Abstract
This study evaluated lumbar spine muscle volume and Muscle Fatty Infiltrate (MFI) across two age groups of healthy adults. Twenty-four participants (young group - YG: age 18-25, n = 12; mature group - MG: age 45-60, n = 12) without low back pain underwent T1-weighted axial MRI. Muscle volume and MFI were obtained from the left and right lumbar erector spinae (ES), multifidus (M), rectus abdominis (RA) and psoas (PS) muscles. For MFI, mean pixel intensity (MPI) of muscles was reported as a percentage of subcutaneous fat MPI. Within-group comparison of left and right side muscle volume was not significantly different in the YG. In the MG, right RA and ES were significantly smaller than left (RA p = 0.049; ES p = 0.03). In both groups, left PS, M and ES MFI was significantly smaller compared to the right side and left RA MFI was significantly greater compared to right side (all p <= 0.001). For M volume, 81.7-84.6% of variance was explained by age, height and Body Mass Index (BMI). For ES volume, 81.6-82.8% of variance was explained by height and BMI. Age explained 18.1%-36.0% of variance in M and ES right MFI. Therefore, age and BMI are relevant factors for extensor muscle volume, but not for flexor muscle volume. Also, age significantly influences MFI for right-sided extensors only. The age effect is apparently independent of full subjective back functionality. For future spinal muscle research, the side and muscle-specific effect of age on muscle morphology should be considered.
Keywords: MRI, Muscle volume, Fatty infiltration, Lumbar spine
Introduction
As muscle atrophy is known to occur with ageing due to a reduction in the number and size of muscle fibres [1,2], the effect of age on lumbar muscle CSA has been investigated in healthy individuals and in patients with LBP [3–8]. The majority of these studies reported a significant effect of age on CSA [4–8], although one LBP study did not [4]. In patients with LBP, extensor muscle CSA has received a lot of attention as atrophy and side to side asymmetry have been identified [6,8–11].
Increased MFI of the spinal muscles has also been associated with ageing and with LBP [9,12,13]. However, measures of MFI in the lumbar muscles have produced inconsistent results [11]. Some studies have demonstrated an association with LBP [6,13], whilst others showed no association [14]. Such discrepancies could be related to disparate measures of MFI quantification with MRI or even varying levels of pain-related disability among the population of subjects. Only two studies were found where the effect of age on lumbar MFI was investigated, with one study showing modest and inconsistent effects of age on muscle composition [6], while in the other study age was associated with multifidus MFI at L3-L4 level [8]. Therefore, the effect of age on lumbar spinal morphometry warrants further investigation.
The relationship between cervical muscle morphometry, age and symptoms of neck pain has been investigated. Following whiplash, an increase in CSA in the anterior and posterior neck muscles was demonstrated [15,16] while no such association was found in other studies [17,18]. In the subjects with increased CSA [15,16], age was inversely related to MFI [16] while in asymptomatic females, age was not found to influence MFI in the upper cervical extensor muscles [19].
Long-term prospective studies across the age spectrum detailing lumbar spine muscle changes over time are limited in number, as are cross sectional studies which investigate the effect of age. Although Fortin and colleagues [6,8] investigated a comprehensive set of factors which may affect lumbar spine muscle asymmetry including age, they only included male participants and muscle assessment was limited to the lumbar spine extensors. Other studies which investigated the effects of age on lumbar spine muscle morphometry only reported CSA and not MFI [3,5,7]. Furthermore, only a small number of studies reported the effects of age on lumbar flexor muscle morphology [3,5]. Going forward, muscle morphology of a comprehensive set of lumbar muscles across different age groups in a healthy male and female adult population is required. As lumbar muscle asymmetry occurs naturally in healthy adults [20], outcomes for the left and right sides should be reported separately. While most of the studies on muscle morphometry use CSA as a measure, the use of several adjacent CSAs as volumetric information is preferable as it is more closely related to muscle function [21]. Therefore the purpose of this study was to evaluate lumbar spine muscle volume and MFI across two age groups of healthy adults, using a standard and widely available T1-weighted MRI sequence. Furthermore, a between-side comparison of bilateral measurements was conducted.
Materials and Methods
Participants
Axial MRI scans of 24 healthy (12 male and 12 female) participants who were recruited as part of a larger study were included. Participants were conveniently recruited from a University population and by word of mouth, and were included if they were either 18-25 (young group) or 45-60 (mature group) years of age, had a Body Mass Index (BMI) of 25 or less (thus not classified as being overweight), and did not have current low back pain or a history of back pain in the last 12 months, previous spinal surgery or spinal fracture, neurological or orthopaedic disease, or open abdominal surgery. Participants were excluded if they were determined, by institutional standards, to be unsuitable for undergoing an MRI exam, for example due to implants which are non-compatible with MRI. All participants received a participant information sheet and gave their written informed consent. Ethical approval for this study was given by the Medical University of Vienna Ethics Committee (1609/2012).
Data collection
Participant age, height, weight, BMI, upper and lower limb dominance and general information regarding weekly exercise were collected for each participant. Lumbar spine axial T1-weighted magnetic resonance images were obtained (1.5T Philips Achieva, Best, The Netherlands), using a slice thickness of 10mm, gap 1mm, repetition time (TR) of 9.3ms, echo time (TE) of 4.6ms, flip angle 15 degrees, field of view (FOV) 345mm, and rectangular FOV of 78%. Participants were positioned supine in the magnetic bore. Images were stored as DICOM format for processing.
Data analysis
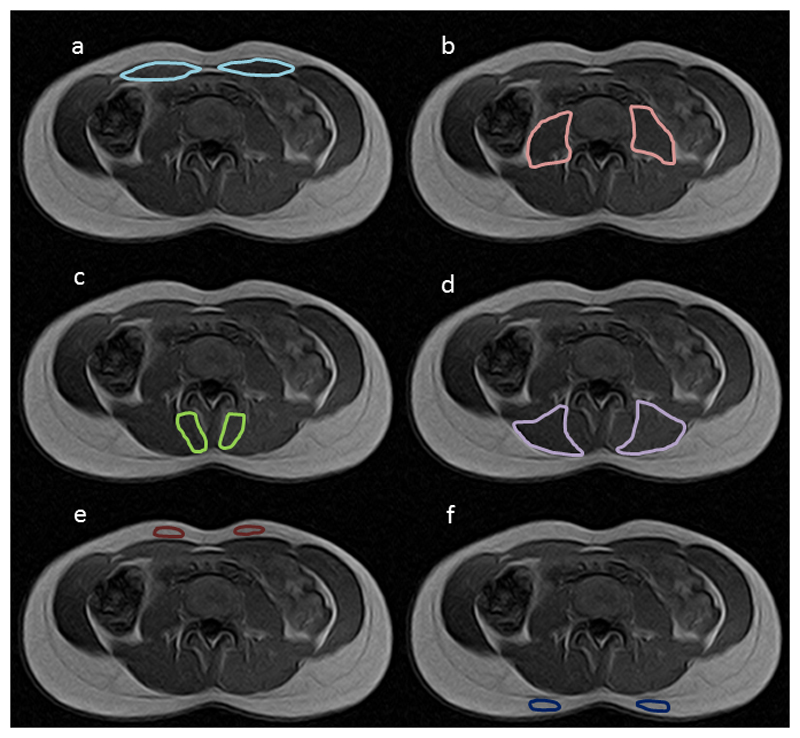
Analyze software (Mayo Clinic, Rochester MN, Version 11.0) was used for data analysis. Axial slices from the most caudal aspect of the fifth lumbar vertebral body (L5) to the most cranial aspect of the first lumbar vertebral body (L1) were included for each participant. These respective slices were identified based on published CT and MRI axial images [22]. The left and right sides of the erector spinae (ES), multifidus (M), rectus abdominis (RA), and psoas (PS) muscles of the lumbar spine were manually traced and the Region of Interest (ROI) quantified, thus providing a CSA for each axial image (Fig 1). As CSA is really a volumetric measure due to it containing partial volumes [23,24] and because volumetric measures are more meaningful functionally [21], all axial images CSAs were interpolated to obtain 3-dimensional volume across L1-L5 for each muscle. For MFI, mean pixel intensity (MPI) from the left and right sides of each muscle across all included slices was reported as a percentage of MPI of an area of abdominal or back fat from the left or right side of the body, taken from an axial slice located between L4 and L5. RA fat was determined relative to abdominal fat and ES, M and PS were determined relative to back fat (Fig 1). All data analysis was conducted by one assessor and muscle volume and fat measures calculated twice from the same scans to allow intra-rater reliability to be reported. For the second assessment after an interval of one month, the assessor was blinded to the outcomes of the first assessment. Due to the variation in height of the participants, the number of axial slices included in the data set varied from 14 to 18.
Figure 1.
Example of Region of Interest (ROI) trace in one axial slice showing (a) rectus abdominis (b) psoas (c) multifidus (d) erector spinae (e) abdominal fat and (f) back fat
Statistical Analysis
Statistical analysis was performed using SPSS (IBM, version 19). Normal distribution of muscle volume and MFI data were verified using the Shapiro-Wilk test. An independent t-test was used to identify statistical differences between groups for the participant characteristics of height, weight, BMI and hours of weekly exercise. Intra-rater reliability was evaluated using an intra-class correlation coefficient (3,1). A paired t-test was used to determine differences between left and right muscle outcomes within each group. On the combined groups data set, multiple linear regression was performed to investigate the effects of age, height, weight and BMI on muscle volume and MFI for each muscle using the enter method. After inspection of the variance inflation factors (VIF), the variable weight was removed in each model as the VIFs had exceeded acceptable levels and the variable weight had the highest VIF. Upon removal of this variable, VIFs were within an acceptable range. Non-significant variables were subsequently removed and the regression model re-calculated with the remaining significant variables.
Results
Participant characteristics for the young and mature group are shown in table 1. In both groups, 11 out of 12 participants were right handed and right footed, and one participant in each group was left handed and left footed. Intra-class correlations of muscle volume and fat percentage were high (range 0.882- 0.996) for all muscles, indicating excellent intra-rater reliability (table 2). No significant side-differences were found in the young group however in the mature group, RA and ES were significantly reduced on the right side compared to the left side (RA p=0.049; ES p=0.03). In both groups, PS, M, and ES MFI was significantly higher on the left side, however MFI was significantly smaller on the left side in RA (all p<0.001) (Table 3). The results of the final multiple regression models are shown in table 4. This shows that for muscle volume, height was a significant factor in all muscles investigated (all p<0.001). Age was a significant factor for M muscle volume and M and ES right MFI (all p<0.05). The factor BMI was significant for M and ES (all p<0.05).
Table 1.
Participant characteristics for age, height, weight, Body Mass Index (BMI) and weekly exercise of the young and mature group, shown as mean and standard deviation (±). Significant differences are indicated by * and non-significance by ns.
| Young group (n=12) | Mature group (n=12) | Significance | |
|---|---|---|---|
| Age (years) | 22.4 (± 1.24)* | 50.2 (± 4.95) * | p=0.001 |
| Height (m) | 1.75 (± 0.14) | 1.73 (± 0.11) | ns |
| Weight (kg) | 67.5 (± 16.6) | 70.2 (± 10.9) | ns |
| BMI (kg/m2) | 21.7 (± 2.2)* | 23.4 (± 1.3)* | p=0.026 |
| Hours of exercise per week | 3.13 (±1.71) | 5.00 (±3.69) | ns |
Table 2.
Intra-class correlation (ICC) of one assessor with two data analysis time points for muscle volume and Muscle Fatty Infiltrate (MFI). RA = rectus abdominis, PS = poas major, M= Multifidus, ES = erector spinae. 95% Confidence Intervals (CI) are shown.
| muscle volume | MFI | ||||||
|---|---|---|---|---|---|---|---|
| ICC | 95% CI | ICC | 95% CI | ||||
| lower | upper | lower | upper | ||||
| RA | left | 0.986 | 0.969 | 0.994 | 0.946 | 0.875 | 0.977 |
| right | 0.989 | 0.976 | 0.995 | 0.967 | 0.925 | 0.986 | |
| PS | left | 0.993 | 0.984 | 0.997 | 0.944 | 0.870 | 0.976 |
| right | 0.996 | 0.991 | 0.998 | 0.948 | 0.871 | 0.978 | |
| M | left | 0.882 | 0.232 | 0.965 | 0.918 | 0.813 | 0.964 |
| right | 0.866 | 0.467 | 0.953 | 0.962 | 0.912 | 0.983 | |
| ES | left | 0.991 | 0.977 | 0.996 | 0.927 | 0.830 | 0.968 |
| right | 0.991 | 0.980 | 0.996 | 0.952 | 0.787 | 0.984 | |
Table 3.
Mean and standard deviation of muscle volume and Muscle Fatty Infiltrate (MFI) values for left and right side rectus abdominis (RA), psoas (PS), multifidus (M) and erector spinae (ES) in the young and mature group. Pairs of matching superscripts indicate within-group left and right significant differences (a,b p<0.05, c–j p<0.001).
| Muscle | Volume (cm3) | MFI (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| young | mature | young | mature | ||||||
| mean | stdev | mean | stdev | mean | stdev | mean | stdev | ||
| RA | left | 13.78 | 3.85 | 12.43a | 2.87 | 37.88c | 3.59 | 39.35g | 2.21 |
| right | 13.39 | 4.05 | 11.70a | 2.25 | 42.99c | 4.51 | 43.64g | 3.11 | |
| PS | left | 16.67 | 6.35 | 14.47 | 5.38 | 39.62d | 3.71 | 41.25h | 3.89 |
| right | 15.82 | 5.33 | 14.11 | 5.10 | 31.87d | 2.90 | 32.91h | 1.84 | |
| M | left | 10.89 | 3.53 | 9.97 | 1.68 | 44.63e | 3.41 | 47.30i | 4.00 |
| right | 11.00 | 3.32 | 10.25 | 1.57 | 36.79e | 3.83 | 40.41i | 3.73 | |
| ES | left | 29.00 | 10.03 | 29.17b | 6.76 | 44.65f | 3.33 | 45.52j | 3.59 |
| right | 28.24 | 9.41 | 28.19b | 6.51 | 34.52f | 2.80 | 37.99j | 1.92 | |
Table 4.
Significant factors, their coefficients and confidence intervals for volume and muscle fatty infiltrate (MFI) of the muscles rectus abdominis (RA), psoas (PS), multifidus (M) and erector spinae (ES). The variance explained by the factors in each model is reflected in R2 and the standardised beta (ß) indicates the relative weighting of each factor.
| Muscle | Model | Significant factors | ||||
|---|---|---|---|---|---|---|
| Signf (p-value) | R2 | Name | Coefficient (Confidence Interval) |
Signf (p-value) | Standardised ß | |
| Muscle volume | ||||||
| RA left | <0.001 | 0.663 | height | 219523.70 (150399.36 to 288648.05) |
<0.001 | 0.815 |
| RA right | <0.001 | 0.581 | height | 200880.28 (125525.96 to 276234.61) |
<0.001 | 0.763 |
| PS left | <0.001 | 0.712 | height | 393023.15 (282606.60 to 503439.70) |
<0.001 | 0.844 |
| PS right | <0.001 | 0.689 | height | 341160.46 (239777.85 to 442543.07) |
<0.001 | 0.830 |
| M left | <0.001 | 0.846 | age | -540.78 (-962.08 to -119.48) |
0.014 | -0.288 |
| height | 142598.06 (91117.28 to 194078.84) |
<0.001 | 0.654 | |||
| BMI | 5433.52 (1695.15 to 9171.88) |
0.007 | 0.386 | |||
| M right | <0.001 | 0.817 | age | -450.34 (-880.39 to -20.29) |
0.041 | -0.256 |
| height | 134014.02 (81464.20 to 186563.83) |
<0.001 | 0.657 | |||
| BMI | 4795.34 (979.34 to 8611.33) |
0.016 | 0.364 | |||
| ES left | <0.001 | 0.828 | height | 480298.29 (331899.05 to 628697.53) |
<0.001 | 0.723 |
| BMI | 12327.61 (2748.83 to 21906.39) |
0.014 | 0.287 | |||
| ES right | <0.001 | 0.816 | height | 465168.68 (320286.89 to 610050.48) |
<0.001 | 0.741 |
| BMI | 10328.94 (977.20 to 19680.68) |
0.032 | 0.255 | |||
| MFI | ||||||
| RA left | 0.467 | |||||
| RA right | 0.260 | |||||
| PS left | 0.140 | |||||
| PS right | 0.573 | |||||
| M left | 0.090 | |||||
| M right | 0.038 | 0.181 | age | 0.12 (0.01 to 0.23) | 0.038 | 0.425 |
| ES left | 0.066 | |||||
| ES right | 0.002 | 0.360 | age | 0.12 (0.05 to 0.19) | 0.002 | 0.600 |
Discussion
In the mature group, but not in the young group, significant differences were observed between the left and right sides of RA and ES muscle volume in the present study. As the vast majority of the participants in the present study were right hand and right foot dominant, left and right sides were compared rather than dominant and non-dominant sides. The RA findings from the young group are in agreement with other studies [25,26]. Idoate et al [26] compared dominant and non-dominant side RA volume using MRI in non-active healthy controls and in Spanish first division soccer players of similar ages (control group 27.5 ± 8.1 years, soccer group 26.2 ± 5.2 years). Their results showed that, although CSA asymmetries occurred at certain muscle levels between the groups, overall dominant and non-dominant sides of RA muscle volume was not significantly different in either group. Kubo et al [25] did not find a dominance asymmetry in RA or ES CSA in young amateur (age 16.8 ± 0.6 years) and young professional Japanese soccer players (age 23.7 ± 3.1 years). No studies were identified which reported RA symmetry values within a group of mature participants, as only averaged left and right side RA CSA has been reported in studies including mature adults [5,27]. In contrast to the present study, Fortin et al [6] did not find age to significantly affect CSA of ES at the levels of L3-L4 and L5-S1 in a cross-sectional study. However, in a longitudinal study by those same authors, age was found to be significant for ES asymmetry but at L5-S1 only, although there was no side-indication [8]. Therefore, the present study reports for the first time differences in RA in mature healthy adults. This may reflect an age-related adaptation in muscle function and structure, and this may have implications for investigating the efficacy of targeted exercise programs in older patients with LBP.
In patients with LBP, M atrophy as identified using MRI has been shown to be side-and level specific to pain source or pathology, with a CSA decrease at the symptomatic side ranging from 2 to 62%, which was positively associated with duration of symptoms [3]. Further, 78% of patients in a LBP subgroup with lumbosacral radiculopathy showed multifidus asymmetry on MRI, compared to only 10% in an asymptomatic control group [28]. An asymmetry greater than 10% between left and right sides in lumbar multifidus has been suggested as a possible indicator of dysfunction or pathology, based on ultrasound images [10]. However, one study identified a multifidus asymmetry on MRI greater than the suggested 10% in more than 40% of their study population of 126 healthy males with a mean age of 49.8 ± 7.7 years [20]. None of the participants in that study reported experiencing low back pain in the 12 months prior to study participation. In addition, no muscle asymmetry was found between those reporting never having back pain greater than 1 day in duration, compared to those reporting back pain in the 12 months prior to study participation. Therefore, in the present study, muscle volume and MFI were reported separately for left and right sides, in order to further explore the potential of muscle asymmetry in healthy participants.
In the present study, there was a lack of significant differences between left and right side M muscle volume in the young or the mature group. This is in contrast to the results of other studies. Niemenläinen et al [20] showed a significantly larger M muscle volume on the right side with MRI in up to 68% of participants without LBP. Further, ES CSA asymmetry ranged from of 8.2% to 18.8% from L3 to L5 respectively, which was considerably greater than the significant difference in ES volume in the present study as it was reduced by 3% in the left side compared to the right side. For the PS muscle, left and right asymmetries have been identified in healthy individuals without LBP, with left side PS CSA being significantly greater than the right side by 5% [29]. The present study also demonstrated a larger muscle size on the left side of PS compared to the right side, although this increase was not statistically significant and the clinical meaningfulness of such asymmetry is unknown at this time.
In the present study, left and right M volume were significantly influenced by age, whereas ES was not. This is in contrast to other studies, which showed ES volume to be moderately negatively correlated with age (r=0.53) in females (mean age 44 ± 11 years, range 23-59) without back pain on MRI [7]. The volumes of each of the two flexor muscles investigated in the present study were also not significantly affected by age. This is in agreement with Rankin et al [30], who also did not find a correlation between RA thickness and age using ultrasound imaging in their investigation of thickness of various abdominal muscles in 123 healthy male and female participants. In contrast, several other studies have shown significant reductions in muscle volume associated with ageing. Takahashi et al [4] showed a significant reduction in PS CSA with MRI in ageing in females. In their study, participants were grouped according to age by decade (age range of 20 to 79) and their results demonstrated that participants in their 20s had the greatest psoas major CSA, which significantly reduced in people in their 50s and was lowest in people in their 70s. In the present study, the mature group included an equal number of males and females with mean age of 50.2 years (± 4.95). This may be as the mature group in the present study were too young for significant age-related changes, or because the present study sample included an equal number of males and females, whereas Takahashi et al [4] and Meakin et al [7] included female participants only.
In the present study, MFI was significantly greater in the left side when compared to the right side of PS, M and ES for both the young and mature group, and significantly greater in the right side compared to the left side of RA. Fortin and colleagues [6] also reported asymmetries in muscle composition in the lumbar extensors in a large group of subjects with and without LBP. These asymmetries were more often observed at L5-S1 and were significantly affected by handedness, by lower sport participation and by an increase in LBP severity for ES and by handedness and disc narrowing for M. At the level of L3-L4, handedness was the only significant factor for both these muscles. Interestingly, this study did not show a significant effect for age or BMI on muscle composition (i.e. MFI) for any muscle at any level. In the present study, age was found to significantly affect M and ES although this was applicable only to the right side. As the study by Fortin et al [6] did not identify MFI per side but calculated the difference in muscle composition asymmetry, it is not clear whether side-specific differences would have been present. Furthermore, differences in MRI sequencing (T1 vs T2) and differences in the method of MFI determination (MFI vs ratio of lean muscle CSA to total CSA) between the present study and the studies by Fortin and colleagues [6,8] may also explain the disparity in study outcomes.
As BMI can influence muscle composition [2], the BMI of study participants has been reported in some studies investigating lumbar spine muscle morphology, with the majority reporting a mean or range of BMI indicating a mix of normal weight and overweight participants [6,8,12,13,31,32]. However, Kjaer et al [13] did not find an effect of BMI on M MFI, and similarly, Fortin et al [6] also did not find BMI to influence asymmetries in muscle composition or size. In a longitudinal study however, a significant effect of BMI on ES change in CSA over time, but not for changes in muscle composition exists [8]. The findings from the present study also support that BMI does not influence MFI in the lumbar spinal muscles, although it was a significant factor for muscle volume in the extensor muscle group, but not the flexor muscles. However, our study sample only included participants of normal weight, which may not be representative of the average population, in particular in the mature group and differs from study samples in other investigations.
With regard to the actual fat percentage values obtained in the present study, our MFI values were higher than those found in other studies. In illustration, studies involving participants with LBP have reported MFI in healthy control participants [12,31], where the mean M MFI, measured with MR spectroscopy, was 14.5% in control subjects [12] and ranged from 2.1 – 11.5% in healthy controls without swayback postures using conventional T2-weighted MRI [31]. These differences in reported values are likely due to methodological differences, warranting future work comparing muscle volume/MFI on MRI to the gold-standard muscle biopsy with histological quantification of muscle fat.
In this study, the results should be interpreted considering some methodological constraints and limitations. This study includes self-reported healthy individuals without any LBP at the time or within the last 12 months prior to study inclusion. Due to the high incidence of lifetime reported LBP with point prevalence as high as 58% [33], the identification of study participants who have never experienced LBP, particularly in mature individuals, may be limited. Regarding LBP history, criteria for the inclusion of asymptomatic participants vary between studies, ranging from no history of LBP [34], no LBP of more than one day in duration [10], to no reports of activity-limiting LBP [29]. Therefore, although the healthy participants included in the present study did not report any LBP at the time of measurement or 12 months prior, they should still nevertheless be considered as ‘functional and asymptomatic controls’.
Another methodological constraint of the present study may be the inclusion of only one assessor for the evaluation of the muscle parameters. A measure of inter-rater reliability has been performed on a subset of the data presented in this study (manuscript currently under review), which showed good to excellent reliability between assessor. Similarly, in the cervical spine, Elliott et al [35] also showed excellent inter-rater reliability of multifidus diffusion properties at C5 using diffusion weighted MRI, and Kilgour et al [36] showed a high inter-reliability for the quantification of cervical spine muscles CSA on T1 weighted MRI. In the lumbar spine, a good inter-rater reliability for the quantification of multifidus thickness has been shown even in ultrasound measurements [37]. Therefore, we established an intra-rater reliability metric for the quantification of muscle volume and MFI in this study, which showed excellent reliability outcomes for volume and fat percentage of all muscles investigated.
A number of imaging assessment methods are available to gain an appreciation of skeletal muscle size/shape at the micro- and macro-scopic levels, including chemical shift MRI [34], opposed phase MRI (multi-echo fat/water separation) [14, 38], and proton-density fat fraction MRI [39], therefore the method of MFI evaluation used in the present study may not be considered a gold-standard method. However, T1 weighted MRIs are frequently and routinely obtained in the clinical assessment of patients with LBP, therefore the approach used in this study may be a clinically and economically viable method for muscle volume and MFI quantification.
Future studies should consider the inclusion of additional age groups with larger participant numbers, for a more detailed definition of expected age-related changes in lumbar spine muscle size, shape and structure. Additionally, participants with different activity types and intensities should also be further investigated. This information could then build a ‘muscle profile’ of representative data for certain age groups and activity levels, which could serve as comparative data for LBP with respect to gender, age and activity. The use of longitudinal study designs would provide even more definitive information regarding any temporal changes in muscle volume and MFI in healthy and symptomatic individuals.
Conclusion
This study showed an age effect on the differences between the left and right sides of muscle volume. Age was found to significantly affect multifidus muscle volume only and right-sided extensor MFI only. This would imply that the effect of age cannot be generalized across all muscles. The results of this study should be used to inform future studies evaluating changes in muscle morphology.
Highlights.
Side-differences in muscle volume were found only in the mature group.
Height significantly increases volume of all muscles investigated.
Muscle volume significantly increased with BMI in the extensors only.
Multifidus muscle volume decreased with age.
With age, but not with BMI, fat increased in the right extensor muscles.
References
- [1].Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. Journal of Applied Physiology. 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- [2].Sions JM, Tyrell CM, Knarr BA, Jancosko A, Binder-Macleod SA. Age- and stroke-related skeletal muscle changes: A review for the geriatric clinician. Journal of Geriatric Physical Therapy. 2012;35:155–61. doi: 10.1519/JPT.0b013e318236db92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barker KL, Shamley DR, Jackson D. Changes in the cross-sectional area of multifidus and psoas in patients with unilateral back pain. Spine. 2004;29:E515–519. doi: 10.1097/01.brs.0000144405.11661.eb. [DOI] [PubMed] [Google Scholar]
- [4].Takahashi K, Takahashi HE, Nakadaira H, Yamamoto M. Different changes of quantity due to aging in the psoas major and quadriceps femoris muscles in women. Journal of Musculoskeletal and Neuronal Interactions. 2006;6:201–205. [PubMed] [Google Scholar]
- [5].Anderson DE, D’Agostino JM, Bruno AG, Manoharan RK, Bouxsein ML. Regressions for estimating muscle parameters in the thoracic and lumbar trunk for use in musculoskeletal modelling. Journal of Biomechanics. 2012;45:66–75. doi: 10.1016/j.jbiomech.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fortin M, Yuan Y, Battié Factors associated with paraspinal muscle asymmetry in size and composition in a general population sample of men. Physical Therapy. 2013;93:1540–1550. doi: 10.2522/ptj.20130051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Meakin JR, Fulford J, Seymour R, Welsman JR, Knapp KM. The relationship between sagittal curvature and extensor muscle volume in the lumbar spine. Journal of Anatomy. 2013;222:608–614. doi: 10.1111/joa.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fortin M, Videman T, Gibbons LE, Battié MC. Paraspinal muscle morphology and composition: A 15-yr longitudinal magnetic resonance imaging study. Medicine & Science in Sports & Exercise. 2014;46:893–901. doi: 10.1249/MSS.0000000000000179. [DOI] [PubMed] [Google Scholar]
- [9].Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, De Cuyper HJ. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. European Spine Journal. 2000;9:266–272. doi: 10.1007/s005860000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hides J, Gilmore C, Stanton W, Bohlscheid E. Multifidus size and symmetry among chronic LBP and healthy asymptomatic subjects. Manual Therapy. 2008;13:43–49. doi: 10.1016/j.math.2006.07.017. [DOI] [PubMed] [Google Scholar]
- [11].Fortin M, Macedo LG. Multifidus and paraspinal muscle group cross-sectional areas of patients with low back pain and control patients: A systematic review with a focus on blinding. Physical Therapy. 2013;7:873–888. doi: 10.2522/ptj.20120457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mengiardi B, Schmid MR, Boos N, Pfirrman CWA, Brunner F, Elfering A, Hodler J. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers: Quantification with MR spectroscopy. Radiology. 2006;240:786–792. doi: 10.1148/radiol.2403050820. [DOI] [PubMed] [Google Scholar]
- [13].Kjaer P, Bendix T, Sorensen JS, Korsholm L, Leboeuf-Yde C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Medicine. 2007;5:2. doi: 10.1186/1741-7015-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Paalanne N, Niinimäki J, Karppinen J, Taimela S, Mutanen P, Takatalo J, Korpelainen R, Tervonen O. Assessment of association between low back pain and paraspinal muscle atrophy using opposed-phase magnetic resonance imaging – A population-based study among young adults. Spine. 2011;36:1961–1968. doi: 10.1097/BRS.0b013e3181fef890. [DOI] [PubMed] [Google Scholar]
- [15].Elliott J, Jull G, Noteboom JT, Galloway G. MRI study of the cross-sectional area for the cervical extensor musculature in patients with persistent whiplash associated disorders (WAD) Manual Therapy. 2008;13:258–265. doi: 10.1016/j.math.2007.01.012. [DOI] [PubMed] [Google Scholar]
- [16].Elliott JM, O’Leary S, Sterling M, Hendrikz J, Pedler A, Jull G. Magnetic Resonance Imaging Findings of Fatty Infiltrate in the Cervical Flexors in Chronic Whiplash. Spine. 2010;35:948–954. doi: 10.1097/BRS.0b013e3181bb0e55. [DOI] [PubMed] [Google Scholar]
- [17].Matsumoto M, Ichihara D, Okada E, Chiba K, Toyama Y, Fujiwara H, Momoshima S, Nischiwaki Y, Takahata T. Cross-sectional area of the posterior extensor muscles of the cervical spine in whiplash injury patients versus healthy volunteers - 10year follow-up MR study. Injury. 2012;43:912–6. doi: 10.1016/j.injury.2012.01.017. [DOI] [PubMed] [Google Scholar]
- [18].Ulbrich E, Aeberhard R, Wetli S, Busato A, Boesch C, Zimmerman H, Hodler J, Anderson SE, Sturzenegger M. Cervical Muscle Area Measurements in Whiplash Patients: Acute, 3, and 6 Months of Follow-up. Journal of Magnetic Resonance Imaging. 2012;36:1413–1420. doi: 10.1002/jmri.23769. [DOI] [PubMed] [Google Scholar]
- [19].Elliott JM, Galloway GJ, Jull GA, Noteboom JT, Centeno CJ, Gibbon WW. Magnetic resonance imaging analysis of the upper cervical spine extensor musculature in an asympomatic cohort: an index of fat within muscle. Clinical Radiology. 2005;60:355–363. doi: 10.1016/j.crad.2004.08.013. [DOI] [PubMed] [Google Scholar]
- [20].Niemeläinen R, Briand MM, Battié MC. Substantial asymmetry in paraspinal muscle cross-sectional area in healthy adults questions its value as a marker of low back pain and pathology. Spine. 2011;36:2152–2157. doi: 10.1097/BRS.0b013e318204b05a. [DOI] [PubMed] [Google Scholar]
- [21].Boom HPW, van Spronsen PH, van Ginkel FC, van Schindel RA, Castelijns JA, Tuinzing DB. A comparison of human jaw muscle cross-sectional area and volume in long- anf short-face subjects, using MRI. Archives of Oral Biology. 2008;53:273–281. doi: 10.1016/j.archoralbio.2007.08.013. [DOI] [PubMed] [Google Scholar]
- [22].Möller TB, Reif E. Taschenatlas der Schnittbildanatomie Band II: Thorax, Herz, Abdomen, Becken. Stuttgart: Georg Thieme Verlag; 2011. pp. 124–140. [Google Scholar]
- [23].Elliott JM, Kerry R, Flynn T, Parrish TB. Content not quantity is a better measure of muscle degeneration in whiplash. Manual Therapy. 2013;18(6):578–582. doi: 10.1016/j.math.2013.02.002. [DOI] [PubMed] [Google Scholar]
- [24].Elliott JM, Pedler AR, Jull GA, Van Wyk L, Galloway GG, O’Leary SP. Differential changes in muscle composition exist in traumatic and nontraumatic neck pain. Spine. 2014;39:39–47. doi: 10.1097/BRS.0000000000000033. [DOI] [PubMed] [Google Scholar]
- [25].Kubo T, Muramatsu M, Hoshikawa Y, Kanehisa H. Profiles of trunk and thigh muscularity in youth and professional soccer players. Journal of Strength & Conditioning Research. 2010;24:1472–1479. doi: 10.1519/JSC.0b013e3181d32eb1. [DOI] [PubMed] [Google Scholar]
- [26].Idoate F, Calbet JAL, Izquierdo M, Sanchis-Moysi J. Soccer attenuates the asymmetry of rectus abdominis muscle observed in non-athletes. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0019022. art.no.e19022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Asaka M, Usui C, Ohta M, Takai Y, Fukunaga T, Higuchi M. Elderly oarsmen have larger trunk and thigh muscles and greater strength than age-matched untrained men. European Journal of Applied Physiology. 2010;108:1239–1245. doi: 10.1007/s00421-009-1337-6. [DOI] [PubMed] [Google Scholar]
- [28].Hyun JK, Lee JY, Lee SJ, Jeon JY. Asymmetric atrophy of multifidus muscle in patients with unilateral lumbosacral radiculopathy. Spine. 2007;32:E598–E602. doi: 10.1097/BRS.0b013e318155837b. [DOI] [PubMed] [Google Scholar]
- [29].Marras WS, Jorgensen MJ, Granata KP, Wiand B. Female and male trunk geometry: size and prediction of the spine loading trunk muscles derived from MRI. Clinical Biomechanics. 2001;16:38–46. doi: 10.1016/s0268-0033(00)00046-2. [DOI] [PubMed] [Google Scholar]
- [30].Rankin G, Stokes M, Newham DJ. Abdominal muscle size and symmetry in normal subjects. Muscle & Nerve. 2006;34:320–326. doi: 10.1002/mus.20589. [DOI] [PubMed] [Google Scholar]
- [31].Pezolato A, de Vasconcelos EE, Defino HLAD, Nogueira-Barbosa MH. Fat infiltration in the lumbar multifidus and erector spinae muscles in subjects with sway-back posture. European Spine Journal. 2012;21:2158–2164. doi: 10.1007/s00586-012-2286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Arbanas J, Pavlovic I, Marijancic V, Vlahovic H, Stracevic-Klasan G, Peharex S, Bajek S, Miletic D, Malnar D. MRI features of the psoas major muscle in patients with low back pain. European Spine Journal. 2013;22(9):1965–1971. doi: 10.1007/s00586-013-2749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hoy D, Brooks P, Blyth F, Buchbinder R. The Epidemiology of low back pain. Best Practice & Research Clinical Rheumatology. 2010;24:769–781. doi: 10.1016/j.berh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- [34].Yanik B, Keyik B, Conkbayir I. Fatty degeneration of multifidus muscle in patients with chronic low back pain and in asymptomatic volunteers: quantification with chemical shift magnetic resonance imaging. Skeletal Radiology. 2012;42:771–778. doi: 10.1007/s00256-012-1545-8. [DOI] [PubMed] [Google Scholar]
- [35].Elliott JM, Pedler A, Beattie P, McMahon K. Diffusion-Weighted Magnetic Resonance Imaging for the Healthy Cervical Multifidus: A Potential Method for Studying Neck Muscle Physiology Following Spinal Trauma. Journal of Orthopaedic & Sports and Physical Therapy. 2010;40:722–728. doi: 10.2519/jospt.2010.3423. [DOI] [PubMed] [Google Scholar]
- [36].Kilgour AHM, Subedi D, Gray CD, Deary IJ, Lawrie SM, Wardlaw JM, Starr JM. Design and validation of a novel method to measure cross-sectional area of neck muscles included during routine MR brain volume imaging. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0034444. art.no.e34444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wallwork T, Hides JA, Stanton WR. Intrarater and interrater reliability of assessment of lumbar multifidus muscle thickness using rehabilitative ultrasound imaging. Journal of Orthopaedic & Sports and Physical Therapy. 2007;37:608–612. doi: 10.2519/jospt.2007.2418. [DOI] [PubMed] [Google Scholar]
- [38].Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153:189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- [39].Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: A standardized mr-based biomarker of tissue fat concentration. Journal of Magnetic Resonance Imaging. 2012;36:1011–1014. doi: 10.1002/jmri.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]