Abstract
Purpose
Goblet cells in the conjunctiva secrete mucin into the tear film protecting the ocular surface. The proresolution mediator resolvin D1 (RvD1) regulates mucin secretion to maintain homeostasis during physiological conditions and in addition, actively terminates inflammation. We determined the signaling mechanisms used by RvD1 in cultured rat conjunctival goblet cells to increase intracellular [Ca2+] ([Ca2+]i) and induce glycoconjugate secretion.
Methods
Increase in [Ca2+]i were measured using fura 2/AM and glycoconjugate secretion determined using an enzyme-linked lectin assay with the lectin Ulex Europaeus Agglutinin 1. Signaling pathways activated by RvD1 were studied after goblet cells were pretreated with signaling pathway inhibitors before stimulation with RvD1. The results were compared with results when goblet cells were stimulated with RvD1 alone and percent inhibition calculated.
Results
The increase in [Ca2+]i stimulated by RvD1 was blocked by inhibitors to phospholipases (PL-) -D, -C, -A2, protein kinase C (PKC), extracellular signal-regulated kinases (ERK)1/2 and Ca2+/calmodulin-dependent kinase (Ca2+/CamK). Glycoconjugate secretion was significantly inhibited by PLD, -C, -A2, ERK1/2 and Ca2+/CamK, but not PKC.
Conclusions
We conclude that RvD1 increases glycoconjugate secretion from goblet cells via multiple signaling pathways including PLC, PLD, and PLA2, as well as their signaling components ERK1/2 and Ca2+/CamK to preserve the mucous layer and maintain homeostasis by protecting the eye from desiccating stress, allergens, and pathogens.
Keywords: resolvin, goblet cells, secretion, mucins
The conjunctiva is a mucous membrane that covers the sclera and the inner surface of the eyelid. A major cell type in the conjunctival epithelium is the goblet cell, which produces mucins, especially the mucin MUC5AC.1,2 Mucin glycoconjugates produced from the corneal epithelia and conjunctival goblet cells form the inner mucus layer of the tear film. The tear film plays a crucial role in innate immune defense of the eye creating a barrier between the environment and the ocular surface.3–6 However, the production of mucin from conjunctival goblet cells may be altered in inflammatory diseases, such as dry eye disease, allergic conjunctivitis, and bacterial and viral conjunctivitis.1–3
Inflammation can be actively terminated by proresolution mediators.7,8 One of the proresolution mediators is resolvin D1 (RvD1), which is biosynthesized from the ω-3-fatty acid, docosahexaenoic acid. Resolvins are biosynthesized during inflammation when there is a class-switch of enzymes, decreasing prostaglandin and leukotriene production, and enhancing biosynthesis of specialized proresolving mediators.8 In experimental systems, RvD1 limits neutrophil infiltration, stimulates macrophage phagocytosis, and reduces the level of inflammatory pain and fibrosis.9
Not only do the proresolution mediators function during disease, but they are also active during normal, noninflamed conditions. RvD1 is present in human peripheral blood, lymphoid tissue, urine, and tears under physiological conditions.10–12 In addition, RvD1 maintains homeostasis in bone marrow by enhancing macrophage removal of apoptotic osteoblasts.13 We previously found that another proresolving mediator, lipoxin A4 (LXA4), is an active mediator of mucin secretion in cultured rat conjunctival goblet cells14 and could function to maintain homeostasis in the conjunctiva.
In the eye, RvD1 functions during inflammation by blocking glycoconjugate secretion stimulated by the proinflammatory mediators leukotriene D4 and histamine in cultured human and rat conjunctival goblet cells.15,16 RvD1 binds to the G protein-coupled receptors ALX/FPR2 (mouse and human) and human DRV1 (identified earlier as GPR32).17–19 Both receptors are present in human conjunctival goblet cells while ALX/FPR2 has been found in rat conjunctival goblet cells.14 Under noninflamed conditions, activation of these receptors leads to an increase in intracellular [Ca2+] ([Ca2+]i), extracellular regulated kinase (ERK) 1/2 activation, and stimulation of glycoconjugate secretion from cultured conjunctival goblet cells.16 In the present study, three signaling pathways, known to be activated by ALX/FPR2, were investigated with resolvin D1 as ligand: phospholipase C (PLC), phospholipase D (PLD), and phospholipase A2 (PLA2).20 PLC breaks down phosphatidylinositol 4,5 bisphosphate (PIP2), producing inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 binds to the IP3 receptor on the intracellular calcium store, the endoplasmic reticulum, opening a ligand-gated Ca2+ channel, increasing [Ca2+]i.21 Ca2+ and DAG together activate protein kinase C (PKC). Using inhibitors to these different pathways, we found that all three of the phospholipases were activated by RvD1 in rat conjunctival goblet cells and that they activate the downstream signaling molecules ERK1/2 and Ca2+/calmodulin-dependent kinase (Ca2+/CamK) to increase [Ca2+]i and stimulate mucin secretion.
Methods and Materials
RPMI-1640 cell culture medium, penicillin/streptomycin, and L-glutamine were purchased from Lonza (Walkerville, IL, USA). Fetal bovine serum (FBS) was from Atlanta Biologicals (Norcross, GA, USA). Fura-2/AM and Amplex Red were from ThermoFisher (Waltham, MA, USA). Inhibitors and suppliers are listed in the Table.
Table.
List of Inhibitors Used and Inhibitory Effects

Resolvin D1 (7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid, RvD1) was from Cayman Chemical (Ann Arbor, MI, USA). RvD1 dissolved in ethanol as supplied by the manufacturer was stored at −80°C with minimal exposure to light. Immediately before use, RvD1 was diluted with either RPMI or Krebs-Ringer bicarbonate buffer with HEPES (KRB-HEPES, 119 mM NaCl, 4.8 mM KCl, 1.0 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 10 mM HEPES, and 5.5 mM glucose [pH 7.45]) to the desired concentration (10−8 M) and added to the cells.
Animals
For all of the experiments, 4- to 8-week-old male Sprague-Dawley rats (Taconic Farms, Germantown, NY, USA) were used. The rats were euthanized with CO2 and decapitated. Bulbar and forniceal conjunctiva were surgically removed. All experiments were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by Schepens Eye Research Institute Animal Care and Use Committee.
Cell Culture
Goblet cells from rats were grown in organ culture as described previously.6,15,22–24 The conjunctival explants were grown in 6-well plates containing RPMI 1640 medium supplemented with 10% FBS, 2-mM glutamine, and 100-μg/mL penicillin-streptomycin. The explant was removed before trypsinization of the cells for first passage. First passage goblet cells were seeded in either glass bottom petri dishes (for Ca2+ measurements) or 24 well plates (for mucin secretion measurements) were used in all experiments. Cultured cells were identified by evaluating staining with the lectin UEA-1, which detects goblet cell secretory product, and the antibody to cytokeratin 7, which detects goblet cell bodies, to ensure that goblet cells predominated.6,15,16,22–25
Measurement of [Ca2+]i
Cultured rat conjunctival goblet cells were plated onto 35-mm glass bottom culture dish and incubated overnight. Cells were then incubated for 1 hour at 37°C with KRB-HEPES plus 0.5% bovine serum albumin containing 0.5-μM fura-2/AM (Invitrogen, Grand Island, NY, USA), 8-μM pluronic acid F127 (Sigma-Aldrich, St. Louis, MO, USA) and 250 μM sulfinpyrazone (Sigma-Aldrich) followed by washing in KRB-HEPES containing sulfinpyrazone. Calcium measurements were made with a ratio imaging system (In Cyt Im2; Intracellular Imaging, Cincinnati, OH, USA) using wavelengths of 340 and 380 nm and an emission wavelength of 505 nm.26 At least five cells were selected in each experimental condition, and the experiments were repeated in at least three separate animals. The goblet cells were incubated with inhibitors before RvD1 (10−8 M) was added. The cholinergic agonist carbachol (Cch, 10−4 M) was used as a positive control. The inhibitors and the pathways they affect are listed in the Table. BOC-2, BAPTA, autocamtide-2-related inhibitory peptide (AIP), and U0126 were added 30 minutes prior to RvD1- or Cch-stimulation. U73122, the negative control U73343, 1-butanol (1-but), and its negative control t-butanol (t-but) were added 15 minutes before RvD1- or Cch-stimulation. 2-Aminoethyl diphenylborate (2-APB) and Ro 31-8220 were added 10 minutes in advance of RvD1- or Cch-stimulation. Aristolochic acid (aris) was added 10 minutes prior to stimulation. Inhibitors were initially dissolved in DMSO and diluted to working concentrations in KRB-HEPES. We have previously shown that DMSO, at the concentrations used, did not have any effect on basal [Ca2+]i.23
The data are presented as the actual [Ca2+]i with time or as the change in peak [Ca2+]i. Change in peak [Ca2+]i was calculated by subtracting the average of the basal value, the average [Ca2+]i value before addition of RvD1, from the peak [Ca2+]i. If no peak occurred, the maximum value obtained in the timeframe in which the control (agonist alone) peaked was used.
Glycoconjugate Secretion
Cultured rat conjunctival goblet cells were trypsinized, passaged in 24-well plates, and grown to approximately 75% confluence. The cells were serum starved in serum free RPMI 1640 containing 0.5% BSA for 2 hours before they were preincubated for 30 minutes with the inhibitors. Inhibitors were initially dissolved in DMSO and diluted to working concentrations in RPMI 1640 medium. We have previously shown that DMSO, at the concentrations used, did not have any effect on basal secretion.23 Thereafter, the cells were incubated with RvD1 (10−8 M) for 2 hours. To determine the amount of goblet cell glycoconjugate secretion, the lectin horseradish peroxidase-conjugated Ulex Europaeus Agglutinin I (UEA-1), which binds to glycoproteins secreted from goblet cells was used in an enzyme-linked lectin assay. The standards and supernatant were spotted onto Nunc microplates and dried overnight at 60°C. The UEA-I was then detected using Amplex Red, which when oxidized by peroxidase in the presence of hydrogen peroxide produces a highly fluorescent molecule. Quantification of fluorescence was done using a fluorescence ELISA reader (model FL600; Bio-Tek, Winooski, VT, USA) with an excitation wavelength of 530 nm and an emission wavelength of 590 nm. The cells were scraped, and the cell homogenate analyzed for the total amount of protein using the Bradford protein assay. Secretion was normalized to the amount of protein in each well and expressed as fold increase above basal. Please note that experiments with Ro31-8220, 1-butanol (1-but), tertiary butanol (t-butanol), and aris were performed in the same experiments.
Statistical Analysis
The data are presented as fold-increase above basal as average ± SEM. Student's t-test was used to perform statistical analysis and P less than 0.05 was set as statistically significant.
Results
ALX/FPR2 Receptors Are Activated by RvD1 in Rat Conjunctival Goblet Cells
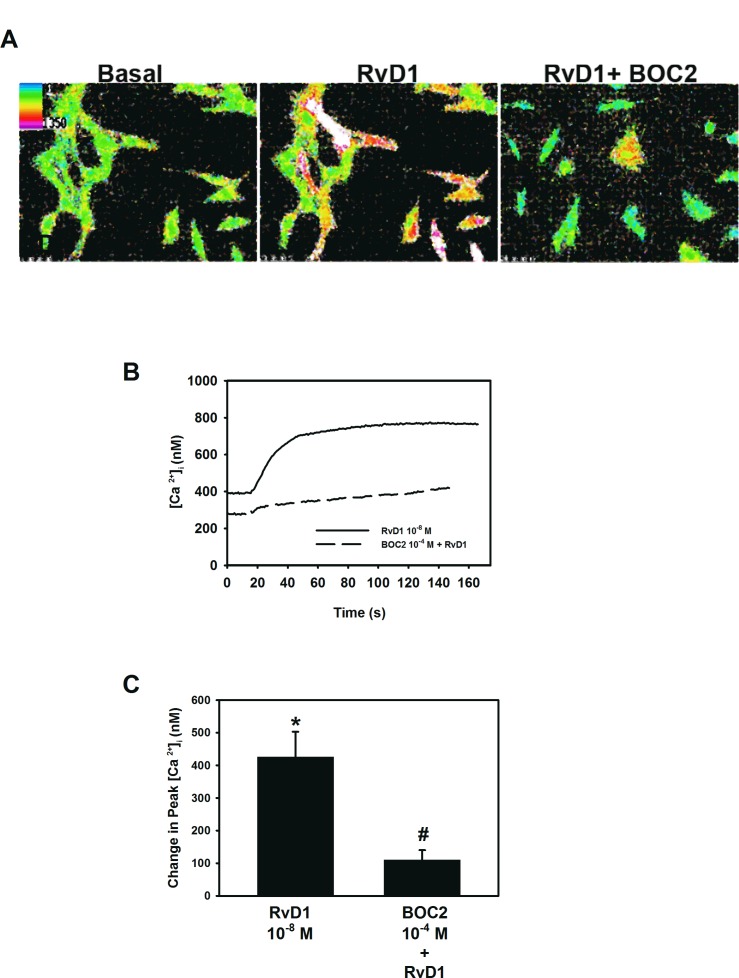
RvD1 can activate two receptors in human cells, ALX/FPR2 and DRV1. An earlier study, where the ALX/FPR2 receptor was knocked down with siRNA in cultured rat conjunctival goblet cells, showed that RvD1 uses the ALX/FPR2 receptor to increase [Ca2+]i.16 To support these findings and further investigate RvD1 intracellular signals, first passage goblet cells cultured from rat conjunctiva were preincubated with the ALX/FPR2 receptor antagonist BOC-2 (10−4 M) for 30 minutes prior to addition of RvD1 (10−8 M) and [Ca2+]i levels over time were measured (Figs. 1A, 1B). RvD1 by itself increased [Ca2+]i by maximum 425.3 ± 77.4 nM (P = 0.003). Inhibition of the ALX/FPR2 receptor gave a significant decrease in RvD1-stimulated [Ca2+]i increase, giving a 71.4 ± 7.2% inhibition (P = 0.2 × 10−6) (Fig. 1C). Thus, RvD1 exerts its actions primarily via the ALX/FPR2.
Figure 1.
Activation of ALX/FPR2 in rat conjunctival goblet cells. Pseudocolor images of goblet cells incubated with BOC2 10−4 M for 30 minutes and stimulated with RvD1 10−8 M are shown in (A). Effect over time is shown in (B). Change in peak [Ca2+]i is shown in (C). Data are mean ± SEM from six individual experiments. *Significant difference from basal. #Significant difference from RvD1 alone.
RvD1-Stimulated Glycoconjugate Secretion Depends on Intracellular Calcium
To determine if RvD1 uses [Ca2+]i to increase glycoconjugate secretion, goblet cells were incubated with BAPTA/AM, which is an intracellular calcium chelator. RvD1 stimulated glycoconjugate secretion by 2.4 ± 0.4-fold above basal (P = 0.002) (Fig. 2). BAPTA (10−5 M) blocked RvD1 (10−8 M)-induced glycoconjugate secretion by 88.9 ± 8.8% (P = 0.03). This indicates that RvD1 uses intracellular calcium to secrete mucin in rat conjunctival goblet cells.
Figure 2.
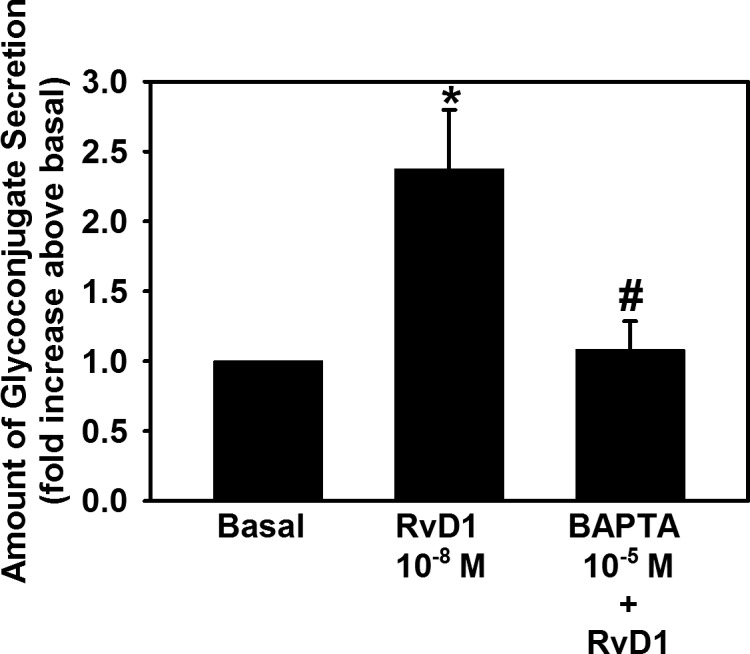
Chelation of extracellular [Ca2+] blocks RvD1-stimulated increase in glycoconjugate secretion. Goblet cells were preincubated for 30 minutes with BAPTA/AM (10−5 M) and stimulated with RvD1 (10−8 M). Glycoconjugate secretion was measured. Data are mean ± SEM from four independent experiments. *Significant difference from basal. #Significant difference from RvD1 alone.
RvD1 Activates PLC to Increase [Ca2+]i and Stimulate Glycoconjugate Secretion in Cultured Goblet Cells
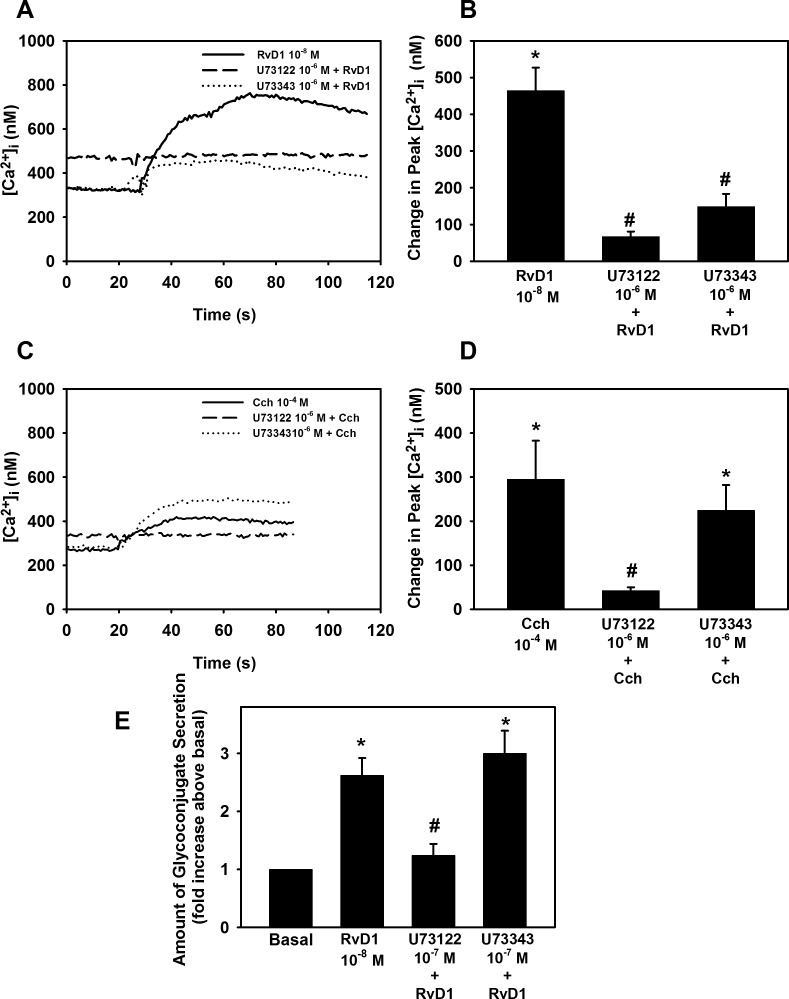
To determinate whether RvD1 activates PLC, cultured rat conjunctival goblet cells were preincubated with the PLC inhibitor U73122 or the negative control U73343 both at 10−6 M. After incubating the cells with U73122, RvD1 (10−8 M) was added and [Ca2+]i over time was measured (Fig. 3A). RvD1 by itself significantly increased [Ca2+]i (P = 0.0003), by 464.1 ± 63.0 nM. When the rat conjunctival goblet cells were treated with U73122, the RvD1-stimulated increase in [Ca2+]i was significantly blocked. PLC inhibition decreased RvD1-stimulated [Ca2+]i increase by 85.8 ± 2.1% (P = 0.0008) (Fig. 3B). The inactive analog of U73122, U73343, also affected RvD1-stimulated [Ca2+]i increase by a 65.3 ± 10.9% blockage (P = 0.005).
Figure 3.
Inhibition of PLC blocks RvD1-stimulated increase in [Ca2+]i and glycoconjugate secretion. Goblet cells were preincubated for 15 minutes with the PLC inhibitor U73122 (10−6 M) or its inactive isomer U73343 (10−6 M), and stimulated with either RvD1 (10−8 M, [A, B]) or carbachol (Cch 10−4 M, [C, D]). [Ca2+]i over time is shown in (A, C) and change in peak [Ca2+]i is shown in (B, D). Glycoconjugate secretion was measured and is shown in (E). Data are mean ± SEM from four (A, B, E) or five (C, D) independent experiments. *Significant difference from basal. #Significant difference from either RvD1 or Cch alone.
The cholinergic agonist carbachol (Cch) is known to increase [Ca2+]i. As many of the signaling pathways for Cch are known,14,27 Cch was used as a positive control for the experiments. Cch gave an increase in [Ca2+]i of 287.5 ± 68.2 nM (P = 0.003) (Fig. 3C). As expected, U73122 blocked Cch-stimulated [Ca2+]i increase. A maximum decrease of 85.9 ± 3.0% was obtained (P = 0.007) (Fig. 3D). U73343 did not affect Cch-stimulated [Ca2+]i increase (P = 0.84).
We next investigated the effect of PLC on glycoconjugate secretion. RvD1 alone significantly stimulated glycoconjugate secretion 2.6 ± 0.3-fold above basal (P = 0.0016). Treatment with U73122 significantly blocked RvD1-stimulated glycoconjugate secretion (P = 4.3 × 10−6) (Fig. 3E). RvD1-stimulated glycoconjugate secretion was inhibited by 90.8 ± 5.8% with U73122. Unlike its effect on [Ca2+]i the control, U73343, did not significantly affect RvD1-stimulated glycoconjugate secretion (P = 0.9). Thus, RvD1 uses PLC to increase [Ca2+]i and stimulate glycoconjugate secretion from rat conjunctival goblet cells.
RvD1 Uses the IP3 Receptor to Increase [Ca2+]i and Stimulate Glycoconjugate Secretion in Cultured Goblet Cells
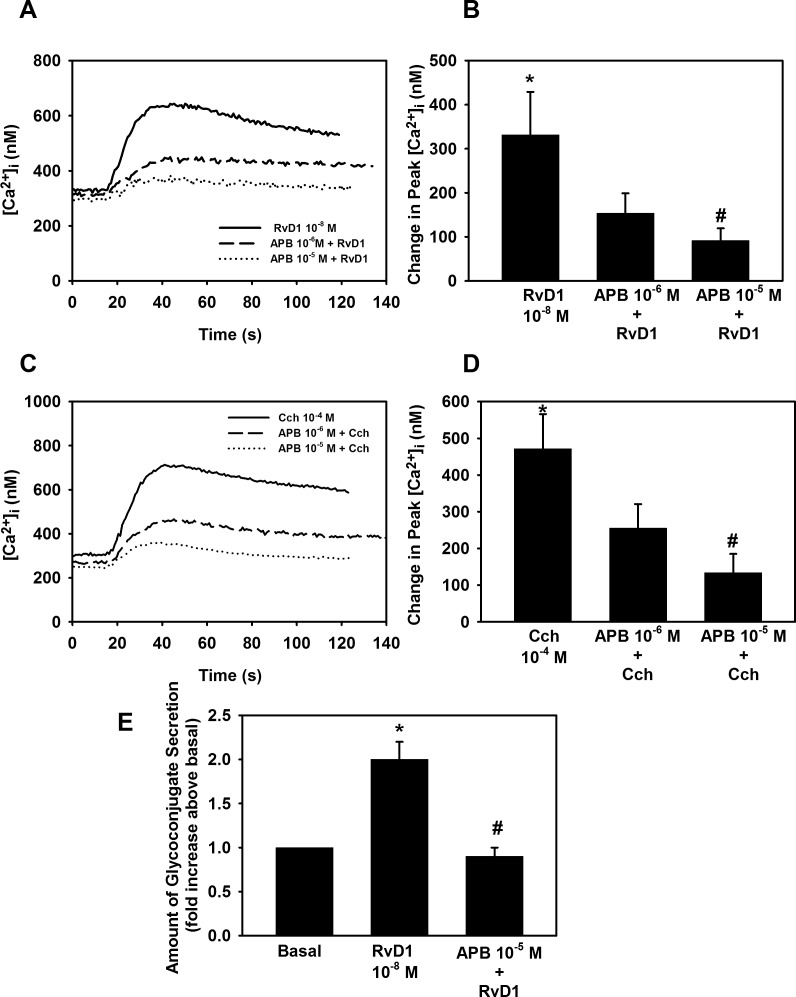
We investigated the PLC signaling pathway further by using the IP3 receptor antagonist, 2-aminoethyl diphenylborinate (2-APB).28 RvD1 10−8 M alone significantly increased [Ca2+]i (P = 0.007), with a value of maximum 331.2 ± 97.5 nM (Figs. 4A, 4B). Cultured goblet cells were incubated with 2-APB at 10−6 M or 10−5 M for 10 minutes before addition with RvD1 (10−8 M). 2-APB gave a 46.7 ± 15.0% inhibition (P = 0.01) at 10−6 M and 66.2 ± 8.3% inhibition (P = 1 × 10−5) at 10−5 M of RvD1-stimulated increase in [Ca2+]i (Figs. 4A, 4B). Cch stimulated an increase in [Ca2+]i by 471.6 ± 94.4 nM (P = 0.0004) (Figs. 4C, 4D). Cch-stimulated increase in [Ca2+]i was inhibited 41.7 ± 14.2% (P = 0.01) and 67.6 ± 13.2% (P = 0.0003) when pretreated with 2-APB 10−6 M and 10−5 M, respectively (Fig. 4D).
Figure 4.
Inhibition of IP3 receptor blocks RvD1-stimulated increase in [Ca2+]i and glycoconjugate secretion. Goblet cells were preincubated for 10 minutes with the IP3 receptor inhibitor APB (10−6 to 10−5 M) and stimulated with either RvD1 (10−8 M, [A, B]) or carbachol (Cch 10−4 M, [C, D]). [Ca2+]i over time is shown in (A, C) and change in peak [Ca2+]i is shown in (B, D). Glycoconjugate secretion was measured and is shown in (E). Data are mean ± SEM from six (A–D) or four (E) independent experiments. *Significant difference from basal. #Significant difference from RvD1 or Cch alone.
We examined if the inhibition of the IP3 receptor blocked RvD1-stimulated glycoconjugate secretion using 2-APB 10−5 M, which blocked the RvD1-stimulated [Ca2+]i increase. RvD1 alone increased glycoconjugate secretion by 2.0 ± 0.20-fold above basal (P = 0.003). Incubation with 2-APB 10−5 M completely blocked RvD1-stimulated glycoconjugate secretion inhibiting it by 98.1 ± 1.8% (P = 2.5 × 10−9) (Fig. 4E).
RvD1 Uses PKC to Increase [Ca2+]i, but Not to Stimulate Glycoconjugate Secretion in Cultured Goblet Cells
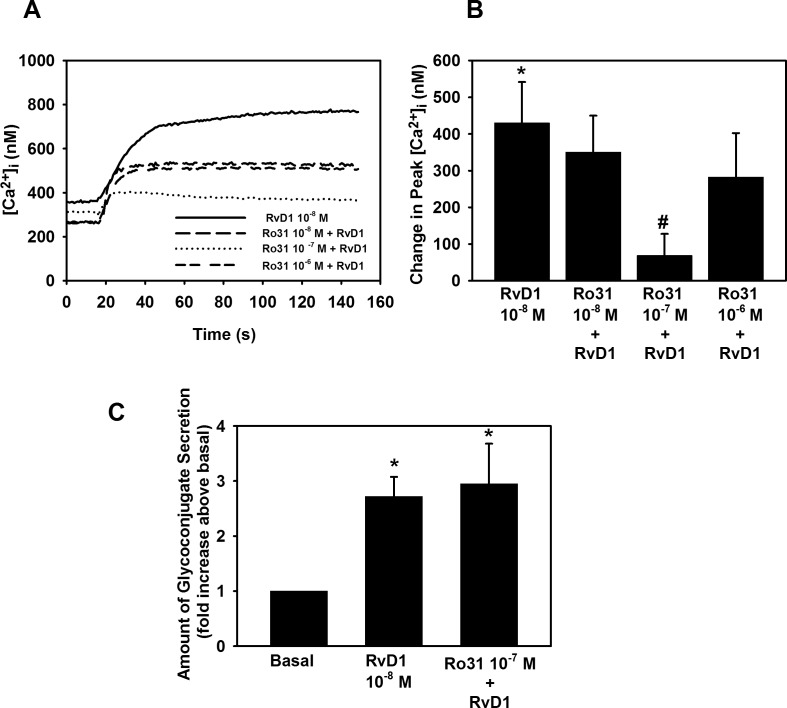
In addition to producing IP3, PLC also activates PKC. We determined if RvD1 uses PKC to increase [Ca2+]i in cultured rat conjunctival goblet cells by using the PKC inhibitor Ro 31-8220. When comparing the increase in [Ca2+]i in goblet cells stimulated with RvD1 alone, which had a peak [Ca2+]i increase of 430.0 ± 111.7 (P = 0.008) (Figs. 5A, 5B), to cells who were pretreated with Ro 31-8220, we found that Ro 31-8220 gave a 31.0 ± 17.4% inhibition (P = 0.61), a 88.6 ± 8.0% inhibition (P = 0.03), and a 43.9 ± 18.3% inhibition (P = 0.40) at 10−8 M, 10−7 M, and 10−6 M, respectively. Thus, RvD1 can activate PKC to increase [Ca2+]i.
Figure 5.
Inhibition of PKC effected RvD1-stimulated increase in [Ca2+]i, but not glycoconjugate secretion. Goblet cells were preincubated for 10 minutes with the PKC inhibitor Ro 31-8220 (10−8 − 10−6 M) and stimulated with RvD1 (10−8 M). [Ca2+]i over time is shown in (A) and change in peak [Ca2+]i is shown in (B). Glycoconjugate secretion was measured and is shown in (C). Data are mean ± SEM from four independent experiments. *Significant difference from basal. #Significant difference from RvD1.
To study whether RvD1 activates PKC in order to stimulate glycoconjugate secretion, we used Ro 31-8220. RvD1 10−8 M increased glycoconjugate 2.7 ± 0.4-fold above basal (P = 0.003). Incubation with Ro 31-8220 did not have any effect on RvD1-stimulated secretion (Fig. 5C). Thus, RvD1 activates PKC to increase [Ca2+]i, but not to stimulate glycoconjugate secretion.
RvD1 Uses Ca2+/CamK to Increase [Ca2+]i and Stimulate Glycoconjugate Secretion in Cultured Goblet Cells
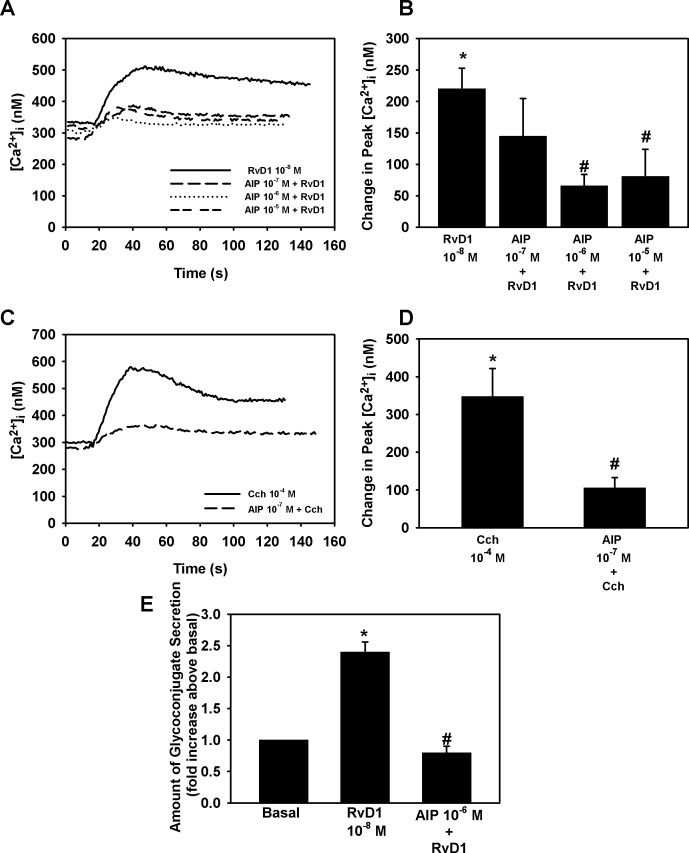
RvD1-stimulated [Ca2+]i increase through Ca2+/CamK was studied using the inhibitor AIP. RvD1 by itself significantly increased [Ca2+]i, by 220.2 ± 32.6 nM (P = 0.0001) (Figs. 6A, 6B). When rat conjunctival goblet cells were treated with AIP, the RvD1-stimulated goblet cell increase in [Ca2+]i was reduced significantly by 46.9 ± 21.9% (P = 0.02), 72.3 ± 9.3% (P = 5 × 10−5), and 62.0 ± 24.6% (P = 0.009) at 10−7 M, 10−6 M, and 10−5 M, respectively (Fig. 6B).
Figure 6.
Inhibition of Ca2+/CamK blocks RvD1-stimulated increase in [Ca2+]i and glycoconjugate secretion. Goblet cells were preincubated for 30 minutes with the Ca2+/CamK inhibitor AIP (10−7 to 10−5 M) and stimulated with either RvD1 (10−8 M, [A, B]) or carbachol (Cch 10−4 M, [C, D]). [Ca2+]i over time is shown in (A, C) and change in peak [Ca2+]i is shown in (B, D). Glycoconjugate secretion was measured and is shown in (E). Data are mean ± SEM from five (A–D) or three (E) independent experiments. *Significant difference from basal. #Significant difference from either RvD1 or Cch alone.
As the positive control, Cch alone significantly increased [Ca2+]i (P = 0.002) by 347.6 ± 74.2 nM. Incubating the goblet cells with Ca2+/CamK inhibitor AIP at 10−7 M, gave a decrease of Cch-stimulated [Ca2+]i increase of 63.2 ± 33.9% (P = 0.004) (Fig. 6C).
The actions of Ca2+/CamK on RvD1-stimulated glycoconjugate secretion were studied using AIP. By itself, RvD1 increased glycoconjugate secretion by 2.4 ± 0.2-fold above basal (P = 0.001). When rat conjunctival goblet cells were preincubated with AIP 10−6 M for 30 minutes before addition of RvD1 for 2 hours and glycoconjugate secretion measured, we found that AIP decreased RvD1-stimulated glycoconjugate secretion by 96.2 ± 3.8% (P = 1.4 × 10−5) (Fig. 6E). These data indicate that RvD1 uses Ca2+/CamK to increase [Ca2+]i and stimulate glycoconjugate secretion from rat conjunctival goblet cells.
RvD1 Uses PLD to Increase [Ca2+]i and Stimulate Glycoconjugate Secretion in Cultured Goblet Cells
The PLD-inhibitor, 1-but (0.3%), was used to study whether RvD1 activates a PLD pathway in rat conjunctival goblet cells. RvD1 10−8 M alone stimulated a [Ca2+]i increase by 381.2 ± 31.1 nM (P = 1.8 × 10−5) (Figs. 7A, 7B). RvD1-stimulated increase in [Ca2+]i was significantly decreased by 70.0 ± 23.5% (P = 0.05) by 1-but. The negative control for 1-butanol, t-but (0.3%), gave a small but significant 33.9 ± 12.0% (P = 0.03) inhibition of RvD1-stimulated increase in [Ca2+]i.
Figure 7.
Inhibition of PLD blocks RvD1-stimulated increase in [Ca2+]i and glycoconjugate secretion. Goblet cells were preincubated for 15 minutes with the PLD inhibitor 1-butanol (1-but) or the negative control tertiary butanol (t-but) (0.3%) and stimulated with either RvD1 (10−8 M, [A, B]) or carbachol (Cch 10−4 M, [C, D]). [Ca2+]i over time is shown in (A, C) and change in peak [Ca2+]i is shown in (B, D). Glycoconjugate secretion was measured and is shown in (E). Data are mean ± SEM from four independent experiments. *Significant difference from basal. #Significant difference from RvD1 or Cch alone.
A similar experiment was conducted using Cch as the positive control. Cch 10−4 M stimulated a maximum [Ca2+]i increase by 244.0 ± 64.2 nM (P = 0.009) (Fig. 7C). Cch-induced increase of [Ca2+]i was blocked by 66.5 ± 22.4% (P = 0.02) by 1-but. t-but did not affect the [Ca2+]i increase stimulated by Cch (P = 0.5).
The actions of PLD-inhibition on RvD1-stimulated glycoconjugate secretion from cultured rat goblet cells were determined. RvD1 (10−8 M) increased glycoconjugate secretion by 2.7 ± 0.4-fold above basal (P = 0.003). When glycoconjugate secretion was measured, 1-but significantly decreased RvD1-induced secretion by 71.9 ± 8.6% (P = 0.02) (Fig. 7E). t-but did not give a significant decrease in RvD1-stimulated glycoconjugate secretion (P = 0.8). These data show that in rat goblet cells, RvD1 uses PLD to increase [Ca2+]i and stimulate glycoconjugate secretion.
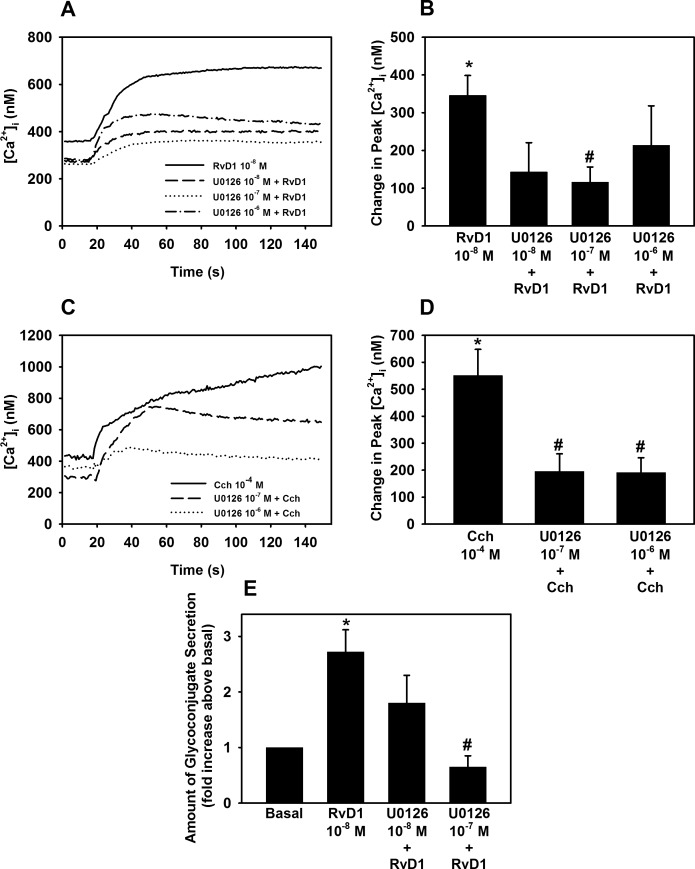
RvD1 Activates ERK 1/2 to Increase [Ca2+]i and Stimulate Glycoconjugate Secretion in Cultured Rat Goblet Cells
The ERK 1/2 pathway can be activated through G protein-coupled receptors by transactivating the EGF receptor.29,30 In goblet cells RvD1 increased ERK 1/2 activity in a concentration and time-dependent manner.16 Therefore, we wanted to investigate the effects of ERK 1/2 on RvD1-stimulated [Ca2+]i increase and mucin secretion. RvD1 by itself increased [Ca2+]i by 345.8 ± 52.9 nM (P = 3.9 × 10−6) (Figs. 8A, 8B). U0126 significantly inhibited RvD1-stimulated [Ca2+]i increase by 67.8 ± 7.7% (P = 6 × 10−8), with U0126 10−7, respectively.
Figure 8.
Inhibition of ERK 1/2 blocks RvD1-stimulated increase in [Ca2+]i and glycoconjugate secretion. Goblet cells were preincubated for 30 minutes with the PLD inhibitor U0126 (10−7 to 10−6 M) and stimulated with either RvD1 (10−8 M, [A, B]) or carbachol (Cch 10−4 M, [C, D]). [Ca2+]i over time is shown in (A, C) and change in peak [Ca2+]i is shown in (B, D). Glycoconjugate secretion was measured and is shown in (E). Data are mean ± SEM from six (A–D) and four (E) independent experiments. *Significant difference from basal. #Significant difference from either RvD1 or Cch alone.
The positive control Cch 10−4 M increased [Ca2+]i by 551 ± 108.0% nM (P = 0.0002) (Figs. 8C, 8D). When the goblet cells were incubated with U0126 10−7 M and U0126 10−6 M, Cch-induced [Ca2+]i increase was inhibited by 64.2 ± 13.8% (P = 0.0009) and 64.8 ± 10.9% (P = 0.0001), respectively.
We also found that U0126 10−7 M, but not 10−8 M, completely blocked RvD1-stimulated glycoconjugate secretion (P = 1.6 × 10−10) (Fig. 8E).
Our studies indicate that RvD1 leads to phosphorylation and activation of ERK1/2 that increases [Ca2+]i in rat conjunctival goblet cells and stimulates secretion of glycoconjugates into the tear film.
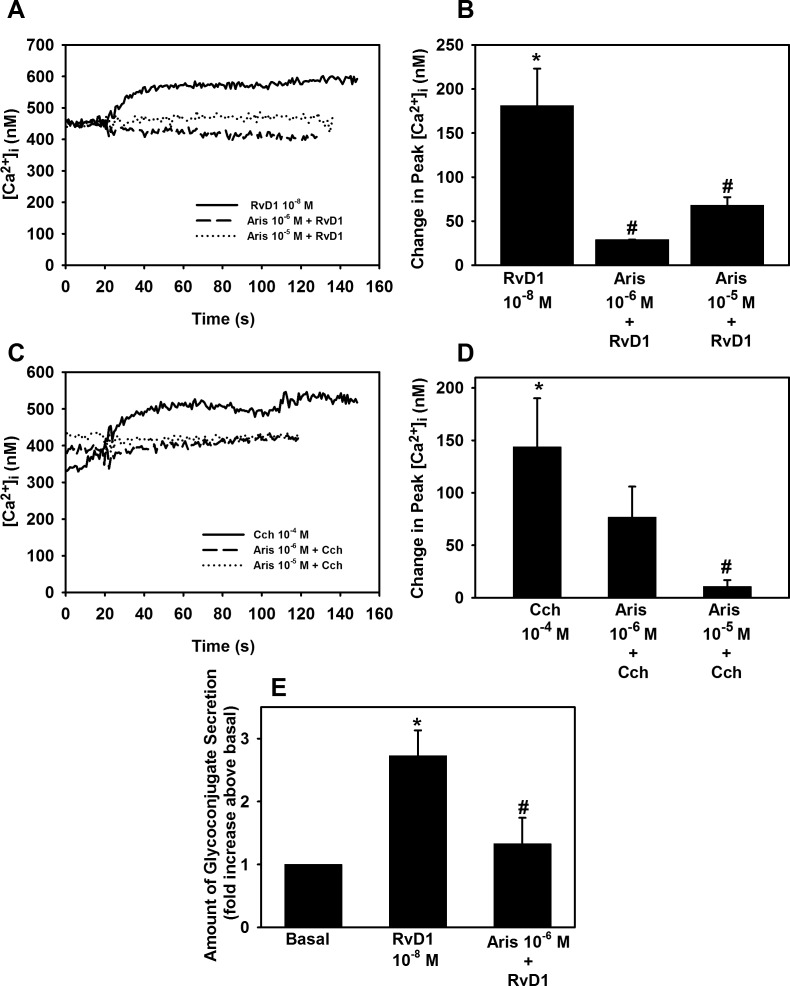
RvD1 Activates PLA2 to Increase in [Ca2+]i and Stimulate Glycoconjugate Secretion in Cultured Goblet Cells
To determine if PLA2 plays a role in RvD1-stimulated increase in [Ca2+]I, cultured rat goblet cells the PLA2 inhibitor Aris (10−6 M and 10−5 M) was used. RvD1 (10−8 M) increased [Ca2+]i by 184.3 ± 37.8 nM (P = 0.01). As shown in Figures 9A and 9B, Aris 10−6 M and 10−5 M significantly inhibited RvD1-stimulated [Ca2+]i increase by a 79.7 ± 2.8 (P = 1.6 × 10−5) and 61.0 ± 11.5% inhibition (P = 0.006), respectively.
Figure 9.
Inhibition of PLA2 blocks RvD1-stimulated increase in [Ca2+]i and glycoconjugate secretion. Goblet cells were preincubated for 10 minutes with the PLD2 inhibitor aristolochic acid (Aris, 10−6 to 10−5 M) and stimulated with either RvD1 (10−8 M, [A, B]) or carbachol (Cch 10−4 M, [C, D]). [Ca2+]i over time is shown in (A, C) and change in peak [Ca2+]i is shown in (B, D). Glycoconjugate secretion was measured and is shown in (E). Data are mean ± SEM from five (A–D) and four (E) independent experiments. *Significant difference from basal. #Significant basal from either RvD1 or Cch alone.
The positive control Cch 10−4 M increased [Ca2+]i by 143.8 ± 46.0 nM (P = 0.02). We found a 50.6 ± 22.1% inhibition (P = 0.11) of Cch-stimulated increase in [Ca2+]i by Aris at 10−6 M and a 70.7 ± 15.3% inhibition (P = 0.016) by Aris at 10−5 M (Figs. 9C, 9D).
To determine if RvD1 uses PLA2 to stimulate high molecular weight glycoconjugate secretion Aris was used. RvD1 (10−8 M) increased glycoconjugate secretion by 2.7 ± 0.4-fold above basal (P = 0.003). Aris (10−6 M) significantly decreased glycoconjugate secretion by 67.9 ± 22.6% (P = 0.02) compared with RvD1-stimulated glycoconjugate secretion without the inhibitor (Fig. 9D).
Discussion
Herein, we report that RvD1 activates ALX/FPR2 in rat conjunctival goblet cells by stimulating PLC, PLD, and PLA2 (Fig. 10). PLC via IP3 production activates the IP3 receptor to release intracellular Ca2+. In turn activation of PLC also produces diacylglycerol (DAG) that activates PKC and PKC increases [Ca2+]i. Stimulation of PLD and PLA2 likewise increases [Ca2+]i. As activation of ERK1/2 and Ca2+/CamK elevate [Ca2+]i ERK1/2 and Ca2+/CamK could be in the signaling pathways activated PLC, PLD, or PLA2. Glycoconjugate secretion is dependent upon Ca2+ as chelation of Ca2+ with BAPTA blocked RvD1-stimulated glycoconjugate secretion. This is similar to LXA4-stimulated secretion, which was also blocked by BAPTA. Use of these pathways to regulate [Ca2+]i, and stimulate secretion strengthens our previous findings that resolvins and the proresolution mediator LXA4 preserve ocular surface homeostasis during noninflamed, normal physiological processes in conjunctival goblet cells by stimulating mucin secretion.15,16,23
Figure 10.

Schematic diagram of signaling pathways of RvD1 in conjunctival goblet cells. RvD1 binds to the ALX/FPR2 receptor and activates the signaling pathways of PLA2, PLC, and PLD. PLA2 and PLD are believed to activate ERK 1/2 to increase [Ca2+]i and Ca2+/CamK. PLC increases IP3 and DAG. IP3 releases Ca2+ while DAG activates PKC. Activation of these pathways leads to mucin secretion.
In human tissues including goblet cells, RvD1 is known to bind to two receptors, DRV1 and ALX/FPR2.17 In the present study, inhibition of ALX/FPR2 blocked RvD1-stimulated increase in [Ca2+]i by over 70%. This indicates that the majority of actions stimulated by RvD1 are mediated thorough ALX/FPR2. In contrast to rat goblet cells, in goblet cells cultured from human conjunctiva, RvD1-stimulated increase in [Ca2+]i was not altered by inhibition of ALX/FPR2.24 Additionally, RvD1 and LXA4, another proresolution mediator that also binds to the ALX/FPR2 receptor, desensitize each other when added sequentially in rat conjunctival goblet cells whereas in human goblet cells, RvD1 does not desensitize the LXA4 response.14,24 These data support the hypothesis that in human cells RvD1 preferentially binds to the DRV1 receptor, while in rats RvD1 preferentially acts via ALX/FPR2 receptor to increase [Ca2+]i and stimulate glycoconjugate secretion. Given the important role of ALX/FPR2 in rat compared human goblet cells stimulated by RvD1, the role of rat DRV1 in goblet cells is likely limited.
In the conjunctiva, glycoconjugate secretion was measured 2 hours after addition of RvD1 while Ca2+ is measured within minutes after addition. Glycoconjugate secretion is the end result of the activation of numerous signal transduction pathways, movement of granules, and fusion of membranes,31 whereas the release of Ca2+ is one of the earliest events that occur after agonist addition. Glycoconjugate release is directly linked to the increase in [Ca2+]i as chelation of Ca2+ blocks it. While it is well-established that MUC5AC, the primary glycoconjugate present in conjunctival goblet cells is stored in granules and secreted upon appropriate stimuli,32–35 it is possible that the release of glycoconjugates could be due to protein synthesis and discharged in an unregulated manner. The rate of MUC5AC synthesis in conjunctival goblet cells has not been determined, the amount of MUC5AC mRNA in response to hypoxia in nasal epithelial cells was not significantly increased for 6 hours,36 and in mouse stomach, newly synthesized MUC5AC did not appear at the cell surface for 5 hours.37 In addition, in previous studies release of gylcoconjugates stimulated by RvD1 and other stimuli occurs in a time-dependent manner, which were blocked by receptor antagonists.15,16,23,38 Therefore, it is unlikely that in the 2-hour time point at which release of glycoconjugates was measured in this study, new protein synthesis plays a significant role. The time frame required to detect the glycoconjugates most likely reflect the sensitivity of this method versus that of the high sensitivity of the Ca2+ fluorescent dye Fura 2.
The signaling pathways activated upon binding of RvD1 to the ALX/FPR2 receptor are similar to the pathways stimulated by LXA4 when it interacts with the ALX/FPR2 receptor.17,39 Both proresolution mediators activate the same signaling pathways to induce glycoconjugate secretion. RvD1 and LXA4 have also shown the same effects in disease.40,41 For example, in a mouse animal model, RvD1 and the LXA4 analog, aspirin-triggered LXA4, both significantly reduced neovascularization in inflamed cornea.41
In the present study, conjunctival goblet cells from rats were used for all the experiments. As RvD1 appears to bind to DRV1 preferentially over ALX/FPR2 in human cells, a comparison between signaling pathways activated by RvD1 in human compared with rat goblet cells could be warranted.17,18 On the other hand, it is plausible that RvD1 activates similar pathways in rat and human goblet cells, as the same receptors are found in both species and RvD1 stimulates an increase in [Ca2+]i and glycoconjugate secretion in both human and rat conjunctival goblet cells.16 In addition, RvD1 blocks LTD4- and histamine-stimulated mucin secretion in goblet cells of both species.15,16
In order to examine whether RvD1 activates a PLC pathway in goblet cells, we used the PLC inhibitor U73122, which gave a significant blockage of RvD1-stimulated [Ca2+]i increase and mucin secretion. However, the inactive analog of U73144, also decreased RvD1-stimulated [Ca2+]i increase. This could indicate that the decrease in [Ca2+]i levels with U73122 was not due to PLC inhibition. However, U73144 did not affect either the RvD1-stimulated mucin secretin, Cch-stimulated increase in [Ca2+]i or Cch-stimulated glycoconjugate secretion. In addition, 2-APB, the IP3 receptor inhibitor, significantly decreased RvD1-stimulated [Ca2+]i increase and glycoconjugate secretion, implying that RvD1 does indeed activate a PLC pathway in rat conjunctival goblet cells.
We found that RvD1 stimulated mucin secretion through an increase in intracellular Ca2+ via multiple signaling pathways. This illustrates the importance of RvD1 in stimulating mucin secretion, including MUC5AC. MUC5AC is a gel-forming protein and is believed to be involved in the clearance of allergens, pathogens, and debris to protect the ocular surface and maintain a clear vision. Lack of sufficient MUC5AC secretion, as seen in atopic keratoconjunctivitis, can lead to corneal ulcers and decreased lubrication.42 Decreased levels of MUC5AC is also seen in dry eye disease.43–46
Treatment with RvD1 has been studied in several animal models, and shows promising results regarding resolution of inflammation.41,47–54 In addition, resolvins decrease the level of pain,55–58 and lower the need for antibiotics,59 which may be a significant benefit, considering the spread of antibiotic resistance. In contrast to glucocorticoids, used as topical treatment in ocular inflammatory diseases, resolvins are not immunosuppressive.
In the eye, ALX/FPR2 receptors are synthesized by uninjured corneal epithelium60 and play a role in corneal wound healing in mice.61 We recently demonstrated that several proresolving mediators including RvD1 are present in human emotional tears from males but not females.12 Interestingly, Gao et al.62 demonstrated that the ALX/FPR2 receptors play a role in autoimmune dry eye that occurs more prominently in females than males.
In conclusion, RvD1 functions under noninflamed conditions to maintain homeostasis of the ocular surface by regulating mucin secretion through activation of ALX/FPR2 and perhaps the DRV1 receptors. Activation of these receptors leads to an increase in [Ca2+]i and stimulation of secretion through stimulation of phospholipases -D, -C, and -A2, as well as the signaling intermediates ERK1/2 and Ca2+/CamK; thus, maintaining the mucin layer of the tear film that is critical for ocular surface health and disease prevention.
Acknowledgments
The authors thank Marie Shatos for her helpful discussions.
Supported by The Norwegian Research Council (ML; Oslo, Norway), National Institutes of Health Grants EY019470 (DAD), R01GM038765 (CNS), and P30 EY00379035 (Bethesda, MD, USA).
Disclosure: M. Lippestad, None; R.R. Hodges, None; T.P. Utheim, None; C.N. Serhan, None; D.A. Dartt, None
References
- 1. Hodges RR, Dartt DA. . Tear film mucins: front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucins. Exp Eye Res. 2013; 117: 62– 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mantelli F, Argueso P. . Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. 2008; 8: 477– 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dartt DA, Masli S. . Conjunctival epithelial and goblet cell function in chronic inflammation and ocular allergic inflammation. Curr Opin Allergy Clin Immunol. 2014; 14: 464– 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGilligan VE, Gregory-Ksander MS, Li D,et al. Staphylococcus aureus activates the NLRP3 inflammasome in human and rat conjunctival goblet cells. PLoS One. 2013; 8: e74010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Contreras-Ruiz L, Ghosh-Mitra A, Shatos MA, Dartt DA, Masli S. . Modulation of conjunctival goblet cell function by inflammatory cytokines. Mediators Inflamm. 2013; 2013: 636812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garcia-Posadas L, Hodges RR, Li D,et al. Interaction of IFN-gamma with cholinergic agonists to modulate rat and human goblet cell function. Mucosal Immunol. 2016; 9: 206– 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Serhan CN. . Novel pro-resolving lipid mediators in inflammation are leads for resolution physiology. Nature. 2014; 510: 92– 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Serhan CN, Chiang N. . Resolution phase lipid mediators of inflammation: agonists of resolution. Curr Opin Pharmacol. 2013; 13: 632– 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Serhan CN, Chiang N, Dalli J, Levy BD. . Lipid mediators in the resolution of inflammation. Cold Spring Harbor Perspect Biol. 2015; 7: a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. . Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol. 2014; 307: C39– C54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sasaki A, Fukuda H, Shiida N,et al. Determination of omega-6 and omega-3 PUFA metabolites in human urine samples using UPLC/MS/MS. Anal Bioanal Chem. 2015; 407: 1625– 1639. [DOI] [PubMed] [Google Scholar]
- 12. English JT, Norris PC, Hodges RR, Dartt DA, Serhan CN. . Identification and profiling of specialized pro-resolving mediators in human tears by lipid mediator metabolomics. Prostaglandins Leukot Essent Fatty Acids. 2017; 117: 17– 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCauley LK, Dalli J, Koh AJ, Chiang N, Serhan CN. . Cutting edge: parathyroid hormone facilitates macrophage efferocytosis in bone marrow via proresolving mediators resolvin D1 and resolvin D2. J Immunol. 2014; 193: 26– 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hodges RR, Li D, Shatos MA,et al. Lipoxin A4 activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion. Mucosal Immunol. 2017; 10: 46– 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dartt DA, Hodges RR, Li D, Shatos MA, Lashkari K, Serhan CN. . Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J Immunol. 2011; 186: 4455– 4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li D, Hodges RR, Jiao J,et al. Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol. 2013; 6: 1119– 1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krishnamoorthy S, Recchiuti A, Chiang N,et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010; 107: 1660– 1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. . Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol. 2012; 180: 2018– 2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M. . Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arterioscler Thromb Vasc Biol. 2012; 32: 1970– 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Migeotte I, Communi D, Parmentier M. . Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006; 17: 501– 519. [DOI] [PubMed] [Google Scholar]
- 21. Berridge MJ. . Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta. 2009; 1793: 933– 940. [DOI] [PubMed] [Google Scholar]
- 22. Shatos MA, Rios JD, Tepavcevic V, Kano H, Hodges R, Dartt DA. . Isolation, characterization, and propagation of rat conjunctival goblet cells in vitro. Invest Ophthalmol Vis Sci. 2001; 42: 1455– 1464. [PubMed] [Google Scholar]
- 23. Hodges RR, Li D, Shatos MA,et al. Lipoxin A4 activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion. Mucosal Immunol. 2017; 10: 46– 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodges RR, Li D, Shatos MA, Serhan CN, Dartt DA. . Lipoxin A4 counter-regulates histamine-stimulated glycoconjugate secretion in conjunctival goblet cells. Sci Rep. 2016; 6: 36124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shatos MA, Rios JD, Horikawa Y,et al. Isolation and characterization of cultured human conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003; 44: 2477– 2486. [DOI] [PubMed] [Google Scholar]
- 26. Grynkiewicz G, Poenie M, Tsien RY. . A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985; 260: 3440– 3450. [PubMed] [Google Scholar]
- 27. Hodges RR, Bair JA, Carozza RB, Li D, Shatos MA, Dartt DA. . Signaling pathways used by EGF to stimulate conjunctival goblet cell secretion. Exp Eye Res. 2012; 103: 99– 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. . 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002; 16: 1145– 1150. [DOI] [PubMed] [Google Scholar]
- 29. Luttrell LM, Daaka Y, Lefkowitz RJ. . Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999; 11: 177– 183. [DOI] [PubMed] [Google Scholar]
- 30. Kanno H, Horikawa Y, Hodges RR,et al. Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. Am J Physiol Cell Physiol. 2003; 284: C988– C998. [DOI] [PubMed] [Google Scholar]
- 31. Perez-Vilar J, Mabolo R, McVaugh CT, Bertozzi CR, Boucher RC. . Mucin granule intraluminal organization in living mucous/goblet cells. Roles of protein post-translational modifications and secretion. J Biol Chem. 2006; 281: 4844– 4855. [DOI] [PubMed] [Google Scholar]
- 32. Paz HB, Tisdale AS, Danjo Y, Spurr-Michaud SJ, Argueso P, Gipson IK. . The role of calcium in mucin packaging within goblet cells. Exp Eye Res. 2003; 77: 69– 75. [DOI] [PubMed] [Google Scholar]
- 33. Forstner G. . Signal transduction, packaging and secretion of mucins. Annu Rev Physiol. 1995; 57: 585– 605. [DOI] [PubMed] [Google Scholar]
- 34. Mitrovic S, Nogueira C, Cantero-Recasens G,et al. TRPM5-mediated calcium uptake regulates mucin secretion from human colon goblet cells. eLife. 2013; 2: e00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu R, Li Q, Zhou X, Perelman JM, Kolosov VP. . Annexin II mediates the neutrophil elastase-stimulated exocytosis of mucin 5ac. Mol Med Rep. 2014; 9: 299– 304. [DOI] [PubMed] [Google Scholar]
- 36. Kim YJ, Cho HJ, Shin WC, Song HA, Yoon JH, Kim CH. . Hypoxia-mediated mechanism of MUC5AC production in human nasal epithelia and its implication in rhinosinusitis. PLoS One. 2014; 9: e98136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Navabi N, Johansson ME, Raghavan S, Linden SK. . Helicobacter pylori infection impairs the mucin production rate and turnover in the murine gastric mucosa. Infect Immun. 2013; 81: 829– 837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hayashi D, Li D, Hayashi C, Shatos M, Hodges RR, Dartt DA. . Role of histamine and its receptor subtypes in stimulation of conjunctival goblet cell secretion. Invest Ophthalmol Vis Sci. 2012; 53: 2993– 3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chiang N, Serhan CN, Dahlen SE,et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006; 58: 463– 487. [DOI] [PubMed] [Google Scholar]
- 40. Zhu M, Wang X, Hjorth E,et al. Pro-resolving lipid mediators improve neuronal survival and increase Abeta42 phagocytosis. Mol Neurobiol. 2016; 53: 2733– 2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jin Y, Arita M, Zhang Q,et al. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Invest Ophthalmol Vis Sci. 2009; 50: 4743– 4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dogru M, Okada N, Asano-Kato N,et al. Atopic ocular surface disease: implications on tear function and ocular surface mucins. Cornea. 2005; 24: S18– S23. [DOI] [PubMed] [Google Scholar]
- 43. Mantelli F, Moretti C, Micera A, Bonini S. . Conjunctival mucin deficiency in complete androgen insensitivity syndrome (CAIS). Graefes Arch Clin Exp Ophthalmol. 2007; 245: 899– 902. [DOI] [PubMed] [Google Scholar]
- 44. Zhang J, Yan X, Li H. . Analysis of the correlations of mucins, inflammatory markers, and clinical tests in dry eye. Cornea. 2013; 32: 928– 932. [DOI] [PubMed] [Google Scholar]
- 45. Berry M, Pult H, Purslow C, Murphy PJ. . Mucins and ocular signs in symptomatic and asymptomatic contact lens wear. Optom Vis Sci. 2008; 85: E930– E938. [DOI] [PubMed] [Google Scholar]
- 46. Gipson IK, Hori Y, Argueso P. . Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf. 2004; 2: 131– 148. [DOI] [PubMed] [Google Scholar]
- 47. Duffield JS, Hong S, Vaidya VS,et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006; 177: 5902– 5911. [DOI] [PubMed] [Google Scholar]
- 48. Dmitrieva N, Suess G, Shirley R. . Resolvins RvD1 and 17(R)-RvD1 alleviate signs of inflammation in a rat model of endometriosis. Fertil Steril. 2014; 102: 1191– 1196. [DOI] [PubMed] [Google Scholar]
- 49. Luo B, Han F, Xu K,et al. Resolvin D1 programs inflammation resolution by increasing TGF-beta expression induced by dying cell clearance in experimental autoimmune neuritis. J Neurosci. 2016; 36: 9590– 9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang X, Qu X, Sun YB,et al. Resolvin D1 protects podocytes in adriamycin-induced nephropathy through modulation of 14-3-3beta acetylation. PLoS One. 2013; 8: e67471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xie W, Wang H, Wang L, Yao C, Yuan R, Wu Q. . Resolvin D1 reduces deterioration of tight junction proteins by upregulating HO-1 in LPS-induced mice. Lab Invest. 2013; 93: 991– 1000. [DOI] [PubMed] [Google Scholar]
- 52. Liu Y, Zhou D, Long FW,et al. Resolvin D1 protects against inflammation in experimental acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2016; 310: G303– G309. [DOI] [PubMed] [Google Scholar]
- 53. Gilbert K, Bernier J, Godbout R, Rousseau G. . Resolvin D1, a metabolite of omega-3 polyunsaturated fatty acid, decreases post-myocardial infarct depression. Mar Drugs. 2014; 12: 5396– 5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rogerio AP, Haworth O, Croze R,et al. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J Immunol. 2012; 189: 1983– 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang L, Wang CF, Serhan CN, Strichartz G. . Enduring prevention and transient reduction of postoperative pain by intrathecal resolvin D1. Pain. 2011; 152: 557– 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xu ZZ, Zhang L, Liu T,et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010; 16: 592– 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xu ZZ, Ji RR. . Resolvins are potent analgesics for arthritic pain. Br J Pharmacol. 2011; 164: 274– 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ji RR, Xu ZZ, Strichartz G, Serhan CN. . Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011; 34: 599– 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chiang N, Fredman G, Backhed F,et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012; 484: 524– 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, . Laniado Schwartzman M. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005; 280: 15267– 15278. [DOI] [PubMed] [Google Scholar]
- 61. Leedom AJ, Sullivan AB, Dong B, Lau D, Gronert K. . Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am J Pathol. 2010; 176: 74– 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gao Y, Min K, Zhang Y, Su J, Greenwood M, Gronert K. . Female-specific downregulation of tissue polymorphonuclear neutrophils drives impaired regulatory T cell and amplified effector T cell responses in autoimmune dry eye disease. J Immunol. 2015; 195: 3086– 3099. [DOI] [PMC free article] [PubMed] [Google Scholar]