Abstract
Background/Objectives
Severe Crohn’s disease impacts negatively on individual quality of life, with treatment options limited once conventional therapies have been exhausted. The aim of this study was to explore factors influencing decision-making and expectations of people considering or participating in the Autologous Haematopoietic Stem Cell Treatment trial.
Methods
An international, cross-sectional qualitative study, involving semistructured face to face interviews across five sites (four UK and one Spain). 38 participants were interviewed (13 men, 25 women; age range 23–67 years; mean age 37 years). The mean age at diagnosis was 20 years. Interviews were audio recorded and transcribed verbatim and transcripts were analysed using a framework approach.
Results
Four themes emerged from the analysis: (1) ‘making your mind up’—a determination to receive stem cell treatment despite potential risks; (2) communicating and understanding risks and benefits; (3) non-participation—your choice or mine? (4) recovery and reframing of personal expectations.
Conclusions
Decision-making and expectations of people with severe Crohn’s disease in relation to autologous haematopoietic stem cell treatment is a complex process influenced by participants’ histories of battling with their condition, a frequent willingness to consider novel treatment options despite potential risks and, in some cases, a raised level of expectation about the benefits of trial participation. Discussions with patients who are considering novel treatments should take into account potential ‘therapeutic misestimation’, thereby enhancing shared decision-making, informed consent and the communication with those deemed non-eligible.
ASTIC trial EudraCT Number
2005-003337-40: results.
Keywords: crohn’s disease, autologous haematopoietic stem cell treatment, qualitative research, decision-making
Strengths and limitations of this study.
This paper provides new knowledge about the factors that may influence decision-making and expectations of people considering and receiving autologous haematopoietic stem cell treatment for Crohn’s disease (CD).
We focused on the factors influencing decision-making and expectations of individuals with severe CD, an important yet often excluded group of participants in studies due to the severity of their disease. We also examined decision-making by participants and non-participants in a trial, the latter being often excluded from study results or the impact that non-participation may have on their experiences.
Findings from this international, qualitative study can inform future research that develops decision-making and information support tools for future participants in ‘radical’ or ‘frontier’ clinical trials such as Haematopoietic Stem Cell Treatment (HSCT).
Face-to-face semistructured Interviews were conducted at only one time-point and did not explore in-depth the cultural and specific healthcare service factors that may have influenced participants’ decision-making and trial experiences across study sites. Future research should harness the strengths of longitudinal study designs taking greater account of the impact of individual, socioeconomic, cultural and health service factors to capture outcomes in relation to those receiving HSCT and for those for whom trial participation proved not to be an option.
In response to stakeholder recommendations, interview settings involved a choice between undertaking an interview at home, in a hospital interview room or via Skype. Although this had strengths in allowing participation for those who lived in rural settings, or for who travel was not desired, we cannot exclude the impact that different settings may have had on the impact of the quality of data collected and the impact of the researcher when using such diverse methods.
Introduction
Providing effective healthcare services for people affected by chronic illness is an established global priority.1 European data on the most frequently recorded chronic illnesses identifies that, while an ageing population is a key factor in the increasing incidence of single and multiple conditions, chronic illness increasingly affects younger populations and experiences of care may be impacted by national and regional variations in care provision.2
In England for example, there are approximately 15.4 million people living with a long-term condition, affecting people of all ages and accounting for 70% of the total health and social care budget. Individuals commonly face challenges relating to physical disability, reduced employment opportunities and an increased likelihood of experiencing depression and anxiety disorders.3
This paper reports on a qualitative exploration of decision-making and expectations of people living with Crohn’s disease (CD) in the context of autologous Haematopoietic Stem Cell Transplantation (HSCT). CD is a life-long, chronic relapsing inflammatory condition predominantly affecting the gastrointestinal tract and is commonly associated with abdominal pain, fever, clinical signs of bowel obstruction or diarrhoea with passage of blood and/or mucus.4 5
The potential impact of living with CD can have on individuals is recognised in validated tools measuring quality of life,6 disease-related concerns7 and personal control.8 Researchers have identified a negative association with quality of life and increased clinical disease activity.9 Common concerns about living with CD including managing uncertainty, the effects of medication, reduced energy levels and fatigue,10 having surgery and being a burden on others.11
The incidence of CD is increasing almost worldwide with increasing trends in industrialised developing countries.12 13 An estimated 1.6 million people in Europe are living with CD with the highest prevalence rates in northern countries.14 There is growing evidence of European wide approaches to treatment interventions and management strategies;15 however, variation exists in the delivery of healthcare systems, for example, regional variation of provision in Spain16 which may have relevance to the current study’s generalisability.
Haematopoietic stem cell transplantation
Immunosuppressive drugs are standard treatment for people living with CD; however, for those that do not respond, or lose response to this therapy, treatment solutions become more challenging to address.17 The Autologous Stem Cell Transplantation International CD trial (ASTIC) commenced in 2008, building on previous non-randomised studies that investigated the impact of Autologous HSCT in CD.18 19 The ASTIC trial was conducted in 11 European transplant units from July 2007 to September 2011, with follow-up through March 2013. Patients were aged 18 to 50 years with impaired quality of life from refractory CD not amenable to surgery despite treatment with three or more immunosuppressive or biological agents and corticosteroids.17 The benefit of autologous HSCT has been examined at length in autoimmune conditions including rheumatoid arthritis and lupus erythematosus20 and the ASTIC trial presented the first international parallel-group randomised clinical trial evaluating its effect in patients with refractory CD, with the primary end point being assessed after 1 year.17
Trial outcome measures included health-related quality of life,21 clinical activity, mucosal healing22 and medication use. However, anecdotal feedback from those who had undertaken HSCT, and those who had considered taking part, suggested there were other factors that had influenced their expectations and decision-making about the trial, including how they viewed the personal benefits gained from taking part. This has particular importance in relation to decision-making as, while the benefits of HSCT are acknowledged,23–25 it has the potential to induce significant side effects in comparison to conventional therapies, including death.26–28
Decision-making
The evidence base for effective shared decision-making between patients and clinicians, informed consent and appropriate feedback of results is well established in relation to clinical trial participation.29 30 Best practice is one where a sense of alliance is developed between patients and clinical staff, where information is presented using appropriate language and one that accounts for the needs of the individual.31 Previous research shows the importance of providing support for patient decision-making in addition to using decision aids and tools.32 However, little was known about key influences on patient decision-making in CD and the sources of information they use to assist them to understand personal benefits and risks, with no previous studies investigating this in relation to HSCT. It is essential to understand more fully the decision-making process and how patients balance risk when considering participation in novel treatments with uncertain and potentially significant risks.33
Methods
The study was informed by pragmatic philosophy, acknowledging the importance of the research question in choosing the best research approach that interests and is of value to the researcher and studying it in the different ways thought to be most appropriate.34 The study employed the strengths of qualitative research methodology to fill an important gap in the understanding of decision-making and expectations in CD. We acknowledge that qualitative research embodies diverse, even conflicting theoretical positions.35 However, it was the broad principles of qualitative inquiry that were adopted within this study, namely the ability to uncover social processes, opinions and experiences of the decision-making process and how this reflected initial expectations of participants.36
Study design and context
The overall aim of this study was to explore, describe and understand peoples’ decision-making and expectations in relation to autologous HSCT for severe CD. In addition to interviews with ASTIC trial participants, this study also aimed to explore decision-making and expectations of those for whom initial trial assessment resulted in ineligibility or those who declined participation by personal choice.
Sampling and recruitment
This study was conducted at five ASTIC study sites, four sites in the UK and one Spanish regional site. UK and Spanish hospitals were publicly funded, acute, inner city university teaching hospitals, providing specialist gastrointestinal and haematological services across a healthcare region. It was acknowledged that variations between healthcare provision models and cultures between UK and Spanish sites was a potential influencing factor; however, the number of Spanish participants recruited to the study made effective exploration of cultural contexts a limiting factor to this study.
Participants were eligible if they were 18 and over, were identified as having severe CD by their specialist inflammatory bowel disease (IBD) consultant (who were also principal investigators in this study) and had taken part or had considered participation in the ASTIC trial. Fifty-eight prospective participants were identified by the ASTIC clinical trial coordinator and principal investigators. Recruitment was conducted in a staged process, using blocks of 10 participants at a time. This was done so as to avoid having to withdraw invitation to any participant who indicated they were willing to do so.
Invitation letters in both English and Spanish were addressed from principal investigators (CJH, JS, ST JL, ER), were accompanied by a copy of the participant information sheet and consent forms. Reminder letters were sent after 21 days if no response was received. Willing participants were asked to return the consent form using a freepost envelope, after which the clinical researcher (IB), chief investigator (JC) or Spanish researcher (AL) contacted them to arrange an interview. General practitioners were informed by letter of the patient’s participation in study.
Participants were categorised into three groups, distinguishing Group 1 ‘ASTIC participants’, or those who had received HSCT, from ‘Non-ASTIC’ participants. ‘Non-ASTIC’ participants (Groups 2 and 3) were separated according to the reason for their non-participation to aid clarity and depth of analysis and discussion (table 1).
Table 1.
Summary of group categories
| Group 1 ‘ASTIC participants’ |
Group 2 ‘Non-ASTIC’ participants (by choice) |
Group 3 ‘Non-ASTIC participants (by external factors)’ |
| Participated in ASTIC trial (including those unable to complete the whole trial) | Participants who did not take part in the trial because they made the decision not to participate themselves or there was another more suitable treatment option available to them | Participants who did not take part in the trial due external factors, for example, non-eligibility, lack of funding, trial halting recruitment. |
ASTIC, Autologous Stem Cell Transplantation International Crohn’s disease.
Ethical considerations
Prior to commencement of the study, ethical approval for the UK was gained from the Nottingham 2 REC committee (Reference number 13/EM/0176). During the study, ethical approval gained participants from La Agencia Española de Medicamentos y Productos Sanitarios in Spain (Madrid) before interviewing Spanish participants. The study was conducted in accordance with the principles of Good Clinical Practice and the Research Governance Framework for English Health and Social Care.37 A protocol was devised directing participants to relevant sources of support should they were to become distressed when talking about their experiences; however, this did not become necessary to enact.
Data collection
Interviews were conducted by three female researchers (IB, JC, AL). All interviewers had experience of conducting qualitative research interviews. JC had conducted qualitative research previously with participants with IBD in her prior role as an IBD nurse specialist38; however, had no prior contact with participants in this study. Only IB and AL (clinical researchers) conducted the Spanish interviews. IB is bilingual in English and Spanish, AL is a native Spanish researcher.
A provisional interview topic guide (see online supplementary material appendix 1) was devised with topic themes informed by a patient and public involvement group with expertise in gastrointestinal conditions. The topic guide included additional probes for in-depth explorations of perceived expectations of HSCT, decision-making and living with severe CD. It was piloted with two UK participants including ongoing revision of the schedule as interviews progressed and concurrent data analysis undertaken.
bmjopen-2016-015201supp001.pdf (212.8KB, pdf)
Face-to-face interviews were conducted with participants according to their preferred method—in their usual IBD clinic, at their home or via Skype, where participants were at home and the researchers interviewing from the hospital. While this introduced varied social contexts in which the interviews were conducted, they reflected recommendations of the study patient and public advisory group and allowed participation of individuals who identified this as a preference, including participants who lived large distances from their hospital and for whom ongoing physical symptoms of CD limited their willingness for home visits or travel. Lo Iacono et al39 acknowledge the challenges such forms of communication can bring on the ability to interpret non-verbal cues language and rapport, they also work well as a viable alternative or complimentary data collection tool for qualitative researchers.
Interviews were conducted between August 2013 and July 2014. Signed consent was obtained prior to starting all interviews except for those by Skype where verbal consent was confirmed and recorded before commencing.
The interviews lasted between 29 min, and 1 hour and 52 min, with the majority of interviews lasting around 45 min. All interviews were audio recorded. Of the 38 interviews conducted, 15 took place in participants’ homes, 16 at their usual clinic and 7 via Skype. On two occasions, immediate family members accompanied participants during their interview at participant’s request; however, they did not contribute to the discussion. Data were collected until no new themes emerged, and therefore we assumed data saturation was reached (n=38).40
Data analysis
All UK interviews were transcribed verbatim by a professional transcriber. Spanish interviews were conducted in Castellano (Spanish) (by AL and IB) and transcribed into an English summary by IB. Transcripts were anonymised of identifiable information prior to analysis being undertaken, pseudonyms applied and then analysed using a framework approach.41
Framework analysis has five key stages:
Familiarisation—immersion in the data began from the time of the first interview. Interviews were read and reread until a broad framework of themes was identified, reflecting key issues of commonality or diversity emerging from the data. Transcripts were analysed either by single sentence or paragraph. A computerised qualitative data management package (QSR NVivo V.10) and Microsoft Excel 2010 were used to assist data management.
Identifying a thematic framework—key issues, concepts and themes were identified from in-depth examination of the data. The analytic framework was guided by (although not restricted to) the key themes identified in the published literature relating to living with chronic illness,42 experiencing HSCT43 and recommendations for effective decision-making.44 As themes emerged, subsequent interviews were adapted to explore specific areas of importance, for example, fertility and early menopause, understanding around the concept of ASTIC treatment as a potential ‘cure’45 and therapeutic misestimation.46 47
Indexing—indices were developed with terms that reflected the language used by participants. This thematic framework was then applied systematically to each transcript.
Charting—the data were sorted according to the appropriate part of the thematic framework to which it relates. Each theme was focused on in detail, returning to the context in which the participants’ statements were made. Charting of the data was done by organising and arranging the data into categories using headings and subheadings.
Mapping and interpretation—the charts were then used to illustrate and define the concepts, map the range and nature of expectations and decision-making that participants described.
Rigour
To enhance rigour, interview transcripts were analysed separately by IB and JC to maximise transparency, accuracy and concordance when developing themes.48 Prior to further discussion and presentation of the analysis with other members of the research team, transcripts were anonymised to remove identifiable information.
Preliminary findings were presented to the study advisory group which included experts of qualitative research and two members of the patient and public involvement group. This group acted as ‘critical friends’ to the ongoing research process. An in-depth description of the research analysis process, in addition to a reflective diary, was maintained to promote transparency of the data collection and analysis and later transferability of the findings. Transcripts were actively analysed for ‘deviant cases,48 such as experiences and expectations that did not concur with the majority to promote dependability of the data.
Results
Forty initial responses were received indicating agreement to participate; however, 2 subsequently withdrew without specific explanation, and therefore 38 participants were interviewed in the study. The mean age was 37 years (range 23–67), 66% (n=25) were female and 58% (n=22) had participated in the ASTIC trial. Table 2 illustrates the basic demographics of the sample used in the study.
Table 2.
Basic demographic and study characteristics (n=38)
| Characteristics | n |
| Gender | |
| Male | 13 |
| Female | 25 |
| Mean age (years) | 37 |
| Range | 23–67 |
| Mean age at diagnosis (years) | 20 |
| Study groups | |
| Group 1 ASTIC participants | 22 |
| Group 2 non-ASTIC participants (by personal choice) Group 3 non-ASTIC participants (by external factors) |
6 10 |
| UK centre study groups | |
| Group 1 ASTIC participants | 16 |
| Group 2 non-ASTIC participants (by personal choice) Group 3 non-ASTIC participants (by external factors) |
6 10 |
| Spanish centre study group Group 1 ASTIC participants |
6 |
ASTIC, Autologous Stem Cell Transplantation International Crohn’s disease.
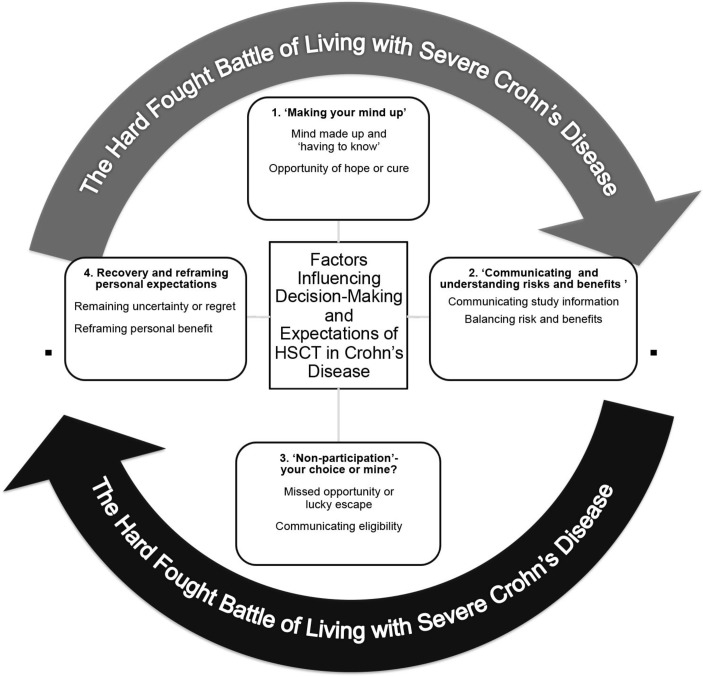
Four major themes collectively captured the factors influencing participants’ decision-making and expectations in relation to HSCT (see figure 1). Irrespective of UK or Spanish context, these were described within contextual background of the ‘hard fought battle of living with Crohn’s disease’ which summarised a unanimous description of the challenges and battles participants had experienced preceding their consideration of trial participation and what was an ongoing experience for many at the time of their interview. Commonalities among the group included the uncertain nature of CD and lack of control over physical symptoms, rapid and significant weight loss, stomach pain and cramps, bloody diarrhoea and nausea. Less common symptoms included mouth and lip ulceration, bruising on their legs and anaemia.
Figure 1.
Summary of factors influencing decision-making and expectations of HSCT in CD. CD, Crohn’s disease; HSCT, Haematopoietic Stem Cell Treatment.
Participants reported a range of treatment side effects due to long-term use of medications for their CD, including corticosteroids. This had resulted in secondary health conditions including damage to the central nervous system, osteopaenia, liver scarring, vertigo, delayed recovery from surgery, wound dehiscence, short bowel syndrome, intestinal failure and a dysfunctional stoma.
Living with severe CD was described negatively in relation to its impact on education and employment. Participants reported constantly ‘preplanning’ and described a lack of spontaneity and resulting social isolation. Prolonged absences from school or work due to fatigue and pain were common, in addition to missing exams, leaving university or work or being medically retired. CD commonly impacted on their social lives causing them to withdraw from social activities, often maintaining a small network of close friends and readjusting their expectations for the future.
Participants shared how over time they have learnt to adapt to living with severe CD, as the gravity of living with such a debilitating physical disease was often described as having a profound impact on life. For all participants, living with severe CD was described as a continual battle for control over its physical, psychological and emotional impact, for some with anger, frustration and for many, with periods of limited success.
The following four themes therefore describe aspects of decision-making and expectations that emerged from the data within the background of this hard-fought battle and are supported by excerpts from the transcripts. Participants are identified by patient identification and study group number. Theme three ‘Non-participation—your choice or mine?’ presents analysis of data from interviews of all study groups 2 and 3, that is, those that did not receive HSCT as part of the ASTIC trial. All other themes present analysis of data from all three study groups.
Theme 1: ‘Making your mind up’
The term ‘making your mind up’ illustrates a common predetermination to undertake treatment by the majority of participants, often due to the limited alternative options described as being available to them but also an opportunity to maximise the potential benefits they may gain from trial participation.
Mind made up and ‘having to know’
All participants described having reached a point where they had either exhausted all treatment options, including medications or surgery, or that other potential options were less preferable to HSCT, for example having a stoma formed. Participants described having ‘no other choice’ for many that this treatment was a ‘last hope’.
I never, ever thought I didn’t want to do it…. But, before I’d even started, I’d made up my mind that I’d wanted to do it….Obviously, I took every—I spoke to a lot of people and I took in their opinion but I, no matter what they said, I still was adamant I was going to do it. (Patient 30, Group 1).
I was at a desperate stage, a real dead end and needed a drastic option. I was facing other health threats and looked like I might need a stoma. (Patient 8, Group 3).
Opportunity of hope or cure
Participants reflected on their expectations of the benefits of HSCT when making their decision to participate. Most participants saw trial participation as an opportunity for hope and a substantial improvement to their condition. Expectations about personal benefits of the trial were viewed as an opportunity to improve their condition and responsiveness to conventional treatment.
I think that was one of the main things, I kind of felt like, if I don’t go for this, you know, in a couple of years’ time, if my Crohn’s is exactly the same and my doctor’s saying, Oh, there’s nothing new we can give you or anything, am I going to be thinking, I wish I’d done it? (Patient 6, Group 3)
For others, expectations were greater, and the trial was described as offering a potential cure.
Definitely, yeah. I mean, when I first heard about it, I thought it was going to be this like, cure…I wouldn’t say it was the radical cure I was hoping for but, I mean, it’s certainly helped a lot. (Patient 26, Group 1)
It’s just all a bit, I got excited that I might go on the stem cell one because it looked like it, you know, from what they’d said that I was hoping that it was going to be my miracle cure, so I was gutted when I couldn’t go on that (Patient 12, Group 2)
Theme 2—communicating and understanding risks and benefits
Participants from all groups described using a variety of sources of information and guidance that informed their decisions about trial participation. Key factors included support from, and duty to family members, trust and communication with specialist clinicians and perceived personal benefits despite the risks involved.
Communication of study information
Participants described having received information about the ASTIC trial study from a variety of sources, including internet searches and online CD forums.
I went on Crohn’s forums and things and, and looked at, you know, the experiences of people, other people who’d done it. Although there wasn’t too many of them around, but there was a few people who were talking about it and considering it and I think, eventually, I said, Okay, you know, I’ll go ahead with it, it’s worth a try.(Patient 6, Group 3)
I read the literature, that, you know, that gets supplied when you’re thinking of a new drug, talk it over with my husband, and we always say, Well, anything’s worth a go. (Patient 14, Group 3)
In contrast, four participants were clear that they had actively avoided thinking about the risks to a great extent and that that to fully understand them is difficult, as exemplified in the quote below:
Obviously, I was, but I just don’t think you can take them on, again, you can’t take them on board because if you do, it’s too scary to take them on board, isn’t it?.… My father, certainly, I think, found that aspect of it quite hard, the amount of risks that came with it. (Patient 24, Group 1)
However, the predominant form of communication and guide for decision-making was based on communications with their specialist inflammatory bowel disease medical consultant. Trust in the expertise of the specialist consultant emerged as key influencing factor in decision-making to consider participation in the trial. This was a therapeutic relationship that had been developed over the course of their condition and their view on the potential risks or benefits were frequently described as influencing decision-making, and most predominantly in Group 1 participants.
I know it’s weird to say but he takes so much time with a patient, it’s kind of like, well, you obviously know what you’re talking about. I mean, I wouldn’t trust any other doctor. I’ve got a kind of close bond with him but, if he says something, he says that, you know, it probably will work, I probably will trust him anyway, you know, because he is that nice.(Patient 25, Group 1)
And I think that if, if he thought it wasn’t going to do me any good, he would never offer that to me…You know what I mean? So, I think if that anything that’s offered from him, I would recommend as well, to anybody else, if he’s recommending it. (Patient 29, Group 1)
Balancing the risks and benefits
Participants described varied concerns and perceptions about potential risks of the treatment. Physical risks such as potential hair loss, impact on fertility, the severity of the treatment, the association of chemotherapy with cancer and even death were described. Fertility and risks to future parenthood emerged as a key issue and is examined in more depth below; however, overall risks relating to treatment for participants were outweighed by the possibility of responding well to the treatment within the context of a hard-fought battle and trust in the expertise of specialist clinical staff.
There was nothing there that stopped me in my tracks… I know there was risks but, there’s risks in everything, isn’t there?…I mean, I, I suppose, I was aware there were some risks but I generally…I trust the people that are looking after me, and, you know, that, sometimes, things don’t work out. (Patient 19, Group 1)
In the past, it was …… I was more, shall I say, I was more critical of treatments and I’d weigh it up. Now, I just think I’ve got nothing to lose so the process is pretty easy, you know, it’s, let’s give it a go, let’s give it a go and I’ll put up with the side effects. (Patient 18, Group 3)
For five participants, one of whom was male, the issue of fertility and parenthood formed a key element of their discussion about the ASTIC trial and understanding of risk to future parenthood. Risks to fertility were described as an important concern and commonly not an issue that they had considered in-depth until the topic arose during their initial consultations, and for some who stated that earlier discussions and preparation would have been beneficial.
Decisions about fertility and future parenthood reflected a varying degree of understanding about the impact that the treatment could have. For some this presented significant anxiety as illustrated below:
And then you start thinking, like, you always think of the pros and the cons, and obviously, I don’t know, it’s just crazy, when I’ve been told that, like, you possibly couldn’t have kids, that’s probably the thing that’s the scariest thing you could be told, because you just want to be, again, you, you’re coming back to this, I want to be normal. (Patient 7, Group 3)
Concerns about potential risks to fertility were also evident in the tension faced by some due to a strong desire to participate and undertake treatment as part of the early randomisation arm of the study. This was in addition to having sufficient time to make choices about freezing eggs or future impacts on parenthood rather than just getting ‘on with the trial’ treatment:
Yeah, I did freeze my eggs. They didn’t actually give that much advice on sort of that. Because I think, from the doctor’s point of view, he wanted me to just like, get on with kind of the trial… obviously fertility was quite important so that I actually delayed starting because I wanted to do the fertility treatment first but it was very much me, really, that pushed for that, rather than them offering to do it. (Patient 30, Group 1)
Three participants described experiencing early onset menopause following trial participation however qualified this decision further by stating that they had not desired to have a family:
Lucky for me, I don’t want children but if I suddenly woke up tomorrow thinking, Actually, I want a child, I now can’t. (Patient 9, Group 1)
Theme 3: ‘Non-participation’—your choice or mine?
This theme considers the data of groups 2 and 3 only, representing the 16 participants who did not receive HSCT. Group 2 represented those who chose not to participate in the trial (n=6), whereas group 3 (n=10) comprised those who were unable to participate due to external factors such as the trial halting, non-eligibility or lack of funding.
When detailing their decision-making not to participate, participants in group 2 most commonly described positive reflections on their decision. For those in group 3 who had been unable to influence this decision however, this was frequently reported as a missed opportunity and where effective communication of trial eligibility was paramount in ensuring effective decision-making and expectations in relation to HSCT.
Missed opportunity or lucky escape
Participants in group 3 (n=10) particularly reflected on how they felt on learning that they were no longer able to participate in the trial. The majority described accepting that the participation in the trial was probably not the best option for them, and while some had initially been disappointed, they described later feeling more accepting.
Erm. … I felt like, it was mixed feelings. It was like, is it a missed opportunity or a lucky escape? (Patient 7, Group 3)
So yeah, I was a bit devastated when they told me that the trial was stopped…It was like, Yeah, I’ll do it. So yeah, a bit sort of deflated when I got that, because there was all the fighting for the funding and, you know, all the rest of it, and I thought, maybe, maybe that would work. (Patient 18, Group 3)
I felt disappointed, but also relieved because what I wouldn’t want is for them to put me through something that wasn’t going to help, because I’d already been there with other things. (Patient 5, Group 3)
Decision-making not to participate for the six participants in group 2 was commonly influenced by the potential isolation that undergoing HSCT would entail, in addition to being treated a long distance from family and home. These are illustrated in the excerpts below and represents data from UK participants only (all Spanish participants were group 1 participants):
Being up there by myself, with nobody, you know, and, I came back and I went to see my consultant gastroenterologist, and that. I just, I was frightened. If I could have had it done in [local hospital] I would have done it in a heartbeat. (Patient 12, Group 3)
Yeah. But also, of the quarantine as well, where I’d have to spend a lot of time down there on my own, away from the kids, away from my husband. And, there wasn’t any guarantee that it could work and also…It wasn’t actually, you know, because of infection, if you got infection and pneumonia and again, that sort of put me off. (Patient 17, Group 2)
For another participant, their age and the additional risks they perceived this to bring also influenced their decision:
So it was total isolation. You know, it’s not like it used to be where you’re in that bubble, and, nobody can come in and, it’s not like that anymore. From what I’ve seen on, on the news, you know, as long as you’re sort of clean and…You know, so, that was obviously age factor again played a big massive part in it. (Patient 12, Group 2)
Communicating eligibility
Of particular pertinence to participants in group 2, the way in which potential eligibility was communicated throughout their assessment stage was important. For those for who viewed HSCT as a ‘last chance’ to improve their hard-fought battle, later ineligibility proved highly disappointing. Participants from all groups had described terms being used such as, ‘you’re a likely candidate’, ‘you’ll be a good candidate’ or ‘you’re severe enough’, during initial trial discussions. This had relevance for how some participants interpreted their likely eligibility and the personal benefits that the treatment could bring due to the significant demands of the trial itself.
While some participants were aware of an earlier death related to the trial, this did not reduce their willingness to participate or expectations of personal eligibility by the language used:
Well, considering the person before died from it….yeah, I’d have still done it, I’d have done it, if they’d have said, Look, you know, there is a risk but you can go ahead. (Patient 23, Group 3)
For one participant who had placed great hope on receiving HSCT as a potential ‘cure’ the way in which their ineligibility was communicated had a strong impact on her reflections on the trial and experience of considering participation.
So, there was messages there already that somebody had died on it, but I was still willing to go, I mean, a main doctor, tells me that I’m a good candidate for it, that gave me too much hope I’d get it….You tell your mum and you tell your daughters that you might be able to have the stem cell transplant and it means a cure, my eldest daughter was with me, when I went for that interview at the hospital… We got married earlier, we rushed to buy a house, we wanted to get settled, he (husband) got a new job, moved area, he took a less paid job because we were made to believe I was going to be having the treatment and it all fell through with just that letter [detailing her non-eligibility]. (Patient 16, Group 3)
Theme 4: Recovery and reframing personal expectations
The final theme represents participants’ reflections on their decision-making and how they viewed this in light of their current experience of living with CD. Participants representing all groups detailed ongoing uncertainties about their condition even if they were content with their current experience of CD.
Remaining uncertainty or regret
Participation in HSCT had not resulted in disease remission for the majority of group 1 participants who described continued uncertainties or regret, including reflections on the value of their decision-making and the expectations they had held in relation to personal benefit as exemplified in the following quotations:
The Crohn’s has returned, and that’s, that’s just, I suppose, incredibly frustrating, disappointing, you know, just totally gutted that you feel you’ve been through so much, you think, you know, is this the one that’s going to get my life back on track? (Patient 19, Group 1).
It’s, my Crohn’s is still bad, it’s still severe, it’s still, well, there in two places, the same places it was before and then again also in my colon. (Patient 2, Group 1)
For groups 2 and 3 participants, ongoing uncertainty also remained, but only with significant regret for four participants. Patient 10, for example, who after much deliberation in decision-making had personally chosen another treatment option (ileostomy) rather than HSCT, described ongoing regret as having the ileostomy had made her experience of CD substantially worse:
I got offered the opportunity to do stem cell which went all the way through to the point of virtually going to do it, made the decision…(but) they thought the only thing left to do then was to give me the ileostomy bag….so, now, I had the ileostomy, regrettably for me now because I don’t actually think it’s done anything to help me…. It’s made me worse. And, I, I regret the day I ever had it, I just wish that I’d had the stem cell done. (Patient 10, Group 2)
Reframing personal benefit
The majority of participants across groups reflected positively on the decisions they had taken, although described doing so after adjusting their expected personal benefits at the outset to the experiences at the time of interview.
Particularly for group 1 participants, reflecting on the benefits of participating in HSCT had brought involved a sense of gratitude at being given the opportunity to participate in the trial and being able to fulfil their ‘need to know’. All spoke about their life after HSTC. Five participants were very positive about their condition, including substantial remission of symptoms and ability to ‘get on with life as normal’. Furthermore, the majority identified positive benefits, even though no disease remission, such as being able to reduce the amount of medication they required and/or allowing them to receive conventional treatments to which they had previously become intolerant:
But the biggest change has got to be from the stem cell trial where after all of that heavy medication I’ve had, I was pretty much free of ninety percent of disease. (Patient 4, Group 1)
But I have managed to take myself off a couple of my medication. So I have, for me, as I look at it, I have got something out of it. (Patient 2, Group 1)
Participants also detailed how the treatment had enabled them to increase spontaneity and to re-engage in activities due to a greater degree of energy and reduced fatigue:
It’s like actually being able to make plans and I started to do my courses and everything like that which was incredible, to actually be able to start to study and to be able to exercise was incredible…I can still work and earn money and pay rent and everything like that……You know, I’m studying and I can actually go and do an exercise class before I study, which you could never, never do before, so it was quite good… even now, like, I, I’m teaching seven (spin) classes a week now. (Patient 24, Group 1)
I got back to relatively normal life straightaway, and it was like again, do what you feel you need to do. I had the transplant in October and then I returned to work, sort of, a phased return, in January. I was back doing full time work 6 weeks after the treatment. (Patient 32, Group 1)
Discussion
This study provides new knowledge about the factors affecting decision-making for people living with severe CD in relation to HSCT as part of the ASTIC study. Uniquely, it provides insight into the views of non-participants, and for whom this outcome was not a result of personal choice and control. Previous research has identified the importance of supporting patients with CD to manage the often uncertain and unpredictable nature of their condition,38 this study further expands this to the context of clinical trial participation, both for recipients of trial treatment, those deemed ineligible and those who declined participation.
Participants identified living with severe CD as a ‘hard-fought battle’ involving a complex balance of adaptation, self-management and acceptance of the restrictions to daily life. It involved prolonged periods of relapse or, for some, having never felt in remission since diagnosis. These findings are congruent with the broader literature on living with chronic illness,49–51 concerns about IBD and its impact on quality of life52 53 and findings from other IBD qualitative investigations.38 54–57. However for the first time, this study has identified how the ‘hard-fought battle’ was a key driving factor when considering ASTIC trial participation and its associated risks and benefits.
Decision-making and clinical trial participation
Decision-making in relation to clinical trials is guided by organisational, professional and clinical trial ethics and legislation, including regulation provided by the European Union.58 While best practice in research recommends that informed consent includes an understanding of the treatment involved, its risks, benefits, treatment alternatives and the opportunity to withdraw, many participants in this study described a prior determination to undertake the ASTIC trial. This was often taken before meeting with trial clinicians, based on a limited understanding of information about the nature of HSCT, yet guided dominantly by trust in specialist clinicians with whom they had developed a relationship over a number of years. While written and verbal information about the benefits and risks were sought and provided, for many this was of lesser importance than an expectation that this treatment may provide direct personal benefit and that they were a ‘likely’ or ‘good candidate’ for eligibility as communicated to them. This decision also reflected their ‘hard-fought battle with CD’ including limited options for future treatment or that alternative options such as surgery were less desirable.
Shannon-Dorcy and Drevdahl59 identified a similar decision-making strategy in their qualitative study of HSCT in cancer, exploring the views of both patients (n=25) and caregivers (n=20). Key influencing factors were having no other option, seeking a cure and trusting the recommendations of home oncologists. Similarly, Snowden et al’s60 survey of patients with rheumatoid arthritis, described a willingness to take mortality-related risks from HSCT to return to normality off all drugs, particularly those with significant disability. While participants in the current study were not facing a cancer diagnosis (and potential end of life outcomes), they had experienced significant disability and impact due to CD and it is noteworthy that decisions about risk and likelihood of individual success may not be considered sufficiently in some cases.
The balancing of risks associated with the treatment process and future outcomes was strongly evident in relation to decisions about fertility and ‘freezing of eggs’ or sperm. Concerns about pregnancy and fertility were consistent with those identified by Kane61 and Alstead and Nelson-Piercy62; however, the challenges were heightened for participants in this study due to the tensions between sufficient information and time to fully consider impacts on fertility and future parenthood and their desire to go ahead with treatment as fast as possible due to potential personal benefits.
The concept of therapeutc misconception has much to inform this study in relation to decision-making and perceptions of risk in HSCT and CD.
Therapeutic misconception and misestimation
Although not considered specifically at the outset of this study, therapeutic misconception and in particular, therapeutic misestimation emerged as an influencing factor on how a number of participants described their decision-making and expected personal outcomes in relation to HSCT and ASTIC study participation.
Coined by Appelbaum and colleagues in 1982,63 64 therapeutic misconception refers to a phenomenon where individuals do not understand that the core objective of clinical trial research is to produce generalisable knowledge rather than direct personal benefit, thereby conflating the aims of research with clinical care. Researchers have stressed the importance of allowing for therapeutic misconception to ensure effective decision-making and informed consent to trials.65 66
Expanding on the concept of therapeutic misconception, Horng and Grady47 outline a related concept, ‘therapeutic misestimation’, where there is disconnect between the likelihood of personal benefit or risk from individual participation (summarised in table 3).
Table 3.
Summary of therapeutic misconception and misestimation (adapted from Horng and Grady47)
| Concept | Definition | Ethical significance |
| Therapeutic misconception | The research participant conflates research with clinical care | Rarely tolerable because understanding the nature of research is necessary for an autonomous decision to participate in research |
| Therapeutic misestimation | The research participant underestimates risk, overestimates benefit or both | Sometimes tolerable because understanding the exact probability of harm and benefit may not be necessary for an autonomous decision to participate in research |
Daugherty et al67 suggest that research participants may experience difficulty in distinguishing the differences between the therapeutic and research components of a trial, highlighting the vulnerability of trial participants when faced with limited treatment options.60 It is noteworthy therefore that decisions about risk and likelihood of individual success in novel treatments for severe CD may be influenced by therapeutic misestimation.
Although expectations about personal benefits and outcomes varied across the three groups, the ASTIC trial was viewed by the majority as an opportunity to improve symptom control or treatment tolerance and by some as a potential cure. As Cho and Magnus66 identify, the extent of therapeutic misconception in clinical trials is extensive and particularly pertinent to stem cell research which may be perceived as frontier research. Appelbaum et al68 found that 31% of research participants had inaccurate beliefs about the nature of their treatment (eg, presuming that they would definitely receive the active treatment rather than the placebo), and 51% had unrealistic beliefs about the nature or likelihood of benefit to themselves of participating in the study. Similar to findings in the current study, Lidz et al69 identified that 24% of participants reported no risks or disadvantages to participation, even though they had been informed about such risks. This may go some way to explain why a number of participants focused predominantly on the positive likelihood of benefit from trial participation.
For group 1 participants in particular, reflections on individual benefits were positively reframed or reprioritised.70 Having extinguished their ‘need to know’, participants were often able to make greater sense of the future, despite ongoing health-related uncertainties and limited quantitative benefits in some cases.
Coolbrandt and Grypondck’s71 mixed-model qualitative study, specifically identified the courage and continued hope for a positive outcome in HSCT treatment. The recurring theme of ‘being out of options’ as part of the hard-fought battle again provided a driving force for decisions to participate in treatment that required the challenges of cancer treatment, including coping with isolation, treatment side effects and maintaining beliefs in a happy ending. Nevertheless, the majority of those not participating in ASTIC also reflected positively on not undergoing treatment as in hindsight the treatment risks (including awareness of one death) and the demands of isolation during the treatment regimen were deemed excessive.
Supporting decision-making
Agrawal and Emanuel72 stated that there is no gold standard or a specific criterion that determines the reliability or validity of trial information comprehension by potential participants—and that it is to be judged on face validity. Studies have examined the use of language in consent forms and identified numerous inclusions of broad statements such as ‘you may or may not benefit’ with statements such as ‘the hope is that we can improve your symptoms and prolong your life with this treatment’ and used terms such as ‘research’ and ‘treatment’ interchangeably.73 These studies suggest that researchers can and should guard against encouraging the therapeutic misconception, both in informed consent forms and in publications. Findings from this current study also identify the importance of minimising potential therapeutic misconception and misestimation resulting from all communication interventions, including face-to-face consultations and during the eligibility stages of clinical trial participation. Agreement about the communication of trial results, including the outcome of tests for eligibility is a key factor in this experience and identifies the benefit that individualised communication plans and tailored decision-making aids could offer.31 74
Strengths and limitations
This study has several strengths and limitations. We focused on the factors influencing decision-making and expectations of individuals with severe CD, an important yet often excluded group of participants in studies due to the severity of their disease. We also examined decision-making by participants and non-participants in a trial, the latter being often excluded from study results or the impact that non-participation may have on their experiences. Findings from this international, qualitative study can inform future research that develops decision-making and information support tools for future participants in ‘radical’ or ‘frontier’ clinical trials such as HSCT. The study is limited however as we conducted face-to-face semistructured interviews at only one time-point and did not explore in-depth the cultural and specific healthcare service factors that may have influenced participants’ decision-making and trial experiences across study sites. Future research should harness the strengths of longitudinal study designs taking greater account of the impact of individual, socioeconomic, cultural and health service factors to capture outcomes in relation to those receiving HSCT and for those for whom trial participation proved not to be an option. In response to stakeholder recommendations, interview settings involved a choice between an interview at home, hospital interview room or via Skype. Although this varied approach to data collection had strengths in widening access to study participation, for example, enhanced recruitment of those living in rural settings, we cannot exclude the impact that different settings may have had on the impact of the quality of data collected, including non-verbal cues, and the impact of the researcher when using such diverse methods.
Conclusions and implications
Decision-making and expectations of people with severe CD in relation to HSCT is a complex process, involving a history of battling with the condition, a willingness to consider novel treatment options and a raised level of expectation about the benefits of trial participation by many participants in this study. Decision-making processes often begin well in advance of formal clinical consultation and are influenced by physical, psychological, socioeconomic and relational aspects of a person’s life as captured in the ‘hard-fought battle of living with CD’. Benefits described by participants receiving the treatment may be more subtle than those captured on standard quality of life questionnaires, where ‘improved quality’ may be related to having extinguished the doubt of knowing whether the treatment would have direct personal benefit. The development of decision-making and information support tools for future participants in clinical trials, such as HSCT, are recommended, subject to further research that takes greater account of individual and sociocultural influencing factors on decision-making over time.
Supplementary Material
Acknowledgments
We are indebted to the individuals who freely gave their time to participate in this research. Sincere thanks also to Dr Jack Satsangi (JS); Dr Simon Travis (ST); Dr Eleanor Ricart (ER); Dr Alicia Lopez (AL) who provided support in recruiting participants to this study. Also thanks to Dr Alicia Lopez (AL) for assistance in data collection of the Spanish participant study group.
Footnotes
Contributors: JC: study chief investigator. Main author of the paper, leading each section from grant capture, study design, data collection, analysis and each section of this paper. IB: clinical researcher. Second author of the paper, involved in data collection, analysis, interpretation and drafting each section of this paper. JOL: study principal investigator at Barts Health NHS Trust. Contributed to participant recruitment, and overall review of the paper. CJH: study principal investigator at Nottingham University Hospitals NHS Trust. Senior advisor to the study (also chief investigator of the ASTIC trial). Contributed to grant capture for this study, participant recruitment, data interpretation and overall review of the paper.
Funding: This work was funded by Crohn’s and Colitis UK Living with IBD Award.
Competing interests: None declared.
Ethics approval: Nottingham 2 REC committee (Reference number- 13/EM/0176) and La Agencia Española de Medicamentos y Productos Sanitarios.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: A podcast is under development using additional participant statements which will be available free of charge once completed and via request to the study CI. Otherwise no additional data are available due to the consent taking at the time of participant recruitment.
References
- 1.WHO. Preventing chronic diseases: a vital investment. Geneva, Switzerland: World Health Organisation, 2005. [Google Scholar]
- 2.Nolte E, McKee M. European observatory on health systems and policies series. Caring for people with chronic conditions: a health system perspective. World Health Organization 2008 on behalf of the European Observatory on Health Systems and Policies 2008. [Google Scholar]
- 3.Department of Health. Long term conditions compendium of information. 3rd edition London: DH, 2012. [Google Scholar]
- 4.Baumgart DC, Sandborn WJ, disease Crohn's. Lancet 2012;380:1590–605. [DOI] [PubMed] [Google Scholar]
- 5.Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011;60:571–607. 10.1136/gut.2010.224154 [DOI] [PubMed] [Google Scholar]
- 6.Carlsen K, Munkholm P, Burisch J. Baumgart DC, Evaluation of quality of life in Crohn’s disease and ulcerative colitis: what is health-related quality of life, in Crohn's disease and ulcerative colitis: from epidemiology and immunobiology to a rational diagnostic and therapeutic approach: Springer International Publishing: Cham, 2017:279–89. [Google Scholar]
- 7.Clement C, Rapport F, Seagrove A, et al. Healthcare professionals' views of the use and administration of two salvage therapy drugs for acute ulcerative colitis: a nested qualitative study within the CONSTRUCT trial. BMJ Open 2017;7:e014512 10.1136/bmjopen-2016-014512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keefer L, Kiebles JL, Taft TH. The role of self-efficacy in inflammatory bowel disease management: preliminary validation of a disease-specific measure. Inflamm Bowel Dis 2011;17:614–20. 10.1002/ibd.21314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casellas F, Alcalá MJ, Prieto L, et al. Assessment of the influence of disease activity on the quality of life of patients with inflammatory bowel disease using a short questionnaire. Am J Gastroenterol 2004;99:457–61. 10.1111/j.1572-0241.2004.04071.x [DOI] [PubMed] [Google Scholar]
- 10.Norton C, Czuber-Dochan W, Bassett P, et al. Assessing fatigue in inflammatory bowel disease: comparison of three fatigue scales. Aliment Pharmacol Ther 2015;42:203–11. 10.1111/apt.13255 [DOI] [PubMed] [Google Scholar]
- 11.Drossman DA, Leserman J, Li ZM, et al. The rating form of IBD patient concerns: a new measure of health status. Psychosom Med 1991;53:701–12. 10.1097/00006842-199111000-00010 [DOI] [PubMed] [Google Scholar]
- 12.Behzadi P, Behzadi E, Ranjbar R. The incidence and prevalence of Crohn’s disease in global scale. SOJ Immunol 3 2015:1–6. [Google Scholar]
- 13.Economou M, Zabmbeli E, Michopoulos S. Incidence and prevalence of Crohn's disease and its etiological influences. Annals of Gastroenterology 2009;22:158–67. [Google Scholar]
- 14.Burisch J, Jess T, Martinato M, et al. The burden of inflammatory bowel disease in Europe. J Crohns Colitis 2013;7:322–37. 10.1016/j.crohns.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 15.Gionchetti P, Dignass A, Danese S, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: part 2: surgical management and special situations. J Crohns Colitis 2017;11:135–49. 10.1093/ecco-jcc/jjw169 [DOI] [PubMed] [Google Scholar]
- 16.García-Armesto S , et al. World Health Organization, on behalf of the European Observatory on Health Systems and Policies. Spain: Health System Review 2010, 2010. [Google Scholar]
- 17.Hawkey CJ, Allez M, Clark MM, et al. Autologous hematopoetic stem cell transplantation for Refractory crohn disease: a Randomized clinical trial. JAMA 2015;314:2524–34. 10.1001/jama.2015.16700 [DOI] [PubMed] [Google Scholar]
- 18.Oyama Y, Craig RM, Traynor AE, et al. Autologous hematopoietic stem cell transplantation in patients with refractory crohn's disease. Gastroenterology 2005;128:552–63. 10.1053/j.gastro.2004.11.051 [DOI] [PubMed] [Google Scholar]
- 19.Hommes DW, Duijvestein M, Zelinkova Z, et al. Long-term follow-up of autologous hematopoietic stem cell transplantation for severe refractory crohn's disease. J Crohns Colitis 2011;5:543–9. 10.1016/j.crohns.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 20.Deane S, Meyers FJ, Gershwin ME. On reversing the persistence of memory: hematopoietic stem cell transplant for autoimmune disease in the first ten years. J Autoimmun 2008;30:180–96. 10.1016/j.jaut.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 21.Irvine EJ, Feagan B, Rochon J, et al. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn's Relapse Prevention Trial Study Group. Gastroenterology 1994;106:287–96. [DOI] [PubMed] [Google Scholar]
- 22.Daperno M, D'Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for crohn's disease: the SES-CD. Gastrointest Endosc 2004;60:505–12. 10.1016/S0016-5107(04)01878-4 [DOI] [PubMed] [Google Scholar]
- 23.Al-toma A, Nijeboer P, Bouma G, et al. Hematopoietic stem cell transplantation for non-malignant gastrointestinal diseases. World J Gastroenterol 2014;20:17368–75. 10.3748/wjg.v20.i46.17368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanzoni G, Roda G, Belluzzi A, et al. Inflammatory bowel disease: moving toward a stem cell-based therapy. World J Gastroenterol 2008;14:4616–26. 10.3748/wjg.14.4616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duran NE, Hommes DW. Stem cell-based therapies in inflammatory bowel disease: promises and pitfalls. Therap Adv Gastroenterol 2016;9:533–47. 10.1177/1756283X16642190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawkey CJ. Stem cells as treatment in inflammatory bowel disease. Dig Dis 2012;30:134–9. 10.1159/000342740 [DOI] [PubMed] [Google Scholar]
- 27.García-Bosch O, Ricart E, Panés J. Review article: stem cell therapies for inflammatory bowel disease - efficacy and safety. Aliment Pharmacol Ther 2010;32:939–52. 10.1111/j.1365-2036.2010.04439.x [DOI] [PubMed] [Google Scholar]
- 28.Hawkey CJ, Hommes DW. Is stem cell therapy ready for prime time in treatment of inflammatory bowel diseases? Gastroenterology 2017;152:389–97. 10.1053/j.gastro.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 29.Cox K, Moghaddam N, Bird L, et al. Feedback of trial results to participants: a survey of clinicians' and patients' attitudes and experiences. Eur J Oncol Nurs 2011;15:124–9. 10.1016/j.ejon.2010.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stacey D, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2011;10:CD001431. [DOI] [PubMed] [Google Scholar]
- 31.Siegel CA. Review article: explaining risks of inflammatory bowel disease therapy to patients. Aliment Pharmacol Ther 2011;33:23–32. 10.1111/j.1365-2036.2010.04489.x [DOI] [PubMed] [Google Scholar]
- 32.Volk RJ, Llewellyn-Thomas H, Stacey D, et al. Ten years of the International Patient Decision Aid Standards Collaboration: evolution of the core dimensions for assessing the quality of patient decision aids. BMC Med Inform Decis Mak 2013;13:S1. 10.1186/1472-6947-13-S2-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keohane J, Shanahan F. Are patients with IBD knowledgeable about the risks of their medications? Inflamm Bowel Dis 2008;14:S70–S71. 10.1097/00054725-200810001-00037 [DOI] [PubMed] [Google Scholar]
- 34.Biesta G : Tashakkori C, Tashakkori C, Pragmatism and the philosophical foundations of mixed methods research, in SAGE handbook of mixed methods in social & behavioural research (2nd edition). Thousand Oaks, California: Sage Publications Inc, 2010:95–117. [Google Scholar]
- 35.Thorne S. Toward methodological emancipation in applied health research. Qual Health Res 2011;21:443–53. 10.1177/1049732310392595 [DOI] [PubMed] [Google Scholar]
- 36.Ritchie J, Lewis J, Practice QR. A guide for social science students and researchers. London: Sage Publications Limited, 2003. [Google Scholar]
- 37.Department of Health. Research governance framework for health and social care. London: DH, 2005. [Google Scholar]
- 38.Cooper JM, Collier J, James V, et al. Beliefs about personal control and self-management in 30-40 year olds living with inflammatory bowel disease: a qualitative study. Int J Nurs Stud 2010;47:1500–9. 10.1016/j.ijnurstu.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 39.Lo Iacono V, Symonds P, Brown DHK, et al. Skype as a tool for qualitative research interviews. Sociol Res Online 2016;21:12 http://www.socresonline.org.uk/21/2/12.html 10.5153/sro.3952 [DOI] [Google Scholar]
- 40.Guest G, Bunce A, Johnson L. How many interviews are enought?: an experiment with data saturation and variability. Field Methods 2006;18. [Google Scholar]
- 41.Gale NK, Heath G, Cameron E, et al. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13:117. 10.1186/1471-2288-13-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor D, Bury M. Chronic illness, expert patients and care transition. Sociol Health Illn 2007;29:27–45. 10.1111/j.1467-9566.2007.00516.x [DOI] [PubMed] [Google Scholar]
- 43.Baker JN, Barfield R, Hinds PS, et al. A process to facilitate decision making in pediatric stem cell transplantation: the individualized care planning and coordination model. Biol Blood Marrow Transplant 2007;13:245–54. 10.1016/j.bbmt.2006.11.013 [DOI] [PubMed] [Google Scholar]
- 44.Vlemmix F , et al. Decision aids to improve informed decision-making in pregnancy care: a systematic review. 120 BJOG: An International Journal of Obstetrics & Gynaecology, 2013:257–66. [DOI] [PubMed] [Google Scholar]
- 45.Fiocchi C. Towards a 'cure' for IBD. Dig Dis 2012;30:428–33. 10.1159/000338148 [DOI] [PubMed] [Google Scholar]
- 46.Belkin GS. Misconceived bioethics?: the misconception of the "therapeutic misconception". Int J Law Psychiatry 2006;29:75–85. 10.1016/j.ijlp.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 47.Horng S, Grady C. Misunderstanding in clinical research: distinguishing therapeutic misconception, therapeutic misestimation, & therapeutic optimism. 25 Ethics and Human Research: IRB, 2003:11–16. [PubMed] [Google Scholar]
- 48.Murphy E , et al. Qualitative research methods in health techonology assessment: a review of the literature. Health Techol Assessment. 2, 1998. [PubMed] [Google Scholar]
- 49.Charmaz K. Loss of self: a fundamental form of suffering in the chronically ill. Sociol Health Illn 1983;5:168–95. 10.1111/1467-9566.ep10491512 [DOI] [PubMed] [Google Scholar]
- 50.Charmaz K, days G. Bad days. the self in chronic illness and time. New Jersey: Rutgers University Press, 1991. [Google Scholar]
- 51.Bury M. Chronic illness as biographical disruption. Sociol Health Illn 1982;4:167–82. 10.1111/1467-9566.ep11339939 [DOI] [PubMed] [Google Scholar]
- 52.Czuber-Dochan W, Dibley LB, Terry H, et al. The experience of fatigue in people with inflammatory bowel disease: an exploratory study. J Adv Nurs 2013;69:1987–99. 10.1111/jan.12060 [DOI] [PubMed] [Google Scholar]
- 53.Casellas F, López-Vivancos J, Badia X, et al. Influence of inflammatory bowel disease on different dimensions of quality of life. Eur J Gastroenterol Hepatol 2001;13:567–72. 10.1097/00042737-200105000-00017 [DOI] [PubMed] [Google Scholar]
- 54.Norton BA, Thomas R, Lomax KG, et al. Patient perspectives on the impact of crohn's disease: results from group interviews. Patient Prefer Adherence 2012;6:509–20. 10.2147/PPA.S32690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hall NJ, Rubin GP, Dougall A, et al. The fight for 'health-related normality': a qualitative study of the experiences of individuals living with established inflammatory bowel disease (ibd). J Health Psychol 2005;10:443–55. 10.1177/1359105305051433 [DOI] [PubMed] [Google Scholar]
- 56.Saibil F, Lai E, Hayward A, et al. Self-management for people with inflammatory bowel disease. Can J Gastroenterol 2008;22:281–7. 10.1155/2008/428967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Devlen J, Beusterien K, Yen L, et al. The burden of inflammatory bowel disease: a patient-reported qualitative analysis and development of a conceptual model. Inflamm Bowel Dis 2014;20:545–52. 10.1097/01.MIB.0000440983.86659.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trials Regulation EU. EU Regulation No 536/2014 on clinical trials on medicinal products published in Official Journal of the European Union - See more at. 2014. http://www.hra.nhs.uk/resources/before-you-apply/types-of-study/clinical-trials-of-investigational-medicinal-products/clinical-trials-investigational-medicinal-products-ctimps-eu-legislation/#sthash.9SKQCooE.dpuf (accessed 26 Sep 2014).
- 59.Shannon-Dorcy K, Drevdahl DJ. "I had already made up my mind": patients and caregivers' perspectives on making the decision to participate in research at a US cancer referral center. Cancer Nurs 2011;34:428–33. 10.1097/NCC.0b013e318207cb03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Snowden JA, Nivison-Smith I, Biggs JC, et al. Risk taking in patients with rheumatoid arthritis: are the risks of haemopoietic stem cell transplantation acceptable? Rheumatology 1999;38:321–4. 10.1093/rheumatology/38.4.321 [DOI] [PubMed] [Google Scholar]
- 61.Kane S. Gender issues in the management of inflammatory bowel disease and irritable bowel syndrome. Int J Fertil Womens Med 2002;47:136–42. [PubMed] [Google Scholar]
- 62.Alstead EM, Nelson-Piercy C. Inflammatory bowel disease in pregnancy. Gut 2003;52:159–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Appelbaum PS, Anatchkova M, Albert K, et al. Therapeutic misconception in research subjects: development and validation of a measure. Clin Trials 2012;9:748–61. 10.1177/1740774512456455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Appelbaum PS, Roth LH, Lidz C. The therapeutic misconception: informed consent in psychiatric research. Int J Law Psychiatry 1982;5:319–29. 10.1016/0160-2527(82)90026-7 [DOI] [PubMed] [Google Scholar]
- 65.Pentz RD, White M, Harvey RD, et al. Therapeutic misconception, Misestimation, and optimism in participants enrolled in phase 1 trials. Cancer 2012;118:4571–8. 10.1002/cncr.27397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cho MK, Magnus D. Therapeutic misconception and stem cell research. Nat Rep Stem Cells 2007. http://www.nature.com/stemcells/2007/0709/070927/full/stemcells.2007.88.html (accessed 11 Apr 2017). 10.1038/stemcells.2007.88 [DOI] [Google Scholar]
- 67.Daugherty C, Ratain MJ, Grochowski E, et al. Perceptions of cancer patients and their physicians involved in phase I trials. J Clin Oncol 1995;13:1062–72. 10.1200/JCO.1995.13.5.1062 [DOI] [PubMed] [Google Scholar]
- 68.Appelbaum PS, Roth LH, Lidz C. The therapeutic misconception: informed consent in psychiatric research. Int J Law Psychiatry 1982;5:319–29. 10.1016/0160-2527(82)90026-7 [DOI] [PubMed] [Google Scholar]
- 69.Lidz CW, Appelbaum PS, Grisso T, et al. Therapeutic misconception and the appreciation of risks in clinical trials. Soc Sci Med 2004;58:1689–97. 10.1016/S0277-9536(03)00338-1 [DOI] [PubMed] [Google Scholar]
- 70.Stephens M. The lived experience post-autologous haematopoietic stem cell transplant (HSCT): a phenomenological study. Eur J Oncol Nurs 2005;9:204–15. 10.1016/j.ejon.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 71.Coolbrandt A, Grypdonck MH. Keeping courage during stem cell transplantation: a qualitative research. Eur J Oncol Nurs 2010;14:218–23. 10.1016/j.ejon.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 72.Agrawal M, Emanuel EJ. Ethics of phase 1 oncology studies: reexamining the arguments and data. JAMA 2003;290:1075–82. 10.1001/jama.290.8.1075 [DOI] [PubMed] [Google Scholar]
- 73.King N , et al. Consent forms and the therapeutic misconception: the example of Gene transfer research. 27 Ethics & Human Research: IRB, 2005:1–8. [PubMed] [Google Scholar]
- 74.Cox K, Moghaddam N, Bird L, et al. Feedback of trial results to participants: a survey of clinicians' and patients' attitudes and experiences. Eur J Oncol Nurs 2011;15:124–9. 10.1016/j.ejon.2010.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-015201supp001.pdf (212.8KB, pdf)