Abstract
The bifunctional protein HtrA displays chaperone and protease activities, enabling bacteria to cope with environmental stress conditions such as heat shock or extreme pH by orchestrating protein folding or degradation. Recently, we added a novel aspect to HtrA functions by identifying HtrA of the human pathogen and class I carcinogen Helicobacter pylori (Hp) as a secreted virulence factor that cleaves the cell adhesion molecule and tumor suppressor E-cadherin. In this study, we analyzed the structural integrity and activity of oligomeric HtrA from Hp under stress conditions. Examining different parameters, HtrA oligomers were investigated by casein zymography and HtrA activity was further analyzed in in vitro cleavage assays using E-cadherin as a substrate. HtrA showed temperature-dependent disintegration of oligomers. Denaturing agents targeting hydrogen bonds within HtrA destabilized HtrA oligomers while reducing agents disrupting disulfide bonds had no effect. Optimal proteolytic activity was dependent on a neutral pH; however, addition of mono- and divalent salts or reducing agents did not interfere with proteolytic activity. These data indicate the HtrA is active under stress conditions which might support Hp colonizing in the gastric environment.
Keywords: HtrA, Helicobacter pylori, E-cadherin, oligomer stability
Introduction
In Gram-negative bacteria, high temperature requirement A (HtrA) is a periplasmic protein, displaying chaperone and serine protease activities in crucially important cellular processes like stress responses, protein quality control and protein maturation [1]. Upon removal of its N-terminal signal peptide, the 48 kDa HtrA consists of a trypsin-like protease domain and one or two C-terminal PDZ (Post synaptic density, Discs large, Zonula occludens-1) domains. The PDZ domains are involved substrate binding and mediate homo-oligomerization into large complexes consisting of up to 24 subunits composed of multitudes of HtrA trimers as basic subunits [1]. Escherichia coli expresses the three HtrA homologs DegP, DegQ and DegS of which DegP (protease Do) is the most characterized and well-studied protease. DegP forms hexameric oligomers which represent the inactive resting state of the protease that can be converted into active 12- and 24-mers by a temperature-dependent switch mechanism or upon recognition of unfolded substrates [2,3]. Structurally, reversible oligomer assembly and conversion of DegP is generated by a number of flexible loops in the protease domain and PDZ1 domain. Trimeric and hexameric structures are stabilized by loops L1, L2 and LA with the latter one protruding from an opposite HtrA monomer into the active center of the adjacent monomer, thus forcing the catalytic triad in an inactivated state [4,5]. Upon protease activation, the interplay of L1, L2 and LA is weakened to create flexibility for intermolecular rearrangement, leading to oligomerization. These newly formed complexes are now stabilized by a tight interaction of loop L3 from the protease domain with PDZ1 domain [4,5]. Among several relevant substrates identified for HtrA proteases, CpxP, α-amylase (MalS) and acylated precursor of Colicin A lysis protein have been described as targets for DegP [3,6,7].
Importantly, HtrA has been implicated to play a role in bacterial pathogenicity, by processing virulence factors and promoting cellular invasion or biofilm formation [8] which was mainly attributed to its periplasmic serine protease and chaperone functions. However, HtrA expressed by the pathogen Helicobacter pylori (Hp) was also extracellularly localized as an active secreted protease [9,10]. Additionally, HtrA was identified as a structure-bound Hp protein [11] indicating that HtrA might also be presented on the bacterial cell surface where it acts as an active protease. Recently, we identified a novel role of secreted HtrA in bacterial pathogenesis of the class-I carcinogen and human pathogen Hp [9,10]. In Hp infections, HtrA cleaves the extracellular domain of the adhesion molecule and tumor suppressor E-cadherin on infected epithelial host cells, thus allowing Hp to subvert epithelial barrier functions and to transmigrate across epithelial monolayers [12].
Hp is a Gram-negative, microaerophilic bacterium that colonizes the human gastric mucosa of about 50% of the world’s population leading to development of severe chronic gastritis and its sequelae such as gastric adenocarcinoma and MALT (mucosa-associated lymphoid tissue) lymphoma [13,14]. The gastric environment is absolutely unique and represents a hostile niche preventing persistence of other pathogens. Thus, Hp needs highly sophisticated mechanisms for adaption allowing colonization at low pH or high salt concentrations. Hp expresses factors such as urease and amidases to locally increase the gastric pH, a finely tuned pH sensing system ArsS and flagella for ensuring bacterial motility inside the mucus layer [15,16].
In this study, we analyzed the stability and activity of recombinant Hp HtrA oligomers in response to different stress conditions. In zymography and in vitro cleavage assays, HtrA activity was tested at different temperatures, pH, salt concentrations and reducing as well as denaturing conditions to obtain more information on the functional role of HtrA in maintaining protein structure and function during Hp infections.
Methods
Expression and purification of recombinant HtrA
Cloning of Hp HtrA into the pGEX-6P-1 plasmid (GE Healthcare Life Sciences) has been previously described [10]. For overexpression and purification, transformed E. coli BL21 was grown in TB medium and the expression of GST-HtrA was induced by 0.1 mM isopropylthiogalactosid (IPTG) for 3 h. After centrifugation at 4000 x g for 30 min, bacteria were lysed in PBS by sonification. The lysate was then incubated with glutathione sepharose (GE Healthcare Life Sciences) at 4°C overnight. Finally, the fusion protein GST-HtrA was cleaved with 20 U Prescission protease (GE Healthcare Life Sciences) per mg fusion protein for 16 h at 4°C to remove the GST tag. Protein concentrations were determined by Bradford assay (Lactan Chemikalien).
In vitro cleavage assays, SDS PAGE and Western blotting
In vitro cleavage assays have been described earlier [12]. Here, 250 ng recombinant HtrA was incubated with 50 ng recombinant E-cadherin or 100 ng casein in 50 mM Hepes pH 7.5 for 4 h at 37°C unless otherwise stated. Where indicated, 50 mM dithiothreitol (DTT), 5% (v/v) β-mercaptoethanol (β-ME), 10 mM NaCl, 10 mM KCl, 10 mM CaCl2, 10 mM MgCl2, or 10 mM MnCl2 were added. Reactions were stopped by boiling in SDS sample buffer. Proteins were separated by SDS PAGE followed by Western Blot analysis using the ChemiDoc Imager (BioRad). Proteins were detected using specific antibodies against HtrA [12], against the extracellular domain of E-cadherin (H108, Santa Cruz) or casein (Acris). Where indicated, Western blots were quantified using the ChemiDoc XRS system (BioRad). All experiments were repeated at least three times.
Oligomer stability assays and zymography
100 ng HtrA were dissolved in 50 mM Hepes pH 7.5 and either incubated at indicated temperatures, for different time periods, or in the presence of 1-6 M urea, and guanidine hydrochloride (GHC). Then, non-reducing SDS sample buffer without β-mercaptoethanol was added to the reaction, which was subsequently used for zymography [10]. Samples were separated by a SDS PAGE containing 0.1% casein (Invitrogen) under non-reducing conditions. Afterwards, the gel was renatured in 2.5% Triton-X-100 for 2 x 30 minutes and subsequently equilibrated in developing buffer (50 mM Tris-HCl, pH 7.4, 200 mM NaCl, 5 mM CaCl2, 0.02% Brij35) to refold proteins at 37 °C for 16 h under gentle agitation. Caseinolytic activity in zymograms was visualized by staining with 0.5% Coomassie Blue R250. To detect oligomers by SDS PAGE, HtrA was separated as described for zymograms excluding casein. All experiments were repeated at least three times.
Results
The gastric stomach is an extreme niche unique for colonizing Hp. The functionality of the serine protease and chaperone HtrA might contribute to persistent infection of Hp. Hence, we investigated the integrity and activity of recombinant HtrA targeting casein or E-cadherin as protease substrates under different stress conditions in this study.
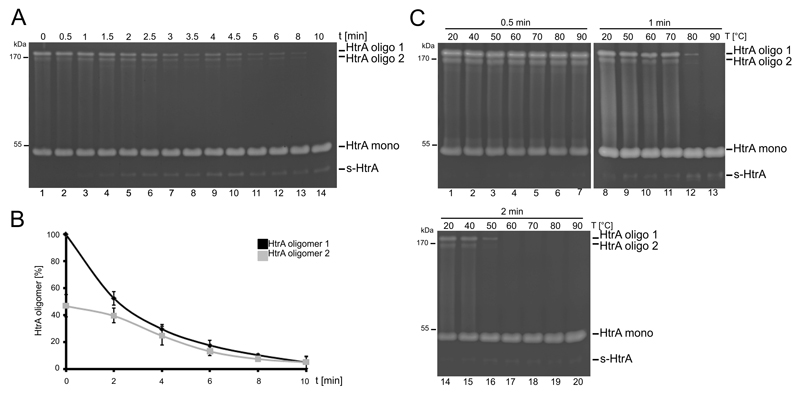
The influence of different temperatures on the integrity of HtrA oligomers was analyzed by boiling recombinant HtrA in Hepes buffer at 95°C for the indicated time periods prior to zymography (Fig. 1A). Untreated HtrA migrated as 45 kD and >170 kD proteins (Fig. 1A, lane 1) which has been identified as monomeric and oligomeric HtrA in our previous study [10]. Incubation at 95°C for approximately 1.5 minutes had only marginal effects on the activity of the HtrA oligomer und monomer. However, extension of boiling time to 10 minutes strongly decreased the activity of HtrA oligomers, while the amount of active HtrA monomers increased, as expected (Fig. 1A). However, even after 8 minutes boiling at 95°C we still observed a little amount of HtrA oligomers suggesting that HtrA oligomers are relatively stable under those conditions. This is supported by the observation that monomeric HtrA could be easily renatured within the SDS gel during zymography procedure (Fig. 1A). We also reproducibly detected two oligomeric forms of Hp HtrA, which might be the result of aggregation of different numbers of HtrA trimers. To monitor the activity of HtrA oligomer 1 and 2, discovered proteolytic activities in zymograms were quantified (Fig. 1B). A ratio of 40% HtrA oligomer 2 to 60% HtrA oligomer 1 was determined in untreated sample preparations (Fig 1B). 50% of both oligomer populations were denatured after approximately 2 minutes at 95°C and after 10 minutes nearly no oligomers could be detected (Fig 1B). However, we also found a short HtrA form (s-HtrA), which resulted from intramolecular autocleavage [10]. During boiling, the amount of s-HtrA increased suggesting that oligomer 1 and/or 2 also contain s-HtrA (Fig. 1A). Another plausible explanation for the increase of Hp s-HtrA might be given by the observation that after oligomer disintegration excessive monomeric HtrA is removed by autocleavage [17].
Figure 1. Thermo-stability and activity of HtrA oligomers.
(A) 100 ng recombinant HtrA was incubated for indicated time periods (0-10 min) at 95°C and proteolytic activity of HtrA oligomers (HtrA oligo 1 and HtrA oligo 2) and monomers (HtrA mono and s-HtrA) was analyzed by casein zymography. (B) Quantification of active HtrA oligomers 1 ( ) and 2 (
) and 2 ( ) from three independent experiments. (C) 100 ng recombinant HtrA was incubated for 0.5, 1 and 2 minutes at indicated temperatures in the presence of non-reducing sample buffer and analyzed by casein zymography.
) from three independent experiments. (C) 100 ng recombinant HtrA was incubated for 0.5, 1 and 2 minutes at indicated temperatures in the presence of non-reducing sample buffer and analyzed by casein zymography.
To explore the thermostability in more detail, HtrA was incubated at different temperatures for the indicated time periods in non-reducing SDS sample buffer instead of Hepes buffer to avoid possible refolding prior to zymography analyses (Fig. 1C). Temperatures between 20°C to 90°C for 0.5 minutes did not affect HtrA oligomer activity (Fig. 1C, upper left panel). However, incubation of HtrA at 80°C for 1 minute drastically decreased oligomerization of HtrA (Fig. 1C, upper right panel). This effect was further potentiated after treating HtrA for 2 minutes at indicated temperatures as 50°C efficiently disrupted HtrA oligomer 1 and oligomer 2 (Figure 1C, lower panel).
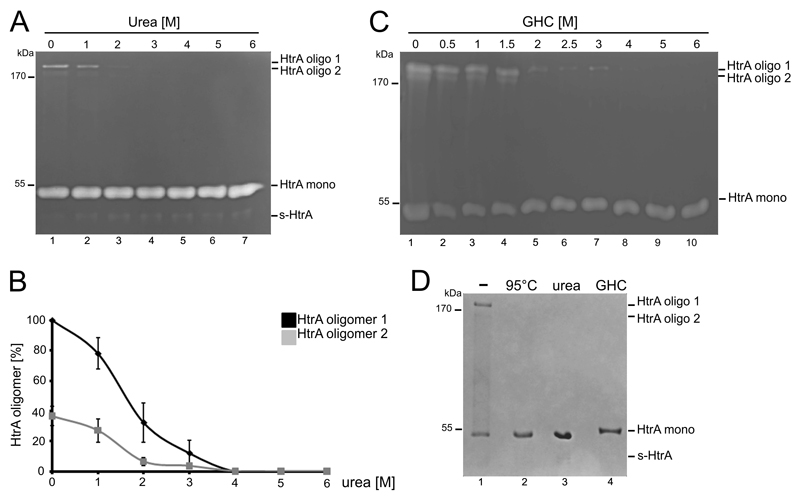
Protein structures and oligomeric assembly are determined by non-covalent forces within the molecule which can be disrupted using high concentrations of urea or guanidine hydrochloride (GHC). HtrA oligomer stability was initially tested using increasing concentrations of urea. Addition of 1 M urea slightly decreased HtrA oligomers, while 2-3 M urea was sufficient to destabilize HtrA completely. Conversely, the amount of monomeric HtrA and s-HtrA increased (Fig. 2A). The intensities of proteolytic activity of HtrA oligomer 1 and 2 are shown in Fig. 2B. Similar results were obtained by incubating HtrA with GHC (Fig 2C). Using 0.5-1.5 M GHC, HtrA oligomer stability was only slightly affected (Fig. 2C, lanes 1-4). However, incubation of HtrA with 2-3 M GHC partially destabilized HtrA oligomers while higher concentrations of GHC completely abolished HtrA oligomerization (Fig. 2C, lanes 5-10). To verify that urea and GHC do not only target oligomer activity, but oligomerization, HtrA assembly into oligomers was verified in SDS PAGEs under non-reducing conditions. Monomers and oligomeric complexes of HtrA were detected in untreated samples. Upon prolonged boiling or treatment with 6 M denaturing urea or GHC, oligomers were destroyed and HtrA enriched in the monomer fraction. A faint band of s-HtrA was also detectible (Fig. 2D).
Figure 2. Stability and activity of HtrA oligomers under denaturing conditions.
(A) 100 ng recombinant Hp HtrA was incubated with increasing concentrations of urea for 10 minutes and analyzed by casein zymography (B). Quantification of HtrA oligomers 1 ( ) and 2 (
) and 2 ( ) from five independent experiments. (C) 100 ng recombinant HtrA was incubated with increasing concentrations of guanidine hydrochloride (GHC) for 10 minutes and analyzed by casein zymography. (D) 1 µg recombinant HtrA was boiled for 10 minutes at 95°C or incubated with 6 M urea or GHC for 10 minutes, separated under non-reducing conditions by SDS PAGE and stained with Coomassie blue G250.
) from five independent experiments. (C) 100 ng recombinant HtrA was incubated with increasing concentrations of guanidine hydrochloride (GHC) for 10 minutes and analyzed by casein zymography. (D) 1 µg recombinant HtrA was boiled for 10 minutes at 95°C or incubated with 6 M urea or GHC for 10 minutes, separated under non-reducing conditions by SDS PAGE and stained with Coomassie blue G250.
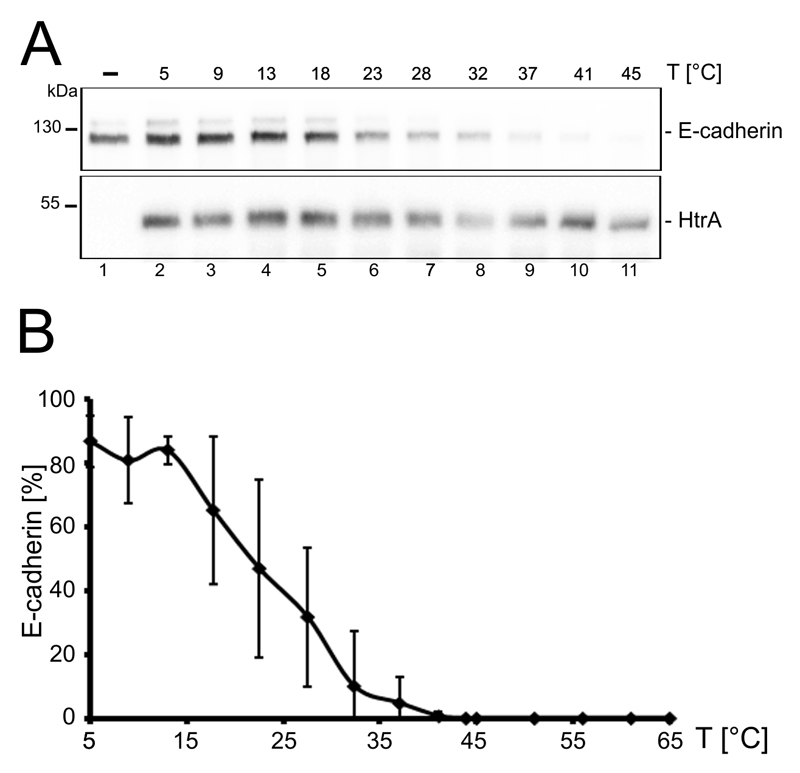
In our previous work, we identified HtrA-mediated ectodomain shedding of E-cadherin as an important step in Hp pathogenesis allowing bacterial entry into the intracellular space of epithelial cells upon cleavage [12]. Hence, E-cadherin was included in this study to investigate a more physiological aspect of HtrA function. In in vitro cleavage assays HtrA activity was analyzed using E-cadherin as a substrate for 4 h at different temperatures. Loss of full length E-cadherin detected by Western blot analyses reliably monitored HtrA-mediated E-cadherin cleavage (Fig. 3A) as previously demonstrated [12,18]. Additionally, the quantity of full length E-cadherin was determined, normalized to HtrA amounts and presented as a graph (Fig. 3B). Low temperatures between 5°C to 13°C inhibited HtrA cleavage activity (Fig. 3A, lanes 1-5, Fig. 3B), while increased temperatures to 37°C efficiently enhanced HtrA activity (Fig. 3A, lanes 6-9, Fig. 3B). Remarkably, HtrA was still active at 65°C (Fig. 3B). Extreme temperatures were also tested, but proteins were not stable for 4 h at temperatures higher than 65°C (data not shown). Half maximal activity was observed at approximately 22°C (Fig 3B).
Figure 3. Temperature-dependent HtrA activity using E-cadherin as a substrate.
(A) Recombinant E-cadherin was incubated with HtrA for 4 h at different temperatures as indicated. Cleavage of full length E-cadherin was analyzed by Western blot analysis detecting the extracellular domain of E-cadherin using a specific antibody (upper panel). To demonstrate equal HtrA amounts, blots were reprobed with an antibody recognizing HtrA (lower panel). (B) Western blots of three independent experiments were quantified and HtrA activity was displayed as the ratio of full length E-cadherin normalized to HtrA amounts.
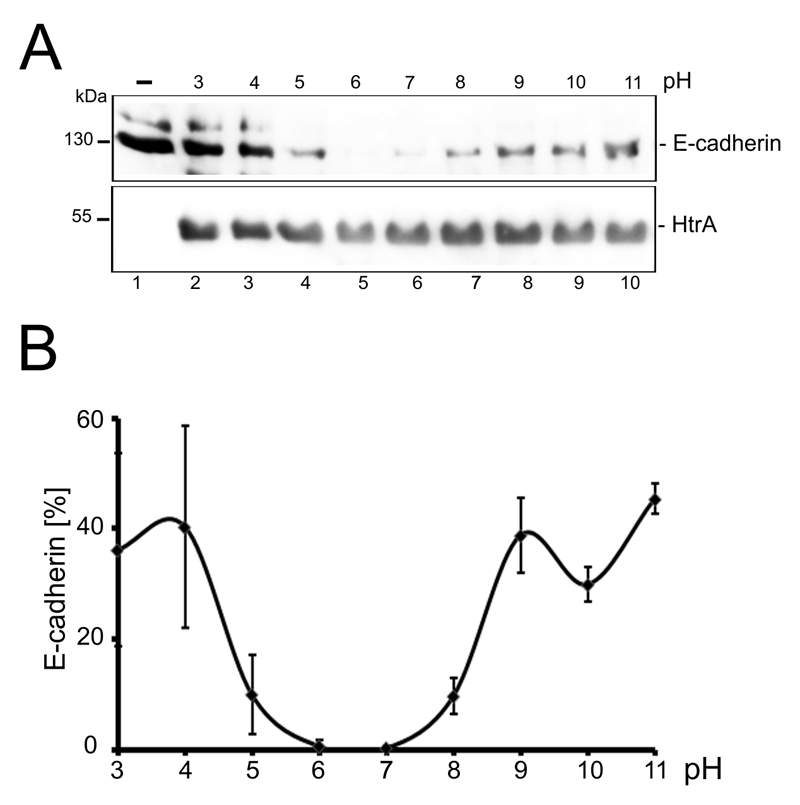
The pH in the gastric stomach ranges from highly acidic (pH 1-2) in the gastric juice to pH ~7 at the mucosal lining [19]. Therefore, the influence of different pHs on HtrA-mediated E-cadherin cleavage was analyzed by incubating HtrA with E-cadherin for 4 h at 37°C in Hepes buffers with pHs ranging from 3 to 11 (Fig 4A). Western Blots of more than 3 independent experiments were quantified and compared to an untreated E-cadherin control (Fig 4B). HtrA showed the strongest activity between pH 6 and 7 (Fig 4A and 4B) indicating an optimal pH in the neutral range. However, HtrA also tolerated extreme pH values from pH 3-11 resulting in a decrease of approximately 40% of full length E-cadherin (Fig. 4B).
Figure 4. pH-dependent HtrA activity.
(A) Recombinant E-cadherin was incubated with HtrA in Hepes buffer ranging a pH from 3 to 11 for 4 h. Cleavage of full length E-cadherin was analyzed by Western blot analysis detecting the extracellular domain of E-cadherin using a specific antibody (upper panel). To demonstrate equal HtrA amounts, blots were reprobed with an antibody recognizing HtrA (lower panel). (B) Western blots of three independent experiments were quantified and HtrA activity was displayed as the ration of full length E-cadherin normalized to HtrA amounts.
Dithiothreitol (DTT) and beta-mercaptoethanol (β-ME) represent commonly used reducing agents for testing the structural influence by disulfide bonds on protein functionality. For E. coli DegP, the formation of a disulfide bond between C83 and C95 has been proposed to increase oligomer stability and localization to the inner periplasmic membrane [17]. Reducing substances with final concentrations of 25 mM DTT or 5% (v/v) (β-ME) were tested on HtrA-mediated E-cadherin cleavage in vitro for 4 h at 37°C (Fig 5A). Neither DTT nor β-ME influenced HtrA-mediated E-cadherin cleavage, implying that disulfide bonds were not essential for HpHtrA activity (Fig 5A, lanes 3-4) and oligomerization (Fig. 5B). HtrA oligomers were detectable after incubation with DTT as well as β-ME, but not after boiling at 95°C (Fig. 5B).
Figure 5. HtrA-mediated E-cadherin cleavage under reducing or high salt conditions.
(A) In vitro cleavage of recombinant E-cadherin using HtrA for 4 h under reducing conditions. Final concentrations of 25 mM of DTT and 5 % (v/v) β-mercaptoethanol (β-ME) were used. Cleavage of full length E-cadherin was analyzed by Western blot analysis detecting the extracellular domain of E-cadherin using a specific antibody (upper panel). To demonstrate equal HtrA amounts, blots were reprobed with an antibody recognizing HtrA (lower panel). (B) To demonstrate the integrity of HtrA oligomers, 1 µg recombinant HtrA was incubated for 10 minutes at 95°C or in presence of 50 mM DTT or 5% (v/v) β-ME, separated under non-reducing conditions by SDS PAGE and HtrA monomers and oligomers were stained by Coomassie blue G250. (C) In in vitro cleavage experiments using E-cadherin or casein as substrates for HtrA for 4 h was performed in presence of either 10 mM NaCl, KCl, CaCl2, MgCl2, or MnCl2. Cleavage of full length E-cadherin or casein was analyzed by Western blot analysis using specific antibody detecting extracellular domain of E-cadherin or casein. To demonstrate equal HtrA amounts, blots were reprobed with an antibody recognizing HtrA.
The gastric salt concentration might also influence the functionality of HtrA since it has been shown for HtrA from cyanobacteria that calcium increases the protease activity [20]. To determine the impact of salt on HtrA activity, 10 mM chloride salts of sodium (Na), potassium (K), calcium (Ca), magnesium (Mg) and manganese (Mn) were added to the protease reaction for 4 h at 37°C. Interestingly, monovalent cations did not influence E-cadherin cleavage efficiency (Fig 5C, lanes 3-4), whereas no or only partial degradation was observed using divalent ions (Fig 5C, lanes 5-7). This might be explainable that calcium ions might stabilize E-cadherin presumably leading to a certain kind of resistance against proteolytic cleavage [21]. However, it is well established that DegP and its homologue DegQ are fully active in presence of various ions [22,23] which could be confirmed for Hp HtrA by using casein, as alternative substrate that was efficiently degraded in the presence of all ions tested (Fig 5C, lanes 3-7).
Discussion
Without treatment with antibiotics, Hp persists life-long in the human stomach where it can induce severe gastric disorders. This is facilitated by the coordinated action of several virulence factors allowing colonizing under stress conditions. Among those factors, flagella for the bacterial motility or the enzyme urease to neutralize the gastric acid are important for persistent infections with Hp [24]. The chaperone and serine protease HtrA cleaves the cell adhesion protein E-cadherin in host cells enabling the bacteria to enter the intercellular space of the epithelium [12] indicating it is exposed to the gastric environment. Investigation whether HtrA could contribute to survival and pathogenesis in this hostile gastric niche is difficult because, in contrast to many other bacteria, HtrA expression is absolutely essential for Hp [12,25] supporting our hypothesis that it evolutionary developed properties to undertake crucially important functions in pathogenesis. In this study, we analyzed the influence of several parameters to define physiologic properties of Hp HtrA and we found that HtrA tolerates denaturing conditions, extreme pH values, high salt concentrations, and revealed a highly thermostable integrity that might contribute to Hp’s adaption to its gastric niche on a molecular level.
HtrA-mediated E-cadherin cleavage was highly efficient at physiological and high temperatures and pH 5 - 8, which is a broader range of activity than reported for other HtrAs [22,23]. Possibly, owing to the fact that HtrA is the only Deg homolog in Hp, the molecule might have adopted functions of DegQ which has a pH optimum at 5.5, which is significantly lower than that observed for DegP [26]. In contrast, the pH optimum of DegP and mycobacterial HtrA-like proteases has been found to be at less acidic conditions between pH 7 and 9 [23,27]. There are studies available, analyzing Hp viability at extreme pH or high temperatures [28]. Further it is known, that two other species from the genus Helicobacter, H. heilmannii and H. mustelae, also tolerate temperatures to 42 °C [29]. Additionally, H. heilmannii is able to colonize gastric parietal cells, which are responsible for acid production [30] implying specific adaptions of the genus to their respective hosts. Whether HtrA expression contributes to those adaptations needs to be investigated in future.
In our study, reducing conditions and elevated concentrations of mono- and divalent ions did not interfere with HtrA activity that has been confirmed for other HtrAs [23]. Interestingly, contrary to the HtrA substrate casein, divalent ions obviously enhanced E-cadherin resistance against proteolytic cleavage, which is contrast to other studies on HtrA functions [22,23]. This could be explained by the effect of calcium on the substrate E-cadherin, which contains binding sites for calcium important for dimerization [21] making E-cadherin inaccessible for the active center of HtrA.
Chemicals reducing disulfide bonds had neither an impact on HtrA activity or on the E-cadherin substrate recognition. On the one hand this is obviously due to the lack of cysteines within the HtrA molecule. On the other hand misfolded E-cadherin as a substrate might have a higher accessibility for the active pocket of HtrA. Although HpHtrA does not form disulfite bonds, it generates remarkably stable oligomers.
In contrast to reducing agents, disrupting hydrophobic contacts and hydrogen bonds strongly affected HtrA protease activity presumably through disrupting oligomers. Oligomeric HtrA complexes have been attributed to encapsulate substrates either for degradation, transport or protection against unfolding [1]. Interplay between loop L3 and PDZ1 has been shown to scaffold multimeric associations. However, molecular details of oligomeric rigidity under elevated temperatures and highly unfolding conditions have not been analyzed so far for HpHtrA.
In line with general functions of HtrA under cellular stress conditions, HpHtrA has been recently shown to be upregulated upon oxidative stress [31] and in accordance with this, HtrA expression was 2.5-4 fold upregulated upon environmental acidification [32]. This observation is interesting since it suggests that HtrA secretion plays an active role in bacterial survival under harsh conditions. In conclusion, Hp HtrA displays some remarkably unique physicochemical features, compared to DegP, which might be explainable with the different life styles of E. coli and Hp, the adaption to colonize their respective niche and, moreover, due to the fact that Hp HtrA seems to exert functions additional to “classical” stress response regulators, by being a secreted virulence factor.
Acknowledgements
We thank Philip Wurm from the Goethe University Frankfurt for scientific advices and critical reading of the manuscript.
Abbreviations
- HtrA
High temperature requirement A
- Hp
Helicobacter pylori
Footnotes
Conflict of interest
All authors read and approved the final manuscript. The authors declare that they have no competing interests.
References
- [1].Clausen T, Kaiser M, Huber R, Ehrmann M. HTRA proteases: regulated proteolysis in protein quality control. Nat Rev Mol Cell Biol. 2011;12:152–162. doi: 10.1038/nrm3065. [DOI] [PubMed] [Google Scholar]
- [2].Krojer T, Sawa J, Schafer E, Saibil HR, Ehrmann M, et al. Structural basis for the regulated protease and chaperone function of DegP. Nature. 2008;453:885–890. doi: 10.1038/nature07004. [DOI] [PubMed] [Google Scholar]
- [3].Spiess C, Beil A, Ehrmann M. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell. 1999;97:339–347. doi: 10.1016/s0092-8674(00)80743-6. [DOI] [PubMed] [Google Scholar]
- [4].Sawa J, Heuck A, Ehrmann M, Clausen T. Molecular transformers in the cell: lessons learned from the DegP protease-chaperone. Curr Opin Struct Biol. 2010;20:253–258. doi: 10.1016/j.sbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- [5].Krojer T, Sawa J, Huber R, Clausen T. HtrA proteases have a conserved activation mechanism that can be triggered by distinct molecular cues. Nat Struct Mol Biol. 2010;17:844–852. doi: 10.1038/nsmb.1840. [DOI] [PubMed] [Google Scholar]
- [6].Isaac DD, Pinkner JS, Hultgren SJ, Silhavy TJ. The extracytoplasmic adaptor protein CpxP is degraded with substrate by DegP. Proc Natl Acad Sci USA. 2005;102:17775–17779. doi: 10.1073/pnas.0508936102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cavard D, Lazdunski C, Howard SP. The acylated precursor form of the colicin A lysis protein is a natural substrate of the DegP protease. J Bacteriol. 1989;171:6316–6322. doi: 10.1128/jb.171.11.6316-6322.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ingmer H, Brondsted L. Proteases in bacterial pathogenesis. Res Microbiol. 2009;160:704–710. doi: 10.1016/j.resmic.2009.08.017. [DOI] [PubMed] [Google Scholar]
- [9].Bumann D, Aksu S, Wendland M, Janek K, Zimny-Arndt U, et al. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect Immun. 2002;70:3396–3403. doi: 10.1128/IAI.70.7.3396-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Löwer M, Weydig C, Metzler D, Reuter A, Starzinski-Powitz A, et al. Prediction of extracellular proteases of the human pathogen Helicobacter pylori reveals proteolytic activity of the Hp1018/19 protein HtrA. PLoS One. 2008;3:e3510. doi: 10.1371/journal.pone.0003510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Backert S, Kwok T, Schmid M, Selbach M, Moese S, et al. Subproteomes of soluble and structure-bound Helicobacter pylori proteins analyzed by two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2005;5:1331–1345. doi: 10.1002/pmic.200401019. [DOI] [PubMed] [Google Scholar]
- [12].Hoy B, Lower M, Weydig C, Carra G, Tegtmeyer N, et al. Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO Rep. 2010;11:798–804. doi: 10.1038/embor.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321–333. doi: 10.1172/JCI20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Peek RM, Jr, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233–248. doi: 10.1002/path.1868. [DOI] [PubMed] [Google Scholar]
- [15].Zanotti G, Cendron L. Functional and structural aspects of Helicobacter pylori acidic stress response factors. IUBMB Life. 2010;62:715–723. doi: 10.1002/iub.382. [DOI] [PubMed] [Google Scholar]
- [16].Sachs G, Weeks DL, Melchers K, Scott DR. The gastric biology of Helicobacter pylori. Annu Rev Physiol. 2003;65:349–369. doi: 10.1146/annurev.physiol.65.092101.142156. [DOI] [PubMed] [Google Scholar]
- [17].Skorko-Glonek J, Zurawa D, Tanfani F, Scire A, Wawrzynow A, et al. The N-terminal region of HtrA heat shock protease from Escherichia coli is essential for stabilization of HtrA primary structure and maintaining of its oligomeric structure. Biochim Biophys Acta. 2003;1649:171–182. doi: 10.1016/s1570-9639(03)00170-5. [DOI] [PubMed] [Google Scholar]
- [18].Lower M, Geppert T, Schneider P, Hoy B, Wessler S, et al. Inhibitors of Helicobacter pylori Protease HtrA Found by 'Virtual Ligand' Screening Combat Bacterial Invasion of Epithelia. PLoS One. 2011;6:e17986. doi: 10.1371/journal.pone.0017986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Forssell H. Gastric mucosal defence mechanisms: a brief review. Scand J Gastroenterol Suppl. 1988;155:23–28. doi: 10.3109/00365528809096277. [DOI] [PubMed] [Google Scholar]
- [20].Huesgen PF, Miranda H, Lam X, Perthold M, Schuhmann H, et al. Recombinant Deg/HtrA proteases from Synechocystis sp. PCC 6803 differ in substrate specificity, biochemical characteristics and mechanism. Biochem J. 2011;435:733–742. doi: 10.1042/BJ20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pokutta S, Herrenknecht K, Kemler R, Engel J. Conformational changes of the recombinant extracellular domain of E-cadherin upon calcium binding. Eur J Biochem. 1994;223:1019–1026. doi: 10.1111/j.1432-1033.1994.tb19080.x. [DOI] [PubMed] [Google Scholar]
- [22].Lipinska B, Zylicz M, Georgopoulos C. The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J Bacteriol. 1990;172:1791–1797. doi: 10.1128/jb.172.4.1791-1797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang WW, Sun K, Cheng S, Sun L. Characterization of DegQVh, a serine protease and a protective immunogen from a pathogenic Vibrio harveyi strain. Appl Environ Microbiol. 2008;74:6254–6262. doi: 10.1128/AEM.00109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wroblewski LE, Peek RM, Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Salama NR, Shepherd B, Falkow S. Global transposon mutagenesis and essential gene analysis of Helicobacter pylori. J Bacteriol. 2004;186:7926–7935. doi: 10.1128/JB.186.23.7926-7935.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sawa J, Malet H, Krojer T, Canellas F, Ehrmann M, et al. Molecular adaptation of the DegQ protease to exert protein quality control in the bacterial cell envelope. J Biol Chem. 2011;286:30680–30690. doi: 10.1074/jbc.M111.243832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ribeiro-Guimaraes ML, Marengo EB, Tempone AJ, Amaral JJ, Klitzke CF, et al. Cloning, expression and characterisation of an HtrA-like serine protease produced in vivo by Mycobacterium leprae. Mem Inst Oswaldo Cruz. 2009;104:1132–1138. doi: 10.1590/s0074-02762009000800010. [DOI] [PubMed] [Google Scholar]
- [28].Velazquez M, Feirtag JM. Helicobacter pylori: characteristics, pathogenicity, detection methods and mode of transmission implicating foods and water. Int J Food Microbiol. 1999;53:95–104. doi: 10.1016/s0168-1605(99)00160-9. [DOI] [PubMed] [Google Scholar]
- [29].Andersen LP, Boye K, Blom J, Holck S, Norgaard A, et al. Characterization of a culturable “Gastrospirillum hominis” (Helicobacter heilmannii) strain isolated from human gastric mucosa. J Clin Microbiol. 1999;37:1069–1076. doi: 10.1128/jcm.37.4.1069-1076.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Heilmann KL, Borchard F. Gastritis due to spiral shaped bacteria other than Helicobacter pylori: clinical, histological, and ultrastructural findings. Gut. 1991;32:137–140. doi: 10.1136/gut.32.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huang CH, Chiou SH. Proteomic analysis of upregulated proteins in Helicobacter pylori under oxidative stress induced by hydrogen peroxide. Kaohsiung J Med Sci. 2011;27:544–553. doi: 10.1016/j.kjms.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Merrell DS, Goodrich ML, Otto G, Tompkins LS, Falkow S. pH-regulated gene expression of the gastric pathogen Helicobacter pylori. Infect Immun. 2003;71:3529–3539. doi: 10.1128/IAI.71.6.3529-3539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]