of both machine-read and manually read ECGs for all participants, and analysis of relevant variables in both categorical and continuous formats. Our study has several limitations. First, a low amplitude P wave may not have been detected by the ECG algorithm, potentially leading to systematic measurement error. Second, despite extensive adjustment for multiple covariates, residual confounding is still possible, through under-reported diseases (e.g., valvular diseases, diastolic dysfunction, thyroid diseases, intermittent AF). We speculate that the prevalence of such conditions is likely low in our community-based study. Third, the cross-sectional design precludes inferences about the causality of the associations observed, although it is unlikely that P-wave indices could lead to obesity or HTN. The cross-sectional design with a single measurement also precludes exploring changes in these indices over time and the relation of these changes in the context of obesity and HTN. While from an epidemiological perspective, this design poses an inferential limitation, from a clinical perspective, similar to a cross-sectional study examination, a routinely performed ECG in a primary care visit setting may raise awareness on the importance of atrial remodeling in patients with obesity and/or HTN. Fourth, automatically read ECGs provided measurements on a limited number of ECG indices. P-wave indices such as P-wave terminal force, mean P-wave area, P-wave durations in various leads (II, III, avF), or P-wave morphology were not available. It is unclear whether this limitation led to an underestimation of the observed effects. In the ARIC Study, P-wave duration in lead II was the strongest predictor of AF, followed by maximum and mean P-wave duration (13). In the same cohort, advanced interatrial block was associated with a threefold increase in AF risk (34). While the lack of additional P-wave indices limits our ability to refine our analysis, our findings have potential “real life” generalizability, as this type of digital ECG readings are commonly used during routine medical exams.
Lastly, our study population consisted of community-recruited individuals and the aim of our study is primary prevention. Thus, we did not perform echocardiography or cardiac magnetic resonance to measure LA size. LA size is a strong AF risk factor, and whether the association of P-wave indices with obesity/HTN is mediated by LA enlargement remains outside the scope of our study. However, recent studies have shown that P-wave can be longer irrespective of LA size. For instance, independently of LA size, prolonged P-wave duration was associated with higher recurrence rates of AF (35) and higher LA pressure (36) Impaired LA function such as early LA strain was found in patients with HTN and diabetes who had normal LA sizes (37). Complex molecular pathways may be at play. For instance, atrial fibrosis and/or autonomic atrial remodeling can both independently affect the atrial conduction (38,39). Considerable evidence has been accumulating recently about the arrhythmogenic effects of cardiac fat. For instance, increased LA fat was associated with AF burden independent of age, BMI, or LA area (40). Thus, the prolongation of P-wave indices in patients with obesity/HTN is likely complex and may involve LA size along with other multifactorial pathways.
Our findings have potential implications for clinical practice. Several previous studies investigating P-wave indices have used advanced methods and computing platforms to analyze the digitized ECGs in specialized central reading laboratories. Yet such advanced methods are rarely available in primary care. Automated measurements of the P-wave duration and PR interval, such as those used in our study, are available with many standard, digital, office-based ECG machines. Such easily obtained measures provide an opportunity to increase the awareness of unrecognized AF risk factors or markers. Our study suggests that even among ECGs visually interpreted by cardiologists as “normal,” automatically read P-wave duration and PR interval may signal early atrial electrophysiological remodeling among patients with HTN, overweight, or obesity. Future studies are needed to investigate whether incorporating serial P-wave measures in risk assessment is valuable in monitoring the AF risk in these patients.
Conclusion
Our study suggests that P-wave indices increase not only in general obesity, but also in central obesity, even among those who do not have obesity based on BMI alone. We showed a gradual increase of P-wave indices across blood pressure categories, including pre-HTN. Our findings suggest an incremental effect of HTN in people with overweight but not in people with obesity. Our study furthers our understanding of atrial remodeling in the setting of major AF risk factors.
Supplementary Material
Acknowledgments
Funding agencies: Data collection was supported by the Polish-Norwegian Research Fund, Grant PNRF-228-AI-1/07.
The authors thank Dr. Witold A. Zatoński, Dr. Lars J.Vatten, and Dr. Paolo Boffetta for facilitating this study. The authors thank all other PONS investigators, the staff, and the participants of the PONS for their valuable contributions. A full list of PONS investigators and participant institutions can be found at http://www.proj-ectpons.pl.
Footnotes
Disclosure: The authors declared no conflict of interest.
Author contributions: GV designed the current study, carried out the statistical analysis, and wrote the manuscript. MM designed and directed data collection in the main study and contributed to the current study design and analysis. JWM contributed to data analysis and writing of the manuscript. All authors read and contributed to earlier drafts of the manuscript. GV and MM had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. Hyattsville, MD: National Center for Health Statistics; 2015. NCHS data brief, no 219. [Google Scholar]
- 2.National Center for Health Statistics. Health, United States, 2015. Hyattsville, MD: U.S. Department of Health and Human Services; 2016. [Accessed May 1, 2016]. DHHS Publication No. 2016–1232. http://www.cdc.gov/nchs/data/hus/hus15.pdf. Published May 2016. [Google Scholar]
- 3.Magnani JW, Rienstra M, Lin H, et al. Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011;124:1982–1993. doi: 10.1161/CIRCULATIONAHA.111.039677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 5.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 6.Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. doi: 10.1093/eurheartj/eht280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 8.Engdahl J, Andersson L, Mirskaya M, Rosenqvist M. Stepwise screening of atrial fibrillation in a 75-year-old population: implications for stroke prevention. Circulation. 2013;127:930–937. doi: 10.1161/CIRCULATIONAHA.112.126656. [DOI] [PubMed] [Google Scholar]
- 9.Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magnani JW, Hylek EM, Apovian CM. Obesity begets atrial fibrillation: a contemporary summary. Circulation. 2013;128:401–405. doi: 10.1161/CIRCULATIONAHA.113.001840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magnani JW, Lopez FL, Soliman EZ, Maclehose RF, Crow RS, Alonso A. P wave indices, obesity, and the metabolic syndrome: the atherosclerosis risk in communities study. Obesity Silver Spring. 2012;20:666–672. doi: 10.1038/oby.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnani JW, Johnson VM, Sullivan LM, et al. P wave duration and risk of longitudinal atrial fibrillation in persons ≥60 years old (from the Framingham Heart Study) Am J Cardiol. 2011;107:917–921.e1. doi: 10.1016/j.amjcard.2010.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soliman EZ, Prineas RJ, Case LD, Zhang Z, Goff DC. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke J Cereb Circ. 2009;40:1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet Lond Engl. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen JB, Kuhl JT, Pietersen A, et al. P-wave duration and the risk of atrial fibrillation: results from the Copenhagen ECG study. Heart Rhythm. 2015;12:1887–1895. doi: 10.1016/j.hrthm.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Babcock MJ, Soliman EZ, Ding J, Kronmal AR, Goff DC. Pericardial fat and atrial conduction abnormalities in the Multiethnic Study of Atherosclerosis (MESA) Obesity (Silver Spring) 2011;19:179–184. doi: 10.1038/oby.2010.121. [DOI] [PubMed] [Google Scholar]
- 17.Francia P, Ricotta A, Balla C, et al. P-wave duration in lead aVR and the risk of atrial fibrillation in hypertension. Ann Noninvasive Electrocardiol. 2015;20:167–174. doi: 10.1111/anec.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dilaveris PE, Gialafos EJ, Chrissos D, et al. Detection of hypertensive patients at risk for paroxysmal atrial fibrillation during sinus rhythm by computer-assisted P wave analysis. J Hypertens. 1999;17:1463–1470. doi: 10.1097/00004872-199917100-00015. [DOI] [PubMed] [Google Scholar]
- 19.Manczuk M, Boffetta P, Sartori S, Hashim D, Vatten LJ, Zatonski WA. Cohort profile: the Polish-Norwegian Study (PONS) cohort [published online ahead of print May 6, 2015] Int J Epidemiol. doi: 10.1093/ije/dyv037. [DOI] [PubMed] [Google Scholar]
- 20.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 21.Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 22.Casale PN, Devereux RB, Kligfield P, et al. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572–580. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 23.Magnani JW, Williamson MA, Ellinor PT, Monahan KM, Benjamin EJ. P wave indices: current status and future directions in epidemiology, clinical, and research applications. Circ Arrhythm Electrophysiol. 2009;2:72–79. doi: 10.1161/CIRCEP.108.806828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 25.Grundvold I, Skretteberg PT, Liestøl K, et al. Upper normal blood pressures predict incident atrial fibrillation in healthy middle-aged men a 35-year follow-up study. Hypertension. 2012;59:198–204. doi: 10.1161/HYPERTENSIONAHA.111.179713. [DOI] [PubMed] [Google Scholar]
- 26.O’Neal WT, Soliman EZ, Qureshi W, Alonso A, Heckbert SR, Herrington D. Sustained pre-hypertensive blood pressure and incident atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis. J Am Soc Hypertens JASH. 2015;9:191–196. doi: 10.1016/j.jash.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okin PM, Hille DA, Larstorp ACK, et al. Effect of lower on-treatment systolic blood pressure on the risk of atrial fibrillation in hypertensive patients. Hypertension. 2015;66:368–373. doi: 10.1161/HYPERTENSIONAHA.115.05728. [DOI] [PubMed] [Google Scholar]
- 28.Stritzke J, Markus MRP, Duderstadt S, et al. The aging process of the heart: obesity is the main risk factor for left atrial enlargement during aging the MONICA/KORA (monitoring of trends and determinations in cardiovascular disease/cooperative research in the region of Augsburg) study. J Am Coll Cardiol. 2009;54:1982–1989. doi: 10.1016/j.jacc.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 29.Magnani JW, Zhu L, Lopez F, et al. P-wave indices and atrial fibrillation: cross-cohort assessments from the Framingham Heart Study (FHS) and Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2015;169:53–61.e1. doi: 10.1016/j.ahj.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen JB, Pietersen A, Graff C, et al. Risk of atrial fibrillation as a function of the electrocardiographic PR interval: results from the Copenhagen ECG Study. Heart Rhythm. 2013;10:1249–1256. doi: 10.1016/j.hrthm.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Magnani JW, Wang N, Nelson KP, et al. Electrocardiographic PR interval and adverse outcomes in older adults: the Health, Aging, and Body Composition study. Circ Arrhythm Electrophysiol. 2013;6:84–90. doi: 10.1161/CIRCEP.112.975342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chamberlain AM, Agarwal SK, Folsom AR, et al. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2011;107:85–91. doi: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Neal WT, Zhang Z-M, Loehr LR, Chen LY, Alonso A, Soliman EZ. Electrocardiographic advanced interatrial block and atrial fibrillation risk in the general population. Am J Cardiol. 2016;117:1755–1759. doi: 10.1016/j.amjcard.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mugnai G, Chierchia G-B, de Asmundis C, et al. P-wave indices as predictors of atrial fibrillation recurrence after pulmonary vein isolation in normal left atrial size. J Cardiovasc Med (Hagerstown) 2016;17:194–200. doi: 10.2459/JCM.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 36.Kishima H, Mine T, Takahashi S, Ashida K, Ishihara M, Masuyama T. The impact of left atrial pressure on filtered P-wave duration in patients with atrial fibrillation [published online ahead of print January 5, 2016] Heart Vessels. doi: 10.1007/s00380-015-0789-3. [DOI] [PubMed] [Google Scholar]
- 37.Mondillo S, Cameli M, Caputo ML, et al. Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr. 2011;24:898–908. doi: 10.1016/j.echo.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 38.King JH, Huang CL-H, Fraser JA. Determinants of myocardial conduction velocity: implications for arrhythmogenesis. Front Physiol. 2013;4:154. doi: 10.3389/fphys.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lau DH, Schotten U, Mahajan R, et al. Novel mechanisms in the pathogenesis of atrial fibrillation: practical applications. Eur Heart J. 2016;37:1573–1581. doi: 10.1093/eurheartj/ehv375. [DOI] [PubMed] [Google Scholar]
- 40.Batal O, Schoenhagen P, Shao M, et al. Left atrial epicardial adiposity and atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:230–236. doi: 10.1161/CIRCEP.110.957241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.