Abstract
Endometriosis is the abnormal growth of endometrial cells outside the uterus, causing pelvic pain and infertility. Furthermore, adhesion of endometrial tissue fragments to pelvic mesothelium is required for the initial step of endometriosis formation outside uterus. TGF-β1 and adhesion molecules importantly function for adhesion of endometrial tissue fragments to mesothelium outside uterus. However, the function of TGF-β1 on the regulation of adhesion molecule expression for adhesion of endometrial tissue fragments to mesothelium has not been fully elucidated. Interestingly, transforming growth factor β1 (TGF-β1) expression was higher in endome-triotic epithelial cells than in normal endometrial cells. The adhesion efficiency of endometriotic epithelial cells to meso-thelial cells was also higher than that of normal endometrial cells. Moreover, TGF-β1 directly induced the adhesion of endometrial cells to mesothelial cells through the regulation of integrin of αV, α6, β1, and β4 via the activation of the TGF-β1/TGF-βRI/Smad2 signaling pathway. Conversely, the adhesion of TGF-β1-stimulated endometrial cells to mesothelial cells was clearly reduced following treatment with neutralizing antibodies against specific TGF-β1-mediated integrins αV, β1, and β4 on the endometrial cell membrane. Taken together, these results suggest that TGF-β1 may act to promote the initiation of endometriosis by enhancing integrin-mediated cell-cell adhesion.
Keywords: Endometrial cells, Endometriosis, Integrin, Mesothelial cells, TGF-β1
INTRODUCTION
Endometriosis is a common gynecological disorder defined as growth of endometrial tissues outside the uterus. Possible causes include retrograde menstruation, immunological disorders, invasive implantation, and ectopic growth of endometrial tissues (1, 2). However, the precise mechanisms that underlie the initial development and subsequent progression of endometriosis are not clear. At the initial stages of the disease, the attachment of retrograde endometrial tissues onto the pelvic mesothelium is a critical step. Several adhesion molecules, including integrin αvβ3, α4β1, VCAM-1, and Nectin-4, have been suggested to be key factors in regulating the attachment of endometrial and mesothelial tissues (3–6). Furthermore, while previous studies have demonstrated that these adhesion molecules are regulated by cytokines and growth factors (7–12), the mechanisms that underlie this regulation are still not clear.
Transforming growth factor-β (TGF-β) is a 25 kDa peptide that plays key roles in the progression of endometriosis (13). TGF-β expression is higher in the serum, peritoneal fluid, and cyst tissues of patients with endometriosis than in women without endometriosis (14–16). Among the three subtypes of TGF-βs, TGF-β1 is generally considered to be a key player in the pathogenesis of endometriosis, due to its pattern of expression and correlation with disease progression (13, 17). TGF-β1 is involved in the suppression of immune surveillance, cell adhesion and invasion into the peritoneum, and in the growth of implants (13). Peritoneal adhesion of endometrial cells, in particular, is elevated in the presence of TGF-β1 (18, 19). The expression of several integrins in different cells is controlled by TGF-β1 (20–23); therefore it was suggested that cell-cell interactions that are activated by TGF-β1 could be mediated by integrins (13). To date, there have been no reports describing direct evidence for TGF-β1-mediated regulation of integrins and other adhesion molecules in endometrial cells, or its role in peritoneal adhesion of retrograde endometrium.
In the present study, we demonstrated that autocrine secretion of TGF-β1 increased adhesion between endometrial and mesothelial cells through expression of integrin αV, α6, β1, and β4. Moreover, blocking these integrins with neutralizing antibodies suppressed the mesothelial adhesion of endometrial cells. Thus, we suggest that TGF-β1 may act to promote the initiation of endometriosis by enhancing integrin-mediated cell-cell adhesion.
RESULTS
TGF-β1 expression is increased in endometriotic epithelial cells and is associated with the adhesion of endometrial cells to mesothelial cells
It has been reported that TGF-β1 expression is increased in the peritoneal fluid of women with endometriosis (13, 24). In the current study, we compared levels of TGF-β1 expression in normal human endometrial cells (HES cells) and human endometriotic epithelial cells from endometrial lesions (12Z cells). As shown in Fig. 1A, TGF-β1 expression was significantly higher in 12Z cells than in HES cells. Results from several recent studies have suggested that proliferative, secretory, and menstrual endometrial fragments rapidly attach to the peritoneal mesothelium in case of endometriosis (25–28). Therefore, we also investigated differences in the adhesion of HES and 12Z cells to human mesothelial cells (Met-5A). As shown in Fig. 1B, the adhesion ratio of 12Z cells to Met-5A cells was approximately 3 times higher than that of HES cells. Because TGF-β1 expression and adhesion were higher in endometriotic epithelial cells than in normal endometrial cells, we examined whether TGF-β1-mediated signaling is involved in the adhesion of 12Z cells to Met-5A cells.
Fig. 1.
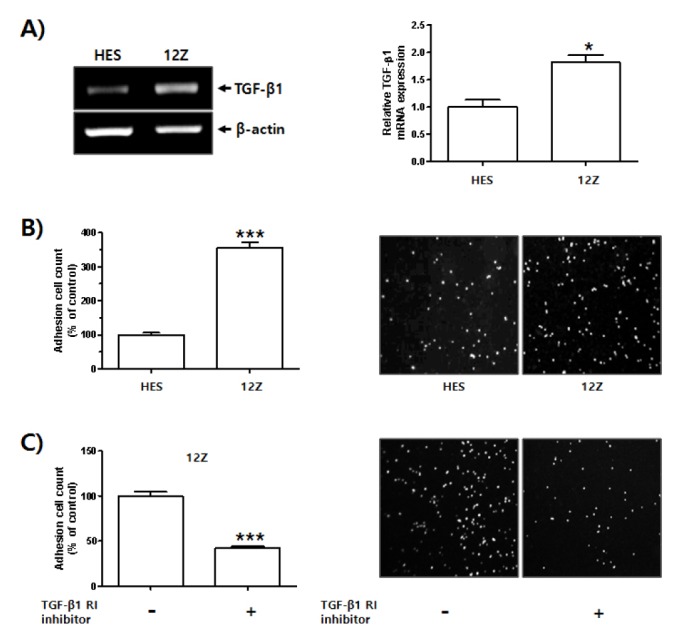
Enhanced TGF-β1 expression in human endometriotic epithelial cells and its function in adhesion of endometrial cells to mesothelial cells. Total RNA was extracted from HES cells and 12Z cells. (A) Levels of TGF-β1 mRNA expression were examined using RT-PCR. β-actin was used as an internal control. Band intensity of TGF-β1 mRNA expression was quantified and normalized to β-actin internal control using densitometry (ImageJ software, NIH). Data obtained from densitometric analyses are shown as bar graph. Data are expressed as fold of control and shown as mean ± SD for three independent experiments (*P < 0.05 in comparison between two groups). Differences between mean values and two groups were evaluated using Student’s t-test and analysis of variance with an unpaired t-test. (B) HES cells (5 × 105 cells) were seeded onto 6-well plate and cultured for 24 h. 12Z cells (3 × 105 cells) were seeded onto 100 π culture dish plate and cultured for 24 h. HES and 12Z cells were labeled with CMFDA for 15 min at 37°C, then washed in 1 × PBS and gently transferred onto a Met-5A cell monolayer. Number of HES and 12Z cells bound to confluent Met-5A cells was manually counted. Four pictures were taken per well and the number of adherent cells was calculated as a percentage of the control cell values and expressed as mean ± SD for three independent experiments (***P < 0.01 in comparison between two groups). Differences between mean values and two groups were evaluated using Student’s t-test and analysis of variance with an unpaired t-test. (C) 12Z cells (3 × 105 cells) were seeded onto 100 π culture dish plate and cultured for 24 h. Medium was replaced and cells were incubated in serum free-medium with or without TGF-βRI inhibitor for 24 h. Cells were then labeled with CMFDA for 15 min at 37°C, then washed in 1 × PBS and gently transferred onto a Met-5A cell monolayer. Number of cells bound to confluent Met-5A cells was manually counted. Four pictures were taken per well and the number of adherent cells was calculated as a percentage of the control cell values and expressed as mean ± SD for three independent experiments (***P < 0.01 in comparison between two groups). Differences between mean values and two groups were evaluated using Student’s t-test and analysis of variance with an unpaired t-test.
Interestingly, adhesion rate of TGF-β receptor I (TGF-βRI) inhibitor (SB-525334, Sigma, St. Louis, MO, USA)-treated 12Z cells to Met-5A cells was lower than that of 12Z cells (Fig. 1C). These results suggest that enhanced expression of TGF-β1 in 12Z cells affects the adhesion of endometrial cells to mesothelial cells, thus playing a role in the progression of endometriosis.
TGF-β1 induces the adhesion of endometrial cells to mesothelial cells through the TGF-βRI/Smad2 signaling pathway
Secretion of TGF-β into the peritoneal fluid plays an important role in the establishment of endometriosis (13, 24). Thus, we investigated whether TGF-β1 played a role in the initial stages of endometriosis formation outside the uterus via direct induction of the adhesion of endometrial cells to mesothelial cells. Adhesion rates of TGF-β1-stimulated HES and 12Z cells to Met-5A cells were clearly higher than that of untreated HES and 12Z cells (Fig. 2A). Furthermore, TGF-β1 significantly induced the adhesion of normal endometrial cells to mesothelial cells through activation of Smad-2 signaling. However, treatment with a TGF-βRI inhibitor markedly suppressed the TGF-β1-induced adhesion of HES cells to Met-5A cells by inhibition of the TGF-βRI/Smad2 signaling pathway (Fig. 2B and C).
Fig. 2.
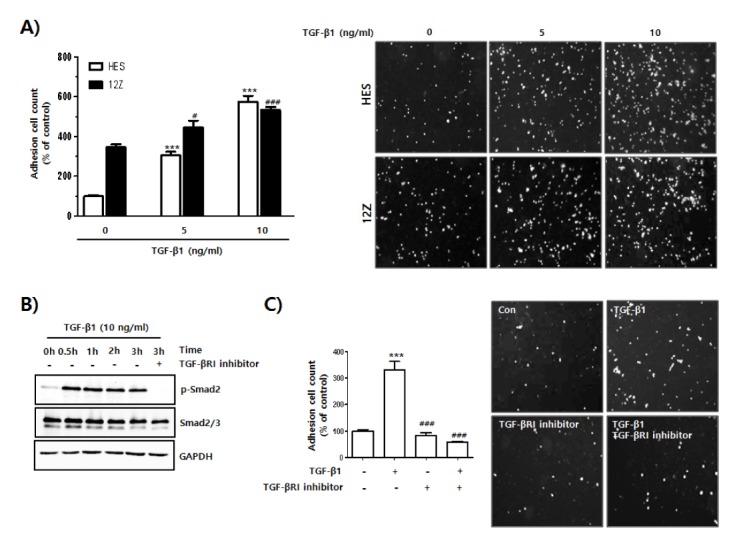
Increased adhesion of endometrial cells to mesothelial cells by activation of TGF-β1-mediated signaling. (A) HES and 12Z cells were seeded and cultured for 24 h. Medium was replaced and cells were incubated in serum free-medium with or without TGF-β1 for 24 h. Cells were labeled with CMFDA for 15 min at 37°C, then washed in 1 × PBS and gently transferred onto a Met-5A cell monolayer. After gentle shaking at 20 rpm for 20 min at 37°C, Cells were washed three times with 1 × PBS to remove unbound cells. Attached cells were visualized using a fluorescent microscope, and quantified using ImageJ software. The number of cells in 4 randomly chosen areas in each well was used for statistical analysis. The results from 3 independent experiments were calculated as a percentage of the control cell values and presented as mean ± SD. ***P < 0.001 compared to control white bar graph (1st column). #P < 0.05 and ###P < 0.001 compared to control black bar graph (1st column). Differences between mean values of experimental groups were determined using one-way analysis of variance (one-way ANOVA) with a Tukey’s post-hoc test, using GraphPad Prism software. (B) HES cells were seeded and cultured for 24 h. Medium was replaced and cells were incubated in serum free-medium with or without TGF-β1 in the presence or absence of TGF-βRI inhibitor for the indicated times. Phosphorylation levels of Smad2 were analyzed using western blot. GAPDH expression was used as an internal control. (C) HES cells were seeded and cultured for 24 h. Medium was replaced and cells were incubated in serum free-medium with or without TGF-β1 in the presence or absence of TGF-βRI inhibitor for the indicated times. Cells were labeled with CMFDA for 15 min at 37°C, then washed in 1 × PBS and then gently transferred onto a Met-5A cell monolayer. Number of cells bound to confluent Met-5A cells was manually counted. Four pictures were taken per well and the number of adherent cells was calculated as a percentage of the control cell values and shown as mean ± SD for three independent experiments. ***P < 0.001 compared to negative control (1st column). ###P < 0.001 compared to positive control (2nd column). Differences between mean values of experimental groups were determined using one-way ANOVA with a Tukey’s post-hoc test, using GraphPad Prism software.
TGF-β1 induces the expressions of cell adhesion molecules in endometrial cells
Adhesion molecules, including integrins, CD44, ICAM-1, L-selectin and E-cadherin (29) and TGF-β1, play pivotal roles in the attachment of endometrial cells outside the uterus i.e., the initiation of endometriosis; however, to the best of our knowledge, there is no direct evidence for a regulatory function of TGF-β1 on expressions of adhesion molecules. As shown in Fig. 3A, TGF-β1 induced the expression of integrins αV, α6, β1, and β4. The expression levels of integrin β5, CD44s, ICAM-1, and L-selectin were not increased by TGF-β1 treatment, and integrin β3 and E-cadherin were not detectable under the same conditions. To further investigate this result, we used a TGF-βRI inhibitor and measured levels of integrin αV, α6, β1, and β4 mRNA expression. Treatment with the TGF-βRI inhibitor clearly reduced integrin expression levels (αV, α6, β1, and β4) in HES cells induced by TGF-β1 (Fig. 3B). Thus, our data showed that TGF-β1 induced adhesion of endometrial cells to mesothelial cells by enhancing the expression of integrins αV, α6, β1, and β4.
Fig. 3.
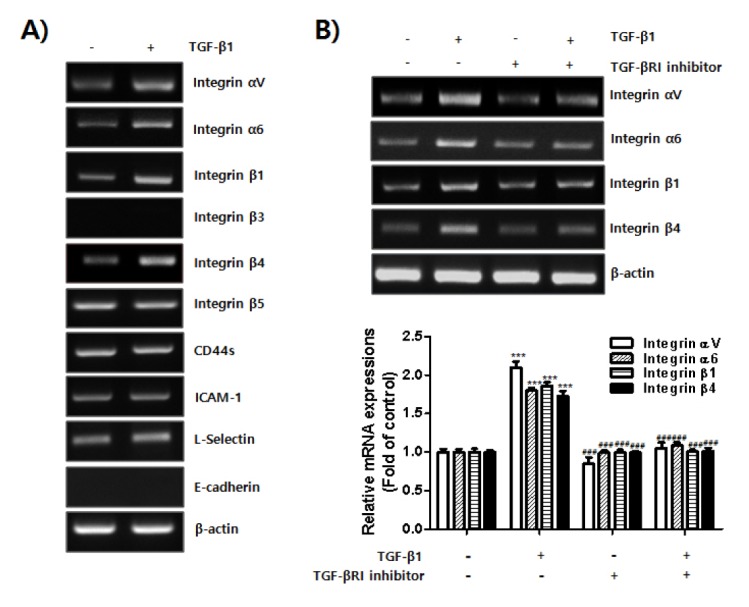
Expressions of integrin αV, α6, β1, and β4 induced by TGF-β1 in endometrial cells. HES cells were seeded and cultured for 24 h. Medium was replaced and cells were incubated in serum free-medium with or without TGF-β1 for 24 h. Total RNA was extracted from the cells. (A) mRNA expression of adhesion molecules was examined using RT-PCR. β-actin was used as an internal control. (B) HES cells were seeded and cultured for 24 h. Medium was replaced and the cells were incubated in serum free-medium with or without TGF-β1 in the presence or absence of TGF-βRI inhibitor for 24 h. Total RNA was extracted from the cells. Expression levels of integrin αV, α6, β1, and β4 were examined using RT-PCR. β-actin was used as an internal control. Band intensity of each integrin mRNA expression was quantified and normalized to β-actin internal control using densitometry. Data obtained from densitometric analyses are shown as bar graph. Data are expressed as fold of control and are shown as mean ± SD for three independent experiments ***P < 0.001 compared to each negative control (1st column). ###P < 0.001 compared to each positive control (2nd column). Differences between mean values of experimental groups were determined using a one-way ANOVA with a Tukey’s post-hoc test, using GraphPad Prism software.
Neutralizing integrin αV, β1, and β4 inhibits adhesion of TGF-β1-stimulated HES cells to Met-5A cells
We then started to investigate whether integrin subunits αV, β1, and β4 regulated the adhesion of endometrial cells to mesothelial cells through TGF-β1-induced expression of integrin heterodimers αVβ1, α6β1, and α6β4 in endometrial cells. We assessed the adhesion of TGF-β1-stimulated endometrial cells to mesothelial cells in the presence of neutralizing antibodies against integrin subunits αV, β1, and β4. As shown in Fig. 4, TGF-β1 greatly enhanced the adhesion of endometrial cells to mesothelial cells. However, this adhesion was significantly reduced when the TGF-β1-mediated expression levels of integrin dimers αVβ1, α6β1, and α6β4 on endometrial cell surfaces were disrupted by neutralizing antibodies (Fig. 4). These results suggested that secreted TGF-β1 may play a role in the adhesion of endometrial fragments generated by menstruation and the passage of these fragments outside the uterus, where endometriosis is initiated through attachment to the mesothelium. The molecular mechanism involves enhanced expression of integrin heterodimers αVβ1, α6β1, and α6β4 in endometrial cells.
Fig. 4.
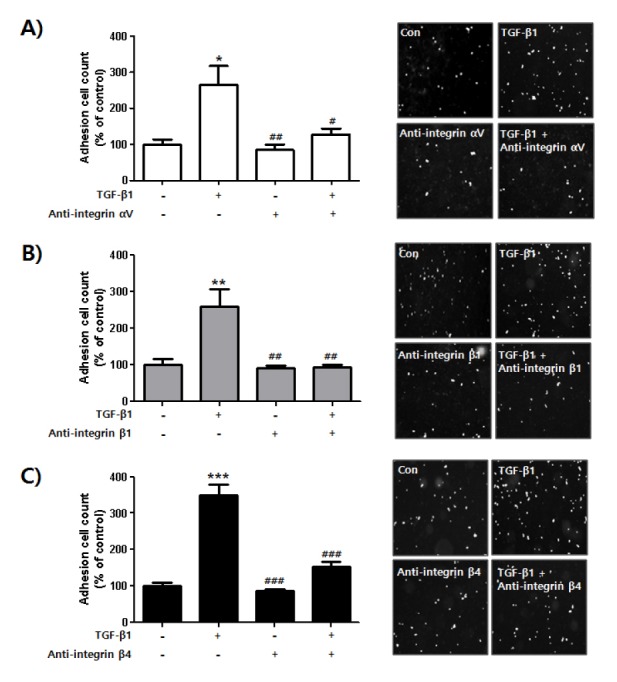
Blocking adhesion of TGF-β1-stimulated endometrial cells to mesothelial cells using integrin αV, β1, and β4 neutralizing antibodies. HES cells were seeded and cultured for 24 h. Medium was replaced and cells were incubated in serum free-medium with or without TGF-β1 in the presence or absence of integrin (A) αV, (B) β1, or (C) β4 antibodies for 24 h. Cells were labeled with CMFDA for 15 min at 37°C, then washed in 1 × PBS and gently transferred onto a Met-5A cell monolayer. Number of cells bound to confluent Met-5A cells was manually counted. Four pictures were taken per well and the number of adherent cells was calculated as a percentage of the control cell values and shown as mean ± SD for three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 compared to each negative control (1st column of each graph). #P < 0.05, ##P < 0.01, ###P < 0.001 compared to each positive control (2nd column of each graph). Differences between mean values of experimental groups were determined using a one-way ANOVA with a Tukey’s post-hoc test, using GraphPad Prism software.
DISCUSSION
Integrins are heterodimeric membrane proteins composed of non-covalently associated α and β subunits, that are essential for linking the extracellular matrix to the cytoskeleton. In mammals, there are 18 α subunits and 8 β subunits that can assemble into 24 different αβ combinations (30). The role of integrins in the reproductive system has been studied for over 20 years. A major focus of these studies has been the involvement of these transmembrane receptors in embryo-endometrial interactions during the implantation window (31). Many researchers have continued to work towards elucidating the function of integrins during endometriosis (32) and have identified potential biomarkers for use in diagnosis and treatment of the disease (33).
Several integrins, including αvβ3, αvβ5, αvβ6, α4β1, and α6β1, have been reported to mediate the attachment of endometrial cells to the mesothelium (4, 34–36). The expression of these integrins is tightly regulated by diverse molecules, such as interleukin (IL)-1, IL-8, macrophage inhibitory factor (MiR)-183, prostaglandin E2, and a cannabinoid receptor agonist (7, 9–11, 37, 38). However, despite the obvious importance of TGF-β1 in the progression of endometriosis (13, 17), there have been no reports of the TGF-β1 function in the regulation of integrins in endometrial cells. Thus, we examined the regulation of integrins by TGF-β1 and their role in the initiation of endometriosis.
First, we confirmed the correlation between endometrial-mesothelial adhesion and TGF-β expression. Our results clearly showed that autocrine expression of TGF-β1 in endometrial cells positively regulated their attachment to mesothelial cells. In human endometriosis lesions, TGF-β1 expression has been found in macrophages, endometrial epithelial cells, endometrial stromal cells, and mesothelial cells (13, 24). Elevated levels of secreted TGF-β1 in peritoneal fluid influenced many steps in the progression of endometriosis, including immune surveillance, cell adhesion and invasion into the peritoneum, angiogenesis, and growth of implants (13, 17). The response of TGF-β1 measured by endometrial-mesothelial adhesion is different between normal endometrial HES cells and ectopic endometrial cells (12Z). Rai et al. (39) reported that expression of adhesion molecules is different between the normal endometrium and endometriosis tissues. Thus, we proposed that it might be caused by elevated expression of TGF-β1 in 12Z cells. We further showed that the use of a specific TGF-β RI inhibitor to inhibit the activity of TGF-β1 activity greatly reduced mesothelial adhesion of TGF-β1-stimulated HES cells. From these results, we postulated that secreted TGF-β1 may act in an autocrine fashion on endometrial-mesothelial interactions.
To identify the factors that mediate TGF-β1-enhanced cell-cell interactions, we analyzed the expression of several adhesion molecules that are known to be important in the development of endometriosis (40, 41). Levels of integrins αv, α6, β1 and β4 mRNA were clearly increased following TGF-β1 treatment. The expression levels of integrin β5, CD44s, ICAM-1, L-selectin were not increased, and integrin β3 and E-cadherin were not detectable under the same conditions. We further suppressed the activity of activity of TGF-β1 by examining whether the expression levels of integrins αV, α6, β1, and β4 were dependent on TGF-β1.
Previous studies reported that several integrins, such as αV, β1, and β3, were positively regulated by TGF-β1 in fibroblast, glioblastoma, and kidney epithelial cells (23, 42, 43). In the present study, expression levels of integrins αV, α6, and β1 were increased in normal endometrial HES cells following TGF-β1 treatment, but integrin β3 expression was not affected by the same conditions. In A549 lung cancer cells, sustained ERK activity induced by TGF-β1 is involved in the induction of integrin β3 (44). In the current study, phosphorylation of Smad2 was found to be a major step in the signaling pathway involved in TGF-β1-stimulated integrin expression and endometrial-mesothelial adhesion. Previous studies demonstrated that integrin β4 is negatively regulated by TGF-β1 in fibroblast and mammary gland cells via epigenetic modifications (45, 46). By contrast, we found that TGF-β1 increased the expression of integrin β4 in endometrial cells. Although the precise molecular machinery involved in the modulation of integrin β4 by TGF-β1 is not fully elucidated, we hypothesize that the differential expression of integrin β4 is mainly due to tissue specificity.
Next, we confirmed the role of induced integrins on endometrial-mesothelial attachment by neutralizing the action of integrins in endometrial cells. Addition of antibodies against integrins αV, β1, and β4 significantly blocked the adhesion of endometrial cells onto mesothelium. To the best our knowledge, this is the first report to show that blocking the functions of integrins αV, β1, and β4 with neutralizing antibodies reduced the development of endometriosis by inhibiting endometrial-mesothelial adhesion.
In conclusion, as illustrated in supplementary Fig. 1, we demonstrated that TGF-β1 increased endometrial-mesothelial adhesion via autocrine regulation. This TGF-β1-stimulated adhesion is mediated by integrins αV, α6, β1, and β4, and blocking these integrins with neutralizing antibodies reduced the mesothelial adhesion of endometrial cells. We therefore propose that TGF-β1-stimulation of integrins αV, α6, β1, and β4 could be a good target for the development of new methods aimed at preventing or treating endometriosis.
MATERIALS AND METHODS
Supplementary Information
ACKNOWLEDGEMENTS
This study was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (MISP), of the Korean Government (Grant no. NRF-2015R1D1A1A01060264 to K.T.H), and a grant of the Traditional Korean Medicine R&D Project, Ministry for Health & Welfare, Republic of Korea (Grant no. HI15C0188 to T.W.C).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting financial interests.
REFERENCES
- 1.Ping S, Ma C, Liu P, et al. Molecular mechanisms underlying endometriosis pathogenesis revealed by bioinformatics analysis of microarray data. Arch Gynecol Obstet. 2016;293:797–804. doi: 10.1007/s00404-015-3875-y. [DOI] [PubMed] [Google Scholar]
- 2.Nothnick W, Alali Z. Recent advances in the understanding of endometriosis: the role of inflammatory mediators in disease pathogenesis and treatment. F1000Res. 2016:5. doi: 10.12688/f1000research.7504.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedir R, Sehitoglu I, Balik G, et al. The role of the adhesion molecule Nectin-4 in the pathogenesis of endometriosis. Clin Exp Obstet Gynecol. 2016;43:463–466. [PubMed] [Google Scholar]
- 4.Schutt AK, Atkins KA, Slack-Davis JK, Stovall DW. VCAM-1 on peritoneum and alpha4beta1 integrin in endometrium and their implications in endometriosis. Int J Gynecol Pathol. 2015;34:85–89. doi: 10.1097/PGP.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 5.Sundqvist J, Andersson KL, Scarselli G, Gemzell-Danielsson K, Lalitkumar PG. Expression of adhesion, attachment and invasion markers in eutopic and ectopic endometrium: a link to the aetiology of endometriosis. Hum Reprod. 2012;27:2737–2746. doi: 10.1093/humrep/des220. [DOI] [PubMed] [Google Scholar]
- 6.Surrey ES, Minjarez DA, Schoolcraft WB. The incidence of aberrant endometrial alphavbeta(3) vitronectin expression in a high risk infertility population: could prolonged GnRH agonist therapy play a role? J Assist Reprod Genet. 2007;24:553–556. doi: 10.1007/s10815-007-9164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez AM, Quattrone F, Pannese M, et al. The cannabinoid receptor CB1 contributes to the development of ectopic lesions in a mouse model of endometriosis. Hum Reprod. 2017;32:175–184. doi: 10.1093/humrep/dew281. [DOI] [PubMed] [Google Scholar]
- 8.Rakhila H, Girard K, Leboeuf M, Lemyre M, Akoum A. Macrophage migration inhibitory factor is involved in ectopic endometrial tissue growth and peritoneal-endometrial tissue interaction in vivo: a plausible link to endometriosis development. PLoS One. 2014;9:e110434. doi: 10.1371/journal.pone.0110434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Banu SK, Burghardt RC, Starzinski-Powitz A, Arosh JA. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 inhibits adhesion of human endometriotic epithelial and stromal cells through suppression of integrin-mediated mechanisms. Biol Reprod. 2013;88:77. doi: 10.1095/biolreprod.112.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khoufache K, Bondza PK, Harir N, et al. Soluble human IL-1 receptor type 2 inhibits ectopic endometrial tissue implantation and growth: identification of a novel potential target for endometriosis treatment. Am J Pathol. 2012;181:1197–1205. doi: 10.1016/j.ajpath.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Arici A. Local cytokines in endometrial tissue: the role of interleukin-8 in the pathogenesis of endometriosis. Ann N Y Acad Sci. 2002;955:101–109. doi: 10.1111/j.1749-6632.2002.tb02770.x. discussion 118, 396–406. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Velasco JA, Arici A. Interleukin-8 expression in endometrial stromal cells is regulated by integrin-dependent cell adhesion. Mol Hum Reprod. 1999;5:1135–1140. doi: 10.1093/molehr/5.12.1135. [DOI] [PubMed] [Google Scholar]
- 13.Omwandho CO, Konrad L, Halis G, Oehmke F, Tinneberg HR. Role of TGF-betas in normal human endometrium and endometriosis. Hum Reprod. 2010;25:101–109. doi: 10.1093/humrep/dep382. [DOI] [PubMed] [Google Scholar]
- 14.Tamura M, Fukaya T, Enomoto A, Murakami T, Uehara S, Yajima A. Transforming growth factor-beta isoforms and receptors in endometriotic cysts of the human ovary. Am J Reprod Immunol. 1999;42:160–167. doi: 10.1111/j.1600-0897.1999.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 15.Pizzo A, Salmeri FM, Ardita FV, Sofo V, Tripepi M, Marsico S. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecol Obstet Invest. 2002;54:82–87. doi: 10.1159/000067717. [DOI] [PubMed] [Google Scholar]
- 16.D’Hooghe TM, Xiao L, Hill JA. Cytokine profiles in autologous peritoneal fluid and peripheral blood of women with deep and superficial endometriosis. Arch Gynecol Obstet. 2001;265:40–44. doi: 10.1007/s004040000126. [DOI] [PubMed] [Google Scholar]
- 17.Dela Cruz C, Reis FM. The role of TGFbeta superfamily members in the pathophysiology of endo-metriosis. Gynecol Endocrinol. 2015;31:511–515. doi: 10.3109/09513590.2015.1018166. [DOI] [PubMed] [Google Scholar]
- 18.Chegini N. TGF-beta system: the principal profibrotic mediator of peritoneal adhesion formation. Semin Reprod Med. 2008;26:298–312. doi: 10.1055/s-0028-1082388. [DOI] [PubMed] [Google Scholar]
- 19.Sandoval P, Jimenez-Heffernan JA, Guerra-Azcona G, et al. Mesothelial-to-mesenchymal transition in the pathogenesis of post-surgical peritoneal adhesions. J Pathol. 2016;239:48–59. doi: 10.1002/path.4695. [DOI] [PubMed] [Google Scholar]
- 20.Chin SL, Johnson SA, Quinn J, et al. A role for alphaV integrin subunit in TGF-beta-stimulated osteoclastogenesis. Biochem Biophys Res Commun. 2003;307:1051–1058. doi: 10.1016/S0006-291X(03)01294-4. [DOI] [PubMed] [Google Scholar]
- 21.Dou Q, Williams RS, Chegini N. Inhibition of transforming growth factor-beta 1 alters the growth, anchor-dependent cell aggregation and integrin mRNA expression in human promonocytes: implications for endometriosis and peritoneal adhesion formation. Mol Hum Reprod. 1997;3:383–391. doi: 10.1093/molehr/3.5.383. [DOI] [PubMed] [Google Scholar]
- 22.Honda E, Yoshida K, Munakata H. Transforming growth factor-beta upregulates the expression of integrin and related proteins in MRC-5 human myofibroblasts. Tohoku J Exp Med. 2010;220:319–327. doi: 10.1620/tjem.220.319. [DOI] [PubMed] [Google Scholar]
- 23.Moyano JV, Greciano PG, Buschmann MM, Koch M, Matlin KS. Autocrine transforming growth factor-{beta}1 activation mediated by integrin {alpha}V{beta}3 regulates transcriptional expression of laminin-332 in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2010;21:3654–3668. doi: 10.1091/mbc.E10-06-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young VJ, Brown JK, Saunders PT, Duncan WC, Horne AW. The peritoneum is both a source and target of TGF-beta in women with endometriosis. PLoS One. 2014;9:e106773. doi: 10.1371/journal.pone.0106773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witz CA, Monotoya-Rodriguez IA, Schenken RS. Whole explants of peritoneum and endometrium: a novel model of the early endometriosis lesion. Fertil Steril. 1999;71:56–60. doi: 10.1016/S0015-0282(98)00400-2. [DOI] [PubMed] [Google Scholar]
- 26.Witz CA, Allsup KT, Montoya-Rodriguez IA, Vaughn SL, Centonze VE, Schenken RS. Culture of menstrual endometrium with peritoneal explants and mesothelial monolayers confirms attachment to intact mesothelial cells. Hum Reprod. 2002;17:2832–2838. doi: 10.1093/humrep/17.11.2832. [DOI] [PubMed] [Google Scholar]
- 27.Witz CA, Thomas MR, Montoya-Rodriguez IA, Nair AS, Centonze VE, Schenken RS. Short-term culture of peritoneum explants confirms attachment of endometrium to intact peritoneal mesothelium. Fertil Steril. 2001;75:385–390. doi: 10.1016/S0015-0282(00)01699-X. [DOI] [PubMed] [Google Scholar]
- 28.Lucidi RS, Witz CA, Chrisco M, Binkley PA, Shain SA, Schenken RS. A novel in vitro model of the early endometriotic lesion demonstrates that attachment of endometrial cells to mesothelial cells is dependent on the source of endometrial cells. Fertil Steril. 2005;84:16–21. doi: 10.1016/j.fertnstert.2004.10.058. [DOI] [PubMed] [Google Scholar]
- 29.Young VJ, Brown JK, Saunders PT, Horne AW. The role of the peritoneum in the pathogenesis of endometriosis. Hum Reprod Update. 2013;19:558–569. doi: 10.1093/humupd/dmt024. [DOI] [PubMed] [Google Scholar]
- 30.Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merviel P, Challier JC, Carbillon L, Foidart JM, Uzan S. The role of integrins in human embryo implantation. Fetal Diagn Ther. 2001;16:364–371. doi: 10.1159/000053942. [DOI] [PubMed] [Google Scholar]
- 32.Umezawa M, Saito Y, Tanaka-Hattori N, Takeda K, Ihara T, Sugamata M. Expression profile of extracellular matrix and adhesion molecules in the development of endometriosis in a mouse model. Reprod Sci. 2012;19:1365–1372. doi: 10.1177/1933719112450340. [DOI] [PubMed] [Google Scholar]
- 33.Gupta D, Hull ML, Fraser I, et al. Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;4:CD012165. doi: 10.1002/14651858.CD012165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klemmt PA, Carver JG, Koninckx P, McVeigh EJ, Mardon HJ. Endometrial cells from women with endometriosis have increased adhesion and proliferative capacity in response to extracellular matrix components: towards a mechanistic model for endometriosis progression. Hum Reprod. 2007;22:3139–3147. doi: 10.1093/humrep/dem262. [DOI] [PubMed] [Google Scholar]
- 35.Koks CA, Groothuis PG, Dunselman GA, de Goeij AF, Evers JL. Adhesion of menstrual endometrium to extracellular matrix: the possible role of integrin alpha(6)beta(1) and laminin interaction. Mol Hum Reprod. 2000;6:170–177. doi: 10.1093/molehr/6.2.170. [DOI] [PubMed] [Google Scholar]
- 36.Puy LA, Pang C, Librach CL. Immunohistochemical analysis of alphavbeta5 and alphavbeta6 integrins in the endometrium and endometriosis. Int J Gynecol Pathol. 2002;21:167–177. doi: 10.1097/00004347-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Khoufache K, Bazin S, Girard K, et al. Macrophage migration inhibitory factor antagonist blocks the development of endometriosis in vivo. PLoS One. 2012;7:e37264. doi: 10.1371/journal.pone.0037264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J, Gu L, Ni J, Hu P, Hu K, Shi YL. MiR-183 Regulates ITGB1P Expression and Promotes Invasion of Endometrial Stromal Cells. Biomed Res Int. 2015;2015;340218 doi: 10.1155/2015/340218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rai V, Hopkisson J, Kennedy S, Bergqvist A, Barlow DH, Mardon HJ. Integrins alpha 3 and alpha 6 are differentially expressed in endometrium and endometriosis. J Pathol. 1996;180:181–187. doi: 10.1002/(SICI)1096-9896(199610)180:2<181::AID-PATH620>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 40.Singh H, Aplin JD. Adhesion molecules in endometrial epithelium: tissue integrity and embryo implantation. J Anat. 2009;215:3–13. doi: 10.1111/j.1469-7580.2008.01034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witz CA. Cell adhesion molecules and endometriosis. Semin Reprod Med. 2003;21:173–182. doi: 10.1055/s-2003-41324. [DOI] [PubMed] [Google Scholar]
- 42.Silginer M, Burghardt I, Gramatzki D, et al. The aryl hydrocarbon receptor links integrin signaling to the TGF-beta pathway. Oncogene. 2016;35:3260–3271. doi: 10.1038/onc.2015.387. [DOI] [PubMed] [Google Scholar]
- 43.Gutierrez J, Droppelmann CA, Contreras O, Takahashi C, Brandan E. RECK-Mediated beta1-Integrin Regulation by TGF-beta1 Is Critical for Wound Contraction in Mice. PLoS One. 2015;10:e0135005. doi: 10.1371/journal.pone.0135005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong SK, Park JR, Kwon OS, Kim KT, Bae GY, Cha HJ. Induction of integrin beta3 by sustained ERK activity promotes the invasiveness of TGFbeta-induced mesenchymal tumor cells. Cancer Lett. 2016;376:339–346. doi: 10.1016/j.canlet.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Yang X, Pursell B, Lu S, Chang TK, Mercurio AM. Regulation of beta 4-integrin expression by epigenetic modifications in the mammary gland and during the epithelial-to-mesenchymal transition. J Cell Sci. 2009;122:2473–2480. doi: 10.1242/jcs.049148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scardigli R, Soddu S, Falcioni R, Crescenzi M, Cimino L, Sacchi A. The beta 4 integrin subunit is expressed in mouse fibroblasts and modulated by transforming growth factor-beta 1. Exp Cell Res. 1996;227:223–229. doi: 10.1006/excr.1996.0271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


