Abstract
Objective
Healthcare faces the continual challenge of improving outcome while aiming to reduce cost. The aim of this study was to determine the micro cost differences of the Glasgow non-operative trauma virtual pathway in comparison to a traditional pathway.
Design
Discrete event simulation was used to model and analyse cost and resource utilisation with an activity-based costing approach. Data for a full comparison before the process change was unavailable so we used a modelling approach, comparing a virtual fracture clinic (VFC) with a simulated traditional fracture clinic (TFC).
Setting
The orthopaedic unit VFC pathway pioneered at Glasgow Royal Infirmary has attracted significant attention and interest and is the focus of this cost study.
Outcome measures
Our study focused exclusively on patients with non-operative trauma attending emergency department or the minor injuries unit and the subsequent step in the patient pathway. Retrospective studies of patient outcomes as a result of the protocol introductions for specific injuries are presented in association with activity costs from the models.
Results
Patients are satisfied with the new pathway, the information provided and the outcome of their injuries (Evidence Level IV). There was a 65% reduction in the number of first outpatient face-to-face (f2f) attendances in orthopaedics. In the VFC pathway, the resources required per day were significantly lower for all staff groups (p≤0.001). The overall cost per patient of the VFC pathway was £22.84 (95% CI 21.74 to 23.92) per patient compared with £36.81 (95% CI 35.65 to 37.97) for the TFC pathway.
Conclusions
Our results give a clearer picture of the cost comparison of the virtual pathway over a wholly traditional f2f clinic system. The use of simulation-based stochastic costings in healthcare economic analysis has been limited to date, but this study provides evidence for adoption of this method as a basis for its application in other healthcare settings.
Keywords: orthopaedics, fracture clinic, outpatients, costs, patient outcomes
Strengths and limitations of the study.
We used a simulation-based costing approach to compare the costs of a service innovation (the virtual fracture clinic or VFC) with the default standard of care, in order to quantify the cost saving associated with the VFC and support decision makers thinking of introducing this new model in their own hospitals.
The data which populates our model is based on operating data from a hospital which has piloted VFC and operated it since 2011.
Our model is explicitly designed to reflect variability in costs as not every patient will have the same needs and the same costs.
Simulated studies involve a great deal of work and necessarily involve making assumptions, both of which could have been avoided had baseline data been collected before the VFC was introduced.
Introduction
An ongoing challenge within healthcare is to improve the quality of care while reducing cost.1–3 A common response to increasing demand is to attempt to increase capacity and activity4 through additional funding.5 6 In the longer term, this is financially unsustainable.7 8 In addition, cost reduction without regard to the outcomes achieved is unsafe and potentially limits effective care.9 When health services are faced with increased demand, service redesign can be presented as the way forward. Instead of providing additional capacity, clinicians and managers should seek to identify activity which shows no demonstrable patient benefit.10 11 These activities may be the result of traditional practices that have not been scrutinised in the light of newer evidence. However, clinicians and managers may fear future cuts in essential resources if volume decreases.12 The term ‘clinical pathway’, first used in 1985, has widespread use in healthcare management.13 Essential to creating value is to understand patient care pathways and to enhance care quality by improving risk adjusted patient outcomes, promoting patient safety and increasing satisfaction with optimum use of resources.14 More recently, computerised algorithmic forms have been called ‘care coordination pathways’ which support a variety of functions.15 These would include benchmark treatment steps with an associated cost and are considered in a different way to clinical pathways.
In healthcare systems, it is relatively simple to measure metrics such as volume16 but more difficult to measure clinical outcome, patient satisfaction and ultimately, value.9 The identification of activities that represent poor clinical and financial value, and obtaining support for redesign, are challenging.17
Case setting
This paper focuses on orthopaedic outpatient fracture clinic pathway redesign which was developed at Glasgow Royal Infirmary (GRI) with the primary aim of improving patient care.18–20 GRI is a large university teaching hospital serving a metropolitan area. It is financed by the National Health Service (NHS) in Scotland. The NHS provides universal coverage, funded from general taxation and free at point of use. In 2014/15, £10.8 billion was allocated by the Scottish Government for operating costs of the NHS.21 This is shared proportionally among the 14 health boards in Scotland and 2 of the 8 special boards, based partially on the Resource Allocation Formula.22
Clinicians identified unnecessary duplication in traditional fracture clinics (TFCs), especially where patients with minor orthopaedic trauma can be managed effectively by emergency medicine clinicians. However, it is common practice to have a further face-to-face (f2f) consultation with an orthopaedic clinician23–25 often within 48 hours of the original emergency department (ED) attendance. The patient does not gain any new information or further treatment during many of these encounters.26 It can be physically arduous and financially penalising for patients to attend during this most painful and functionally restricted period of their recovery. Recent evidence supports encouraging early mobilisation without further routine review by demonstrating good functional outcomes.27–34 In healthcare systems with fee-per-patient visit,35 these patients may be considered as a source of income.
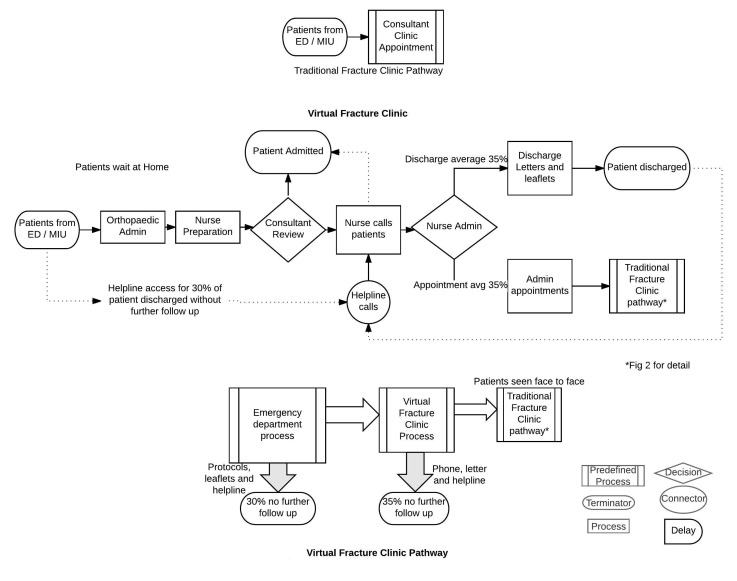
While we recognise that non-operative orthopaedic patient pathways vary and operate according to local conditions, the VFC model pioneered at GRI has attracted significant attention and interest.20 For example, other pathways use Trauma Triage Clinics where all patients with non-operative trauma are virtually reviewed by an orthopaedic consultant, with instructions transmitted electronically for nurse and administrative staff to execute, while others carry out virtual reviews with a larger team. Previously, the only evidence on costing was derived from a relatively broad brush top-down costing,36 which gives relatively little insight into the cost savings and outcome measures. One advantage of the Glasgow virtual fracture pathway (figure 1), promoted by the Scottish Government37 is that it uses agreed evidence-based standard protocols and multidisciplinary review of patients. Agreed protocols between the ED and orthopaedic department have been established for six common/frequent injuries to reduce unnecessary variation.38–40 Protocols include the use of removable Velcro splints for specific injuries and enable direct ED discharge of minor stable fractures and facilitate patient self-care without the need to attend a clinic for removal.41 Those not discharged have their records and X-rays reviewed 7 days a week, by an Orthopaedic consultant and senior nurse in a ‘virtual fracture clinic’ (VFC). Again standard agreed protocols are used during VFC for some injuries to determine further treatment, reducing further unnecessary variation. The resulting management plan is then outlined and agreed with the patient by telephone immediately afterwards. An ‘open-door’ policy is essential to respond to any patient’s concerns after discharge. Patients therefore only attend when necessary, at the optimal time point and with the most relevant specialist. The VFC pathway includes the attendance of patients for their first face to face visit. The standardisation of care along evidence-based guidelines, as applied in the Glasgow pathway, is an important way of increasing value.39 However, is a virtual fracture pathway cost effective?
Figure 1.
Virtual fracture pathway process flow model using the symbols shown to define individual steps in the process. This defines the flow of information and the review process where patients are reviewed without being present. It also includes a predefined process where some patients are seen face to face in a traditional fracture clinic. ED, emergency department; MIU, minor injuries unit.
Methods
The study was performed at GRI. It is a provider of both secondary and tertiary orthopaedic care. Under the terms of the Governance Arrangements for NHS Research Ethics Committees in the UK, the research project was classified as a service evaluation and therefore did not require ethical review.
There are different methods to quantify the costs associated with a healthcare process. A ‘top-down’ approach examining high-level, administrative, financial data has already been performed.36 This does not detail how the cost savings were realised.
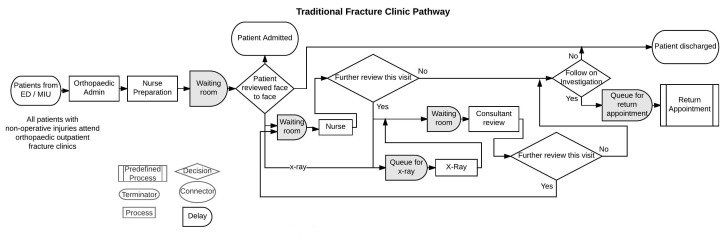
Mapping patient pathways assists in the redesign of healthcare systems42 and this was used to understand the VFC pathway and the TFC pathway (figure 1 and figure 2). Discrete event simulation was chosen as the modelling tool as it is often used for comparison of alternative scenarios. Capturing variability in the parameters defining the pathways was also important.43 It had also been previously used at the case hospital with a focus on improving patient flow. This mapping can then be translated to DES models which have the benefit of incorporating stochastic variability and modelling of patient flows, which has been used extensively in healthcare.44 45 DES has also been used to examine costs in time-driven activity-based costing (TDABC), a ‘bottom-up’ approach to costing. Kaplan et al 9 evaluated the time spent and costs incurred at each step of a complete patient care cycle and summed using clinical resources, material and allocated costs. A pilot project undertaken using TDABC concluded that better management of resource utilisation will ultimately enable providers to deliver the same, or better, outcomes with fewer personnel. These detailed costings can provide evidence to inform decision making46–48 as they investigate the cost of individual episodes by measuring actual resource utilisation.9 49 By using a simplified mapping and modelling approach and including personnel costs, we can report on the value of two different patient pathways. DES for this type of costing approach facilitates comparison between alternative pathways providing managers and clinicians with information to improve the patient experience. DES was also useful as it allowed us to assess costs subject to variations in demand and available resources. The role of DES is often used as a basis for experimentation, especially when there is interest in looking at different scenarios for change and improvement. It is used here retrospectively to show the cost and efficiency saving that are possible from implementing such a virtual pathway. Patient outcomes and satisfaction are also a crucial consideration.
Figure 2.
Traditional fracture pathway process flow model using the symbols shown. This defines individual steps in the process for patients attending an orthopaedic outpatient appointment. It is based on the British Orthopaedic Association Standards for Trauma where all patients attending emergency department with a non-operative orthopaedic injury must attend an outpatient clinic.
Outcomes on the Glasgow pathways
This is reported based on literature published by the case hospital. Audit and publication for specific injury of patient outcomes has been ongoing at the study hospital since the new pathway began operating in 2011. There are six protocols used by ED to discharge stable fractures, with four publications thus far published relating to patient outcomes.26 50–52 Patients are assessed using patient-reported outcome measures appropriate to their injury in addition to patient satisfaction regarding discharge leaflets and the outcome of their injury. The unit has also published more generally on the VFC18–20 and reported on the new pathway from a medicolegal perspective, assessing whether patients have access to as much information as they want.
Measuring cost
This work takes a hospital perspective on costs and DES has been used to develop detailed simulation models allowing application of a stochastic costing approach to staff utilisation within the healthcare setting.53 54 Stochastic costing uses statistical distributions of model activity duration and routings through complex pathways and can measure resource utilisation of each step, and therefore the overall process. It follows three broad steps: (1) identifying relevant patient-specific resources; (2) quantifying the resources consumed and (3) determining the value of each resource consumed. Although this method shares aspects with TDABC,9 it focuses on a single step in the patient care cycle and has the advantage of yielding accurate cost data that can be subject to statistical analysis.54 Capital and consumable costs are not modelled.
An action research methodology,55–57 combined with DES, was used to investigate the Glasgow VFC pathway and compare it with a traditional British Orthopaedic Standards for Trauma pathway. Our study focused exclusively on patients with non-operative trauma attending ED or the minor injuries unit (MIU) and the next step in the patient pathway. Two DES models were developed to determine the staff utilisation and actual staff costs of a VFC model compared with a TFC model using computer simulation software (Simul8).58 These models were developed by close observation of the clinical and administrative processes involved, and interviews with clinical and managerial staff. Conceptual pathways of both the VFC (figure 1) and the TFC (figure 2) were the first steps in the model building process.
Data collection—simulation models
Multiple sources were used to develop the models. Data for 1 year of the new process (2014/15) was extracted from the hospital patient management system (TRAKcare) and orthopaedic electronic database (Bluespier,59). A total of 6291 patients were considered. Within the redesigned VFC pathway, 30% of the patients initially attending ED/MIU with a non-operative fracture injury were discharged home without follow-up at a fracture clinic (figure 1). This did not require any new resources and brought significant benefits to ED.15 A further 35% were discharged from the VFC. These patients would incur a cost if they accessed the telephone helpline and this was modelled. This resulted in only 35% of the initial cohort of patients being reviewed at f2f consultation in a TFC. Within the TFC model: 100% (n=6291) of the patients were referred onto orthopaedics and reviewed f2f in a clinic (figure 2). The time taken for each activity was collected by direct observation over a period of 4 months. This prolonged time period made the ‘Hawthorne Effect’,60 that is, behaviour modification in response to being observed, less likely as the staff became accustomed to the researcher's presence. TFC model activity timings were based on direct observation of f2f consultations over a 2-week period, with a variable case-mix in 10 clinics of 20–32 patients. As no data were available, the routings for a TFC were estimated by multiple experts with over 30 years experience in orthopaedic care. The institution’s finance department provided the mean salary for each staff group, including a standard overhead cost of 23% (table 1). Since the same costs were used in each model and run for 1 year for comparison purposes, discounting of costs and benefits was not used. There are no costs included associated with the discharge of patients from ED18 or from the VFC, as only a small proportion of these patients sought additional help (table 2) and this was beyond the scope of this work. The model includes staff costs incurred if patients contact the helpline. The patient’s journey is based on probability distributions and availability of resources resulting in patients waiting in queues. Both models were simulated for a period of 1 year and output results were collected for 20 trial runs simulated for each model. The number of trial runs was obtained by using the trials calculator.61
Table 1.
Input parameters for simulation models
| Parameter | Resources | Mean and (SD) | Data source/comments | |
| VFC Steps | VFC arrival rates (33% discharged ED) | 12 patients per day | Historical data analysis- patients discharged at ED figure 1 One-third of patients discharged at ED so only 12 at VFC | |
| Admin 1 | Admin | 1.06 min (0.64) | Direct observation | |
| Nurse Prep | Nurse | 1.61 min (0.7) | ||
| VFC consultant review | Consultant | 1.75 min (0.95) | ||
| Nurse | ||||
| Nurse calls | Nurse | 8 min (4) | ||
| Admin 2 | Admin | 2.5 min (1.7) | ||
| Admin letters and appointments | Admin | 2.9 min (1.35) | Around one-third of patients will still follow the traditional pathway. All others have been discharged. | |
| Discharge advice letters | Admin | 2.33 min (0.5) | Direct observation | |
| Helpline call arrival rate | Nurse | 2.6 per day | ||
| Helpline call duration | Nurse | 4.5mins (0.15) | ||
| VFC decision point Discharged | 50% | Historical data analysis | ||
| Referred to consultant clinic | 40% | |||
| Referred to N/L clinic | 10% | |||
| Staffing: Consultants | 1 | |||
| Nurses | 1 | |||
| Admin | 2 | |||
| Typists | 1 | |||
| TFC Steps | ED/MIU arrival rates | 18 patients per day | Historical data—all patients (figure 2) | |
| X-ray | 14 min (5) | Historical data | ||
| Nurse prep | Nurse | 1.61 (0.7) | ||
| Consultant consultation | Consultant | 12 min | Observation | |
| Second consultation | Consultant | 3 min | Expert opinion/observation | |
| Nurse consultation | Nurse | 20 min | ||
| Discharge admin | Admin | 6 min | ||
| Assessment routing: Admitted | 1% | Expert opinion: Much of these routing values are based on the clinical mix of patients and therefore does not warrant sensitivity analysis. As stated in the text, one limitation of this work is that the clinical mix for the new virtual pathway is that these routings are likely to be different as the simplest injuries have been discharged. | ||
| X-ray | 3% | |||
| Treatment | 72% | |||
| Discharge | 24% | |||
| Treatment routing | ||||
| X-ray | 6% | |||
| Second consultation | 93% | |||
| Discharge routing | ||||
| Discharged | 36% | |||
| Return appointment | 64% | |||
| Staffing: Consultants | 3 | Sensitivity analysis was necessary here in terms of the number of staff required to ensure all patients were seen within the allocated session time. These values represent the necessary staffing required. | ||
| Nurses | 3 | |||
| Admin | 2 | |||
| Typists | 4 | |||
| BOTH | Shifts Admin1 (VFC) | 07:30–08:15 | For printing off lists only | |
| Admin 1 (TFC) | 07:45–09:00 | Longer shift as higher volume of patients | ||
| Consultant (14) | 09:00–13:00 | 4-hour consultant session | ||
| Nurse (8) | 08:00–16:00 | Average Full Time nurse working hours | ||
| Typists | 09:00–17:00 | Discharge letters to General Practitioner and patient (Mon–Fri) | ||
| Admin 2 | 11:00–16:00 | For issuing letters only | ||
| Hourly rates GRI consultant | £62.91 | Average for GRI orthopaedic consultants. Obtained from GGC finance dept. for 2014/15. Include 23% employer costs | ||
| Nurse | £20.96 | Based on April 2014 figures. They have 23% employer costs added and are then divided by 42 weeks. GRI staffing levels based on average of 8 nurses (B7, B6 and B5×6). | ||
| Admin | £12.74 | Average | ||
| Admin 2 | £16.22 | Average | ||
| Efficiency | 85% | Sensitivity analysis shows the effects of this on cost | ||
| Clock | Hours | 1 year as we had historical data for this time period | ||
| Warm-up period | 168 hours | Tests completed to ensure model in steady state73 |
ED, emergency department; GRI, Glasgow Royal Infirmary; MIU, minor injuries unit; TFC, traditional fracture clinic; VFC, virtual fracture clinic.
Table 2.
Summary of outcome publications from the case hospital
| Injury | Where protocol used | Study year | Patients/number of appointments Response rate |
PROM | PROM Score | Satisfaction | Comments | Reference |
| Fifth Metatarsal | ED | 2009–2010 | 279 patients/491 appts. Before new pathway 96% attended ortho clinics |
None | – | Retrospective. Satisfaction and outcome not collected prior to 2011 |
No added clinical value of routine follow-up of these patients. | Ferguson et al 51 |
| 2011–2012 | 339 patients/102 appts. After new pathway 57% discharged ED 19.5% attend f2f |
None | – | 78% satisfied outcome 79.6% satisfied leaflet |
3% visited another hospital or GP | |||
| Mallet finger | ED | 2011–2012 | 47 patients 77% response rate Two patients referred to VFC rest discharged ED |
Quick Dash EQ5D VAS EQ5D |
Mean=2.27 (IQR 0–4.55) 90 (IQR 75–90) 0.88 (IQR 0.84–1) |
100% satisfied process 100% satisfied helpline |
Mean follow-up 322 days postinjury 19% visited another hospital or GP |
Brooksbank et al 50 |
| Fifth Metacarpal | ED | 2012 Apr–Oct |
167 patients 59% response rate |
Quick Dash EQ5D |
Mean=2.3 (IQR 0–6.8) 0.87 (IQR 0.74–1) |
80.6% satisfied outcome 84.9% satisfied process |
Retrospective. Mean follow-up time 21 months 9% visited another hospital or GP |
Gamble et al 26 |
| Radial head and neck | ED | 2011–2012 | 202 patients 77% response |
Extensively investigated in the literature. Short-term satisfaction to maximise response. |
96% satisfied- (suspected fracture) 87% satisfied (fracture group) 95% satisfied with information received. |
Retrospective Mason I or II fractures included 90% discharged with no f2f review 3.4% discrepancy rate based on radiologist report 10% visited another hospital or GP |
Jayaram et al 52 |
Two paediatric fractures are also discharged at ED, the Torus/Buckle and Clavicle. PROM’s and satisfaction for trauma pathway not routinely carried out at the case hospital prior to introduction of VFC.
ED, emergency department; f2f, face-to-face PROM, patient-reported outcome measure.
TFC process steps can be unique for a specific hospital; however, our study simplifies this by investigating and simulating only the first step after ED. This model was based on both data collections for timings and expert opinion on some of the routings as this service no longer runs at GRI. Our TFC model has similar administrative preparation, but every patient attends for an f2f review as was the case historically. Several consultants and nurses were required, but there were no weekend clinics. The time for dressings or plaster cast removal was included based on expert opinion. The model included queuing prior to each activity and the FIFO rule was applied. In the TFC, patients are not turned away and additional help would be called in to deal with patients still waiting. This meant clinics could overrun, creating additional pressures on staff.
Validation and verification
The aim of model validation is to provide the modeller and stakeholders with appropriate confidence in the model such that they will use it as an aid for decision making.62 Verification and validation occurred throughout the model development and testing62 63 as a continuous iterative process. The models contained activities proceeded by queues which were based on a first in first out routing. For both models we did micro white box structural verification through regular meetings with clinical and managerial staff who authenticated the structure, assumptions and face validity.
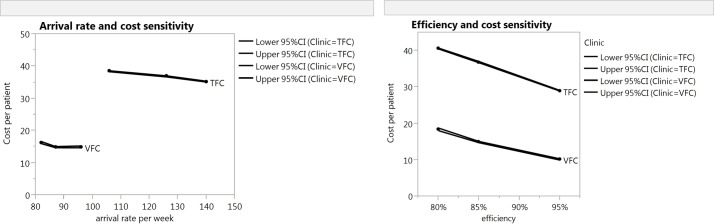
Parameter verification and operational validity of the TFC model was provided by the process experts who delivered some of the data. We also investigated the number of patients completed as it was essential that there was no baulking or reneging within the model and that all patients were seen within allocated session time. Black box validation was challenging as costs are not currently reported based on activity. The reported cost for an outpatient appointment at the case hospital in 2011 was £113 split into £31 allocated and £82 direct. The direct cost includes overheads, allied health professionals costs, lab and pharmacy costs and does not distinguish between trauma and multiple elective pathway patients being seen in an outpatient clinic. To test the model under extreme conditions consultant and nurse-estimated timings of +/−10% and +/−50% was also undertaken. Even making the extreme assumption that the TFC patients take 50% less time for both of the consultant consultations and the treatment time, the TFC pathway still costs more (£25.31 (95% CI 24.67 to 25.09)) than the VFC pathway. The relationship among efficiency, arrival rates and cost is demonstrated in figure 3. We conclude that the models are a good representation of reality that allow questions about the process to be answered and provide outcomes which are reliable. For the TFC, there were limited historical data available to compare with.
Figure 3.
Sensitivity analysis. This shows the sensitivity to cost based on arrival rates and efficiency. It clearly highlights the difference in cost between a TFC and a VFC. TFC, traditional fracture clinic; VFC, virtual fracture clinic.
Results
The output of the models was the staff (medical, nursing and administrative) utilisation and costs for each process step. This allowed a cost per patient to be derived. The reported costs are based on figures for 2013/14, with no corrections for inflation. The performance indicators are reported based on the model output and the 95% CI calculated. The cost for each resource is then summed and divided by the number of patients processed through the model to give a per-patient cost for both the VFC and the TFC. The calculated costs for 20 simulated runs for both the TFC and VFC were compared. A t-test was performed to determine if there was a statistically significant difference between the two pathways.
Effectiveness
A summary of outcome publications from the case hospital on the new pathway is provided in table 2. This includes four upper extremity injuries where patients with simple stable fractures are discharged directly from ED. They all conclude that simple upper extremity fractures can be managed with good patient satisfaction and acceptable functional results without further f2f review. More recently, published analysis of low risk upper extremity fractures64 also found equal outcomes such that these patients should have an optional rather than a scheduled second visit.
The medicolegal aspects of the VFC at the case hospital are addressed65. It states that ‘professional negligence claims can be avoided by the use of robust, up-to-date protocols that are based on national standards’. Since 2011 when the VFC has been in operation at Glasgow, over 30 000 patients have been managed with ‘no complaints or medicolegal actions arising from diagnosis or management in the VFC’ 65. This has been achieved with robust protocols, patient access to information and an open door policy, providing an important safety net giving patients reassurance that they can access expert assistance if required. Advice leaflets for specific injuries can be found on the redesign website,20 which are also provided to patients in paper form at ED.
Efficiency
There is more efficient use of resource with the VFC pathway. The resources required per day were one consultant for 51 min (95% CI 40 to 62), one nurse for 279 min (95% CI 275 to 283) and one typist for 88 min (95% CI 68 to 108). Two administration staff were used (table 1) detailed as admin 1 for 18 min (95% CI 16 to 20) and admin 2 for 28 min (95% CI: 28 to 29) (table 3).
Table 3.
Utilisation and annual cost results for the virtual and traditional fracture clinics
| VFC | Utilisation per staff member | Time worked per day on VFC | TFC | Utilisation per staff member | Time worked per day on TFC | |||||||||||
| Simulation object | Lower 95% | Avg. % | Upper 95% | shift time (min) | Lower 95% | Avg. (min) | Upper 95% | Simulation Object | Lower 95% | Avg % | Upper 95% | Shift time (min) | Lower 95% | Avg. (min) | Upper 95% | |
| Admin 1 | 36 | 40 | 44 | 45 | 16 | 18 | 20 | Admin 1 | 21 | 23 | 24 | 75 | 16 | 17 | 18 | |
| Admin 2 | 9 | 9 | 10 | 300 | 28 | 28 | 29 | Admin 2 | 12 | 14 | 16 | 300 | 36 | 42 | 48 | |
| Typists (1) | 14 | 18 | 23 | 480 | 68 | 88 | 108 | Typists (4) | 16 | 18 | 19 | 480×4 | 307 | 346 | 365 | |
| Nurse (1) | 57 | 58 | 59 | 480 | 275 | 279 | 283 | Nurse (3) | 52 | 54 | 55 | 480×3 | 998 | 1037 | 1056 | |
| Consultant ortho (1) | 17 | 21 | 26 | 240 | 40 | 51 | 62 | Consultant ortho (3) | 74 | 75 | 77 | 240×3 | 533 | 540 | 554 | |
| VFC: Resource | Performance Measure | Lower 95% CI | Average | Upper 95% CI | TFC: Resource | Performance Measure | Lower 95% CI | Average | Upper 95% CI | |
| Consultant ortho | Total cost | 15 199.31 | 19 375.79 | 23 552.26 | Consultant ortho | Total cost | 145 107.76 | 147 754.65 | 150 401.54 | |
| Nurse | Total cost | 34 960.53 | 35 482.45 | 36 004.38 | Nurse | Total cost | 42 756.66 | 43 785.48 | 44 814.29 | |
| Typists | Total cost | 4764.26 | 6181.82 | 7599.38 | Typists | Total cost | 21 665.30 | 23 945.39 | 26 225.47 | |
| Admin 2 | Total cost | 1521.80 | 1572.91 | 1624.01 | Admin 2 | Total cost | 13 230.47 | 15 155.64 | 17 080.82 | |
| Admin 1 | Total cost | 1257.40 | 1393.10 | 1528.80 | Admin 1 | Total cost | 862.75 | 932.78 | 1002.80 | |
| TOTAL COST | 57 703.31 | 64 006.07 | 70 308.83 | TOTAL COST | 223 622.94 | 231 573.93 | 239 524.92 | |||
| GRI VFC referrals | Patients | 4362.21 | 4496.67 | 4631.13 | ED and MIU | Patients | 6273.09 | 6291.00 | 6308.91 | |
| VFC | Per patient | 13.23 | 14.23 | 15.18 | TFC | Per patient | 35.65 | 36.81 | 37.97 |
GRI, Glasgow Royal Infirmary; TFC, traditional fracture clinic; VFC, virtual fracture clinic.
In the TFC model, which required all patients to be seen f2f, more staff were required (table 1). Assumptions were tested by running the model and addressing the build-up of queues. This model required 540 min of consultant time (95% CI 533 to 554), 778 min of nurse time (95% CI 749 to 792), typists for 346 min (95% CI 307 to 365) and two administration staff. Admin 1 staff were used for 17 min (95% CI 15 to 18) and admin 2 for 43 min (95% CI 36 to 48) (table 3).
Cost
The cost of a patient attending a TFC was £36.81 (95% CI 35.65 to 37.97). The cost of the VFC was £14.23 (95% CI 13.23 to 15.18) per patient. In the VFC pathway fewer patients with injuries are referred (70% of TFC model), and half of these proceed to a subsequent f2f encounter. The overall cost per patient of the VFC model was £22.84 (95% CI 21.74 to 23.92) per patient compared with £36.81 (95% C: 35.65 to 37.97), a saving of 38% (table 4). Comparison of these costs using t-tests generated p values that were highly statistically significant (p<0.001).
Table 4.
Summary of costs from model
| Per patient costs £ | −95% | Average | 95% |
| VFC | 13.23 | 14.23 | 15.18 |
| TFC (all patients seen f2f) | 35.65 | 36.81 | 37.97 |
| Virtual pathway (35% seen f2f in TFC) | 21.74 | 22.84 | 23.92 |
| Saving per patient | 13.91 | 13.97 | 14.05 |
f2f, face-to-face; TFC, traditional fracture clinic; VFC, virtual fracture clinic.
Discussion
A new fracture pathway for non-operative fractures instituted at GRI demonstrated clear benefits to the patient. In addition to this, the novel pathway avoids unnecessary follow-up appointments and current evidence demonstrates clinical safety and efficacy.26 50–52 A robust ‘bottom-up’ cost analysis of the VFC demonstrated a cost saving of 38%. There was reduced utilisation of medical and nursing resources: a nurse normally works up to 5 hours per day in VFC duties, whereas in a traditional clinic, several nurses are required to cope with caring for patient’s f2f. To meet demand only one consultant is required in a VFC for up to 1 hour per day, compared with three consultants each working for 3 hours to sustain an equivalent TFC model. The pressures on the outpatient service were greatly eased as the redesign reduced the volume of f2f attendances by 65%. The peaks in demand were smoothed by improving flow and facilitating better patient management. Staff were used for a shorter time in the VFC compared with a TFC, but the actual costs of our VFC process compared with TFC per patient had previously not been established.
There have been both long-term and short-term effects on the service as a result of the somewhat theoretical savings (not reported on end of year financial reports). Shorter term, they reduced pressure on the outpatient service due to the reduction in patient appointments, providing additional capacity long-term. In addition, over the intervening period these savings were gradually reinvested into subspecialist service development, which otherwise would have resulted in significant expenditure for the hospital. In fact, in the context of GRI, 30 hours of consultant-led specialist services were provided without new capital or recurring investment.
The strength of this study was that it provided a simple bottom-up micro costing method approach which analysed the next step after ED/MIU attendance. This allowed the VFC pathway to be observed and timed, providing staff costs based on actual activity. The DES approach allowed us to model the stochastic nature of process flow, incorporating steps that are not normally studied in detail. The use of DES to examine the stochastic nature of the process and model reality in a transparent and simple way was another strength. DES is time-based and takes into account resources, constraints and how the process and activities interact with each other as time passes. DES is often used in stochastic situations where the visual component is very important when planning change.66 67 It is has been widely used in healthcare66 and more recently in health economic evaluation, to gain insight into complex processes.68 69
In terms of the limitations, the TFC simulation was built retrospectively using expert opinion and current observation of a TFC, based on the case hospital, perhaps resulting in a less robust model. One could also argue about the completeness of this data although we have been transparent about how these data were collected. In contrast, the VFC was based on direct observation and historical data analysis and validated against actual performance.
The TFC simulation used the same f2f costing as for 35% of patients in the VFC. A limitation of the study was that the virtually discharged patients in the new system were assumed to use the same clinic resources as those reviewed f2f, whereas the latter may be more complex and costly. We also did not include patients returning for further visits or other cost savings, especially replacing plaster casting with removable splints,70 or the reduction in the use of hospital transport, or societal costs. However, including these factors could only make the VFC seem more attractive and so would not change our conclusion that the VFC is more cost effective. Since this work was a process change across a number of different injuries, there was unfortunately no opportunity to do any randomisation. The process change was made based on existing clinical evidence and with the agreement of experts for each specialty.
One of the biggest opportunities for healthcare improvement is to eliminate unnecessary clinical processes, freeing up time and thereby achieving higher quality of care at lower cost. Healthcare professionals can reduce clinical variation; however, one major barrier to change is that the financial benefits of successful grass-root redesign are frequently unrecognised71 and a decrease in volume may result in a reduction in funding.
Further research could look at the cost effectiveness of other fracture pathways to explore the impact of discharge rates without standard protocols in place at ED and within orthopaedics. It would also be of benefit to understand the impact of the open door policy and the longer term effects of the standardisation on patient care and costs.
Conclusion
The VFC pathway has been in operation at the case hospital for over 5 years and has spread rapidly across the UK and beyond as the evidence of quality improvement has been recognised by other hospitals.20
The financial impact of a virtual process, with patients being reviewed f2f only if necessary, has been highlighted. This opportunity to make savings and increase activity should be of interest to Managers and Clinicians especially due to the benefits for patients. The reported outcome shows that the new pathway is at least as effect as a traditional clinic for a reduced cost. Although reproducing any best practice project on a larger scale is challenging and requires multiple factors to succeed,72 this approach to standardisation and multidisciplinary team virtual review is likely to be useful in many other areas of medicine.
Supplementary Material
Acknowledgments
We gratefully acknowledge the four reviewers for their outstandingly thorough and fair reviews.
The authors alone are responsible for the conclusions reached in this paper and for any mistakes.
Footnotes
Contributors: GHA collected and analysed data, built the models and cowrote the manuscript. PJJ, LAR and MN collected data and cowrote the manuscript. DAM planned the study and cowrote the manuscript. RvdM and AM planned the study and participated in the analysis. LAR and RvdM cowrote the manuscript and act as coguarantors for the study.
Funding: This study was funded by the Scottish Government Quality and Efficiency Support Team.
Competing interests: LAR, PJJ and GHA are in receipt of an unrestricted grant from the Scottish Government, Quality and EfficiencySupport Team as part of the Whole system patient flow programme. DAM works for the Scottish Government which funded the research.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Bradford E. Scottish independence: nhs in Scotland ‘faces £400m funding gap’, 2014. http://www.bbc.co.uk/news/uk-scotland-29213416(accessed 20 Sep 2015).
- 2. Moore A. HSJ100 roundtable: how do you fill a £30bn funding gap? Health Serv J 2015. [Google Scholar]
- 3. Whittington JW, Nolan K, Lewis N, et al. . Pursuing the Triple Aim: The first 7 years. Milbank Q 2015;93:263–300. 10.1111/1468-0009.12122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hurst J, Williams S. Can NHS hospitals do more with less? Nuffield Trust 2012. [Google Scholar]
- 5. Triggle N. Lucrative NHS overtime for consultants questioned, 2011. http://www.bbc.co.uk/news/health-12115669 (accessed 20 Sep 2015).
- 6. Allen P, Wright J, Keen J, et al. . Investigating the governance of NHS Foundation Trusts. NIHR Service Delivery and Organisation Programme, 2010. [Google Scholar]
- 7. Barbour J, Morton A, Schang L. The scottish NHS: meeting the financial challenge ahead. PIIP Policy paper http://strathprints.strath.ac.uk/53563/ (accessed June 2015). [Google Scholar]
- 8. Public Accounts Committee. Thirty fifth report: financial Sustainability of NHS bodies, 2015. http://www.publications.parliament.uk/pa/cm201415/cmselect/cmpubacc/736/73602.htm(accessed Dec 2015).
- 9. Kaplan RS, Witkowski M, Abbott M, et al. . Using time-driven activity-based costing to identify value improvement opportunities in healthcare. J Healthc Manag 2014;59:399–413. [PubMed] [Google Scholar]
- 10. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA 2012;307:1513–6. 10.1001/jama.2012.362 [DOI] [PubMed] [Google Scholar]
- 11. Maughan D, Ansell J. Protecting resources, promoting value: a doctor’s guide to cutting waste in clinical care. Academy of Medical Royal Colleges, Centre for Sustainable Healthcare, 2014. [Google Scholar]
- 12. Tilburt JC, Wynia MK, Sheeler RD, et al. . Views of US physicians about controlling health care costs. JAMA 2013;310:380–8. 10.1001/jama.2013.8278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Bleser L, Depreitere R, de Waele K, et al. . Defining pathways, Nurs. Manag. J 2006;14:553–63. [DOI] [PubMed] [Google Scholar]
- 14. Vanhaecht K, De Witte K, Sermeus. The impact of clinical pathways on the organization of careprocesses. PhD dissertation KULeuven [dissertion]. Leuven: Katholieke Universiteit Leuven, 2007:154. [Google Scholar]
- 15. Zeigler BP, Carter E, Redding SA, et al. . Care coordination: formalization of Pathways for Standardization and Certification. summarized in Innovations Exchange Team In: Zeigler BP, Redding SA, Formalization of the Pathways Model Facilitates Standards and Certification, 2014. [Google Scholar]
- 16. Porter ME. What is value in health care? N Engl J Med 2010;363:2477–81. 10.1056/NEJMp1011024 [DOI] [PubMed] [Google Scholar]
- 17. Brennan N, Cafarella N, Kocot SL, et al. . Improving Quality and value in the US Healthcare System, 2009. http://www.brookings.edu/research/reports/2009(accessed Mar 2015).
- 18. Vardy J, Rymaszewski L, Begbie K, et al. . Fracture pathway redesign improves emergency department efficiency. Emer Med J 2013;30:876.2–876. 10.1136/emermed-2013-203113.25 [DOI] [Google Scholar]
- 19. Jenkins P, Gilmour A, Murray O, et al. . The Glasgow Fracture pathway: a virtual clinic. BJJ News 2014;2:22–4. [Google Scholar]
- 20. Fracture Clinic Redesign. http://www.fractureclinicredesign.org/resources/ (accessed Sep 15 2014)
- 21. National Services Scotland. Scottish Health Service costs: publication summary, 2015. [Google Scholar]
- 22. Audit Scotland. NHS in Scotland, 2015. [Google Scholar]
- 23. British Orthopaedic Association Standards for Trauma. BOAST 7: fracture clinic services. https://www.boa.ac.uk/publications/boa-standards-trauma-boasts/ (accessed Oct 9 2015). [DOI] [PubMed]
- 24. Australian Society of Orthopaedic Surgeons. Orthopaedic Specialities-Trauma. http://www.asos.org.au/trauma.html.
- 25. Orthopaedic Trauma Association- U.S.A. http://ota.org/media/251711/HPOrthopaedic-trauma-organization-Oct-2015.pdf (accessed 9 Oct 2015).
- 26. Gamble D, Jenkins PJ, Edge MJ, et al. . Satisfaction and functional outcome with ‘self-care’ for the management of fifth metacarpal fractures. Hand 2015;10:607–12. 10.1007/s11552-015-9749-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bansal R, Craigen MA. Fifth metacarpal neck fractures: is follow-up required? J Hand Surg Eur Vol 2007;32:69–73. 10.1016/j.jhsb.2006.09.021 [DOI] [PubMed] [Google Scholar]
- 28. Cakir H, Van Vliet-Koppert ST, Van Lieshout EM, et al. . Demographics and outcome of metatarsal fractures. Arch Orthop Trauma Surg 2011;131:241–5. 10.1007/s00402-010-1164-6 [DOI] [PubMed] [Google Scholar]
- 29. Calder JD, Solan M, Gidwani S, et al. . Management of paediatric clavicle fractures--is follow-up necessary? an audit of 346 cases. Ann R Coll Surg Engl 2002;84:331–3. 10.1308/003588402760452457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duckworth AD, Watson BS, Will EM, et al. . Radial head and neck fractures: functional results and predictors of outcome. J Trauma 2011;71:643–8. 10.1097/TA.0b013e3181f8fa5f [DOI] [PubMed] [Google Scholar]
- 31. Egol K, Walsh M, Rosenblatt K, et al. . Avulsion fractures of the fifth metatarsal base: a prospective outcome study. Foot Ankle Int 2007;28:581–3. 10.3113/FAI.2007.0581 [DOI] [PubMed] [Google Scholar]
- 32. Martin AG. Weber B ankle fracture: an unnecessary fracture clinic burden. Injury 2004;35:805–8. 10.1016/j.injury.2003.12.013 [DOI] [PubMed] [Google Scholar]
- 33. Symons S, Rowsell M, Bhowal B, et al. . Hospital versus home management of children with buckle fractures of the distal radius. A prospective, randomised trial. J Bone Joint Surg Br 2001;83:556–60. [DOI] [PubMed] [Google Scholar]
- 34. Hamilton TW, Hutchings L, Alsousou J, et al. . The treatment of stable paediatric forearm fractures using a cast that may be removed at home: comparison with traditional management in a randomised controlled trial. Bone Joint J 2013;95-B:1714–20. 10.1302/0301-620X.95B12.31299 [DOI] [PubMed] [Google Scholar]
- 35. Department of Health. Payment by results: background. 2010. http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/Managingyourorganisation/NHSFinancialReforms/DH_077259 (accessed Aug 15 2015).
- 36. Jenkins PJ, Morton AD, Anderson GH, et al. . Fracture Clinic redesign was associated with a reduced cost of outpatient orthopaedic provision. Bone and Joint 2016;5:33-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. NHS Scotland: quality Improvement hub. http://www.qihub.scot.nhs.uk/quality-and-efficiency/whole-system-patient-flow/fracture-pathway-redesign/how-you-can-do-it.aspx (accessed 15 Aug 2016).
- 38. Rao TS, Radhakrishnan R, Andrade C. Standard operating procedures for clinical practice. Indian J Psychiatry 2011;53:1–3. 10.4103/0019-5545.75542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koenig KM, Bozic KJ. Orthopaedic Healthcare Worldwide: the role of standardization in improving outcomes. Clin Orthop Relat Res 2015;473:3360–3. 10.1007/s11999-015-4492-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Reese S. Will you be pressured to perform ‘cookbook’ medicine? http://www.medscape.com/viewarticle/808258 (accessed Oct 2015).
- 41. Farias M, Jenkins K, Lock J, et al. . Standardized Clinical Assessment And Management Plans (SCAMPs) provide a better alternative to clinical practice guidelines. Health Aff 2013;32:911–20. 10.1377/hlthaff.2012.0667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mould G, Bowers J, Ghattas M. The evolution of the pathway and its role in improving patient care. Qual Saf Health Care 2010;19:e14–16. 10.1136/qshc.2009.032961 [DOI] [PubMed] [Google Scholar]
- 43. Brailsford S. Theoretical comparison of discrete event simulation and system dynamics : Brailsford S, Churilov L, Dangerfield B, Discrete event simulation and system dynamics for management decision making: Wiley &Sons, West Sussex, 2014:P105–24. [Google Scholar]
- 44. Fone D, Hollinghurst S, Temple M, et al. . Systematic review of the use and value of computer simulation modelling in population health and health care delivery. J Public Health Med 2003;25:325–35. 10.1093/pubmed/fdg075 [DOI] [PubMed] [Google Scholar]
- 45. Hulshof PJH, Kortbeek N, Boucherie RJ, et al. . Taxonomic classification of planning decisions in health care: a structured review of the state of the art in OR/MS. Health Syst 2012;1:129–75. 10.1057/hs.2012.18 [DOI] [Google Scholar]
- 46. Mear L, Fulop N, Lamont T. Why the nhs must evaluate complex service changes. Health Serv J 2015;125:16–17. [PubMed] [Google Scholar]
- 47. Cantero L. Getting the measurements right: qipp in 2013. Health Service J http://www.hsj.co.uk/home/innovation-and-efficiency/getting-the-measurements-right-qipp-in-2013/5056500.article#.VhPWO_nF9ps (accessed 13 Jul 2013). [Google Scholar]
- 48. Drummond MF. Methods for the economic evaluation of healthcare programs. Oxford University Press, 2005. [Google Scholar]
- 49. Kolata G. What are a hospitals costs? Utah System is trying to learn. 2015. http://www.nytimes.com/2015/09/08/health/what-are-a-hospitals-costs-utah-system-is-trying-to-learn.html?_r=0 (accessed 15 Jul 2015).
- 50. Brooksbank K, Jenkins PJ, Anthony IC, et al. . Functional outcome and satisfaction with a ‘self-care’ protocol for the management of mallet finger injuries: a case-series. J Trauma Manag Outcomes 2014;8:21. 10.1186/s13032-014-0021-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferguson KB, McGlynn J, Jenkins P, et al. . Fifth metatarsal fractures - Is routine follow-up necessary? Injury 2015;46:31–42. 10.1016/j.injury.2015.05.041 [DOI] [PubMed] [Google Scholar]
- 52. Jayaram PR, Bhattacharyya R, Jenkins PJ, et al. . A new virtual patient pathway for the management of radial head and neck fractures. J Shoulder Elbow Surg 2014;23:297–301. 10.1016/j.jse.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 53. Johnston K, Buxton MJ, Jones DR, et al. . Assessing the costs of healthcare technologies in clinical trials. Health Technol Assess 1999;3:1–76. [PubMed] [Google Scholar]
- 54. Smith KA, Rudmik L. Cost collection and analysis for health economic evaluation. Otolaryngol Head Neck Surg 2013;149:192–9. 10.1177/0194599813487850 [DOI] [PubMed] [Google Scholar]
- 55. Lewin K. Action Research and Minority Problems. J Soc Issues 1946;2:34–46. 10.1111/j.1540-4560.1946.tb02295.x [DOI] [Google Scholar]
- 56. Greenwood DJ, Levin M. Introduction to action research. Thousand Oaks, CASage, 1988. [Google Scholar]
- 57. East L, Robinson J. Change in process: bringing about change in health care through action research. J Clin Nurs 1994;3:57–61. 10.1111/j.1365-2702.1994.tb00359.x [DOI] [PubMed] [Google Scholar]
- 58. Simul8. http://www.simul8.com/products/ (accessed Oct 2014).
- 59. Bluespier software. http://www.bluespier.com/(accessed June 2015)
- 60. Mayo E. The human problems of an industrial civilization. New York, MacMillan 1993;3:53–73. [Google Scholar]
- 61. Hoad K, Robinson S, Davies R. Automated selection of the number of replications for a discrete-event simulation. J Oper Res Soc 2010;61:1632–44. 10.1057/jors.2009.121 [DOI] [Google Scholar]
- 62. Robinson S. Simulation: the practice of model development and use. Palgrave MacMillan, 2014. [Google Scholar]
- 63. Law AM. How to build valid and credible simulation models. presented at: 2009 Winter Simulation Conference (WSC). Austin, TX: Simulation Conference IEEE, 2009. [Google Scholar]
- 64. Finger A, Teunis T, Hageman MG, et al. . Do patients prefer optional follow-up for simple upper extremity fractures: a pilot study. Injury 2016;47:2276–82. 10.1016/j.injury.2016.06.029 [DOI] [PubMed] [Google Scholar]
- 65. Jenkins PJ, Stephenson DA, Rymaszewski LA. Legal aspect of virtual fracture clinics. Journal of Trauma and Orthopaedics 2016;4:36–40. [Google Scholar]
- 66. Brailsford SC, Harper PR, Patel B, et al. . Analysis of the academic literature on simulation and modelling in healthcare. JSimul 2009;3:130–40. [Google Scholar]
- 67. Marshall DA, Burgos-Liz L, IJzerman MJ, et al. . Applying dynamic simulation modelling methods in healthcare delivery research—Part 2: Report of the ISPOR Dynamic Simulation. Value in Health 2015;18:147–60. [DOI] [PubMed] [Google Scholar]
- 68. Karnon J, Stahl J, Brennan A, et al. . Modelling using discrete event simulation: a report of the ISPOR-SMDM Modelling Good Research Practices TaskForce-4. MedDecisMaking 2012;32:701–11. [DOI] [PubMed] [Google Scholar]
- 69. Bayer S. Simulation modelling and resource allocation in complex services. BMJ Qual Saf 2014;23:353–5. 10.1136/bmjqs-2013-002742 [DOI] [PubMed] [Google Scholar]
- 70. Davidson JS, Brown DJ, Barnes SN, et al. . Simple treatment for torus fractures of the distal radius. J Bone Joint Surg Br 2001;83:1173–5. 10.1302/0301-620X.83B8.11451 [DOI] [PubMed] [Google Scholar]
- 71. Kaplan RS, Porter ME. The big Idea: how to solve the cost crisis in Health Care. HBR 2011. [PubMed] [Google Scholar]
- 72. Marshall M, Øvretveit J. Can we save money by improving quality? BMJ Qual Saf 2011;20:293–6. 10.1136/bmjqs.2010.050237 [DOI] [PubMed] [Google Scholar]
- 73. Hoad K, Robinson S, Davies R. Automating warm-up length estimation. J Oper Res Soc 2010;61:1389–403. 10.1057/jors.2009.87 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.